Summary
The amyloid precursor family of proteins are of considerable interest both because of their role in Alzheimer’s disease pathogenesis and because of their normal physiological functions. In mammals, the amyloid precursor protein (APP) has two homologs, amyloid precursor-like protein 1 and amyloid precursor-like protein 2. All 3 proteins undergo ectodomain shedding and regulated intramembrane proteolysis, and important functions have been impunged to the full-length proteins, shed ectodomains, C-terminal fragments and intra-cellular domains (ICDs). One of the proteases known to cleave APP and which is essential for generation of the amyloid β-protein is the β-site APP cleaving enzyme 1 (BACE1). Here we investigated the effects of genetic manipulation of BACE1 on the processing of the APP family of proteins. BACE1 expression regulated the levels and species of full-length APLP1, APP and APLP2, of their shed ectodomains and membrane-bound C-terminal fragments. In particular, APP processing appears to be tightly regulated, with changes in APPsβ being compensated with changes in APPsα. In contrast, the total levels of soluble cleaved APLP1 and APLP2 species were less tightly regulated and fluctuated depending on BACE1 expression. Importantly, the production of ICDs for all three proteins was not decreased by loss of BACE1 activity. These results indicate that BACE1 is involved in regulating ectodomain shedding, maturation and trafficking of the APP family of proteins. Consequently, while inhibition of BACE1 is unlikely to adversely affect potential ICD-mediated signalling it may alter other important facets of APLP/APP biology.
Keywords: APP, APLP1, APLP2, BACE1, Alzheimer’s disease
Introduction
Genetic evidence indicates that the amyloid precursor protein (APP) is centrally involved in Alzheimer’s disease (AD) pathogenesis [1] but it also appears to have important physiological functions. APP is part of an evolutionary conserved family of type-I transmembrane glycoproteins [2], which includes the mammalian homologs amyloid precursor-like protein 1 (APLP1) [3] and 2 (APLP2) [4]. These three proteins share a considerable degree of sequence and domain similarity [5, 6]. Both APP and APLP2 are expressed in a variety of tissues and cell types [4, 7], whereas APLP1 expression is neuron-specific [8]. The APP family of proteins are believed to play important roles in both the peripheral and central nervous system [6]. In the former they are involved in the formation and correct functioning of the neuromuscular junction [9], while in the latter they have been implicated in neurite outgrowth [10], synaptogenesis [11] and neuronal migration during embryogenesis [12]. Knock-out (KO) studies indicate a high degree of functional redundancy between APP, APLP1 and APLP2 [13], with only subtle defects observed in animals with ablation of one member [14]. In contrast, APP/APLP2 and APLP1/APLP2 double KO mice die soon after birth [14] and mice lacking all three proteins die in utero [13]. Surprisingly, APP/APLP1 double KO mice are viable and healthy, thus indicating that APLP2 possesses some functions that cannot be compensated for by APP and APLP1 [13]. There is also considerable evidence that the APP family of proteins have a role in cell-cell and cell-matrix adhesion, and that they can form both cis and trans homo- and hetero-dimers [15, 16]. In addition, the APP family of proteins can interact with a variety of cellular proteins that regulate APP, APLP1 and APLP2 processing. The majority of APP molecules are cleaved at the cell/luminal-surface by α-secretase resulting in the shedding of its ectodomain (APPsα) [17, 18]. α-secretase cleavage is mediated by at least three enzymes, all of which are members of the ADAM (a disintegrin and metalloprotease) family [19]. A smaller fraction of APP molecules are proteolysed by β-secretase in endosomes or at the plasma membrane [20]. The β-secretase activity is attributed to a single protease, BACE1 [21, 22]. BACE1 is an aspartyl protease and an atypical member of the pepsin family [21], and is also referred to as memapsin-2 [23] or Asp-2 [24]. The expression and activity of BACE1 is regulated at multiple levels (reviewed in [25]), including mRNA transcription, mRNA stability, glycosylation, proteolytic maturation, palmitoylation, and by its cellular localization.
Initial reports describing BACE1 KO mice failed to reveal significant defects [22, 26], however recent studies have demonstrated that deletion of BACE1 results in impaired myelination [27, 28] and in the development of behavioural abnormalities reminiscent of Schizophrenia [29, 30]. Both effects have been attributed to the loss of BACE1 cleavage of the neurotrophic factor neuregulin-1 (NRG1). In addition to APP and NRG1, BACE1 has been shown to cleave the type II α-2,6-sialyltransferase [31], the P-selectin glycoprotein ligand-1 [32], the β2-subunit of sodium channels [33] and the interleukin-1 receptor type II [34]. However loss of BACE1 processing of these latter substrates has not yet been shown to have significant adverse consequences.
Like APP, both APLP1 and APLP2 undergo ectodomain shedding and their soluble ectodomains have been detected in the conditioned media of transfected cell lines and in human cerebrospinal fluid (CSF) [35–37]. While substantial data indicates that APLP2 is cleaved by both α- and β-secretases [38, 39], the enzymes involved in APLP1 ectodomain cleavage are less well defined [40, 41]. Irrespective of the identity of the enzymes involved, ectodomain shedding of APP, APLP1 and APLP2 results in the generation of membrane-bound C-terminal fragments (CTFs). These CTFs are further processed by γ-secretase, releasing intracellular domains (ICDs) [42, 43] postulated to be involved in transcriptional regulation [44, 45]. While the transcriptional properties of ICDs are contentious [45–48], there is consensus that the APP family of proteins may function as membrane anchors for a variety of proteins, and when CTFs are cleaved ICDs together with associated proteins are released from the membrane [49].
Here we investigated the effects of genetic manipulation of BACE1 on the processing of APP, APLP1 and APLP2 and on the production of their ICDs. We report that BACE1 knock-out and over-expression affects the steady state levels of full-length APLP1 and APLP2 in a manner similar to APP [50]. BACE1 expression also regulates the levels and species of the shed ectodomains and membrane-bound CTFs. In particular, APP processing appears to be tightly regulated, with the total levels of soluble APP remaining constant irrespective of the presence or absence of BACE1. The levels of APPsα increased to account for the loss of APPsβ in BACE1 KO mice and decreased when APPsβ levels increased due to BACE1 over-expression. In contrast, the total levels of soluble cleaved APLP1 and APLP2 species fluctuated depending on BACE1 expression. Importantly, we show that the production of ICDs for all three proteins is not decreased by a loss of BACE1 activity, indicating that BACE1 inhibition would not adversely affect ICDs production.
Results
BACE1 regulates APP, APLP1 and APLP2 ectodomain shedding and secretion of full-length APLP1
Using murine models of BACE1 over-expression (BACE1 transgenic, Tg) and deletion (BACE1 knock-out, KO) we set out to investigate the role of BACE1 on the processing of APLP1 and APLP2. To do this we employed an extraction procedure capable of separating water-soluble and membrane-associated proteins. First water-soluble parenchymal and cytosolic proteins were extracted in tris-buffered saline (TBS), then the membrane pellet was washed with sodium carbonate and proteins extracted using TBS containing 1% Triton X-100 (TBST). Secreted proteins were detected using ectodomain-specific antibodies while full-length (FL) proteins and C-terminal fragments (CTFs) were detected using antibodies that specifically recognize the C-termini of each protein. The specificity of antibodies for their cognate target proteins was confirmed using brains from APP, APLP1 and APLP2 KO mice (Fig. S1).
In TBS extracts of mouse brains, 22C11, a monoclonal antibody recognizing an epitope between amino acids 66 to 81 of APP (Fig. S1), specifically detected a single band at around 100 kDa in WT, BACE1 KO and BACE1 Tg samples, which roughly co-migrates with a strong band detected in lysates of human APP695 expressing cells and which was absent in the APP KO sample (Fig. 1A). When the same samples were western blotted with C8, an antibody specifically recognizing an epitope at the very C-terminus of APP (Fig. S1), a ~100 kDa band was detected only in the lysate of APP695 expressing cells (Fig. 1E). The fact that the ~100 kDa band detected in the TBS mouse brain extracts is revealed by the ectodomain-directed antibody 22C11 but not by the C-terminal specific antibody C8 indicates that this protein lacks an intact C-terminus and likely represents secreted forms of APP (APPs). The levels of total APPs were not significantly altered by either knock-out or over-expression of BACE1 (Fig. 1A–B). When the same samples were western blotted using a polyclonal antibody capable of detecting APPsα, but not APPsβ (Fig. S1), a single band of ~100 kDa was detected in WT, BACE1 KO and BACE1 Tg mice, but was absent in both the APP KO and in the cell lysate sample (Fig. 1 C). The lack of detection of full-length APP (FLAPP) in APP695 expressing cells is due to the fact that the epitope for the anti-rodent Aβ antibody is not present in human APP (Table 1), while the absence of this band in the APP KO extract confirms the specificity of this band as a true APPs species (Fig. 1C). The levels of this APPsα band were dramatically increased in BACE1 KO (+57.4 ± 3.1 %, P < 0.0001) and decreased in BACE1 Tg (−58.9 ± 1.6 %, P < 0.0001) (Fig. 1 C-D). Given that the total protein loaded for each extract were highly similar (Fig. 1F), and that total APPs levels were unchanged (Fig. 1A–B), these results imply a tight regulation of APP ectodomain shedding, with over-expression of BACE1 causing a compensatory decrease in APPsα and BACE1 ablation causing a compensatory increase in APPsα. These changes are unlikely to be a result of a difference in genetic background as a near identical pattern was seen when other BACE1 KO and BACE1 Tg mouse lines were examined (Fig. S3).
Fig. 1. Levels of total APPs are unaffected by changes in BACE1 expression whereas APPsα levels are dependent on BACE1 activity.
TBS homogenates of brains from wild type (WT), BACE1 knock-out (KO) and BACE1 transgenic (Tg) mice were electrophoresed on 10% tris-glycine polyacrylamide gels and western blotted with a panel of antibodies which allow detection of total APPs (22C11, A), APPsα (anti-Aβ rodent, C) and full-length and C-terminal fragments of APP (C8, E). Western blotting for GAPDH was included to check for equal protein loading (F). Lysates of a cell line over-expressing human wild type APP695 (+), were included as a positive control and TBS homogenates of brains from APP knock-out mice (−) were included as a negative control. The levels of total APPs and of APPsα (B and D respectively) were quantitated by densitometry and values normalized versus WT control are presented as averages ± standard error of duplicate measurements of three animals of each genotype.
Table 1. Antibodies recognizing the APP family of proteins.
Details about the specific target protein, epitope recognized, host, species specificities and source are provided for each antibody used (H = Human, M = Mouse). The amino acid (aa) numbering is for human sequences of APP695, APLP1650 and APLP2751. For anti-Aβ rodent antibody numbering is for the Aβ sequence.
| Antibody | Target | Antigen | Host | Species reactivity |
Source |
|---|---|---|---|---|---|
| 22C11 | APP | aa 66–81 | Mouse | H, M | Chemicon |
| anti-Rodent Aβ | APP | aa 3–16 Aβ | Rabbit | M | Signet |
| C8 | APP | aa 676–695 | Rabbit | H, M | Selkoe lab. |
| W1NT | APLP1 | aa 75–90 | Rabbit | H, M | Walsh lab. |
| W1CT | APLP1 | aa 640–650 | Rabbit | H, M | Walsh lab. |
| D2-II | APLP2 | FL | Rabbit | H, M | Calbiochem |
| W2CT | APLP2 | aa 740–751 | Rabbit | H, M | Walsh lab. |
Western blot analysis of TBS homogenates using W1NT, an antibody directed against the N-terminal domain of APLP1 (Fig. S1), revealed two specific bands in BACE1 KO, WT and BACE1 Tg samples which were not present in the APLP1 KO sample (Fig. 2A). The band migrating at ~94 kDa was present only in the BACE1 KO samples and migrated just below the band from lysates of cells over-expressing human APLP1650, an additional band, which migrated at ~83 kDa, was also present in WT and BACE1 KO samples (Fig. 2A). Moreover, when the same samples were analysed by western blotting with W1CT, a polyclonal antibody raised against the extreme C-terminus of APLP1 (Fig. S1) or a commercial antibody to the C-terminus of APLP1, 171615 (Calbiochem, EMD Biosciences, Merck KGaA, Darmstadt, Germany; not shown), a band migrating at ~94 kDa was detected in all BACE1 KO, WT and BACE1 Tg samples but not in APLP1 KO samples (Fig. 1C). Since the band migrating at ~94 kDa was recognized by antibodies directed to both the ectodomain and to the C-terminus, this band appears to be full-length APLP1. In contrast, the band migrating at ~83 kDa, which is recognized by W1NT and not by W1CT, is likely to be a secreted C-terminally truncated form of APLP1 (hereafter referred to as APLP1s). It is unusual for a transmembrane protein to be found in a detergent-free aqueous environment. One possible explanation for this behaviour may be that FLAPLP1 is present in membrane fractions such as exosomes or microvesicles which are not readily sedimented by centrifugation. Whatever the reason, the levels of APLP1s were dramatically reduced in BACE1 KO samples (−47.1 ± 5.4 %, P < 0.0001) and slightly increased by BACE1 over-expression (+11.4 ± 4.1 %, non significant) (Fig. 2A–B). Since W1NT cannot discriminate between APLP1s produced by α- or β-secretase, we can only assess effects on total APLP1s production. Accordingly, BACE1 seems to be required for the production of at least half of the total amount of APLP1s, since its deletion caused a ~50% decrease in APLP1s (Fig. 2A). Given that over-expression of BACE1 did not lead to a significant increase in the levels of APLP1s (Fig. 2A–B), it would appear that APLP1s production is tightly regulated by factors other than BACE1 expression. A feature of APLP1, unique among the members of the APP family, is its secretion as unprocessed FL protein (compare Fig. 1E, Fig. 2C and Fig. 3C). Moreover this property appears to be modulated by BACE1 as deletion of BACE1 caused a large increase in the levels of FLAPLP1 released (+251 ± 4.7 %, P < 0.0001), while BACE1 over-expression resulted in a sizeable reduction in FLAPLP1 release (−45.6 ± 2.8 %, P < 0.0001) (Fig. 2C–D). Thus expression of BACE1 regulates the release of FLAPLP1 and strongly influences the production of APLP1s. As with APP, these results are independent of genetic background and have been replicated in other BACE1 KO and Tg mice lines (Fig. S4).
Fig. 2. BACE1 deletion decreases the levels of secreted APLP1 (APLP1s) and increases the levels of FLAPLP1.
TBS homogenates of brains from wild type (WT), BACE1 knock-out (KO) and BACE1 transgenic (Tg) mice were electrophoresed on 10% tris-glycine polyacrylamide gels and western blotted with antibodies recognizing the N-terminus (W1NT, A) and C-terminus (W1CT, C) of APLP1. Western blotting for GAPDH was included to check for equal protein loading (E). Lysates of a cell line over-expressing human APLP1650 (+), are included as a positive control and TBS homogenates of brains from APLP1 knock-out mice (−) are included as a negative control. FLAPLP1 and APLP1s bands detected by W1NT are indicated by arrows in A. APLP1s and of FLAPLP1 levels (B and D respectively) were quantitated by densitometry and values normalized relative to WT control are presented as averages ± standard error of duplicate measurements of three animals of each genotype.
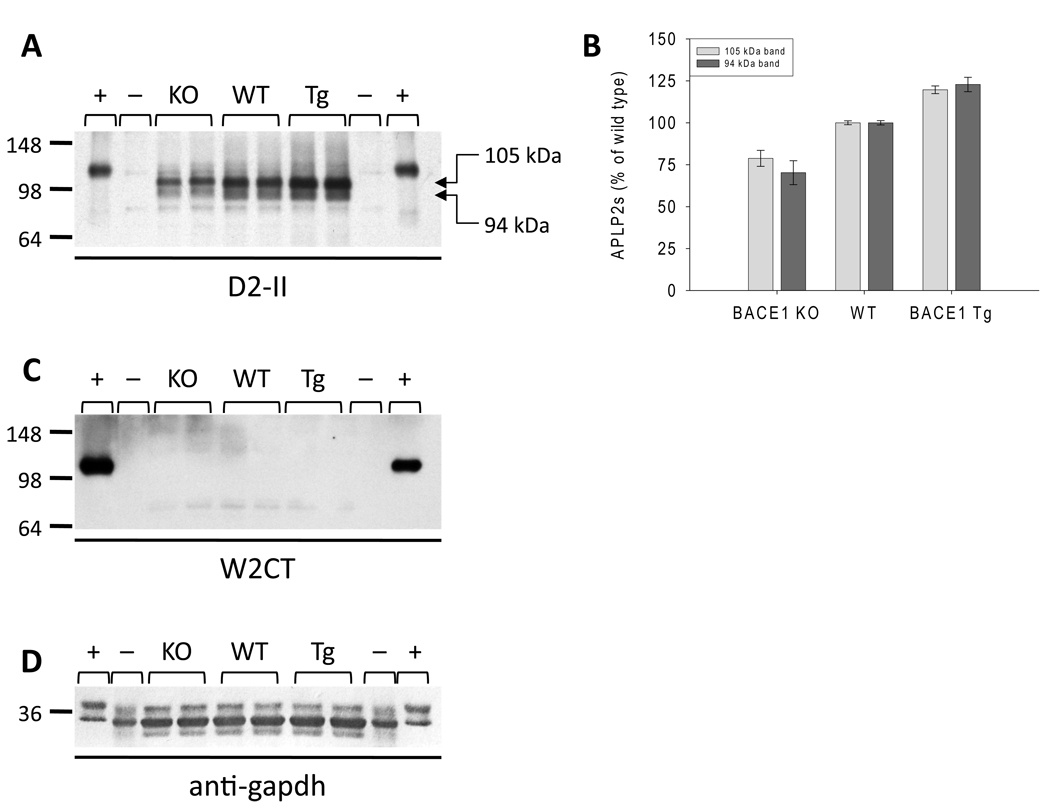
Fig. 3. BACE1 deletion decreases APLP2s levels, whereas BACE1 over-expression increases APLP2s levels.
TBS homogenates of brains from wild type (WT), BACE1 knock-out (KO) and BACE1 transgenic (Tg) mice were electrophoresed on 10% tris-glycine polyacrylamide gels and western blotted with antibodies recognizing either FLAPLP2 (D2-II, A) or the extreme C-terminus of APLP2 (W2CT, C). Western blotting for GAPDH was included to check for equal protein loading (D). Lysates of cell lines over-expressing human wild type APLP2751 (+), are included as positive control and TBS homogenates of brains from APLP2 knock-out mice (−) are included as negative control. APLP2s bands (indicated by arrows, A) were quantitated by densitometry and values normalized versus the WT control are presented as averages ± standard error of duplicate measurements of three animals of each genotype (B).
Western blot analysis of TBS homogenates using D2-II, an antibody raised against the FLAPLP2 protein (Fig. S1), identified two bands migrating at ~105 kDa and ~94 kDa in the WT, BACE1 KO and BACE1 Tg samples but not in APLP2 KO samples (Fig. 3A). Both bands migrated considerably faster than the band detected in the lysate of human APLP2751-expressing cells which migrated at ~111 kDa (Fig. 3A). Western blot analysis with W2CT detected the ~111 kDa band in the lysates of APLP2751-expressing cells, but did not detect any specific bands in TBS extracts of mouse brain (Fig. 3C). Together these data indicate that the ~94 and ~105 kDa bands detected by D2-II but not by W2CT likely represent soluble APLP2 (APLP2s). BACE1 deletion caused a decrease in the levels of both APLP2s species (105kDa: −21.2 ± 4.8 %, P < 0.0001; 94kDa: −29.8 ± 7.1 %, P < 0.0001) while BACE1 over-expression caused an increase (105kDa: +19.7 ± 2.3 %, P < 0.0005; 94kDa: +22.8 ± 4.3 %, P < 0.005) (Fig. 3A–B). As with APP and APLP1, these results were independent of genetic background (Fig. S5), and indicate that BACE1 is responsible for the generation of at least ~20% of APLP2s.
BACE1 manipulation alters the quantity and form of APP, APLP1 and APLP2 CTFs
To examine the effects of BACE1 expression on FL proteins and CTFs, membrane fractions of mouse brains were analysed using C-terminal specific antibodies. Analysis using the APP-specific C8 antibody revealed the presence of two high molecular weight bands in WT, BACE1 KO and BACE1 Tg mice but not in APP KO samples (Fig. 4A). These two bands, which co-migrate with similar bands detected in the lysate of APP695-expressing cells, most probably represent mature (APPm: ~ 96 kDa) and immature (APPi: ~ 91 kDa) forms of APP (Fig. 4A) [51, 52]. The levels of both forms are significantly increased by BACE1 deletion (APPm: +48.4 ± 3.1 %, P < 0.0001; APPi: +35.4 ± 3.3 %, P < 0.0001) and significantly decreased by BACE1 over-expression (APPm: −45.5 ± 1.4 %, P < 0.0001; APPi: −26.7 ± 1.0 % P < 0.0001) (Fig. 4B). These differences did not result from changes in expression of APP, since APP mRNA levels were unchanged in brains of genetically modified animals (Fig. S6A). Although effects on both forms of FLAPP followed the same trend, the ratio of ~96 kDa to ~91 kDa FLAPP was increased in BACE1 KO samples (1.31 ± 0.05 vs 1.19 ± 0.02, P < 0.01) and decreased in BACE1 Tg samples (0.89 ± 0.02 vs 1.19 ± 0.02, P < 0.0001). These results imply that BACE1 expression influences the levels of FLAPP by a mechanism independent of direct proteolysis.
Fig. 4. BACE1 expression decreases FLAPP steady state levels and gives rise to a ~14.3 kDa APP CTF.
TBST homogenates of wild type (WT), BACE1 knock-out (KO) and BACE1 transgenic (Tg) mouse brains were electrophoresed on 10–20% tris-tricine polyacrylamide gels and western blotted with the anti-APP C-terminus specific antibody C8 (A, C). Lysates of a cell line over-expressing human wild type APP695 (+) are included as a positive control; while TBST homogenates of brains from APP knock-out mice (−) are included as a negative control. The asterisk in C indicates a specific band detected in certain WT and Tg samples. FL and CTF bands (indicated by arrows in B and D respectively) were quantified by densitometry and normalized versus the WT control. Results are presented as averages ± standard error of duplicate measurements of three animals for each condition.
Analysis with C8 also revealed a series of low molecular weight species having sizes consistent with CTFs (Fig. 4C). Two CTFs of approximately 13.3 and 12.5 kDa were detected in WT and BACE1 KO samples (Fig. 4C) and the levels of both were increased in the latter (13.3 kDa: +45.0 ± 9.3 %, P < 0.0001; 12.5 kDa: +50.0 ± 8.7 %, P < 0.0001). In contrast, the levels of both ~13.3 and ~12.5 kDa bands were slightly decreased in BACE1 Tg samples (13.3 kDa: −12.2 ± 2.2 %, non significant; 12.5 kDa: −19.1 ± 2.3 %, P < 0.05) and a third CTF band migrating at around 14.3 kDa was detected (Fig. 4C). A faint ~14.3 kDa band was also detected in WT samples (BACE1 Tg +77.3% ± 16.9% vs WT, P < 0.0001) but was not present in BACE1 KO samples. These results indicated that the ~14.3 kDa band is a BACE1 cleavage product (Fig. 4D). An additional faint band migrating at ~15.4 kDa (indicated by an asterisk in Fig. 4C) was occasionally detected in WT and BACE1 Tg mice only. In other experiments using different lines of BACE1 KO and BACE1 Tg mice the changes in FLAPP and APP CTFs were highly similar to those reported above (Fig. S3). In all the mouse lines studied the total amounts of CTFs were not altered by BACE1 expression, a finding in keeping with the fact that total APPs is not altered by BACE1 expression and which suggests that the change in FLAPP is not the result of a net change in APP processing or APP expression (Fig. S6) but which is mediated by a BACE1-regulated change in turn-over or trafficking.
Western blot analysis of TBST homogenates with antibody W1CT revealed two discrete bands migrating at ~88 kDa and ~80 kDa in WT, BACE1 KO and BACE1 Tg mice, which were absent in APLP1 KO samples (Fig. 5A). As revealed by N-glycosidase F treatment, the slower migrating specific band is N-glycosylated APLP1 (Fig. S7), therefore, by analogy with FLAPP (Fig. 4A), these two bands may represent mature and immature APLP1 (Fig. 5A) [37]. Following the trend seen for APP (Fig. 4B) the slower migrating FLAPLP1 band is dramatically increased (+92.2 ± 4.6 %, P < 0.0001) in the BACE1 KO samples and decreased in BACE1 Tg samples (−19.2 ± 2.4 %, P < 0.0005) (Fig. 5B). On the other hand, the ~80 kDa APLP1 band was decreased in the BACE1 KO samples (−65.2 ± 3.0 %, P < 0.0001) and unchanged in BACE1 Tg samples (−0.5 ± 7.0 %, non significant) (Fig. 5B). As was the case for FLAPP, the differences seen in the levels of FLAPLP1 are not due to a difference in the levels of APLP1 mRNA (Fig. S6B). Importantly for APLP1 the ratio of mature to immature FL was drastically shifted towards the mature form in BACE1 KO samples (15.28 ± 1.04 vs 2.78 ± 0.29, P < 0.0001) and was unchanged in the BACE1 Tg samples (2.31 ± 0.33 vs 2.78 ± 0.29), suggesting that BACE1 may regulate the maturation of APLP1. For WT, BACE1 KO and BACE1 Tg samples a single CTF band was detected, the size of which varied depending on BACE1 expression (Fig. 5C). In BACE1 deficient mice this band migrated at ~8.2 kDa, while in samples from WT and BACE1 Tg mice it migrated at ~ 7.8 kDa. These data indicate that deletion of BACE1 precludes the production of normal APLP1 CTF, leading to the production of a slightly longer CTF (Fig. 5C). The effective difference in the molecular weight of APLP1 CTFs form BACE1 KO mice and from WT and BACE1 Tg mice was confirmed by using a 12 cm-long 16% polyacrylamide tris-tricine gel, and, in these gels two tightly migrating bands were detected (not shown). This suggests that two distinct APLP1s forms can be produced, although the relative molecular weight would be too close to be effectively separated on a 10% tris-glycine gel (Fig. 2A). Thus it would appear that BACE1 is the principal sheddase for APLP1 and that only when BACE1 activity is deleted can APLP1 be cleaved by another activity. It is also of note that the amount of APLP1 CTF in BACE1 KO samples tended to be greater than in WT (+35.1 ± 6.4 %, P < 0.0005), whereas APLP1 CTF was slightly decreased in BACE1 Tg samples (−17.9 ± 12.0 %, non significant) (Fig. 5D). A second very faint CTF band migrating around 5.9 kDa was seen in all samples, and its levels are slightly elevated in BACE1 KO (+59.0 ± 23.7 % P < 0.005) and slightly lower in BACE1 Tg (−23.6 ± 10.3 %, non significant) (Fig. 5D). However, whether this band represents an authentic CTF or a membrane-associated ICD is unclear (see below for more details). As with APP, the effects of BACE1-expression were replicated in a distinct set of mouse lines (Fig. S4).
Fig. 5. BACE1 expression decreases FLAPLP1 levels and gives rise to a ~7.5 kDa APLP1 CTF.
TBST homogenates of wild type (WT), BACE1 knock-out (KO) and BACE1 transgenic (Tg) mouse brains were electrophoresed on 10–20% tris-tricine polyacrylamide gels and western blotted with the anti-APLP1 C-terminus specific antibody W1CT (A, C). Lysates of a cell line over-expressing human wild type APLP1650 (+) are included as a positive control; while TBST homogenates of brains from APLP1 knock-out mice (−) are included as a negative control. The FL and CTFs species identified (indicated by arrows in A and C respectively) were quantified by densitometry and normalized versus the WT control and results are presented as averages ± standard error of duplicate measurements of three animals for each condition (FLAPLP1: B; APLP1 CTFs: D).
Analysis of TBST samples with W2CT (Fig. 6A and 6C) revealed a broad ~92 kDa band (that on occasion appeared as a doublet) in WT, BACE1 KO and BACE1 Tg samples (Fig. 6A). The difference in molecular weight observed for FLAPLP2 from mouse brains and from transfected CHO cells likely reflects the presence of different APLP2 isoforms and/or differences in post-translational modifications. The levels of the ~92 kDa FLAPLP2 were increased in BACE1 KO samples (+39.4 ± 2.3 %, P < 0.0001) and decreased in BACE1 Tg samples (−27.4 ± 0.9 %, P < 0.0001) (Fig. 6B). As was the case also for FLAPP and FLAPLP1, differences in FLAPLP2 are not the result of differential expression of APLP2 mRNA (Fig. S6C).
Fig. 6. BACE1 expression decreases FLAPLP2 protein levels and gives rise to a ~14.8 kDa APLP2 CTF.
TBST homogenates of wild type (WT), BACE1 knock-out (KO) and BACE1 transgenic (Tg) mouse brains were electrophoresed on 10–20% tris-tricine polyacrylamide gels and western blotted with the anti-APLP2 C-terminus specific antibody W2CT (A, C). Lysates of a cell line over-expressing human wild type APLP2751 (+) are included as positive controls; while TBST homogenates of hemibrains of APLP2 knock-out mice (−) are included as negative controls. The FL and CTFs species identified (indicated by arrows in A and C respectively) were quantified by densitometry and normalized versus the WT control. Results are presented as averages ± standard error of duplicate measurements of three animals for each condition (FLAPLP2: B; APLP2 CTFs: D).
APLP2 processing generates at least four CTFs, three higher molecular weight bands migrating close together at ~14.8 kDa, ~13.4 kDa and ~12.6 kDa, respectively and a fourth lower molecular weight band migrating at ~9.6 kDa (Fig. 6C). Because of the close migration of APLP2 CTFs, quantitative densitometric analysis of each species was not possible. However, the ~14.8 kDa and ~9.6 kDa bands were quantified separately and the ~13.4 kDa and ~12.6 kDa bands were considered together. The ~14.8 kDa APLP2 CTF is likely the product of BACE1 cleavage as this band was absent in BACE1 KO samples and was increased in BACE1 Tg samples (+80.3 ± 11.6 %, P < 0.0001) (Fig. 6D). The ~9.6 kDa APLP2 CTF was found in all samples, being increased by an average of 84.3 ± 17.6 % (P < 0.0001) in BACE1 KO and unchanged in BACE1 Tg samples (Fig. 6D). The ~13.4 kDa and ~12.6 kDa bands were essentially unchanged in BACE1 Tg and increased in BACE1 KO samples (Fig. 6D). With regard to total CTF levels BACE1 deletion lead to a minor increase, whereas BACE1 over-expression caused a significant increase. The increase in total CTF levels in BACE1 Tg are in keeping with the increase in total APLP2s (Fig. 3B), while this is not the case for BACE1 KO, where we observed a substantial decrease in APLP2s (Fig. 3A–B) and a minor increase in total APLP2 CTFs (Fig. 6D). However, there is a good correspondence between the levels of APLP2s and FLAPLP2, with FLAPLP2 increased and APLP2s decreased in BACE1 KO. The same trends for APLP2s, FLAPLP2 and APLP2 CTFs were confirmed in mice of a different genetic background (Fig. S5). Taken together these data suggest that BACE1 expression largely mediates regulation of APLP2 by direct proteolysis.
BACE1 deletion does not impair ICD production
Since CTFs are the direct precursors of ICD generation and since BACE1 expression alters the size of CTFs, we investigated whether or not BACE1 cleavage was necessary for ICD production. This was accomplished by searching for endogenous ICDs in mouse brain and by the use of an in vitro ICD generation (ICDivg) assay. For all three proteins a single band migrating ~5.8 kDa was produced by microsomes from both BACE1 KO and WT mice (Fig. 7 A–C). When the ICDivg assay was performed in the presence of protease inhibitors we found an increase in the total amount of ICD produced (Fig. 7A–C). This finding is in keeping with prior reports that ICDs produced from the APP family of proteins are degraded by insulin degrading enzyme (IDE) [43, 53], and hence are stabilized in the presence of IDE inhibitors. The levels of ICDs tended to be higher in samples from BACE1 KO brains than in those from WT brains (Fig. 7A–C). Therefore the deletion of BACE1, and consequently the loss of BACE1 processing of APP, APLP1 and APLP2, had no detrimental effect on the de novo production of ICDs (compare lanes 2 and 3 and lanes 1 and 4 for Fig. 7A–C).
Fig. 7. BACE1 deletion does not compromise APP, APLP1 and APLP2 ICD generation.
Microsomes prepared from BACE1 knock-out (KO) or wild type (WT) mouse brains were incubated at 37°C for 2 hours to allow de novo in vitro ICD production (A-C). ICDs were detected by western blot using specific antibodies for APP (C8 antibody, A), APLP1 (W1CT antibody, B) and APLP2 (W2CT antibody, C). Western blots shown in A-C are representative of three different experiments. Generation of ICDs was conducted either in presence (+) or absence (−) of protease inhibitors and insulin (PI mix). Endogenous ICDs were immunoprecipitated from mouse brains with C8, W1CT or W2CT, and immunoprecipitates analysed by western blotting with the same antibodies (D, E, F). Western blots shown in D-F are representative of two different experiments. For comparison, in vitro generated ICDs were electrophoresed alongside endogenous ICDs (D-F).
In a complementary approach we also sought to determine if the physiological production of ICDs was altered by BACE1 deletion. Since ICDs are extremely labile [53, 54], mouse brains were processed in a fashion designed to minimize ICD degradation and the ICDs present in the homogenates were analysed by immunoprecipitation and western blotting using antibodies C8, W1CT and W2CT. A ladder of bands was detected migrating until the 7 kDa marker in all immunoprecipitates (Fig. 7 D–F). These bands likely represents various CTFs, consistent with previous reports [54], and non-specific bands due to the use of the same polyclonal antibody for both immunoprecipitation and western blotting. In addition, less abundant lower molecular weight bands were also detected. For APP two closely migrating bands with estimated molecular weights of ~5.8 and ~6.4 kDa were detected (Fig. 7D). The lower of the two bands perfectly co-migrated with in vitro generated APP ICDs, with the upper band migrating in a manner consistent for phosphorylated APP ICD [55]. Moreover, as with the brain-microsome-generated APP ICDs, the abundance of these two bands were slightly greater in the BACE1 KO samples (Fig. 7D), a result that we observed in two separate experiments using a total of 2 BACE1 KO and 2 WT mouse brains. For APLP1 a similar ladder of bands was detected, together with a single putative ICD band that co-migrated with in vitro generated APLP1 ICD (Fig. 7E). Again the levels of the endogenous APLP1 ICD were slightly higher in extracts of BACE1 KO brains. For APLP2 the pattern was similar to that for APP, that is, a putative ICD band that co-migrated with in vitro generated APLP2 ICD at ~5.8 kDa, together with a slighter slower migrating band at ~6.4 kDa (Fig. 7F).
Discussion
By analogy with APP, both APLP1 and 2 have been proposed to be substrates of BACE1 [41]. Moreover, a previous study found evidence that APLP2 is cleaved by BACE1 in vivo [38], but so far the processing of APLP1 and APLP2 by BACE1 has been mainly studied in transfected cell lines [40, 41]. Since trafficking and interaction with other cellular partners are likely to be altered when a single member of the APP family of proteins is over-expressed [56], we set out to investigate the effects of BACE1 on APLP1 and APLP2 in mouse models where the only variable parameter was expression of BACE1.
All three APP family proteins underwent ectodomain shedding and their soluble ectodomains were detected in the TBS fraction of brain homogenates (for a summary of results see Table S2). Shedding of the APP ectodomain appears to be tightly regulated, since BACE1 levels altered the ratio of APPsα to APPsβ, but did not substantially modify the total levels of APPs or of APP CTFs. This suggests that there is a discrete pool of FLAPP that is directed towards processing, and that α- and β-secretases have access to the same cellular pool. For APLP1 ablation of BACE1 resulted in a near complete loss of APLP1s, suggesting that BACE1 is centrally involved in APLP1 ectodomain cleavage. A notion supported by the finding that FLAPLP1 is increased by deletion of BACE1 and slightly decreased by BACE1 over-expression. However, the current data cannot discriminate between cleavage of APLP1 by BACE1 and cleavage of APLP1 by another enzyme regulated by BACE1. Indeed, prior studies using cell culture systems have found APLP1 to be cleaved by an α-secretase-like activity [40, 57]. Importantly, BACE1 over-expression did not dramatically alter APLP1 processing (as assessed by APLP1s and APLP1 CTF levels) thus suggesting that the ectodomain shedding of APLP1, although not as tightly regulated as APP, is nonetheless closely regulated.
Interestingly, FLAPLP1 was detected in the TBS brain homogenates, an observation consistent with the detection of FLAPLP1 in conditioned media from transfected cells [43]. Moreover, the levels of secreted FLAPLP1 were influenced by BACE1 expression, mirroring the modifications of the levels of FLAPLP1 in the TBST fraction. An attractive explanation for the presence of FLAPLP1 in TBS homogenates is its secretion via vesicles, e.g. exosomes [58]. Indeed, it is interesting to note that the prion protein, which is known to interact with APLP1 [59] and to be a regulator of BACE1 activity [60], can be secreted via exosomes [59]. Whatever the mechanism for secretion of FLAPLP1 the net outcome of these results suggest that BACE1 has a key role in the regulation of APLP1 maturation, trafficking and secretion.
BACE1 expression also regulates the steady state levels of FLAPLP2, in a manner analogous to what has been shown for APP [50] and to what has been presented here for APLP1. Since BACE1 did not alter the levels of APP, APLP1 and APLP2 mRNA, it would appear that BACE1 is an important post-transcriptional regulator of the APP family of proteins. In agreement with previous reports [38, 50] we detected β-cleavage-specific products for both APP and APLP2. In addition, we detected a slightly longer APLP1 CTF when BACE1 was deleted, but we did not see an effect of BACE1 over-expression on the size of this CTF. We found also that the total amounts of APP and APLP2 CTFs were not altered by BACE1 expression, meaning that competing or complementary pathways intervene to balance the loss of BACE1 cleavage. In direct TBST extracts we could not detect ICDs of either APP or APLP2, but we did detect a band consistent with APLP1 ICD, possibly because APLP1 ICDs are more stable than either APP or APLP2 ICDs [43, 53, 54].
Taken together, this characterization of BACE1 effects on APP, APLP1 and APLP2 has highlighted the fact that APP and APLP2 share many similarities, while APLP1 has some unique characteristics. APLP1 is an atypical member of the APP family: it is neuron-specific, whereas APP and APLP2 are ubiquitously expressed, and its subcellular localization and dimerization properties are different from those of APP and APLP2 [16]. Given the similarities between processing of Notch and of the APP family of proteins, it seems plausible that APP, APLP1 and APLP2 ICDs may play a role in transcriptional regulation [42, 43]. The APP ICD has been shown to form a complex with the adaptor protein Fe65 and the histone acetyltransferase Tip60 capable of inducing transcription of reporter genes [44]. The ability to form transcriptionally-active complexes with Fe65 has also been demonstrated for APLP ICDs [42, 43], however a definite physiological relevance of these complexes has yet to be demonstrated.
Since we have found that BACE1 activity regulates the maturation and the processing of the three APP family members, we were interested in understanding if abolishing BACE1 activity had a detrimental effect on APP, APLP1 and APLP2 ICD production, to better characterize the impact of BACE1 inhibition as a putative therapy for the treatment of AD. We found that the de novo production and the endogenous levels of ICDs were not reduced by deletion of BACE1. In fact in most cases deletion of BACE 1 resulted in an increase in ICDs. Indeed treatment of cultured cells with a potent β-secretase inhibitor (Fig. S8) consistently resulted in a slight elevation of ICDs production. However, how genetic or chemical ablation of BACE1 leads to a modest increase in ICDs is unclear. One potential explanation is that due to spatial differences α-secretase derived CTFs are more prone to γ-secretase cleavage than β-secretase derived CTFs. The fact that BACE1 deletion does not dramatically alter ICD production, opens two possible scenarios: one where ICDs produced by γ-secretase cleavage of α- and β-secretase-derived CTFs serve the same function and their levels are tightly regulated by compensatory mechanisms, and another where only ICDs produced by α-secretase cleavage are physiologically relevant and ICDs derived from the amyloidogenic processing of APP are quickly degraded. This latter possibility is corroborated by the fact that over-expression of BACE1, while leading to increased processing of APP and APLP2, does not translate into an increased amount of APP or APLP2 CTFs. Therefore it is reasonable to suppose that the levels of CTFs are regulated, possibly by degradation of excess CTFs.
Together these results demonstrate that BACE1 is involved in ectodomain shedding of APP, APLP1 and APLP2. That BACE1 expression levels regulate the trafficking and maturation of the APP family of proteins, but that BACE1 ablation did not prevent generation of APP, APLP1 and APLP2 ICDs. This suggests that inhibition of BACE1 will not adversely affect the potentially important signaling role of the ICDs released by the APP family of proteins, but may impact upon other functions of this family of proteins.
Material and methods
Reagents
Unless specified, chemicals were from Sigma-Aldrich (Sigma-Aldrich Ireland Ltd., Dublin, Ireland).
Antibodies
Novel rabbit polyclonal antibodies W1NT, W1CT and W2CT were raised to peptide immunogens conjugated to keyhole limpet hemocyanin via an N-terminal cysteine (Table 1). W1NT was raised to residues EPDPQRSRRCLRDPQR of the human APLP1 ectodomain, while W1CT and W2CT were raised to peptides NPTYRFLEERP and NPTYKYLEQMQI corresponding to the extreme C-termini of human APLP1 and human APLP2, respectively (Fig. S1A). The sequences to which W1CT and W2CT were raised are identical in both human and mouse proteins. The sequence to which W1NT was raised differs in 1 out of the 16 amino acids from the corresponding murine region (Arg12Lys, antigen numbering); as expected W1NT recognizes both murine and human APLP1 (Table 1). The specificity of antibodies W1NT, W1CT and W2CT was confirmed by western blotting of brain material from mice null for APP, APLP1 or APLP2 (Fig. S1B). The monoclonal antibody 22C11 (Chemicon, Millipore, Billerica, MA), which recognizes the N-terminus of APP, and the polyclonal antiserum C8, which recognizes the C-terminus of APP, and D2-II, which recognizes the ectodomain of APLP2 (Calbiochem, EMD Chemicals Inc., Gibbstown, NJ), have been described previously [43] (Table 1). The polyclonal anti-BACE1 N-terminal antibody was from Sigma (Dublin, Ireland) and the polyclonal antibody anti Aβ-rodent was from Signet (Signet Covance, Dedham, MA). The monoclonal antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was from Abcam (Cambridge, UK).
Animals
All genetically manipulated mouse lines have been described previously [26, 38, 50, 61] Black Swiss BACE1 knock-out and littermate control wild type mouse brains and C57/BL6 BACE1 transgenic mouse brains were from 4 month-old mice. Additional mouse brains from C57/BL6-OLA129 BACE2 knock-out mice, C57/B6Jx129SVola BACE1 knock-out mice, C57/BL6 wild type and C57/BL6 BACE1 transgenic mice were from 4 month-old mice. Immediately after explant the cerebellum and olfactory bulb were removed, the remaining brain was cut in half along the midline, snap frozen in liquid nitrogen and stored at −80°C until analysis. Levels of BACE1 protein in all the brain tissue analysed was assessed by western blotting (Fig. S2). Brains from APP, APLP1 and APLP2 knock-out mice were provided by Dr. Ulrike Müller (University of Heidelberg, Heidelberg, Germany) [14].
Preparation of mouse brain extracts
Hemibrains were homogenized in 5 volumes of tris-buffered saline (TBS: 20 mM Tris pH 7.4, 150 mM NaCl) containing protease inhibitors (5 mM EDTA, 1 mM EGTA, 5 µg/mL leupeptin, 5 µg/mL aprotinin, 2 µg/mL pepstatin, 120 µg/mL Pefabloc, 2 mM 1,10-phenanthroline) with 40 strokes of a Dounce homogenizer at 5000 rpm. The resulting suspension was then centrifuged at 175,000 g and 4°C for 30 minutes and the upper 75% of the supernatant was collected. Protein content was assessed using a BCA protein assay kit (Pierce, Rockford, IL), and then samples were aliquoted and stored at −80°C until used. The membrane-containing pellet was resuspended by pipetting in 5 volumes of 100 mM sodium bicarbonate pH 11.4 and incubated on a rocking platform for 15 minutes at 4°C. The washed pellet was harvested by centrifugation, and washed in 5 volumes of TBS. The membrane fraction was again pelleted by centrifugation and then resuspended by pipetting in 5 volumes of TBS containing 1% Triton X-100 (TBST) plus protease inhibitors. In order to effectively extract integral membrane proteins this suspension was incubated on a rocking platform at room temperature (RT) for 15 min, homogenized with 40 strokes of a Dounce homogenizer and sonicated with a microtip attached to an XL-2000 sonicator (Misonix Inc, Farmingdale NY) at power setting 4 (~12 W) for 30 seconds. The detergent extract was centrifuged as described above and the upper 75% of the supernatant collected. Protein content was assessed using a BCA protein assay kit (Pierce, Rockford, IL), and then samples were aliquoted and stored at −80°C until used.
Quantitative Real-Time PCR (QRT-PCR) analysis
Total RNA was isolated using TRI Reagent (Ambion, Austin, TX) according to manufacturer’s instructions and quantified using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). 1 µg aliquots of total RNA were treated with Deoxyribonuclease I (Invitrogen, Carlsbad, CA) and used to synthesize first strand cDNA with 200 U SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) in a final reaction volume of 11 µL containing 50 ng random hexamers. 1 µL of the first strand cDNA PCR reaction was used as template for QRT-PCR amplification with primers specific for APP, APLP1 and APLP2 (Sigma-Genosys, Hamburg, Germany, Table S1). Primers specific for the 18S rRNA were used as an internal control. Primer pairs for the APP family of proteins contained one intron-spanning region to avoid amplification of genomic DNA. QRT-PCR reactions were run in duplicate. 1 µl of Taq DNA polymerase and the appropriate primer pair each at a concentration of 0.5 µM, together with the Power SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA), were brought to a final volume of 10 µL and analysed on an ABI Prism 7000 SDS (Applied Biosystems, Darmstadt, Germany). An initial step of 15 minutes at 95°C for polymerase activation was followed by 40 cycles of a standard PCR protocol (15 seconds at 95°C, 30 seconds at 60°C, 30 seconds at 72°C) as described in the supplier’s protocol (Applied Biosystems, Darmstadt, Germany). APP, APLP1 and APLP2 expression was normalized to 18S rRNA levels by the comparative cycle threshold (Ct) method.
ICD in vitro generation (ICDivg) assay from mouse brain-derived microsomes
This method was adapted from an ICD in vitro generation assay previously used with microsomes prepared from cultured cells [62]. Hemibrains were homogenized on ice in 8 volumes of hypotonic lysis buffer (10 mM MOPS pH 7, containing 10 mM KCl, 5 mM EDTA, 1 mM EGTA, 120 µg/mL Pefabloc, 2 mM 1,10-phenanthroline) with 30 passes of a Dounce homogenizer at 6,000 rpm. The resulting homogenate was divided into 1 mL aliquots and centrifuged at 1000 g and 4°C for 15 minutes, the supernatant was then transferred to a new tube and microsomes were harvested by centrifugation at 16000 g and 4°C for 40 minutes. Each pellet derived from 1 mL of homogenate was resuspended in 100 µL of assay buffer (150 mM sodium citrate pH 6.8) either containing or devoid of a cocktail of protease inhibitors (5 mM EDTA, 1 mM EGTA, 2 mM 1,10-phenanthroline and 250 µg/mL human recombinant insulin). Microsomes were then incubated in a water bath at 37°C for 2 hours, after which they were placed on ice for 10 minutes to stop the reaction and centrifuged at 150,000 g and 4ºC for 75 min in an Optima centrifuge using a TLA55 rotor (Beckman Coulter, Fullerton, CA). The upper 90 µL of the supernatant were collected for analysis by western blotting.
Immunoprecipitation of endogenous ICDs from mouse brains
Hemibrains were homogenized on ice in 9 volumes of homogenization buffer (50 mM Tris-HCl pH 7.4, containing 1% SDS, 150 mM NaCl, 5 mM EDTA, 5 µg/mL leupeptin, 5 µg/mL aprotinin, 2 µg/mL pepstatin, 120 µg/mL Pefabloc, 2 mM 1,10-phenanthroline) with 30 passes of a Dounce homogenizer at 6,000 rpm. The homogenates were then boiled at 100ºC for 10 minutes and divided into 1 mL aliquots prior to sonication for 30 seconds at power setting 4 (~12 W) using a XL-2000 sonicator connected to a microtip (Misonix Inc, Farmingdale NY). Following sonication, aliquots were pooled and boiled for a further 10 minutes and then centrifuged at 16000 g for 20 minutes. The supernatant was collected, transferred to a fresh tube and diluted 1:10 with lysis buffer (50 mM Tris Base pH 7.6, containing 150 mM NaCl, 5 mM EDTA, 2% NP-40, 5 µg/mL leupeptin, 5 µg/mL aprotinin, 2 µg/mL pepstatin, 120 µg/mL Pefabloc, 2 mM 1,10-phenanthroline). To immunoprecipitate APP, APLP1 and APLP2 ICDs, 1 mL aliquots of brain extracts were incubated overnight on a rocking platform at 4ºC with either antibody C8, W1CT or W2CT respectively (at a dilution of 1:40) together with 40 µL of protein A-Sepharose. Beads were collected by centrifugation at 6000 g for 5 minutes, washed in subsequent steps of incubation for 20 minutes on a rocking platform at 4ºC with 0.5M STEN buffer (50 mM Tris Base pH 7.6, 500 mM NaCl, 2 mM EDTA, 2% NP-40), SDS-STEN buffer (50 mM Tris Base pH 7.6, 150 mM NaCl, 2 mM EDTA, 2% NP-40, 0.1% SDS) and STEN buffer (50 mM Tris Base pH 7.6, 150 mM NaCl, 2 mM EDTA, 2% NP-40). Captured proteins were eluted with 2X tris-tricine electrophoresis sample buffer containing 10% β-mercaptoethanol (20 µL/sample) [63].
Western blot analysis
TBS homogenates of mouse brains were diluted with 4X tris-glycine sample buffer (1X concentrations: 62.5mM Tris-HCl pH 6.8, 10% glycerol, 2% SDS) and electrophoresed on 10% polyacrylamide tris-glycine gels [64], while TBST homogenates of mouse brains and ICDivg samples were diluted with 4X tris-tricine sample buffer (1X concentrations: 450 mM Tris pH 8.45, 10% glycerol, 4% SDS) and electrophoresed on pre-cast Novex 10–20% polyacrylamide tris-tricine gels (Invitrogen, Carlsbad, CA). Immunoprecipitated endogenous ICDs from mouse brains were also electrophoresed on pre-cast Novex 10–20% polyacrylamide tris-tricine gels (Invitrogen, Carlsbad, CA). Proteins were transferred onto nitrocellulose (0.2 µm pore size, Sigma-Aldrich Ltd., Dublin, Ireland) at 400 mA and 4°C for 2 hours. Membranes were subsequently blocked for 1 hour at room temperature (RT) with 5% skim milk (Fluka, Sigma-Aldrich Ireland Ltd., Dublin, Ireland) in TBS containing 0.05% Tween-20 (TBS-Tw), washed twice for 10 minutes with TBS-Tw to remove traces of blocker and incubated overnight with primary antibodies diluted in TBS-Tw containing 5% skim milk. The following day blots were washed four times for 15 minutes with TBS-Tw, incubated with appropriate HRP-linked secondary antibodies (Amersham, GE Healthcare, Chalfont St. Giles, UK) for 1 hour at RT, washed as above and visualized using an enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL) and Hyperfilm MP (Amersham, GE Healthcare, Chalfont St. Giles, UK).
Data analysis
Band intensities were quantified using Scion Image (Scion Corporation, Frederick, MD) and data were analyzed using one-way ANOVA (SigmaStat, Systat Software Inc, Chicago, IL).
Supplementary Material
Acknowledgements
Authors thank Dr. Ulrike Müller (University of Heidelberg, Heidelberg, Germany) for APP, APLP1 and APLP2 knock-out mouse brains, Prof. Jordan Tang (Protein Studies Program, Oklahoma Medical Research Foundation, University of Oklahoma Health Science Center, Oklahoma City, OK 73104) for the β-secretase inhibitor GRL-8234, Dr. Barry Boland (UCD, Dublin, Ireland) for help with the midi tris-tricine PAGE gels and Dr. Tracy Young-Pearse (CND, Harvard Medical School, Boston) for constructive discussions and critical reading of the manuscript. This work was supported by Wellcome Trust grant 067660 (D.M.W.) and NIH grant AG027443 (D.M.W., & D.J.S.) and by the Foundation for Neurologic Diseases (D.M.W.).
Abbreviations
- Aβ
amyloid β-peptide
- APP
amyloid precursor protein
- APLP1
amyloid precursor-like protein 1
- APLP2
amyloid precursor-like protein 2
- BACE1
β-site APP cleaving enzyme
- CTF
C-terminal fragment
- FL
full-length
- FLAPP
full-length APP
- FLAPLP1
full-length APLP1
- FLAPLP2
full-length APLP2
- ICD
intracellular domain
- ICDivg
ICD in vitro generation
- IDE
insulin degrading enzyme
- KO
knock-out
- TBS
tris-buffered saline
- TBST
TBS with 1% Triton X-100
- TBS-Tw
TBS with 0.05% Tween-20
- Tg
transgenic
- 1,10 PTH
1,10-phenanthroline
Bibliography
- 1.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Coulson EJ, Paliga K, Beyreuther K, Masters CL. What the evolution of the amyloid protein precursor supergene family tells us about its function. Neurochem Int. 2000;36:175–184. doi: 10.1016/s0197-0186(99)00125-4. [DOI] [PubMed] [Google Scholar]
- 3.Wasco W, Bupp K, Magendantz M, Gusella JF, Tanzi RE, Solomon F. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid beta protein precursor. Proc Natl Acad Sci U S A. 1992;89:10758–10762. doi: 10.1073/pnas.89.22.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasco W, Gurubhagavatula S, Paradis MD, Romano DM, Sisodia SS, Hyman BT, Neve RL, Tanzi RE. Isolation and characterization of APLP2 encoding a homologue of the Alzheimer's associated amyloid beta protein precursor. Nat Genet. 1993;5:95–100. doi: 10.1038/ng0993-95. [DOI] [PubMed] [Google Scholar]
- 5.Bayer TA, Wirths O, Majtenyi K, Hartmann T, Multhaup G, Beyreuther K, Czech C. Key factors in Alzheimer's disease: beta-amyloid precursor protein processing, metabolism and intraneuronal transport. Brain Pathol. 2001;11:1–11. doi: 10.1111/j.1750-3639.2001.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh DM, Minogue AM, Sala Frigerio C, Fadeeva JV, Wasco W, Selkoe DJ. The APP family of proteins: similarities and differences. Biochem Soc Trans. 2007;35:416–420. doi: 10.1042/BST0350416. [DOI] [PubMed] [Google Scholar]
- 7.Schmechel DE, Goldgaber D, Burkhart DS, Gilbert JR, Gajdusek DC, Roses AD. Cellular localization of messenger RNA encoding amyloid-beta-protein in normal tissue and in Alzheimer disease. Alzheimer Dis Assoc Disord. 1988;2:96–111. doi: 10.1097/00002093-198802020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kim TW, Wu K, Xu JL, McAuliffe G, Tanzi RE, Wasco W, Black IB. Selective localization of amyloid precursor-like protein 1 in the cerebral cortex postsynaptic density. Brain Res Mol Brain Res. 1995;32:36–44. doi: 10.1016/0169-328x(95)00328-p. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, Zhao NM, Dominguez B, Lee KF, Gan WB, Zheng H. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25:1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small DH, Nurcombe V, Reed G, Clarris H, Moir R, Beyreuther K, Masters CL. A heparin-binding domain in the amyloid protein precursor of Alzheimer's disease is involved in the regulation of neurite outgrowth. J Neurosci. 1994;14:2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herms J, Anliker B, Heber S, Ring S, Fuhrmann M, Kretzschmar H, Sisodia S, Muller U. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. Embo J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heber S, Herms J, Gajic V, Hainfellner J, Aguzzi A, Rulicke T, von Kretzschmar H, von Koch C, Sisodia S, Tremml P, Lipp HP, Wolfer DP, Muller U. Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J Neurosci. 2000;20:7951–7963. doi: 10.1523/JNEUROSCI.20-21-07951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soba P, Eggert S, Wagner K, Zentgraf H, Siehl K, Kreger S, Lower A, Langer A, Merdes G, Paro R, Masters CL, Muller U, Kins S, Beyreuther K. Homo- and heterodimerization of APP family members promotes intercellular adhesion. Embo J. 2005;24:3624–3634. doi: 10.1038/sj.emboj.7600824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaden D, Voigt P, Munter LM, Bobowski KD, Schaefer M, Multhaup G. Subcellular localization and dimerization of APLP1 are strikingly different from APP and APLP2. J Cell Sci. 2009;122:368–377. doi: 10.1242/jcs.034058. [DOI] [PubMed] [Google Scholar]
- 17.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 18.Sisodia SS. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci U S A. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 20.Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 21.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 22.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 23.Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 25.Willem M, Lammich S, Haass C. Function, regulation and therapeutic properties of beta-secretase (BACE1) Semin Cell Dev Biol. 2009;20:175–182. doi: 10.1016/j.semcdb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 27.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 29.Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, Winter PH, Shilliam CS, Hughes ZA, Dawson LA, Gonzalez MI, Upton N, Pangalos MN, Dingwall C. BACE1 (beta-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci. 2003;24:646–655. doi: 10.1016/s1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 30.Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitazume S, Tachida Y, Oka R, Shirotani K, Saido TC, Hashimoto Y. Alzheimer's beta-secretase, beta-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc Natl Acad Sci U S A. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtenthaler SF, Dominguez DI, Westmeyer GG, Reiss K, Haass C, Saftig P, De Strooper B, Seed B. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J Biol Chem. 2003;278:48713–48719. doi: 10.1074/jbc.M303861200. [DOI] [PubMed] [Google Scholar]
- 33.Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ, Kovacs DM. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn PH, Marjaux E, Imhof A, De Strooper B, Haass C, Lichtenthaler SF. Regulated intramembrane proteolysis of the interleukin-1 receptor II by alpha-, beta-, and gamma-secretase. J Biol Chem. 2007;282:11982–11995. doi: 10.1074/jbc.M700356200. [DOI] [PubMed] [Google Scholar]
- 35.Slunt HH, Thinakaran G, Von Koch C, Lo AC, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse beta-amyloid precursor protein (APP) J Biol Chem. 1994;269:2637–2644. [PubMed] [Google Scholar]
- 36.Webster MT, Groome N, Francis PT, Pearce BR, Sherriff FE, Thinakaran G, Felsenstein KM, Wasco W, Tanzi RE, Bowen DM. A novel protein, amyloid precursor-like protein 2, is present in human brain, cerebrospinal fluid and conditioned media. Biochem J. 1995;310(Pt 1):95–99. doi: 10.1042/bj3100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paliga K, Peraus G, Kreger S, Durrwang U, Hesse L, Multhaup G, Masters CL, Beyreuther K, Weidemann A. Human amyloid precursor-like protein 1--cDNA cloning, ectopic expression in COS-7 cells and identification of soluble forms in the cerebrospinal fluid. Eur J Biochem. 1997;250:354–363. doi: 10.1111/j.1432-1033.1997.0354a.x. [DOI] [PubMed] [Google Scholar]
- 38.Pastorino L, Ikin AF, Lamprianou S, Vacaresse N, Revelli JP, Platt K, Paganetti P, Mathews PM, Harroch S, Buxbaum JD. BACE (beta-secretase) modulates the processing of APLP2 in vivo. Mol Cell Neurosci. 2004;25:642–649. doi: 10.1016/j.mcn.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Endres K, Postina R, Schroeder A, Mueller U, Fahrenholz F. Shedding of the amyloid precursor protein-like protein APLP2 by disintegrin-metalloproteinases. Febs J. 2005;272:5808–5820. doi: 10.1111/j.1742-4658.2005.04976.x. [DOI] [PubMed] [Google Scholar]
- 40.Eggert S, Paliga K, Soba P, Evin G, Masters CL, Weidemann A, Beyreuther K. The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves alpha-, beta-, gamma-, and epsilon-like cleavages: modulation of APLP-1 processing by n-glycosylation. J Biol Chem. 2004;279:18146–18156. doi: 10.1074/jbc.M311601200. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Sudhof TC. Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J Biol Chem. 2004;279:10542–10550. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- 42.Scheinfeld MH, Ghersi E, Laky K, Fowlkes BJ, D'Adamio L. Processing of beta-amyloid precursor-like protein-1 and −2 by gamma-secretase regulates transcription. J Biol Chem. 2002;277:44195–44201. doi: 10.1074/jbc.M208110200. [DOI] [PubMed] [Google Scholar]
- 43.Walsh DM, Fadeeva JV, LaVoie MJ, Paliga K, Eggert S, Kimberly WT, Wasco W, Selkoe DJ. gamma-Secretase cleavage and binding to FE65 regulate the nuclear translocation of the intracellular C-terminal domain (ICD) of the APP family of proteins. Biochemistry. 2003;42:6664–6673. doi: 10.1021/bi027375c. [DOI] [PubMed] [Google Scholar]
- 44.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 45.Scheinfeld MH, Matsuda S, D'Adamio L. JNK-interacting protein-1 promotes transcription of A beta protein precursor but not A beta precursor-like proteins, mechanistically different than Fe65. Proc Natl Acad Sci U S A. 2003;100:1729–1734. doi: 10.1073/pnas.0437908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardossi-Piquard R, Petit A, Kawarai T, Sunyach C, Alves da, Costa C, Vincent B, Ring S, D'Adamio L, Shen J, Muller U, St George Hyslop P, Checler F. Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Hebert SS, Serneels L, Tolia A, Craessaerts K, Derks C, Filippov MA, Muller U, De Strooper B. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep. 2006;7:739–745. doi: 10.1038/sj.embor.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen AC, Selkoe DJ, et al. Response to: Pardossi-Piquard. Presenilin-Dependent Transcriptional Control of the Abeta-Degrading Enzyme Neprilysin by Intracellular Domains of betaAPP and APLP. Neuron. 2007;53:479–483. doi: 10.1016/j.neuron.2007.01.023. Neuron 46, 541–554. [DOI] [PubMed] [Google Scholar]
- 49.Bruni P, Minopoli G, Brancaccio T, Napolitano M, Faraonio R, Zambrano N, Hansen U, Russo T. Fe65, a ligand of the Alzheimer's beta-amyloid precursor protein, blocks cell cycle progression by down-regulating thymidylate synthase expression. J Biol Chem. 2002;277:35481–35488. doi: 10.1074/jbc.M205227200. [DOI] [PubMed] [Google Scholar]
- 50.Bodendorf U, Danner S, Fischer F, Stefani M, Sturchler-Pierrat C, Wiederhold KH, Staufenbiel M, Paganetti P. Expression of human beta-secretase in the mouse brain increases the steady-state level of beta-amyloid. J Neurochem. 2002;80:799–806. doi: 10.1046/j.0022-3042.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- 51.Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 52.Weidemann A, Eggert S, Reinhard FB, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G. A novel epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry. 2002;41:2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- 53.Edbauer D, Willem M, Lammich S, Steiner H, Haass C. Insulin-degrading enzyme rapidly removes the beta-amyloid precursor protein intracellular domain (AICD) J Biol Chem. 2002;277:13389–13393. doi: 10.1074/jbc.M111571200. [DOI] [PubMed] [Google Scholar]
- 54.Kimberly WT, Zheng JB, Town T, Flavell RA, Selkoe DJ. Physiological regulation of the beta-amyloid precursor protein signaling domain by c-Jun N-terminal kinase JNK3 during neuronal differentiation. J Neurosci. 2005;25:5533–5543. doi: 10.1523/JNEUROSCI.4883-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki T, Nakaya T. Regulation of APP by phosphorylation and protein interactions. J Biol Chem. 2008 doi: 10.1074/jbc.R800003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neumann S, Schobel S, Jager S, Trautwein A, Haass C, Pietrzik CU, Lichtenthaler SF. Amyloid precursor-like protein 1 influences endocytosis and proteolytic processing of the amyloid precursor protein. J Biol Chem. 2006;281:7583–7594. doi: 10.1074/jbc.M508340200. [DOI] [PubMed] [Google Scholar]
- 57.Minogue AM, Stubbs AK, Frigerio CS, Boland B, Fadeeva JV, Tang J, Selkoe DJ, Walsh DM. gamma-secretase processing of APLP1 leads to the production of a p3-like peptide that does not aggregate and is not toxic to neurons. Brain Res. 2009;1262:89–99. doi: 10.1016/j.brainres.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 58.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 59.Yehiely F, Bamborough P, Da Costa M, Perry BJ, Thinakaran G, Cohen FE, Carlson GA, Prusiner SB. Identification of candidate proteins binding to prion protein. Neurobiol Dis. 1997;3:339–355. doi: 10.1006/nbdi.1997.0130. [DOI] [PubMed] [Google Scholar]
- 60.Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, Baybutt HN, Turner AJ, Hooper NM. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc Natl Acad Sci U S A. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willem M, Dewachter I, Smyth N, Van Dooren T, Borghgraef P, Haass C, Van Leuven F. beta-site amyloid precursor protein cleaving enzyme 1 increases amyloid deposition in brain parenchyma but reduces cerebrovascular amyloid angiopathy in aging BACE x APP[V717I] double-transgenic mice. Am J Pathol. 2004;165:1621–1631. doi: 10.1016/s0002-9440(10)63419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sala Frigerio C, Kukar TL, Fauq A, Engel PC, Golde TE, Walsh DM. An NSAID-like compound, FT-9, preferentially inhibits gamma-secretase cleavage of the amyloid precursor protein compared to its effect on amyloid precursor-like protein 1. Biochemistry. 2009;48:10894–10904. doi: 10.1021/bi901237k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001;276:40288–40292. doi: 10.1074/jbc.C100447200. [DOI] [PubMed] [Google Scholar]
- 64.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.