Abstract
A Phase 1 trial was conducted in malaria-naïve adults to evaluate the recombinant protein vaccine apical membrane antigen 1 – Combination 1 (AMA1-C1) formulated in Montanide® ISA 720 (SEPPIC, France), a water-in-oil adjuvant. Vaccinations were halted early due to a formulation issue unrelated to stability or potency. Twenty-four subjects (12 in each group) were enrolled and received 5 or 20 μg protein at 0 and 3 months and 4 subjects were enrolled and received one vaccination of 80 μg protein. After first vaccination, nearly all subjects experienced mild to moderate local reactions and 6 experienced delayed local reactions occurring at day 9 or later. After the second vaccination, 3 subjects experienced transient grade 3 (severe) local reactions; the remainder experienced grade 1 or 2 local reactions. All related systemic reactogenicity was grade 1 or 2, except 1 instance of grade 3 malaise. Anti-AMA1-C1 antibody responses were dose dependent and seen following each vaccination, with mean antibody levels 2-3 fold higher in the 20 μg group compared to the 5 μg group at most time points. In vitro growth-inhibitory activity was a function of the anti-AMA1 antibody titer. AMA1-C1 formulated in ISA 720 is immunogenic in malaria-naïve Australian adults. It is reasonably tolerated, though some transient, severe, and late local reactions are seen.
1. Introduction
Malaria remains a primary cause of morbidity and mortality in children, with an estimated 881,000 malaria deaths in 2006, most of which were in sub-Saharan Africa [1]. A vaccine that reduces both mortality and morbidity secondary to Plasmodium falciparum infection would be a valuable new resource in the fight against this disease. Apical membrane antigen 1 (AMA1), a surface protein expressed during the asexual and sporozoite stages of P. falciparum, is a leading blood-stage vaccine candidate [2]. AMA1 is highly polymorphic, and immunization with only one form of AMA1 may not protect against parasites expressing different AMA1 alleles [3,4]. For this reason, the AMA1-Combination 1 (AMA1-C1) vaccine contains equal mixtures of the recombinant AMA1 proteins representing the FVO and 3D7 strains of P. falciparum. Previous clinical studies of AMA1-C1 adsorbed to Alhydrogel® showed that the vaccine is well tolerated and moderately immunogenic [5-7]. However a recent Phase 2 field trial in 2-3 year old Malian children failed to demonstrate protection [8], perhaps due to inadequate antibody response, failure to inhibit heterologous parasites, or both. The addition of the adjuvant CPG 7909 to AMA1-C1/Alhydrogel® markedly enhances antibody responses and the in vitro growth inhibition activity against homologous parasites was as high as 96% in some malaria-naive adult volunteers [9,10]. However, CPG 7909 is a novel adjuvant that has not been tested in infants and young children, thus the search for other effective adjuvants and formulations is warranted.
Montanide® ISA 720 (ISA 720) is a water-in-oil adjuvant [11,12] that induces high antibody titers in several animal species, likely due to formation of a depot at the injection site that hypothetically releases the immunogen over time. The manufacturer, Seppic, Inc. recommends formulations with a droplet size of approximately 1 μm as optimal for stability and immunogenicity. It has been shown that the addition of glycine or glycylglycine helps prevent antigen modification and that a droplet size of 1 μm can be reliably obtained by a homogenization method of formulation [13]. ISA 720 is not a component of any approved human vaccine but has been used in many previous trials of candidate malaria vaccines [14-25]. Concerns about severe and delayed local reactions, possibly related to specific formulations, antigen dose, and shorter dosing intervals, have slowed development. This study is the first Phase 1 trial of AMA1-C1 formulated in Montanide® ISA 720 (AMA1-C1/ISA 720). The trial was initially planned for 12 volunteers in each of three dose groups, 5, 20 and 80 μg, to receive three vaccinations at study days 0, 84, and 168. An audit unrelated to this study was conducted during the course of the trial and raised concerns about documentation of procedures at the site where formulation occurred. For this reason, the trial was halted by the sponsor after the 5 and 20 μg groups had received 2 vaccinations and only 4 subjects had received the first vaccination with the 80 μg formulation. This documentation issue did not affect stability or potency of the vaccines, which were shown to be stable and potent in assays conducted every six months through the course of vaccinations.
2. Materials and Methods
2.1 Study Design
This study was conducted by Q-Pharm Pty at the Queensland Institute for Medical Research/Royal Brisbane and Women's Hospital in Brisbane, Australia, and was an open-label Phase 1 clinical trial designed to evaluate the safety and reactogenicity of AMA1-C1/ISA 720 in healthy malaria-naïve adults. Eligible volunteers were sequentially recruited and vaccinated in 3 dose cohorts of 12 volunteers each, with vaccinations planned to be given at Day 0, Day 84 and Day 168. In both the 20 and 80 μg dose groups, a subgroup of 4 volunteers were planned to be vaccinated two weeks before the remainder of the group to add an extra margin of safety. The study was conducted under a protocol reviewed and approved by the Institutional Review Board (IRB) of the National Institute of Allergy and Infectious Disease (NIAID), the Western IRB, and by the Queensland Institute of Medical Research-Human Research Ethics Committee. The study protocol was submitted to the U.S. Food and Drug Administration for review as part of Investigational New Drug (IND) application BB-IND#13381 with Clinical Trials Notification to the Australian Therapeutic Goods Administration, in accordance with local regulations. The study was monitored for regulatory compliance and data quality by Clinical Network Services Pty Ltd, with auditing by the Regulatory Compliance and Human Subjects Protection Branch, NIAID, as the IND sponsor. Written informed consent was obtained from all volunteers prior to screening for eligibility for participation. (Clinicaltrials.gov Identifier NCT00487916)
2.2 Participants
Participants were healthy adults ages 18-45. Exclusion criteria included prior malaria infection or prior residence in a malaria-endemic area. Subjects were required to be in good general health, not pregnant, without known clinically significant medical conditions, and were required to have results within normal limits for screening laboratory tests: complete blood count, alanine aminotransferease (ALT), and creatinine, and no serologic evidence of hepatitis B, hepatitis C, or human immunodeficiency virus infection. Urine pregnancy testing was performed at screening as well as prior to each vaccination for females.
2.3. Vaccines
Recombinant AMA1-FVO and AMA1-3D7 were prepared and purified as described previously (3). AMA1-FVO and AMA1-3D7 bulk antigens (drug substances) were both manufactured at the Walter Reed Army Institute of Research (WRAIR) Bioproduction Facility (Silver Spring, Maryland), according to current Good Manufacturing Practices (cGMP). All doses of AMA1-C1/ISA 720 were produced by the Pharmaceutical Development Section, Department of Pharmacy, National Institutes of Health (NIH). AMA1-C1 refers to the 1:1 mixture of AMA1-FVO and AMA1-3D7. Equal weights of AMA1-FVO and AMA1-3D7 were mixed, diluted to the appropriate concentration in saline/167 mM glycine solution, and homogenized in a 3:7 (volume/weight) ratio with Montanide® ISA 720 (Seppic, NJ, US) to produce a stable emulsion with an average droplet size of ∼1 μm [13]. Assays determining droplet size, protein content, identity and integrity, and potency in mice at ∼6, 12, and 18 months after formulation confirmed that stability and potency of the vaccines remained within specifications throughout the period that vaccinations were given. The AMA1-C1/ISA 720 formulations consisted of a total of 5, 20 or 80 μg of AMA1-C1 per 0.5 mL dose, supplied in single-use vials and labeled “For Investigational Use Only.” Vaccines were kept refrigerated at 2°C to 8°C until just before use. Each 0.5 mL dose of the vaccine was delivered by IM injection in the deltoid muscle. Successive vaccinations were given in alternating arms. For participants who received two doses, vaccinations were given at days 0 and 84.
2.4. Safety
Volunteers were observed for 30 minutes after each vaccination to evaluate immediate adverse events and were given thermometers and diary cards to record events occurring during the first month after vaccination. The diary cards were used as a memory prompt and were reviewed with volunteers at follow-up visits, when adverse events were recorded. Subjects were seen at 1, 3, 7, 14, and 28 days after each vaccination, and then approximately monthly for a total of 11 months (to study Day 336). A phone call was also made 21 days after each vaccination to inquire about adverse reactions since the last visit. Solicited adverse events included injection site pain, erythema, and induration, fever, headache, nausea, myalgia, arthralgia, and rash. Pain and solicited adverse events other than fever and urticaria were graded as follows: 0=absent/none, 1=easily tolerated, 2=interferes with daily activity or treatment given, 3=prevents daily activity. Unless otherwise specified, non-solicited adverse events were graded as 0=none, 1=no effect on activities of daily living and no treatment given, 2=partial limitation in activities of daily living or treatment given, 3=activities of daily living limited to <50% of baseline or medical evaluation required. The size of injection-site reactions was measured using a standardized clear plastic measurement device and recorded in the volunteer symptom diary. Injection site erythema, swelling, and induration were graded based on the maximum diameter as follows: mild = > 0 to ≤ 20mm, moderate = 21 - ≤ 50mm, and severe = > 50mm. Hematological (hemoglobin, white blood cell counts, and platelets) and biochemical (ALT and creatinine) laboratory parameters were measured at screening, on days of immunization, and 3, 14, and 56 days after each vaccination. All adverse events were graded for severity and relationship to study product. Serious adverse events were defined as any adverse event resulting in death, life threatening, requiring hospitalization, resulting in disability or incapacity or congenital anomaly or birth defect, or any other event which required intervention to prevent such outcomes. Safety data were reviewed by an external Safety Monitoring Committee prior to second vaccinations in the 20 μg dose group and prior to first vaccinations in the 80 μg dose group.
2.5 Immunogenicity
The standardized methods for performing the ELISA and the growth inhibition assay (GIA) have been described previously [26, 27]. AMA1-FVO and AMA1-3D7 allele specific IgG antibody levels were assessed by ELISA at baseline (Day 0), then at Days 56, 84, 112, 140, 196, 252 and 336 for the 5 and 20 μg groups, and Days 0, 56, 112 and 168 for the 80 μg group. The minimal detection level of this assay was 31 ELISA units and all data below that limit of detection were assigned a value of one half the limit of detection (i.e., 16 units) for analysis. The following conversion factors were used: 1 ELISA unit=0.0294 μg/mL for AMA1-FVO and 1 ELISA unit=0.0329 μg/mL for AMA1-3D7. GIA was performed using purified IgG from the 5 and 20 μg groups at Days 0 and 112. In this assay, purified antibody was added to the parasite cultures at approximately the same concentration as present in the corresponding serum sample (10 mg/mL in GIA well).
2.6. Statistics
Adverse events were summarized by grade and relationship to vaccination; all subjects receiving any vaccination were included in the analysis. For analysis of ELISA antibody responses, the arithmetic average of the AMA1-FVO and AMA1-3D7 ELISA responses for each subject was used as that subject's AMA1-C1 antibody response for that day, because the ELISA responses for the two allelic AMA1 proteins were highly correlated (data not shown), as in previous studies [5-10]. Wilcoxon-Mann-Whitney tests and the associated Hodges-Lehmann confidence intervals were used at each day to compare AMA1-C1 antibody responses between the 5 μg and 20 μg dose groups. Since only the first subcohort of 4 subjects were vaccinated in the 80 μg dose group and these subjects only received one vaccination, we only compared the 80 μg group to the other groups at day 56.
All non-missing observations were used for each statistical test. However, for the graphical representation of geometric mean antibody over time, subjects who were missing any antibody values were not included. To model the relationship between anti-AMA1 antibodies (X) and growth inhibition (Y), we used a Hill function where a is the Hill coefficient, and b is the Ab50 (amount of antibody needed to give 50% growth inhibition):
We fit the model by nonlinear least squares on only the data from the 5 and 20 μg groups at Day 112. The analyses were done using SAS version 9.1, R Version 2.8.1, and Stat Xact Procs Version 8.0.
3. Results
3.1 Participant Flow
Vaccinations began in July, 2007 and were completed in December, 2007. Twelve subjects in the 5 μg group received the first vaccination and 11 received the second. In the 20 μg group, 12 received the first vaccine and 9 received the second. Withdrawals were due to an exacerbation of asthma (unlikely related to vaccination), a work commitment, elective surgery, and a planned holiday. Four of 12 subjects in the 80 μg cohort received one dose of vaccine, after which point vaccinations were stopped. All vaccinated subjects completed follow up for safety except for two volunteers in the 5 μg group who were lost to follow up at weeks 20 and 28.
3.2 Safety
All subjects experienced mild to moderate local injection site reactions (pain, tenderness, or swelling) after each injection (Table 1). Six subjects (one in the 5 μg, four in the 20 μg, and one in the 80 μg dose groups) experienced delayed local reactions (mild to moderate pain) beginning nine days to 16 days after first injection; there were no delayed local reactions after the second injection. Two subjects from the 5 μg group experienced severe tenderness, pain and swelling at the injection site 1-2 days after second vaccination which resolved within five days, and one subject from the 20 μg group experienced grade 3 erythema 2 days after the second vaccination. One subject in the 20 μg group developed a painless, non-erythematous injection site nodule 7 days after second vaccination that resolved spontaneously in 6 days; the maximum diameter was 4 mm. While no subjects were withdrawn prior to second vaccination due to local adverse events, the two subjects in the 5 μg group who had severe pain and swelling after second vaccination would not have received the third dose had the study continued. All systemic reactogenicity was grade 1 or 2 with the exception of one instance of grade 3 malaise after second vaccination in the 5 μg group (Table 2). Other systemic adverse events judged possibly related to vaccination were mild dizziness, pharyngitis, and upper respiratory infection. No rashes related to vaccination occurred.
Table 1.
Local Reactogenicity
| 1st Vaccination | 2nd Vaccination | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Grade 1 | Grade 2 | Grade 3 | Total | Grade 1 | Grade 2 | Grade 3 | |
| 5 μg | ||||||||
| Pain/Tenderness | 12/12 | 9 | 3 | 0 | 11/11 | 7 | 2 | 2 |
| Erythema | 2/12 | 1 | 1 | 0 | 2/11 | 2 | 0 | 0 |
| Swelling | 2/12 | 0 | 2 | 0 | 4/11 | 0 | 2 | 2 |
| Induration | 2/12 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Nodule | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 μg | ||||||||
| Pain/Tenderness | 12/12 | 5 | 7 | 0 | 9/9 | 6 | 3 | 0 |
| Erythema | 2/12 | 2 | 0 | 0 | 4/9 | 1 | 2 | 1 |
| Swelling | 3/12 | 3 | 0 | 0 | 2/9 | 1 | 1 | 0 |
| Induration | 3/12 | 3 | 0 | 0 | 1/9 | 1 | 0 | 0 |
| Nodule | 0 | 0 | 0 | 0 | 1/9 | 1 | 0 | 0 |
| 80 μg | ||||||||
| Pain/Tenderness | 3/4 | 3 | 0 | 0 | NA | |||
| Erythema | 1/4 | 1 | 0 | 0 | NA | |||
| Swelling | 2/4 | 1 | 1 | 0 | NA | |||
| Induration | 1/4 | 1 | 0 | 0 | NA | |||
| Nodule | 0 | 0 | 0 | 0 | NA | |||
Maximum observed grade for each volunteer after each vaccination
Table 2.
Systemic Reactogenicity
| 1st Vaccination | 2nd Vaccination | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Grade 1 | Grade 2 | Grade 3 | Total | Grade 1 | Grade 2 | Grade 3 | |
| 5 μg | ||||||||
| Fever | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 3/12 | 1 | 2 | 0 | 5/11 | 4 | 1 | 0 |
| Nausea/vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malaise | 3/12 | 3 | 0 | 0 | 1/11 | 0 | 0 | 1 |
| Tiredness | 2/12 | 1 | 1 | 0 | 1/11 | 1 | 0 | 0 |
| Arthralgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 μg | ||||||||
| Fever | 0 | 0 | 0 | 0 | 1/9 | 0 | 1 | 0 |
| Headache | 7/12 | 5 | 2 | 0 | 1/9 | 1 | 0 | 0 |
| Nausea/vomiting | 1/12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malaise | 2/12 | 1 | 1 | 0 | 1/9 | 0 | 1 | 0 |
| Tiredness | 4/12 | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| Arthalgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 80 μg | ||||||||
| Fever | 0 | 0 | 0 | 0 | NA | |||
| Headache | 2/4 | 2 | 0 | 0 | NA | |||
| Nausea/vomiting | 0 | 0 | 0 | 0 | NA | |||
| Myalgia | 1/4 | 1 | 0 | 0 | NA | |||
| Malaise | 0 | 0 | 0 | 0 | NA | |||
| Tiredness | 0 | 0 | 0 | 0 | NA | |||
| Arthralgia | 1/4 | 1 | 0 | 0 | NA | |||
Maximum observed grade for each volunteer after each vaccination
Two serious adverse events occurred. One subject in the 20 μg group experienced an exacerbation of asthma one week after first vaccination; this subject also experienced moderate injection site pain in the two days immediately following vaccination. The asthma event was judged unlikely to be related to vaccination but the subject was withdrawn from further vaccinations. Another volunteer in the 20 μg group developed peritonsillar abscess requiring hospitalization 7 weeks after first vaccination, which was judged to be unrelated; this volunteer was not withdrawn from further vaccinations. One volunteer in the 5 μg group had a grade 1 decrease in platelets one week after first vaccination that was judged to be possibly related to vaccination; this volunteer did not experience a delayed local reaction. No other laboratory abnormalities judged to be related to vaccination occurred.
3.3 Immunogenicity
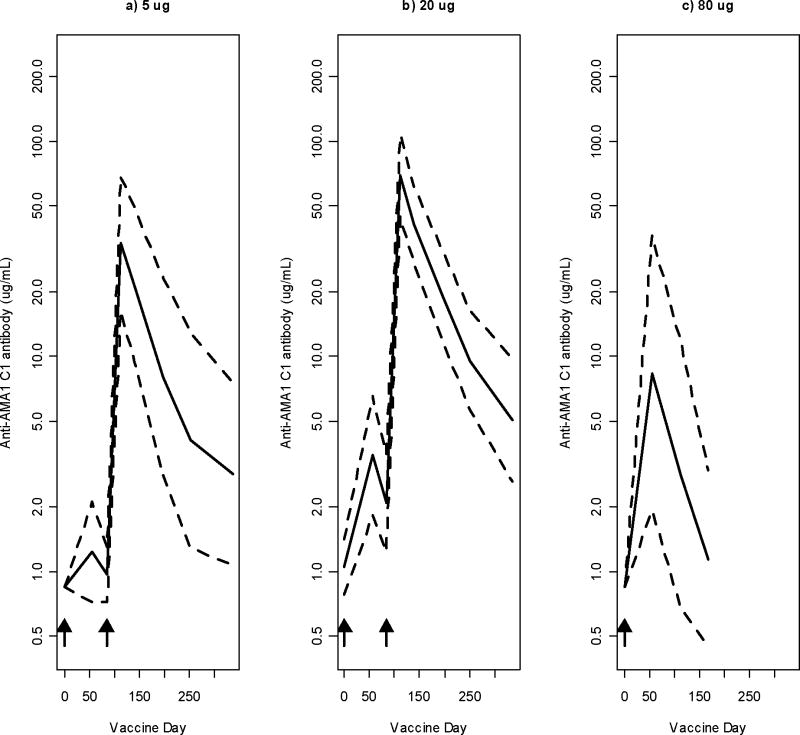
Anti-AMA1-C1 antibody responses for the 3 cohorts are shown in Figure 1. Anti-AMA1 antibody responses were seen following each vaccination. A dose response was observed, with geometric mean antibody levels 2 to 3 fold higher in the 20 μg group than the 5 μg group at all measured times after immunization except day 196, although it was only significantly higher at days 56 (p=0.021) and 84 (p=0.0025) (Table 3). At day 56, after one vaccination, 6.9 (95% CI 2.2, 17.9, p=0.002) and 2.4 (95% CI 0.75, 9.5, p=0.078) fold increases were seen in the 80 μg dose group compared to the 5 μg dose and 20 μg dose groups respectively. These differences were seen even though only 4 subjects received the 80 μg dose. Antibody levels declined rapidly after vaccination.
Figure 1. Antibody Responses of 5, 20, and 80 μg Dose Groups.
Antibody response of volunteers vaccinated with AMA1-C1/ISA 720. Geometric means, with 95% confidence intervals, of anti-AMA1-C1 antibody level are shown for the 5, 20, and 80 μg dose groups (a, b, and c; n=9, 9, and 4 respectively). Arrows indicate the days of immunization; all 3 groups were immunized on Day 0 and the 5 and 20 μg groups were also immunized on Day 84.
Table 3.
Difference in anti-AMA1 antibody between 5 μg and 20 μg dose groups after vaccination
| Study Day | Fold difference (95% CI) | P value |
|---|---|---|
| 56 | 3.11 (1.0 – 5.01) | 0.021 |
| 84 | 3.45 (1.19 - 4.45) | 0.003 |
| 112 | 2.26 (0.99 – 4.66) | 0.056 |
| 140 | 1.95 (0.88 – 4.23) | 0.080 |
| 196 | 1.36 (0.38 – 5.21) | 0.533 |
| 252 | 2.67 (0.78 – 8.95) | 0.931 |
| 336 | 2.30 (0.91 – 5.82) | 0.098 |
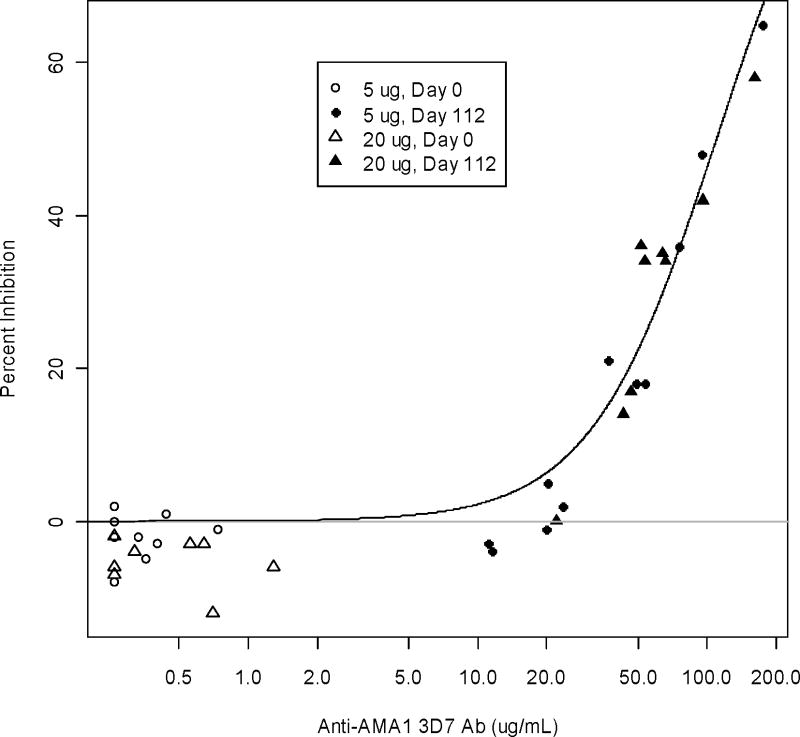
In vitro P. falciparum growth inhibition assays (GIA) were performed using purified IgG isolated from Days 0 and 112 sera from the 5 and 20 μg groups against 3D7 parasites (Figure 2). The growth-inhibitory activity of all subjects at baseline (Day 0) was negligible. At peak antibody response (day 112, 4 weeks after second vaccination), the GIA activity of the 5 μg groups ranged from -4 to 65 % (median 18%) and the 20 μg group ranged from 0 to 58 % (median 34%). As in other studies [9,10], inhibition levels for FVO parasites were slightly less as compared to 3D7 (data not shown).
Figure 2. In Vitro Growth Inhibition.
Biological activity of anti-AMA1 antibodies against P. falciparum 3D7 parasites judged by in vitro growth inhibition assay (GIA). Days 0 and 112 total IgGs from the 5 (n=11) and 20 μg (n=9) cohorts were tested at 10 mg/ml by GIA. The anti-AMA1-3D7 antibody level in the GIA well (x-axis) is plotted against % inhibition (y-axis) to P. falciparum 3D7 parasites. Line is the Hill model fit calculated using only Day 112 data.
As shown in Figure 2, the growth-inhibitory activity was a function of anti-AMA1-3D7 antibody level, regardless of dose of AMA1-C1. The 50% inhibition level (Ab50) for the 3D7 parasites was estimated as 109.9 μg/mL (95% CI 96.5, 129.1) and for FVO parasites was 211.7 μg/mL (95% CI 173.9, 283.6).
4. Discussion
AMA1-C1 is in development as a candidate blood-stage malaria vaccine, and a Phase 2b pediatric trial with the AMA1-C1/Alhydrogel formulation has been completed (8). In that trial the vaccine was only moderately immunogenic and no impact on parasite density or clinical malaria was seen. A more immunogenic formulation of the vaccine may be more likely to be protective; thus, candidate adjuvants are under investigation. Montanide® ISA 720 is an investigational adjuvant that has been shown to improve immune responses. Severe local reactions have been the major drawback to use of this adjuvant, with higher doses and shorter vaccination intervals being more reactogenic [28]. Formulation issues have also limited use of ISA 720, with stability of pre-formulated vaccines an issue in some previous trials, including trials with AMA1 [14,20]. Point-of-injection formulations are another option, however, determining the quality and characterization of bedside formulations, particularly when vortexing or homogenization is required to assure droplet sizes within a narrow range, presents additional problems [13].
In this trial the AMA1-C1/ISA 720 vaccines were pre-formulated and were well characterized, with stability and potency demonstrated throughout the period vaccinations occurred. The vaccines were reasonably tolerated, though some transient, severe, local reactions were seen. Delayed local reactions were also seen, as in other trials with malaria antigens [14,20,23-25]. In a recent Phase 1 trial of AMA1 with three different adjuvants, including ISA 720, 2 of 19 volunteers developed sterile abscesses after a third vaccination with this adjuvant, and contralateral injection site reactions were also reported [25]. In the study described here, one volunteer had a clinically insignificant nodule and no contralateral reactions occurred. Only one subject was removed from further vaccination: a case of asthma thought unlikely to be related to vaccination. However, if three vaccinations had been given as planned, two additional subjects would have been removed from further vaccinations due to the occurrence of severe local reactions.
In two trials in populations where malaria is endemic (Papua New Guinea), malaria vaccines (non-AMA1) containing Montanide® ISA 720 demonstrated fewer and less severe local reactions in the target populations than when tested in malaria naïve adults in Australia [16,17]. The reason for this is not known, but this relative lack of reactogenicity in target populations suggests that if moderate reactogenicity in malaria-naïve adults is accompanied by sustained high-level immunogenicity, the candidate vaccine should be further evaluated in malaria-endemic populations. RTS,S, the malaria vaccine shown to have a consistent benefit in young children in Africa [29,30], is combined with novel emulsion adjuvants (AS02 or AS01) and also has demonstrated some severe local reactions, primarily injection site swelling and erythema. These reactions were transient and generally well tolerated and have not precluded its further development. Severe local reactions have also been seen with another AMA1 vaccine adjuvanted with AS02A that has gone forward to a Phase 2b field trial, although as with RTS,S the local reactions were mostly swelling and erythema and were well tolerated by vaccinees [31].
AMA1-C1/ISA 720 is immunogenic in malaria-naïve Australian adults and demonstrates a dose response. Responses were highly variable, with a wide range of peak antibody levels and growth inhibition seen and with antibody levels declining rapidly after vaccination. Although trials of other formulations of AMA1-C1 have been done at different dosing intervals in different populations and definitive comparisons cannot be made, the antibody level seen here after two vaccinations with 20 μg AMA1-C1/ISA720 at a 12 week vaccination interval was higher than that seen after two vaccinations with 20 μg of AMA1-C1/Alhydrogel at a 4 week interval and was comparable to two vaccinations with 20 μg AMA1-C1/Alhydrogel + CPG 7909 at a 4 week interval (both also in malaria naïve adults) [5, 9]. Given the presumed depot mechanism of action of ISA 720, it was hoped that this adjuvant would lead to more sustained antibody responses, however the responses observed in this study were short-lived. Nevertheless antibody levels at 300 days remained significantly above baseline. Longer follow up would be required to determine if the antibody levels plateau or continue to decline.
While the induced antibody demonstrated correspondingly improved in vitro growth-inhibitory activity in this study, the range of antibody responses and corresponding growth inhibition was wide and antibody levels declined rapidly. Unfortunately the early termination of vaccinations precluded investigation of the highest planned dose and number of vaccinations, so the tolerability and immune response at these higher total doses are not known.
In summary, the blood-stage vaccine AMA1-C1/ISA 720 is reasonably tolerated, though some transient, severe, local reactions were seen. The vaccine is immunogenic in malaria-naïve Australian adults and demonstrates a dose response. The level of antibody response and biologic activity is comparable to other formulations of adjuvanted AMA1-C1. While the vaccines were stable and potent for the duration of this trial, longer-term data showed degradation in droplet size and protein integrity at 18 months (Zhu et al unpublished data). This lack of long-term stability, together with the concerns related to local reactogenicity and the absence of a consistent or prolonged antibody response in the volunteers vaccinated, make further development of this formulation unlikely.
Acknowledgments
This work was supported by the Intramural Division of the National Institutes of Health, National Institute of Allergy and Infectious Diseases and by the PATH Malaria Vaccine Initiative. Thanks to the study participants and the entire study team, especially the Q-Pharm trial staff, Gabrielle McKee, Regina White, and Terrell Carter. Thanks also to the members of the Safety Monitoring Committee: James McCarthy, Kirsten Lyke, and Tony Allworth.
References
- 1.WHO. World Malaria Report. 2008 http://www.who.int/malaria/wmr2008/malaria2008.pdf
- 2.Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends in Parasitology. 2008;24(2):74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infection and immunity. 2002;70(12):6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta S, Lee SY, Batchelor AH, Lanar DE. Structural basis of antigenic escape of a malaria vaccine candidate. PNAS. 2007;104(30):12488–12493. doi: 10.1073/pnas.0701464104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, et al. Phase I clinical trial of AMA1-C1/Alhydrogel®: an asexual blood stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73(6):3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dicko A, Diemert DJ, Sagara I, Sogoba M, Niambele MB, Assadou MH, et al. Impact of a Plasmodium falciparum AMA1 Vaccine on Antibody Responses in Adult Malians. PLoS ONE. 2007;2(10):e1045. doi: 10.1371/journal.pone.0001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicko A, Sagara I, Ellis RD, Miura K, Guindo O, Kamate B, et al. Phase 1 study of a combination AMA1 blood stage malaria vaccine in Malian children. PLoS ONE. 2008 Feb 13;3(2):e1563. doi: 10.1371/journal.pone.0001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagara I, Dicko A, Ellis RD, Fay MP, Diawara SI, Assadou MH, Sissoko MS, et al. A Randomized Controlled Phase 2 Trial of the Blood Stage AMA1-C1/Alhydrogel Malaria Vaccine in Children in Mali. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE. 2008 Aug 13;3(8):e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis RD, Mullen GE, Pierce M, Martin LB, Miura K, Fay MP, et al. A Phase 1 study of the blood-stage malaria vaccine candidate AMA1-C1/Alhydrogel with CPG 7909, using two different formulations and dosing intervals. Vaccine. 2009 Jun 24;27(31):4104–9. doi: 10.1016/j.vaccine.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aucouturier J, Dupuis S, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1:111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence GW, Saul A, Giddy AJ, Kemp R, Pye D. Phase 1 trial in humans of an oil-based adjuvant SEPPIC MONTANIDE ISA 720. Vaccine. 1997;15(2):176–178. doi: 10.1016/s0264-410x(96)00150-8. [DOI] [PubMed] [Google Scholar]
- 13.Miles AP, McClellan HA, Rausch KM, Zhu D, Whitmore MD, Singh S, et al. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine. 2005;23(19):2530–2539. doi: 10.1016/j.vaccine.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Saul A, Lawrence G, Smillie A, Rzepczyk CM, Reed C, Taylor D, et al. Human phase 1 vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA 720 adjuvant. Vaccine. 1999;17:3145–3159. doi: 10.1016/s0264-410x(99)00175-9. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence G, Cheng Q, Reed C, Taylor D, Stowers A, Cloonan N, et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. 2000;18:1925–1931. doi: 10.1016/s0264-410x(99)00444-2. [DOI] [PubMed] [Google Scholar]
- 16.Genton B, Al-Yaman F, Anders R, Saul A, Brown G, Pye D, et al. Safety and immunogenicity of a three-component blood-stage malaria vaccine in adults living in an endemic area of Papua New Guinea. Vaccine. 2000;18:2504–11. doi: 10.1016/s0264-410x(00)00036-0. [DOI] [PubMed] [Google Scholar]
- 17.Genton B, Al-Yaman F, Betuela I, Anders RF, Saul A, Baea K, et al. Safety and immunogenicity of a three component blood stage malaria vaccine (MSP1, MSP2, RESA) against Plasmodium falciparum in Papua New Guinean children. Vaccine. 2003;22:30–41. doi: 10.1016/s0264-410x(03)00536-x. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira GA, Wetzel K, Calvo-Calle JM, Nussenzweig R, Schmidt A, Birkett A, et al. Safety and Enhanced Immunogenicity of a Hepatitis B Core Particle Plasmodium falciparum Malaria Vaccine Formulated in Adjuvant Montanide ISA 720 in a Phase 1 Trial. Infect Immun. 2005;73(6):3587–3597. doi: 10.1128/IAI.73.6.3587-3597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther M, Dunachie S, Keating S, Vuola JM, Berthoud T, Schmidt A, et al. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine. 2005;23:857–864. doi: 10.1016/j.vaccine.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Saul A, Lawrence G, Allworth A, Elliott S, Anderson K, Rzepczyk C, et al. A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA 720 adjuvant. Vaccine. 2005;23:3076–3083. doi: 10.1016/j.vaccine.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Hermsen C, Verhage D, Telgt D, Teelen K, Bousema JT, Roestenberg M, et al. Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine. 2007 Apr 12;25(15):2930–40. doi: 10.1016/j.vaccine.2006.06.081. [DOI] [PubMed] [Google Scholar]
- 22.Audran R, Cachat M, Lurati F, Soe S, Leroy O, Corradin G, et al. Phase 1 Malaria Vaccine Trial with a Long Synthetic Peptide Derived from the Merozoite Surface Protein 3 Antigen. Infect Immun. 2005;73:8017–8026. doi: 10.1128/IAI.73.12.8017-8026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malkin E, Hu J, Li Z, Chen Z, Bi X, Reed Z, et al. A phase 1 trial of PfCP2.9: an AMA1/MSP1 chimeric recombinant protein vaccine for Plasmodium falciparum malaria. Vaccine. 2008 Dec 9;26(52):6864–73. doi: 10.1016/j.vaccine.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Chen Z, Gu J, Wan M, Shen Q, Kieny MP, et al. PLoS ONE. 4. Vol. 3. 2008. Apr 9, Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults; p. e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roestenberg M, Remarque E, de Jonge E, Hermsen R, Blythman H, Leroy O, et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS ONE. 2008;3(12):e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26(2):193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura K, Zhou H, Moretz SE, Diouf A, Thera MA, Dolo A, Doumbo O, Malkin E, Diemert D, Miller LH, Mullen GE, Long CA. Comparison of biological activity of human anti-apical membrane antigen-1antibodies induced by natural infection and vaccination. J Immunol. 2008;181:8776–8783. doi: 10.4049/jimmunol.181.12.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engers H, Kieny MP, Malhotra P, Pink JR. Third meeting on Novel Adjuvants Currently in or Close to Clinical Testing World Health Organization. Vaccine. 2003;21(2526):3503–24. doi: 10.1016/s0264-410x(03)00164-6. [DOI] [PubMed] [Google Scholar]
- 29.Bejon P, Lusingu J, Olutu A, Leach A, Lievens M, Vekemans J, et al. Efficacy of RTS,S/AS01E Vaccine against malaria in Children 5 to 17 Months of Age. NEJM. 2008;359(24):2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 31.Thera MA, Doumbo OK, Coulibaly D, Diallo DA, Kone AK, Guindo Ab, et al. PLoS ONE. 1. Vol. 3. 2008. Safety and Immunogenicity of an AMA-1 Malaria Vaccine in Malian Adults: Results of a Phase 1 Randomized Controlled Trial; p. e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]