Abstract
Background
Patients’ substance use problems are a particularly understudied aspect of psychosocial variables in cancer treatment.
Objectives
The specific hypothesis tested was that lifetime substance use disorders increased the risk of adverse outcome, in the context of other psychosocial and clinical characteristics demonstrated in other studies to have an impact on treatment outcome.
Method
Prospective cohort study of 106 adults with chronic myelogenous leukemia or primary myelodysplastic syndrome. None satisfied criteria for current substance abuse or dependence, but the lifetime rates of substance use disorders in this sample were 28% for alcohol, 12% for cannabis, and 9% for cocaine.
Results
Participants received treatment as directed by their physicians, and were followed until death or the end of the study (median 1.5 years). Twenty-eight died. Multivariate survival analysis identified three predictors of outcome: lifetime cocaine use, associated with a six-fold increased risk of death (p = .04), and two protective variables, baseline hemoglobin (p = .002) and estimated intelligence quotient (IQ) (p = .04).
Conclusion
The results of this study highlight the potential significance of substance use disorders, and lifetime cocaine diagnoses in particular, on treatment outcome for people with chronic myelogenous leukemia or myelodysplastic syndrome. Whereas neither lifetime alcohol nor cannabis use were associated with survival on either the univariate or multivariate models of survival, lifetime cocaine diagnoses were associated with significant six-fold increased risk of death (p =.04).
Keywords: Cancer, cocaine use disorders, stem cell transplantation, survival
INTRODUCTION
Patients’ substance use problems are a particularly understudied aspect of psychosocial variables in cancer treatment. Substance use disorders present a complex set of physical and psychosocial issues that complicate such treatment and may even influence outcome (1). Although the evidence is limited so far, it is hypothesized that patients with substance use disorders are at higher risk for morbidity and mortality while undergoing cancer treatment such as hematopoietic stem cell transplantation (HSCT) (2). While some patients may no longer be actively using alcohol or oher substances as they initiate cancer treatment, it is possible that the longer term medical consequences of lifetime substance use will become manifest.
Appreciation of the predictors of treatment outcome is of potential benefit to patients and their doctors alike. Identification of predictors may signal opportunities to improve and optimize care. Similarly, such knowledge may allow patients to have a more realistic evaluation of their risks that will assist with cancer treatment planning (3). Whereas biomedical variables have been shown to affect HSCT outcome, the contribution of psychosocial variables continues to be an area of active inquiry (4). A possible explanation for the difficulty in evaluating the role of substance use disorders is that patients’ use of alcohol and drugs is only infrequently and incompletely assessed at the inception of treatment (5).
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder first described in 1845. It represents 14% of all leukemias, and there are no known hereditary, familial, geographic, ethnic, or economic associations with CML. CML is considered to be a model in research and management among malignant disorders because of its linkage with a specific chromosomal abnormality (6). Myelodysplastic syndrome (MDS) is a common, acquired, clinically challenging hematologic condition characterized by bone marrow failure and risk of progression to acute leukemia in 20 to 30% of patients. MDS can arise de novo, or less frequently, as a consequence of prior chemotherapy or radiotherapy. The true incidence and prevalence of MDS are not known (7). Neither CML nor MDS involves the central nervous system, and their treatments can be similar, with allogeneic HSCT as a curative possibility.
In a study of mental status changes after hematopoietic stem cell transplantation and other treatment, evaluations of lifetime and current substance abuse disorders were completed in a group of 106 people with CML or primary MDS (8). By study design, none of the people satisfied criteria for current substance abuse or dependence, but it was ascertained that 28% had lifetime alcohol use disorder, 12% had lifetime cannabis use disorder, and 9% had lifetime cocaine use disorder. Recent studies of lifetime rates in the United States indicate 30.3% for alcohol use disorders, 8.5% for cannabis use disorders, and 2.8% for cocaine use disorder (9, 10). Compared to the national rates, it appeared that the study patients had higher rates of both lifetime cannabis and especially cocaine use disorders.
The purpose of this study was to evaluate the predictors of treatment survival in that cohort of 106 patients. The specific hypothesis tested was that lifetime substance use disorders increased the risk of adverse outcome, in the context of other psychosocial and clinical characteristics demonstrated in other studies to have an impact on treatment outcome. These characteristics included type of disease, type of treatment, physical and mental function, hemoglobin at study enrollment, and depression, among other demographic variables (5, 6, 11–14).
METHODS
Participants were 106 adults with CML or primary MDS, enrolled in a study that evaluated their neurocognitive function as they initiated treatment at the Dana Farber/Brigham and Women’s Cancer Care in Boston, MA (84.3%) and other settings (6.5%) including theMassachusetts General Hospital. The remainder responded to Web-based and other study advertisements (9.2%). These 106 participants were enrolled from 336 people screened. Two-hundred thirty were not consented because they were ineligible (30.9%), lived too far away (24.4%), were not interested in participating further (20.0%), were too ill (4.8%), had no time (4.3%), or could not be contacted (3.5%). Other eligibility criteria included reading and listening comprehension of English, and diagnosis within the past year or new treatment plan (e.g., for HSCT) in the year after enrollment. Exclusion criteria included history of significant head injury (resulting in loss of consciousness), or disease or treatment such as intrathecal medication, and current alcohol or substance abuse or dependence. Additional details are available elsewhere (8).
This investigation will focus on characteristics of the participants ascertained at study enrollment and on treatment outcome (dead or alive) over the course of the study. Subjects offered written, informed consent that was reviewed and approved by the Partners Institutional Review Board, which is responsible for the review and approval of all human subject research conducted by the staff of Brigham andWomen’s Hospital and other Partners affiliated hospitals. They received an honorarium of $50.00 for participating in the enrollment interview.
The enrollment interview consisted of: 1) a patient profile to include education, usual occupation, past medical and psychiatric history, and current medications. Usual occupation was coded according to the 1989 General Social Survey prestige scores (15); 2) the Shipley Institute of Living Scale to estimate full-scale intelligence quotient (IQ) (16); 3) the Alcohol and Drug Modules from the Structured Clinical Interview for DSM-IV to establish current and lifetime substance use diagnoses (17); 4) the Medical Outcomes Study 36 Item Short Form (SF-36) to evaluate the physical and mental health of the participant (18); 5) the brief Profile of Mood States (POMS) to provide a summary of general distress or mood with scores ranging from zero to forty-four (19); and 6) the Functional Living Index–Cancer (FLIC), to measure the overall functional quality of the person’s day to day life (20). Each participants’ Hollingshead’s Two-Factor Index of Social position, including occupation and education, was calculated (21).
DATA ANALYSIS
All analyses were carried out using the SAS statistical package (version 9.1). Simple descriptive statistics were calculated and are reported as percentages, means, standard deviations (SD), and ranges, as appropriate.
Time-to-event was calculated from the date of the enrollment interview to the date of the last clinical contact (censoring) or death, whichever came first. Potential predictors of survival were tested independently using the Cox proportional hazards model. The seven variables associated with long-term survival (p < .10) were considered for entry into the final multivariable Cox proportional hazards model. To adjust for potential confounding, six variables associated with long term drug use and socioeconomic status were also forced into the final model, regardless of their significance in the univariate analyses.
A Kaplan Meier curve was generated to illustrate the impact of lifetime cocaine use disorders and estimated total IQ on outcome.
RESULTS
The demographic, clinical, and psychosocial measures are summarized in Table 1. The mean age was 48.1 (SD = 13.4) years, with a range from 21 to 79. There were somewhat more men (55%). The majority was married (57.6%), with the balance being single (22.7%), divorced (11.3%), widowed (3.8%), or other (4.6%).Most were of white, non-Hispanic (86%) background, and nearly half had completed either a 4-year college education (27.4%) or graduate/professional school (20.8%). The mean occupational prestige rating for the group was 47.6 (SD = 21.02), comparable to that for a police officer or public relations specialist (15). The mean Index of Social Position Score from the Hollingshead Two Factor Index was 33.2 (SD = 15.9), or middle class. The range in the sample was 11 to 73, where 11 is the “highest” status, reflecting education and occupation rankings.
TABLE 1.
Participant characteristics at enrollment (N = 106).
| Sociodemographic Variables | |
| Age | 48.1(SD = 1.34) years |
| Gender | |
| Male | 55% |
| Female | 45% |
| Marital Status | |
| Single | 22.7% |
| Married | 57.6% |
| Divorced | 11.3% |
| Widowed | 3.8% |
| Other | 4.6% |
| Race | |
| White, non-Hispanic | 86% |
| Black, non-Hispanic | 6.6% |
| Asian/Pacific Islander | 2.8% |
| Native American | 0.9% |
| Other | 2.8% |
| Estimated IQ | 104.9 (SD = 11.1) |
| Education | |
| Graduate or professional | 20.8% |
| College, 4 year | 27.4% |
| Partial College | 25.5% |
| High school | 22.6% |
| Other | 3.7% |
| Occupational Prestige Score (0–86.05) |
47.6 (SD = 21.02) |
| Hollingshead Two-Factor Index of Social Position | |
| Social Position Score (10–67) | 33.2 (SD = 15.9) |
| Clinical Variables | |
| Disease | |
| CML, stable | 67% |
| CML, accelerated | 19% |
| MDS | 14% |
| Treatment | |
| HSCT | 42% |
| Other Treatment | 58% |
| Baseline Hemoglobin | 11.77(SD = 1.84) |
| Substance Use and Psychosocial Variables | |
| Lifetime Substance Abuse or Dependence | |
| Alcohol | 28% |
| Cocaine | 9% |
| Cannabis | 12% |
| POMS, depression | 9.1 (SD = 8.8) |
| FLIC | 116.3(SD = 23.7) |
| SF-36, Mental Component Score | 45.9 (SD = 10.7) |
| SF-36, Physical Component Score |
45.7 (SD = 9.5) |
Sixty-seven percent had stable phase CML, 19% had accelerated phase CML, and 14% had MDS. The enrollment assessment was completed at a median of 5.6 months after the participants’ diagnosis date; or 6.1 months for the 91 people diagnosed with CML, and 3.8 months for the 15 diagnosed with MDS. Forty-five participants (42%) were treated with an allogeneic HSCT, of which half (53%) used stem cells from a donor related to the patient. Ten of the 45 participants completed their evaluation in the first 8 days prior to stem cell infusion. The remaining 35 HSCT participants completed their evaluation 17 days prior to hospital admission for the procedure. Ninety-four percent of the HSCT recipients received total body irradiation (total dose 14 Gy, in 7 fractions). More people with CML received HSCT than those with MDS (47% vs. 20%, p = .053). Among those with CML treated in other ways, the majority received imantinib mesylate (84.5%); other treatments included hydroxyurea (10.4%) or interferon (4.2%). Treatment other than HSCT for those with MDS included hydroxyurea (17%), supportive treatment (25%), erythropoietin (33%), and azactidine (42%), with some individuals receiving more than one of the listed treatments simultaneously. The mean enrollment Hemoglobin for the participants was 11.77 (SD = 1.84).
None of the participants satisfied criteria for current substance abuse or dependence by design. However, they did fulfill DSM-IV diagnostic criteria for lifetime alcohol abuse or dependence (28%), cocaine abuse or dependence (9%), and cannabis abuse or dependence (12%). There was no difference in rates of lifetime substance use disorders by either disease or treatment group. Participants did not endorse many items consistent with signs or symptoms of depression, averaging a brief POMS score of 9. Scores for the brief POMS range from 0 (no affective signs or symptoms) to 44 (maximum affective signs or symptoms). The mean FLIC score was 116.3; they can range from 22 to 154, where higher scores are consistent with higher function. The mean enrollment SF-36 score for physical (45.7, SD = 9.5) and mental (45.9, SD = 10.7) component summary scales were less than the norms established for a healthy U.S. population with no chronic conditions, but comparable to those with all types of cancer (except skin cancer). The mean total estimated IQ was 104.9 (SD = 11.1), which was average (63rd percentile).
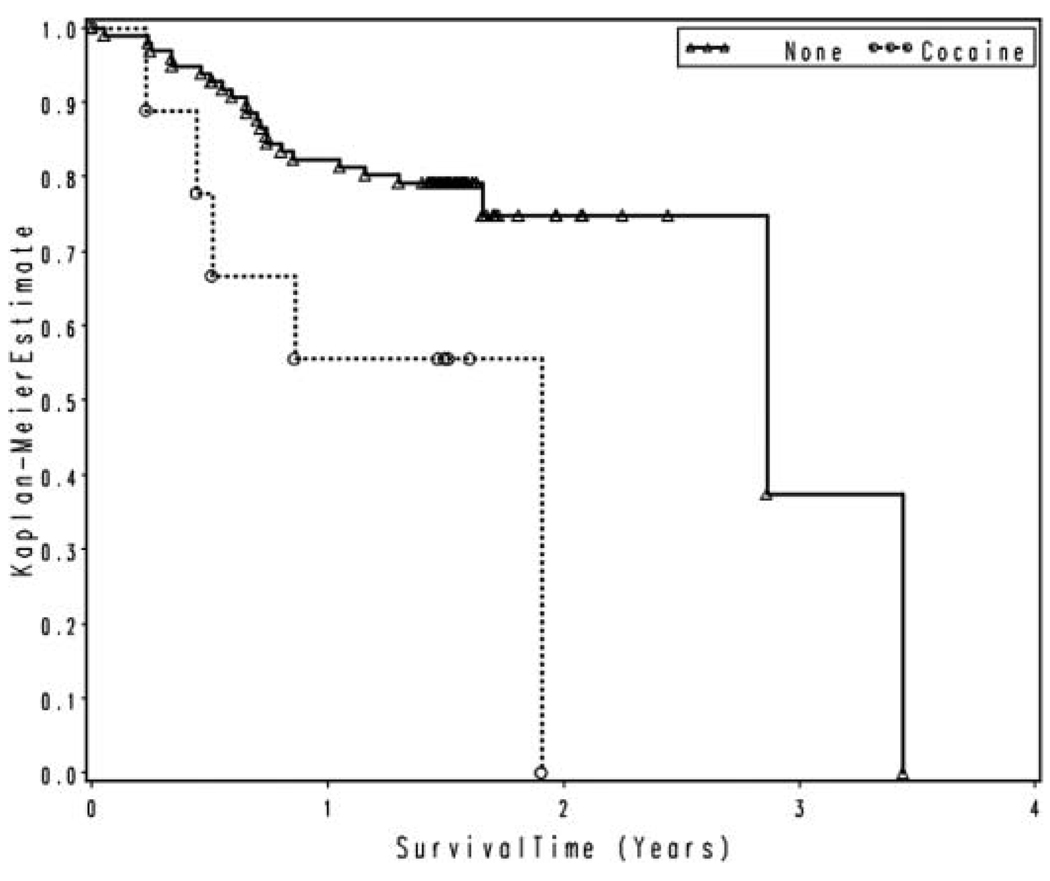
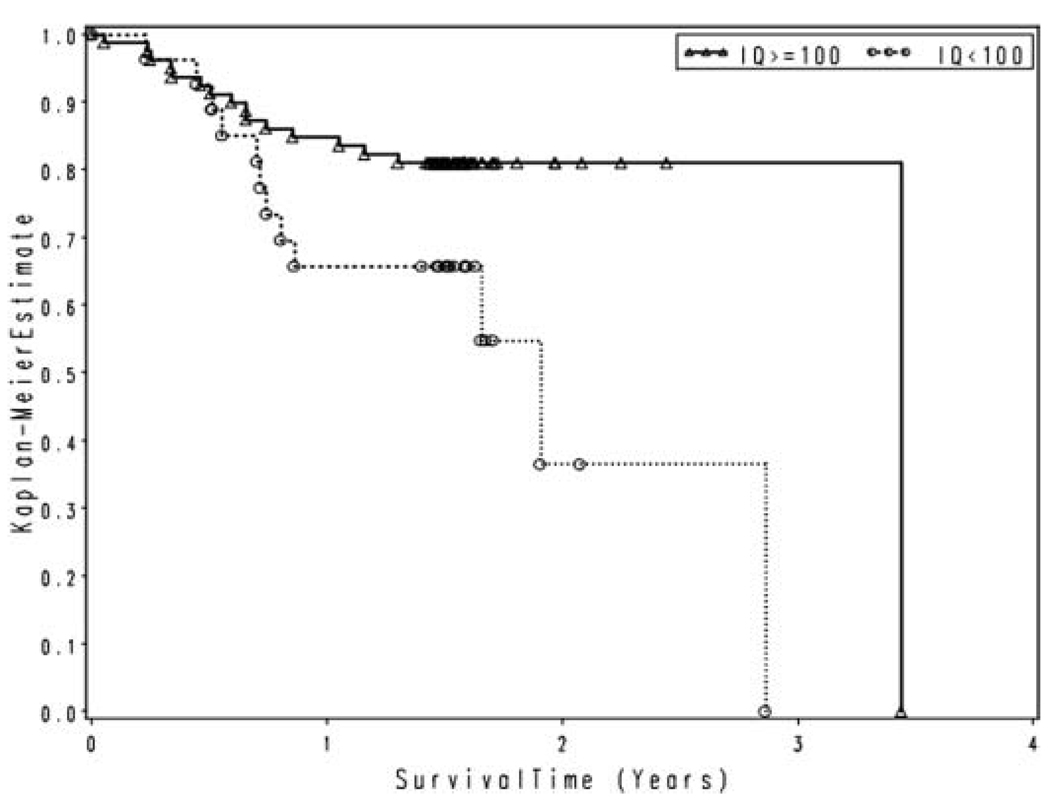
Of the 106 participants enrolled, 28 died in the course of follow-up, with an estimated median time-to-death of 34 months. Among the 78 survivors, median follow-up was approximately 1.5 years. The left-hand side of Table 2 lists the univariate predictors of mortality evaluated at study enrollment. Several predictors significantly increased the risk of mortality (Hazard Ratio, HR > 1.0): type of treatment, (HSCT vs. other treatment, HR = 2.53, p = .022); type of disease (accelerated phase CML vs. stable phase CML, HR = 4.91, p < .0001, and MDS vs. stable phase CML, HR = 2.47, p = .044); and lifetime cocaine abuse or dependence (HR = 3.11, p = .023). Figure 1 compares the estimated survival curves for participants with lifetime cocaine abuse or dependence (23 months median survival) to patients without abuse or dependence (34 months median survival). Univariate predictors with a HR < 1.0 were considered protective against mortality, and included: enrollment hemoglobin (HR = .59, p < .0001); estimated IQ (HR = .96, p = .0084); and physical component score of the SF-36, PCS (HR = .95, p = .0039). Figure 2 compares the estimated survival curves for those participants with an IQ of 100 or more (41 months median survival), with those with an IQ less than 100 (23 months median survival).
Table 2.
Predictors of survival after treatment.
| Variable | Univariate Hazard Ratio | (95% CI) | p-value | Multivariate Hazard Ratio | (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Age | 1 | (.98, 1.04) | 0.54 | 1.01 | (.97, 1.07) | 0.57 |
| Gender | 0.86 | (.4, 1.87) | 0.71 | 0.69 | (.26, 1.85) | 0.46 |
| Race | 0.96 | (.33, 2.8) | 0.94 | 1.21 | (.34, 4.34) | 0.77 |
| Education | 0.99 | (.46, 2.1) | 0.97 | 1.36 | (.46, 4.00) | 0.57 |
| Estimated IQ | 0.96 | (.93, .99) | 0.0084 | 0.95 | (.91, 1.00) | 0.036 |
| Hollingshead | 1.01 | (.98, 1.03) | 0.59 | 1 | (.96, 1.04) | 0.87 |
| POMS | 1.02 | (.98, 1.06) | 0.28 | — | — | — |
| FLIC | 0.99 | (.97, 1.0) | 0.099 | 1.02 | (.99, 1.05) | 0.26 |
| SF-36, MCS | 0.99 | (.96, 1.03) | 0.59 | — | — | — |
| SF-36, PCS | 0.95 | (.91, .98) | 0.0039 | 0.96 | (.90, 1.03) | 0.12 |
| LT Cocaine | 3.11 | (1.17, 8.26) | 0.023 | 6.03 | (1.08, 33.86) | 0.041 |
| LT Alcohol | 1.13 | (.49, 2.6) | 0.78 | 1.37 | (.49, 3.82) | 0.55 |
| LT Cannabis | 0.87 | (.26, 2.92) | 0.93 | 0.45 | (.07, 2.97) | 0.41 |
| CML1 | 8.44 | (3.23, 22.1) | <.0001 | 1.87 | (.48, 7.24) | 0.37 |
| MDS2 | 5.47 | (1.9, 15.7) | 0.0016 | 1.79 | (.34, 9.52) | 0.5 |
| Treatment3 | 2.53 | (1.14, 5.6) | 0.022 | 2.36 | (.79, 7.04) | 0.12 |
| Hemoglobin4 | 0.59 | (.47, .75) | <.0001 | 0.6 | (.43, .83) | 0.0022 |
Comparison between CML-Accelerated Phase and CML-Stable Phase.
Comparison between MDS and CML-Stable Phase.
Comparison between HSCT and other treatment.
Hemoglobin at study enrollment.
FIG. 1.
Survival by lifetime cocaine abuse or dependence.
FIG. 2.
Survival by IQ.
Results of the multivariate survival model are summarized in the right-hand side of Table 2. Both baseline hemoglobin (p = .0022) and estimated IQ (p = .036) at study enrollment were associated with reduced risk of death. For example, for every unit increase in hemoglobin, there was a 40% reduction in risk of death. For every unit increase in IQ, there was a 5% reduction in risk of death. However, lifetime cocaine use disorders were associated with a six-fold increase risk of death (p = .04). Disease, treatment, and other sociodemographic variables, such as quality of life or Hollingshead index of social position, were not associated with outcome. Moreover, neither lifetime alcohol nor cannabis use disorderswere associated with survival on either the univariate or multivariate models of survival.
DISCUSSION
The results of this study highlight the potential significance of substance use disorders, and lifetime cocaine diagnoses in particular, on treatment outcome for people with CML or MDS. The impact of the substance use disorders was evaluated in the context of other psychosocial and clinical variables such as mood, quality of life, enrollment hemoglobin, type of disease, and treatment, all which have been demonstrated in other studies to be independent predictors of outcome. Whereas neither lifetime alcohol nor cannabis use disorders were associated with survival on either the univariate or multivariate models of survival, lifetime cocaine diagnoses were associated with significant six-fold increased risk of death (p = .04).
The prevalence rate of lifetime cocaine use disorders in this sample (9%) was three times the rate reported for the U.S. population (2.8%). Compared to national data, this sample also had a higher rate of lifetime cannabis diagnoses (12% vs. 8.5%), but its rate of lifetime alcohol use was similar (28% vs. 30%). Overall, the participants were among the middle-class, and nearly half had at least a 4-year college education.
The explanation for the association of lifetime cocaine abuse or dependence with increased mortality risk is unknown at present. While few studies in the general population have confirmed that cocaine use has a significant effect on physical health, perhaps reflecting limitations in design, one longitudinal study of over 1000 individuals found that among men, chronic cocaine use increased physical health problems. The longitudinal study controlled for prior health status, current use of cocaine and other drugs, as well as sociodemographic characteristics (22). An early analysis predicted cocaine as a carcinogen in rodents, and more recent work has shown that cocaine augments tumor growth through a cytokine-dependent receptor-mediated mechanism (23, 24).Hence, the explanation for the impact of cocaine on survival in this sample remains to be determined, especially with the increase use of cocaine by older Americans (25).
Other predictors of treatment outcome were evaluated. Estimated IQ and baseline hemoglobin were also identified on the multivariate analysis to be significant predictors of outcome. Both support findings from other research studies. For example, all-cause mortality has been shown to be inversely related to IQ, even after adjustment for social class and deprivation (13). Ideas about the relationship between IQ and survival are speculative, but may include the possibility that better cognitive function is associated with more effective self-care. Similarly, anemia has been shown to be independent prognostic factor in survival after treatment for cancer (12).
Potential limitations to the generalizability of these findings include the design which limited the diseases to CML and MDS, and those without current substance use disorders. The sample size was relatively small, and time of follow-up was limited to the duration of the larger study. Although a thorough substance abuse assessment was completed for each participant, a detailed history of cigarette smoking was not obtained. Toxicology screening to evaluate current use of illicit substances was not obtained; such screening is not a routine practice in cancer treatment at present. Other researchers have found cigarette smoking to be associated with adverse outcome after HSCT (26, 27). Information about compliance with medical recommendations after treatment for the hematologic diseases was not assessed, although patients did return for medical follow-up, and participation rates in the study were very high among survivors: 98% at the initial evaluation, 95% at 12 months, and 89% at 18 months, which reflect their attitudes toward treatment adherence (8). It is possible that non-adherence was greater among certain sub-groups of the participants. Finally, evaluation of all potential, relevant medical variables was beyond the scope of the project. While subsequent research will address these potential limitations, strengths of this study include the systematic evaluation of psychosocial variables in the context of selected medical ones as they related to treatment outcome.
The number of people living with a cancer diagnosis in the United States has tripled in the past three decades, as treatment protocols have resulted in an increased number of survivors (28). Whereas socioeconomic differences in cancer survival are well documented and methods to address such health disparities are areas of active, ongoing inquiry, results of this study suggest that substance use issues may be fruitful areas of future investigation (29).
ACKNOWLEDGMENTS
This study was supported by grants from the American Cancer Society (RSG 01–246–01, GC), and the National Institute on Alcohol Abuse and Alcoholism (K24 AA00289, GC). Alyson Lavigne Dolan and Christina Briegleb were the lead research assistants.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. They alone are responsible for the content and writing of the article.
Contributor Information
Grace Chang, Department of Psychiatry, Harvard Medical School, Boston, Massachusetts, USA, and Department of Psychiatry, Brigham and Women’s Hospital, Boston, Massachusetts, USA
Mary-Ellen Meadows, Department of Psychiatry, Harvard Medical School, Boston, Massachusetts, USA, and Department of Neurology, Division of Cognitive and Behavioral Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA
Jennifer A. Jones, Department of Psychiatry, Brigham and Women’s Hospital, Boston, Massachusetts, USA
Joseph H. Antin, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA, Department of Adult Oncology, Division of Hematologic Malignancies, Dana Farber Cancer Center, Boston, Massachusetts, USA, and Department of Adult Oncology, Division of Hematologic Malignancies, Brigham and Women’s Hospital, Boston, Massachusetts, USA
E. John Orav, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA, and Department of General Medicine and Primary Care, Brigham and Women’s Hospital, Boston, Massachusetts, USA
REFERENCES
- 1.Passik SD, Portenoy RK, Ricketts PL. Substance abuse issues in cancer patients. Part 1: Prevalence and diagnosis. Oncology (Williston Park) 1998;12:517–521. [PubMed] [Google Scholar]
- 2.Stagno SJ, Busby K, Shapiro A, Kotz MM. Patients at risk: Addressing addiction in patients undergoing hematopoetic SCT. Bone Marrow Transplant. 2008;42:221–226. doi: 10.1038/bmt.2008.211. [DOI] [PubMed] [Google Scholar]
- 3.Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J, Christakis N. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–201. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38:255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- 5.Polednak AP. Documentation of alcohol use in hospital records of newly diagnosed cancer patients: A population based study. Am J Drug Alcohol Abuse. 2007;33:403–409. doi: 10.1080/00952990701315236. [DOI] [PubMed] [Google Scholar]
- 6.Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia: Diagnosis and treatment. Mayo Clin Proc. 2006;81:973–988. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 7.Steensma DP, Bennett JM. The myelodysplastic syndromes: Diagnosis and treatment. Mayo Clin Proc. 2006;81:104–130. doi: 10.4065/81.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Chang G, Meadows M-E, Orav EJ, Antin JH. Mental status changes after hematopoietic stem cell transplantation. Cancer. 2009;115:4625–4635. doi: 10.1002/cncr.24496. Published online: 23 June 2009. DOI: 10.1002/cncr.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV Alcohol Abuse and Dependence in the United States. Arch Gen Psych. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 10.Compton WM, Thonas YF, Stinson FW, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States. Arch Gen Psych. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 11.Carey MS, Bacon M, Tu D, Butler L, Bezjak A, Stuart GC. The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecol Oncol. 2008;108:100–105. doi: 10.1016/j.ygyno.2007.08.088. [DOI] [PubMed] [Google Scholar]
- 12.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 13.Hart CL, Taylor MD, Davey-Smith G, Whalley LJ, Starr JM, Hole DJ, Wilson V, Deary IJ, Childhood IQ. social class, deprivation and their relationships with mortality and morbidity risk in later life: Prospective observational study linking the Scottish mental survey 1932 and midspan studies. Psychosom Med. 2003;65:877–883. doi: 10.1097/01.psy.0000088584.82822.86. [DOI] [PubMed] [Google Scholar]
- 14.Crook ED, Peters M. Health disparities in chronic diseases: Where the money is. Am J Med Sci. 2008;335:266–270. doi: 10.1097/maj.0b013e31816902f1. [DOI] [PubMed] [Google Scholar]
- 15.Nakao K, Treas J. Computing 1989 prestige scores. Chicago, IL: NORC; GSS Methological Report No. 70. 1990
- 16.Zachary RA. Revised Manual. Los Angeles, CA: Western Psychological Services; 1991. Shipley Institute of Living Scale. [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. Patient edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. Structured clinical interview for axis I DSM-IV disorders. [Google Scholar]
- 18.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item, short-form health survey (SF-36): Psychometric and clinical uses of validity in measuring physical and mental constructs. Med Care. 1993;331:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Cella DF, Jacobsen PB, Orav EJ, Holland JC, Silberfarb PM, Rafla S. A brief POMS measure of distress for cancer patients. J Chronic Dis. 1987;40:939–942. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 20.Schipper H, Clinch J, McMurray A, Levitt M. Measuring the quality of life in cancer patients: The functional living index—Cancer: Development and validation. J Clin Oncol. 1984;2:472–483. doi: 10.1200/JCO.1984.2.5.472. [DOI] [PubMed] [Google Scholar]
- 21.Miller DC, Salkind NJ, editors. Handbook of Research Design and Social Measurement. 6th Edition. Thousand Oaks, CA: Sage Publications; 2002. Chapter 7.2.5: Hollingshead’s Index of Social Position; pp. 463–469. [Google Scholar]
- 22.Chen K, Scheier LM, Kandel DB. Effects of chronic cocaine use on physical health: A prospective study in a general population sample. Drug Alcohol. 1996;43:23–37. doi: 10.1016/s0376-8716(96)01285-9. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkranz HS, Klopman G. The carcinogenic potential of cocaine. Cancer Lett. 1990;52:243–243. doi: 10.1016/0304-3835(90)90193-2. [DOI] [PubMed] [Google Scholar]
- 24.Garnder B, Zhu LX, Roth MD, Tashkin DP, Dubinette SM, Sharma S. Cocaine modulates cytokine and enhances tumor growth through sigma receptors. J Neuroimmunol. 2004;147:95–98. doi: 10.1016/j.jneuroim.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Aurora T, Gunnerson K, Urrunaga J, Baltarowich L, Muzzin A, Rivers E. Prevalence study of cocaine use in the elderly. Acad Emerg Med. 2000;7(5):499. [Google Scholar]
- 26.Chang G, Orav EJ, McNamara TK, Tong MY, Antin JH. Depression, cigarette smoking, and hematopoietic stem cell transplantation outcome. Cancer. 2004;101:782–789. doi: 10.1002/cncr.20431. [DOI] [PubMed] [Google Scholar]
- 27.Hoodin F, Kallbfleish KR, Thornton J, Ratanatharathorn V. Psychosocial influences on 305 adults’ survival after bone marrow transplantation; depression, smoking, and behavioral self-regulation. J Psychosom Res. 2004;57:145–154. doi: 10.1016/S0022-3999(03)00599-3. [DOI] [PubMed] [Google Scholar]
- 28.Stanton AL. Psychosocial concerns and interventions for cancer survivors. J Clin Oncol. 2006;24:5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 29.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: A review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]