Abstract
Cell envelope compounds of bacteria trigger immune and inflammatory reactions by way of chemokines/cytokines. In this study, we demonstrated that pneumococcal peptidoglycan-polysaccharides (PGPS) induced the production of interleukin (IL)-8 by way of nuclear factor (NF)-κB, nuclear factor interleukin (NF-IL)6, and activation protein (AP)-1 dependent mechanisms in the human bronchial epithelial cells (NL-20) in a dose- and time-dependent manner in vitro, and the mutation of either the NF-κB, NF-IL6, or AP-1 binding sites in the promoter of IL-8 abrogated the IL-8 transcriptional activity. In a similar way, lipopolysaccharides induced the promoter activation of IL-8 in NL-20. However, the PGPS-induced IL-8 promoter activation in rodent middle ear epithelial cells required NF-κB and NF-IL6 but not AP-1.
Keywords: Peptidoglycan-polysaccharides, interleukin-8, bronchial epithelial cell, middle ear epithelial cell, rodent, human
Introduction
Peptidoglycan-polysaccharides (PGPS) are cell envelope components of Gram-positive bacteria exemplified by S. pneumoniae. PGPS, present in middle ear effusion,1 causes chronic inflammation in some tissues: chronic otitis media in the middle ear.2 How PGPS induces the production of interleukin (IL)-8, now referred to as CXCL8 in humans, is not currently fully understood. In inflammatory cells, nuclear factor (NF)-κB (RelA, a central molecule for immune and inflammatory reactions), nuclear factor interleukin (NF-IL)6 (a CCAAT/enhanced binding protein family member frequently responsive to infection),3 and activation protein (AP)-1 (a transcription factor often responding to extracellular signals or extracellular signal-regulated kinases) are involved in the expression of IL-8,4 and it is possible that these molecules are involved in the production of IL-8 in airway and middle ear epithelial cells in response to PGPS.
IL-8 is an important chemokine involved in immune and inflammatory reactions by trafficking leukocytes from the blood stream to inflammatory sites. Knockout of IL-8 receptor in mice diminished infiltration of neutrophils and airway inflammatory and immune responses.5 IL-8 in middle ear effusion is reported as high as 92%6 and is involved in mucin production and chronic changes of otitis media.7
In this study, we demonstrated PGPS stimulated the production of IL-8 by way of an NF-κB, NF-IL6, and AP-1 dependent mechanism in the human bronchial epithelial cells but by way of an NF-κB and NF-IL6 dependent mechanism in the rodent middle ear epithelial cells. Although these two mucosal linings are analogous in many ways, the differential regulation of this pro-inflammatory cytokine may be of pathophysiologic consequence in the airway.
Materials and Methods
Materials
The human bronchial epithelial cell line (NL-20) was purchased from American Type Culture Collection (ATCC, Manassas, VA). Rat and mouse middle ear epithelial cells (rMEECs and mMEECs) were established as previously described.8,9 I kappa B alpha dominant negative mutant (IκBαM), for which serine at position 36 had been changed to alanine, thus preventing phosphorylation and subsequent proteosome degradation in response to stimuli, is a specific inhibitor of NF-κB activity. Pyrrolidine dithiocarbamate (PDTC), an antioxidant that inhibits proteasome-mediated protein degradation, was purchased from Calbiochem (La Jolla, CA) and used as an alternative inhibitor of NF-κB activity. The IL-8 reporter and its mutants (NF-κB mt, AP-1 mt, and NF-IL6 mt) are shown in Figure 4A. β-galactosidase (β-gal) reporter is used as an internal control for luciferase assays in this study. An enzyme-linked immunoabsorbent assay (ELISA) kit for CXCL8 was purchased from R&D Systems (Minneapolis, MN).
Fig. 4.
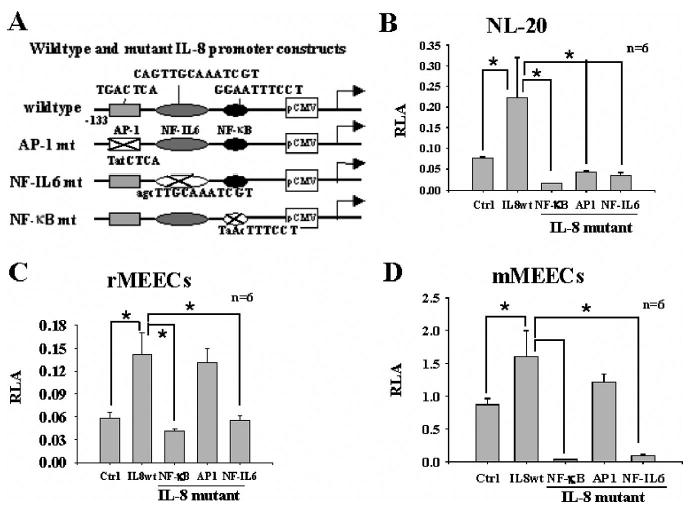
Promoter activity of interleukin (IL)-8 in airway epithelial cells requires nuclear factor (NF)-κB, NF-IL6, or activation protein (AP)-1 activities. Wild-type and mutated IL-8 reporter genes were used (A). (B) Peptidoglycan-polysaccharides (PGPS)-induced IL-8 promoter activity was significantly inhibited when NL-20 cells were transfected with IL-8 mutants (mutations at NF-κB NF-IL6, and AP-1 binding sites, respectively). (C and D) IL-8 promoter activity induced by PGPS was abrogated when rat middle ear epithelial cells (MEECs) and mouse MEECs were transfected with IL-8 mutants (mutations at NF-κB and NF-IL6 binding sites) but not abrogated when cells were transfected with IL-8 mutants (mutations at AP-1 binding site). *P < .05.
Purification and Characterization of Pneumococcal PGPS
PGPS was extracted from S. pneumoniae type 6A (Pn6A) using the method described previously by Tuomanen et al.10 The protein core and carbohydrate moiety of PGPS were measured with protein and hexose assays as described previously.11 To study the biochemistry of PGPS, composition and linkage analysis were performed at the University of Georgia Complex Carbohydrate Research Center using the protocols (gas chromatography/mass spectrometry) described by Merkle and Poppe.12 Lipopolysaccharides (LPS), Gram-positive bacterial envelope metabolites, were purchased from Sigma (St. Louis, MO).
Cell Culture
NL-20 was maintained in Ham's F-12 K culture medium (ATCC) supplemented with 1.5 g/L sodium bicarbonate and 2.0 mmol/L L-glutamine, 10 ng/mL epidermal growth factor, 5 μL/mL insulin-transferrin-sodium selenite (ITS, 100×, Sigma) solution, 2.7 g/L glucose, 500 ng/mL hydrocortisone, 0.1 mmol/L nonessential amino acids, and 4% fetal bovine serum (thereafter referred to as full growth medium [FGM]) with medium change every 3 to 4 days. rMEECs and mMEECs were maintained in the same FGM medium.
Cell Transient Transfection and Luciferase Assay
Cells were cultured to 70% confluence and transfected with IκBαM at 1.4 μg/mL, cotransfected with the reporter genes (IL-8, IL-8 mutants, NF-κB, and β-gal) at 0.6 to 1.4 μg/mL for 16 hours in Opti-MEM (serum-free, Invitrogen, Carlsbad, CA), incubated in FGM for 24 hours, challenged with PGPS at 0.125 to 1.0 μg/mL or LPS at 0.5 to 100 μg/mL for 6 to 24 hours in the presence or absence of PDTC at 10 μmol/L, and harvested for luciferase assays as previously described.9 Cells transfected with empty vector served as controls. Results are presented as a ratio of target luciferase versus β-gal internal control (e.g., relative luciferase activity).
ELISA Assay
Cells were cultured to 40% to 50% confluence and starved for 24 hours in basal medium, namely, Ham's F-12 K supplemented with 1.5 g/L sodium bicarbonate and 2.0 mmol/L L-glutamine but serum free. Cells were incubated with PGPS at 0.125 to 1.0 μg/mL, respectively, in basal medium with 4% fetal bovine serum. Cell culture supernatants were harvested at 6, 12, and 24 hours, respectively, for evaluation of IL-8 protein concentration. Cells incubated with phosphate buffered saline (PBS) served as controls. All experiments were run in triplicate. ELISA was performed as previously described.13
Statistical Analysis
Differences of data between groups in triplicate were analyzed by nonpaired Student's t test, with P < .05 considered statistically significant. Results are presented as mean ± standard deviation (SD).
Results
PGPS Resistant to Host Enzyme Digestion
Pn6A, cell envelope, and PGPS are presented in Figure 1, A to C. PGPS was resistant to the digestion of proteases, trypsins, DNases, and RNases (Fig. 1, C and D) and composed of approximately 8.5% of peptidoglycans and 91.5% of polysaccharides (Fig. 1E). The carbohydrate moiety consisted of 34.9% glucose and 65.1% galactose (Fig. 1E), with a common linkage at 1,2- or 1,4 carbon positions between glucose and galactose residues by linkage analysis (data not shown).
Fig. 1.
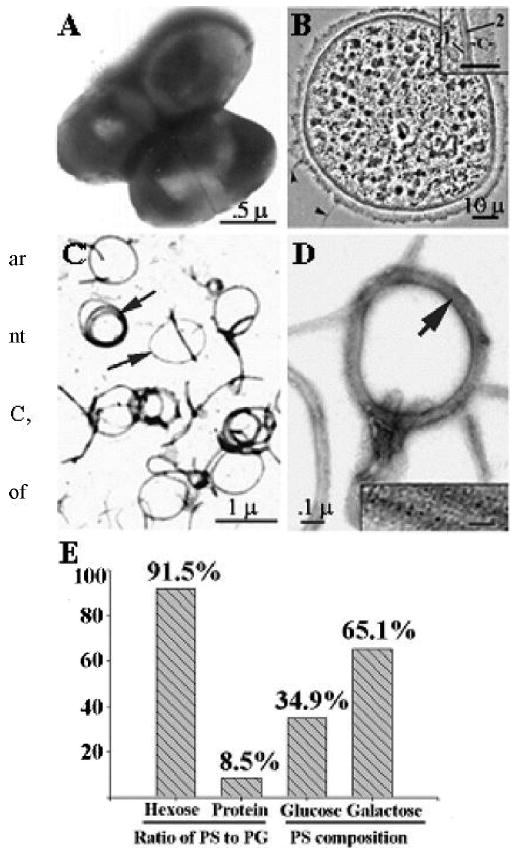
Electron micrograph of intact Pn6A bacteria (A) and cross-sectioned Pn6A bacterium (B) as well as purified peptidoglycan-polysaccharides (PGPS) after treatment with 8% sodium dodecyl sulfate at 100°C for 15 minutes (C, lipid dissolved); 1 = plasma membrane; 2 = cell wall; c = capsular layer. (D) Amplification of C, with insert indicating that peptidoglycan moiety of Pn6A contains filament-like structures (peptidoglycan backbone). Note that polysaccharide moiety of Pn6A is not visible under electron microscopy. Biochemical analysis indicates that ratio of carbohydrate-to-protein of PGPS is approximately 11:1 (91.5% carbohydrates and 8.5% proteins by hexose and protein assays, E). Carbohydrate compositions are mainly glucose (34.9%) and galactose (65.1%) with 1,2- and 1,4-glycosidic linkage as judged by gas chromatography/mass spectrometry.
PGPS Induces the Expression of IL-8 at Both mRNA and Protein Levels
PGPS significantly increased the promoter activity of IL-8 in NL-20 cells at concentrations of 0.25 to 1.0 μg/mL compared with PBS control (Fig. 2A), with PGPS at 0.5 μg/mL being maximal. PGPS at 0.5 μg/mL activated the promoter of IL-8 in a time-dependent manner (Fig. 2B), with PGPS at 6 hours being maximal. Accumulation of IL-8 in tissue culture medium from NL-20 cells in a dose- and time-dependent manner (Fig. 2, C and D) confirmed the luciferase reporter gene findings. It was noted that there was a time lag between the IL-8 promoter activation and peptide release (Fig. 2B vs. 2D). The similar IL-8 promoter activity was observed in rMEECs and mMEECs (Fig. 2, E and F).
Fig. 2.
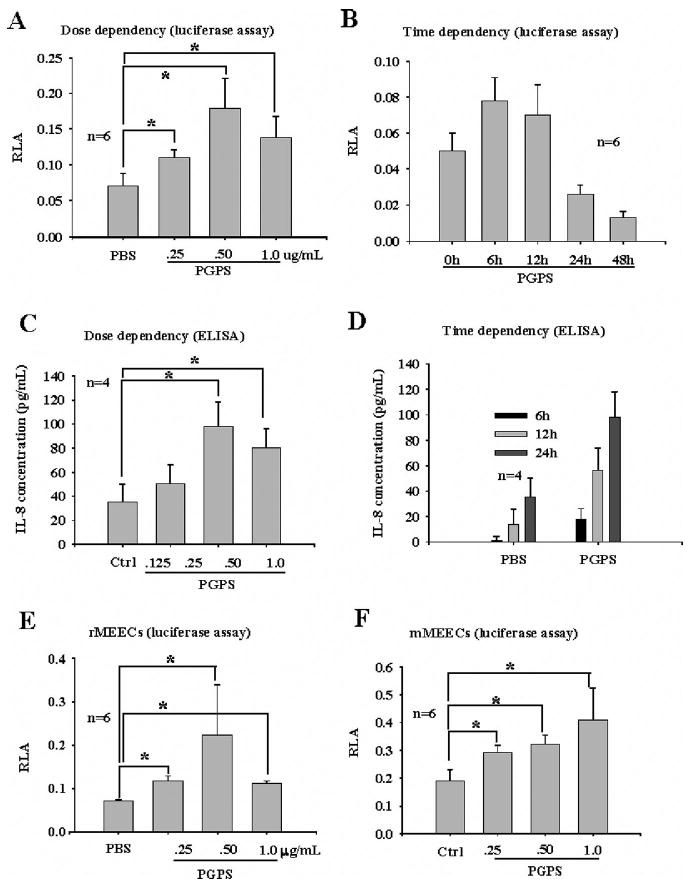
Peptidoglycan-polysaccharides (PGPS) stimulates production of interleukin (IL)-8 in bronchial and middle ear epithelial cells. Luciferase assays indicated that PGPS increased transcription of IL-8 in NL-20 (A and B), rat middle ear epithelial cells (MEECs) (E), and mouse MEECs (F) in dose- and time-dependent manner. Enzyme-linked immunosorbent assay verified that PGPS at 0.5 μg/mL stimulated production of IL-8 peptide in NL-20 in dose- (C) and time-dependent (D) manner. It was noted that normal NL-20 cells produce very little IL-8 (D, phosphate buffered saline), although IL-8 mRNA transcription was detected (A and B). *P < .05.
PGPS Induced-Promoter Activity of IL-8 Dependent on NF-κB in Both Bronchial and Middle Ear Epithelial Cells
PGPS significantly increased the promoter activities of IL-8 and NF-κB in the NL-20 cells (Fig. 3A). To study whether PGPS-induced promoter activity of IL-8 is dependent on NF-κB in NL-20, cells were transfected with IκBαM, cotransfected with the IL-8 reporter gene in the presence or absence of PGPS, and harvested for luciferase assays. In a similar way, cells were transfected with the IL-8 reporter gene, incubated with PGPS in the presence or absence of PDTC at 10 μmol/L, and harvested for luciferase assays. It was demonstrated that both IκBαM at 1.4 μg/mL and PDTC at 10 μmol/L significantly inhibited the PGPS-induced IL-8 luciferase activity in NL-20 (Fig. 3B), rMEECs (Fig. 3C), and mMEECs (Fig. 3D).
Fig. 3.
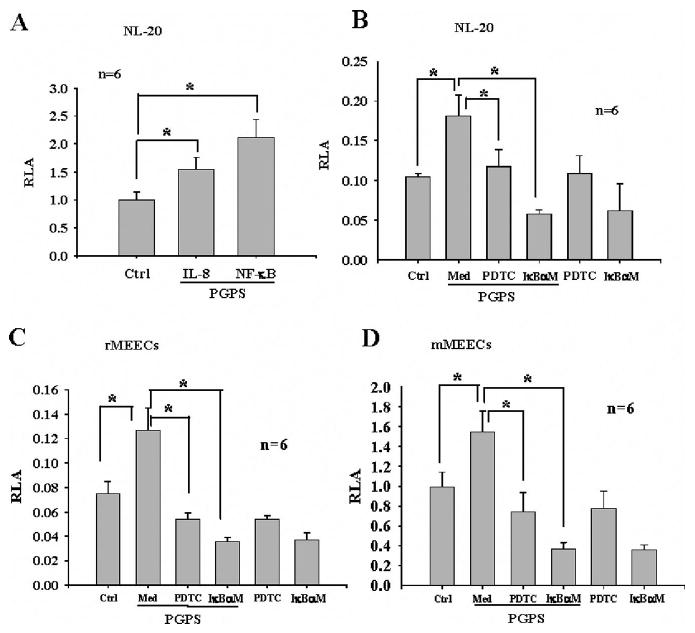
Interleukin (IL)-8 production in NL-20 cells is significantly inhibited by nuclear factor (NF)-κB inhibitors. Peptidoglycan-polysaccharides (PGPS) at 0.5 μg/mL significantly increased activity of both IL-8 and NF-κB reporter genes in NL-20 cells (A, *P < .05). PGPS-induced IL-8 promoter activity was significantly inhibited by pyrrolidine dithiocarbamate and IκBαM in NL-20 cells by luciferase assays (B, *P < .05). Similar results were also observed in rat middle ear epithelial cells (MEECs) (C) and mouse MEECs (D). Med = medium.
PGPS-Induced IL-8 Promoter Activation Abrogated in Bronchial Epithelial Cells but not in Middle Ear Epithelial Cells when AP-1 Binding Site of IL-8 Promoter is Mutated
To study whether AP-1 is required for IL-8 promoter activation in the bronchial and middle ear epithelial cells, the IL-8 promoter with the AP-1 binding site was mutated as indicated in Figure 4A (AP-1 mt). As shown in Figure 4, B to D, the AP-1 binding site mutation in the IL-8 promoter affected the luciferase activity in the NL-20 cells (Fig. 4B, IL-8 mutant, AP-1) but not in rMEECs (Fig. 4C, IL-8 mutant, AP-1) and mMEECs (Fig. 4D, IL-8 mutant, AP-1).
PGPS-Induced Promoter Activity of IL-8 Abrogated in Both Bronchial and Middle Ear Epithelial Cells when NF-IL6 Binding Site is Mutated
The NF-IL6 binding site in the IL-8 promoter was mutated as indicated in Figure 4A (NF-IL6 mt). The results demonstrated that the IL-8 promoter activity was significantly reduced with the NF-IL6 binding site mutation (IL-8 mutant, NF-IL6) in NL-20 (Fig. 4B), rMEECs (Fig. 4C), and mMEECs (Fig. 4D) compared with wild-type controls.
LPS Induces Promoter Activity of IL-8 in Bronchial Epithelial Cells in a Similarly to PGPS
Similar studies were performed using LPS, instead of PGPS, for stimulating IL-8 transcription. As expected, LPS stimulated the promoter activity of IL-8 in a dose-dependent manner in the NL-20 cells (Fig. 5A). The response of cells to LPS was dual. LPS at a range of 0.5 to 1.0 μg/mL significantly increased the promoter activity of IL-8, but the IL-8 promoter activity was decreased at 100 μg/mL. The action was fully dependent on the NF-κB, NF-IL6, and AP-1 activities. Mutations of either NF-κB, AP-1, or NF-IL6 binding sites in the IL-8 promoter fully abrogated the LPS-induced promoter activation of IL-8 in the NL-20 cells (Fig. 5B).
Fig. 5.
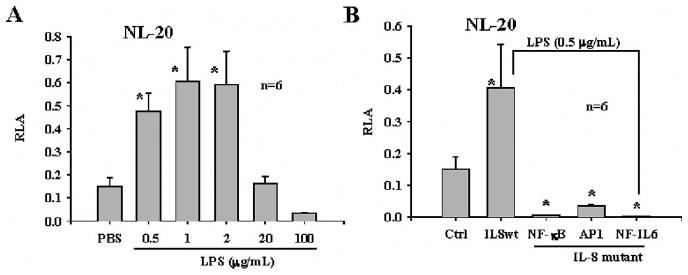
Lipopolysaccharide (LPS) regulates transcription of interleukin (IL)-8 in NL-20 cells in similar way to peptidoglycan-polysaccharides (PGPS). (A) NL-20 cells responded to challenge of LPS in dose-dependent manner, from a range of 0.5 to 2 μg/mL of LPS. Higher concentrations of LPS, 20 to 100 μg/mL resulted in low activity of IL-8 transcription in NL-20 cells. (B) LPS stimulated IL-8 transcription in similar way to PGPS. With nuclear factor (NF)-κB, NF-IL6, and activation protein-1 mutations, LPS-induced IL-8 transcription was abrogated in NL-20 cells.
Discussion
IL-8 is one of the cytokines/chemokines associated with airway inflammation and chronic otitis media. PGPS is difficult to discharge from tissues because it is resistant to digestion by the host enzymes. Our data indicate that the peptidoglycan (the “core” of PGPS) is highly wrapped by carbohydrates, making it difficult to attack by host enzymes, and possibly present in the body for a long-term basis. In this study, we demonstrated that PGPS induced the IL-8 production in rodent middle ear epithelial cells.
PGPS also increased the promoter activity of NF-κB in this study. This is probably by way of toll-like receptors (TLRs, perhaps TLR1 and TLR2) because the referenced study clearly demonstrated that bacteria activate NF-κB by way of TLRs in a variety of epithelial cells, including airway epithelial cells.14 Because the IL-8 promoter area contains the potential NF-κB binding site,15 it is logical to assume that PGPS triggers IL-8 production through the NF-κB transcription factor. Indeed, blockage of NF-κB activity by PDTC (an antioxidant inhibiting proteasome-mediated protein degradation) or IκBαM (a dominant negative mutant of NF-κB) abrogated IL-8 promoter activity. The fact that mutation of the NF-κB binding site in the IL-8 promoter abrogated the IL-8 transcriptional activation in NL-20, rMEEC, and mMEECs (Fig. 4, B to D) verifies the above conclusion. Taken together, the data add support to the notion that PGPS activates NF-κB by way of TLRs, and NF-κB in turn, activates the transcription of IL-8.
In addition to the NF-κB pathway, the IL-8 transcriptional activation by pneumococcal PGPS in the airway epithelial cells involves the NF-IL6 and AP-1 pathways. In bronchial epithelial cells, the activation of the IL-8 gene requires coordinated and simultaneous activation of NF-κB NF-IL6, and AP-1 for maximal reporter gene activity. However, in rMEECs and mMEECs, both NF-κB and NF-IL6 are required for activation, but the mutation at the AP-1 site does not decrease transcriptional activation compared with NL-20 cells. It appears that in bronchial epithelial cells, the three transcription factors work in concert for IL-8 activation. In the middle ear epithelial cells, at least two pathways are needed for IL-8 promoter activation. This may be attributed to cell type or species differences. Understanding the production of IL-8 in the airway and middle ear epithelial cells through early response gene activation provides a theoretical basis for development of innovative therapeutic means to intervene inflammatory reactions induced by pneumococcus or its products.
Acknowledgments
This study is in part supported by the National Institute of Health (grant # DC03433; DC005846; DC04660; CA107989) and Lions 5M Multiple District Hearing Foundation.
Footnotes
Some data included in this paper was presented at the Association of Research in Otorhinolaryngology (ARO) meeting, February 2004, St. Petersburg Beach, Florida, U.S.A.
Bibliography
- 1.Karma P, Sipila P, Virtanen T, et al. Pneumococcal bacteriology after pneumococcal otitis media with special reference to pneumococcal antigens. Int J Pediatr Otorhinolaryngol. 1985;10:181–190. doi: 10.1016/s0165-5876(85)80031-8. [DOI] [PubMed] [Google Scholar]
- 2.Ripley-Petzoldt ML, Giebink GS, Juhn SK, et al. The contribution of pneumococcal cell wall to the pathogenesis of experimental otitis media. J Infect Dis. 1988;157:245–255. doi: 10.1093/infdis/157.2.245. [DOI] [PubMed] [Google Scholar]
- 3.Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 4.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 5.Shi ZO, Fischer MJ, De Sanctis GT, et al. IFN-gamma, but not Fas, mediates reduction of allergen-induced mucous cell metaplasia by inducing apoptosis. J Immunol. 2002;168:4764–4771. doi: 10.4049/jimmunol.168.9.4764. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell KS, Fitzgerald JE, Burleson JA, et al. Interleukin-8 expression in otitis media. Laryngoscope. 1994;104:989–995. doi: 10.1288/00005537-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Smirnova MG, Birchall JP, Pearson JP. In vitro study of IL-8 and goblet cells: possible role of IL-8 in the aetiology of otitis media with effusion. Acta Otolaryngol. 2002;122:146–152. doi: 10.1080/00016480252814144. [DOI] [PubMed] [Google Scholar]
- 8.Toyama K, Kim Y, Paparella MM, Lin J. Temperature-sensitive SV40 immortalized rat middle ear epithelial cells. Ann Otol Rhino Laryngol. 2004;113:967–974. doi: 10.1177/000348940411301206. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya K, Kim Y, Ondrey FG, Lin J. Characterization of a temperature-sensitive mouse middle ear epithelial cell line. Acta Otolaryngol. 2005;125:823–829. doi: 10.1080/00016480510031533. [DOI] [PubMed] [Google Scholar]
- 10.Tuomanen E, Liu H, Hengstler B, et al. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Haruta A, Kawano H, et al. Induction of mucin gene expression in middle ear of rats by tumor necrosis factor-α potential cause for mucoid otitis media. J Infect Dis. 2000;182:882–887. doi: 10.1086/315767. [DOI] [PubMed] [Google Scholar]
- 12.Merkle RK, Poppe I. Carbohydrate composition analysis of glycoconjugates by gas-liquid chromatography/mass spectrometry. Methods Enzymol. 1994;230:1–15. doi: 10.1016/0076-6879(94)30003-8. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Tsuprun V, Kawano H, et al. Characterization of mucins in human middle ear and Eustachian tube. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1157–1167. doi: 10.1152/ajplung.2001.280.6.L1157. [DOI] [PubMed] [Google Scholar]
- 14.Li JD. Exploitation of host epithelial signaling networks by respiratory bacterial pathogens. J Pharmacol Sci. 2003;91:1–7. doi: 10.1254/jphs.91.1. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Kartha S, Iasvovskaia S, et al. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am J Physiol Lung Cell Mol Physiol. 2002;283:L690–699. doi: 10.1152/ajplung.00060.2002. [DOI] [PubMed] [Google Scholar]


