Abstract
(1) Purpose
The purpose of this study was to modify the method of platelet-rich plasma (PRP) preparation for obtaining optimal angiogenic potential and accelerate bone healing. Also, the potential synergistic effect of a suboptimal concentration of bone morphogenic protein-2 (BMP-2) and modified PRP (mPRP) on bone healing was evaluated in vivo.
(2) Materials and Methods
The angiogenic factor-enriched PRP which includes peripheral blood mononuclear cells (mostly lymphocytes and monocytes fraction, excluding polymorphonuclear leukocyte, PMNs) was achieved by lowering concentrations of thrombin and CaCl2, after pre-activation with shear stress using a table-top vortex machine and collagen. In vitro, endothelial cell migration activity in the mPRP group was compared to conventional PRP preparation using a modified Boyden chamber assay. In an animal study, PGA scaffold, PGA scaffold + mPRP, PGA scaffold + mPRP + rhBMP-2, and PGA scaffold + rhBMP-2 were applied to 28 NIH nude rats’ critical size calvarial defects. At 2 weeks, periosteal blood flow was measured using LDPI, and bone formation was evaluated at 8 weeks by histology, DEXA, and μCT.
(3) Results
mPRP induced faster migration of cord blood-derived outgrowth endothelial-like cells. In vivo, mPRP with low dose rhBMP-2 group showed significantly increased numbers of blood vessels at 2 weeks, and notable synergistic effect on bone healing at 8 weeks as evaluated with histology, bone mineral density (BMD) and bone mineral content (BMC, and μCT.
(4) Conclusion
mPRP used in this study improved vascular perfusion around the defect, and resulted in enhanced bone healing. Also, combining mPRP with a suboptimal dosage of rhBMP-2 improved bone formation and enhanced bone density.
Keywords: angiogenic potential, rhBMP-2, athymic rat, calvarial defect
INTRODUCTION
Fast and predictable healing of bone grafts would provide great benefits for implant dentistry. One of the recent strategies to accelerate bone graft healing includes the use of platelet-rich plasma (PRP). Since Balk reported in 1971 that growth factors may be preserved in platelets,1 platelets have been identified as containing factors such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF)-like protein, and connective tissue activating peptide-III (CTAP-III).2,3 Most of these factors are involved in angiogenesis and osteogenesis. Moreover, Sipe et al.,4 reported evidence for the presence of bone morphogenetic proteins (BMPs)-2, 4, and 6, potent osteogenic inducers, within megakaryocytes and platelets. With this composition, autogenous PRP could be an attractive and potent material for bone graft procedures, as it has additional advantages including hydrogel formation suitable for cellular migration and proliferation, while being immunologically inactive. Also, recent studies5, 6 indicate that combinations of pure factors seem to work better than single factors, supporting the promise of PRP.
The genuine effect of PRP on osteogenesis is, however, very controversial, and PRP has failed to show advantages in promoting healing of bone grafts in some recent animal and human applications.7, 8 Possible causes for such unsatisfactory results include an underestimation of the importance of the various factors involved in PRP activation and function. For example, thrombin and calcium, potent platelet activators, could induce immediate growth factor release from platelets in a dose-dependent manner. If their concentration is too high, however, vascular endothelial proliferation could be inhibited9 and the fate of stem cells altered.10 Also, PRP normally exerts its effects in the very early healing period, as the life span of a platelet in a wound and the period of direct influence of its growth factors are less than 5 days.11 Therefore, the mismatch between the long-term process of bone healing and the mode of action of PRP suggests that the angiogenesis effects of PRP may be more relevant than its direct effects on osteogenesis. Angiogenesis, a process of new vessel formation, plays a critical role in bone development. Reconstruction of the circulatory system is one of the earliest events during bone healing. The early establishment of a functionally intact vascular network appears not only to precede the event of bone formation,12 but also to have a substantial influence on the quality of the resulting bone.13
In the present study, the method of PRP activation and preparation were modified to maximize its angiogenic potential, and its bone healing effect was evaluated in vivo. We hypothesized that incorporation of a suboptimal concentration of BMP-2 into the angiogenic factor-enriched PRP would show a synergistic effect on bone healing. To evaluate the osteoconductive effect of angiogenic factor-enriched PRP, fibronectin-coated woven polyglycolic acid (PGA) scaffolds were used as a carrier in a critical-size rat calvarial defect model.
MATERIALS AND METHODS
Preparation of the angiogenic factor-enriched PRP
Fresh human adult whole blood was purchased from AllCells (Emeryville, CA, USA). Peripheral blood mononuclear cells (PBMNCs) and platelets were separately isolated by a density gradient centrifugation (Histopaque-1077, Sigma, St. Louis, MO, USA) according to the manufacturer’s instruction. PBMNCs and platelet concentrate were collected by additional centrifugation (200 g and 1400 g, respectively). Degranulation from platelets was induced with 10 U/ml of thrombin (Sigma) and 0.2 mg/ml CaCl2·H2O after pre-activation with shear stress and 20 μg/ml of collagen, using a table-top vortex machine. The rotation rate was 100 rpm for the first 15 seconds and then, increased to 3500 rpm for 5 minutes. Angiogenic factor-enriched PRP was prepared by incorporation of PBMNCs into the PRP.
Endothelial Migration into Fibrin Gel
Vascular endothelial cell migration activity was measured using a modified Boyden chamber assay described by Osusky et al.14 Cord blood-derived endothelial-like outgrowth cells (CBOECs) having progenitor characteristics, were cultured in our laboratory and used for the experiment. At 80% confluence in culture dishes, cells were starved in EBM-2 medium for 24 hours. 2.0 × 105 CBOECs in 1 ml of serum-free starvation medium were placed into the transwell fibrin gel-coated insert (24 mm diameter, 3.0 μm pore size, Corning Incorporated Costar, Corning, NY, USA). 100 μl of fibrinogen solution (4 mg/ml, Sigma) was applied to the insert and polymerized with 2 kinds of thrombin and calcium solution (10 U/ml of thrombin and 0.2 mg/ml CaCl2·2H2O, and 142.8 U/ml of thrombin and 14.3 mg/ml CaCl2·2H2O) for 30 minutes at 37°C. Fresh EBM-2 medium (3 ml) with the modified PRP or the conventional PRP (300 μl) was added to the lower chamber, and incubated overnight. The insert chamber membrane was fixed with 100% cold methanol and was cut-off around the edge and stained with 0.2% of DAPI (4′-6-Diamidino-2-phenylindole) for 5 minutes. The lower surface of the membrane, which contained the migrated cells, was placed face-up on a histological slide, and covered with a cover slide mounted with PBS/Glycerol solution. The migrated cells were quantified in five random fields for each membrane, using fluorescence microscopy. Assays were performed in triplicate.
Implantation of fibronectin-coated PGA scaffolds loaded with PRP or rhBMP-2
Harvard University and National Institute of Health (NIH) animal care guidelines were followed in all procedures. Anesthesia and pain control followed recommended routines for the species. 28 NIH nude rats were anaesthetized using isoflurane inhalation anesthesia (E-Z Anesthesia, Euthanex Corp., Palmer, PA, USA). Buprenorphine HCl, 0.02–0.03 mg/kg, was administered pre-surgically. After a midline skin incision, a critical size calvarial through-and-through defect, 8.0 mm in diameter, was trephined into the central portion of the cranium using a dental handpiece and trephine bur (Ace Surgical Supply Co. Inc., Brockton, MA, USA) under constant irrigation with sterile saline. Using an aseptic technique, 50μl of angiogenic factor-enriched PRP (1×107 PBMNCs + 1×109 activated platelet) was soaked into the fibronectin-coated woven polyglycolic acid (PGA) scaffold. Ready-made PGA mesh, 1.2 mm in thickness (Synthecon, Houston, TX) was cut into Ø 7.5 mm in diameter and then the circular scaffolds were soaked in 100 μg/ml fibronectin solution (Sigma) for 30 minutes and dried overnight in clean bench under ultraviolet lamp for sterilization. The cell-loaded scaffolds were kept at 37°C during the site preparation to maintain cell viability in the experimental group. Certain angiogenic factor-enriched PRP/BMP-2 scaffolds also contained 200 ng of recombinant human BMP-2 (rhBMP-2, R&D Systems, Minneapolis, MN, USA). The constructs were inserted into the defects and the margins of the wound were closed using nylon sutures. The animals were monitored until they had recovered from anesthesia. In addition, the same scaffolds without PRP or additional factors (blanks) and scaffolds containing either PRP or rhBMP-2 only were applied in the same manner.
Laser Doppler Analysis and Immunohistochemistry
At 2 weeks, periosteal blood flow over the cranial defect was measured using laser Doppler perfusion imaging (LDPI) (PeriScan, Perimed AB, Stockholm, Sweden) in three animals per each group. After a skin incision and meticulous supraperiosteal dissection under inhalation anesthesia, consecutive perfusion measurements were obtained by scanning the size of ROI; 10×10 mm2 above the defects. After LDPI measurements, the animals were euthanized and the cranial bones including the defects were retrieved, fixed in 10% zinc-buffered formalin, and decalcified. Paraffin tissue sections were immunostained for von Willebrand factor (vWF) using a commercial vessel staining kit (Chemicon, Temecula, CA, USA) and imaged by means of a Nikon Eclipse E800 light microscope and a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA). Blood vessels, marked by vWF staining, were counted manually at 100× magnification in randomly selected sites of the defect and normalized to the tissue area with the use of NIH Image J Software.
New Bone Formation
At 8 weeks, four animals per each group were euthanized, and the implants were retrieved and fixed in 10% zinc-buffered formalin. The implants were scanned for bone mineral content (BMC, in grams) and bone mineral density (BMD, in g/cm2) in the region of interest (ROI) sized 8 × 8 mm2 using dual energy x-ray absorptiometry (DEXA, ODR2000+, Hologic Inc., Waltham, MA, USA). The specimens were also imaged two dimensionally with microradiography (Faxitron, Hewlett Packard, McMinnville, OR, USA) and three dimensionally with micro-computed tomography (μ CT40, ScanoMedical, Bassersdorf, Switzerland). The samples were then decalcified, embedded with paraffin and sectioned from the center of the defects. Sections were stained with haematoxylin and eosin.
Statistical analysis
Wilcoxon Rank Sum test was applied to compare the conventional method to the modified method, and also compare experimental groups (PRP only, rhBMP-2 only, and PRP + rhBMP-2) to the control group. The level of significance was set at 5%.
RESULTS
Angiogenic Effects
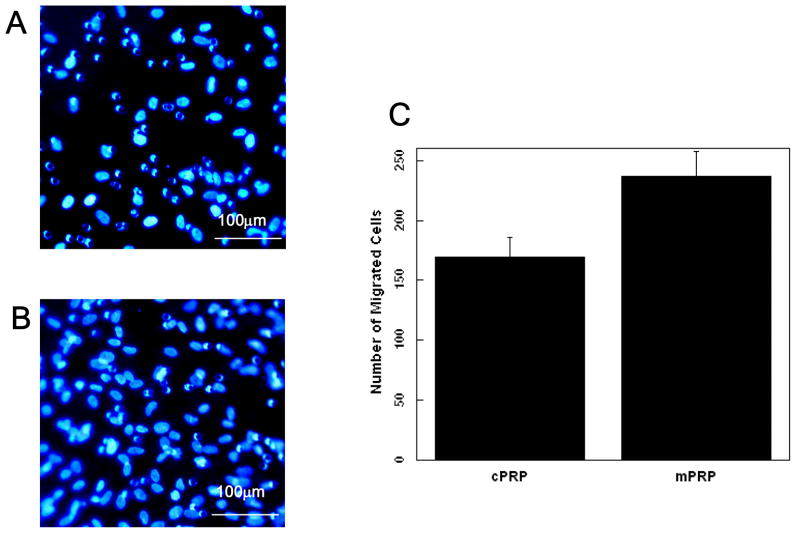
Angiogenic factor-enriched PRP induced faster migration of cord blood-derived outgrowth endothelial-like cells (or differentiated endothelial progenitor cells) in an in vitro model. (Fig. 1)
Fig. 1.
In vitro fibrin gel migration of cord blood-derived outgrowth endothelial-like cells after 48 hours. A. fibrin gel formed with PRP activated by 142.8 U/ml thrombin and 4.3 mg/ml CaCl2 (Conventional PRP), B. fibrin gel formed with PRP activated by 10 U/ml thrombin and 0.2 mg/ml CaCl2 (Modified PRP) (p<0.05), C. Quantification of the number of cells that migrate in response to the conventional PRP preparation (cPRP) and modified PRP (mPRP). Values represent mean and standard deviation.
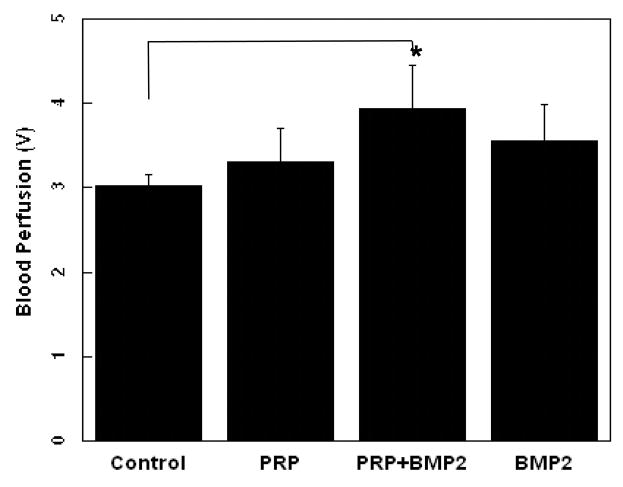
The blood vessel density within defects was analyzed 2 weeks post-implantation. Compared to the control group (42 ± 8 vessels/cm2, Fig. 2A), PRP (66 ± 12 vessels/cm2), rhBMP-2 (72 ± 16 vessel/cm2) and PRP + rhBMP-2 (92 ± 13 vessel/cm2) showed significantly increased numbers of blood vessels. (p < 0.05) (Fig. 2). Supraperiosteal blood perfusions was also improved in the defects grafted with PRP, PRP + rhBMP-2, and rhBMP-2 compared to the control group, but there was no statistically significant difference except for the PRP + rhBMP-2 condition. (p < 0.05, Fig. 3)
Fig. 2.
Staining for vWF in histologic sections from critical size (8 mm) rat craniotomy defects following implantation of fibronectin-coated PGA scaffold only (A) and PGA scaffold + angiogenic factors-enriched PRP (B) at 2 weeks. Blood vessels are denoted with arrows. Quantitative analysis of the number of blood vessels in all groups (C) (p < 0.05). Values represent mean and standard deviation.
Fig. 3.
Supraperiosteal blood perfusion after implantation of fibronectin-coated PGA scaffolds containing angiogenic factor-enriched PRP, and rhBMP-2 at 2 weeks. LDPI Values represent mean and standard deviation.
New Bone Formation
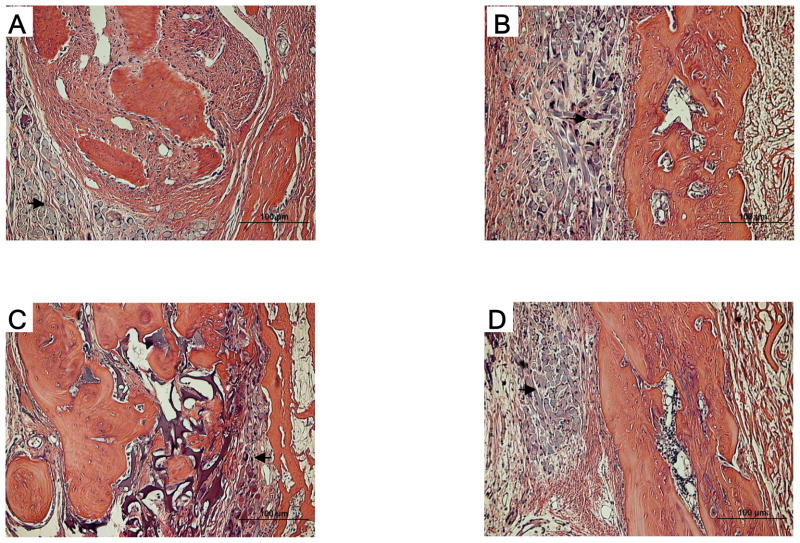
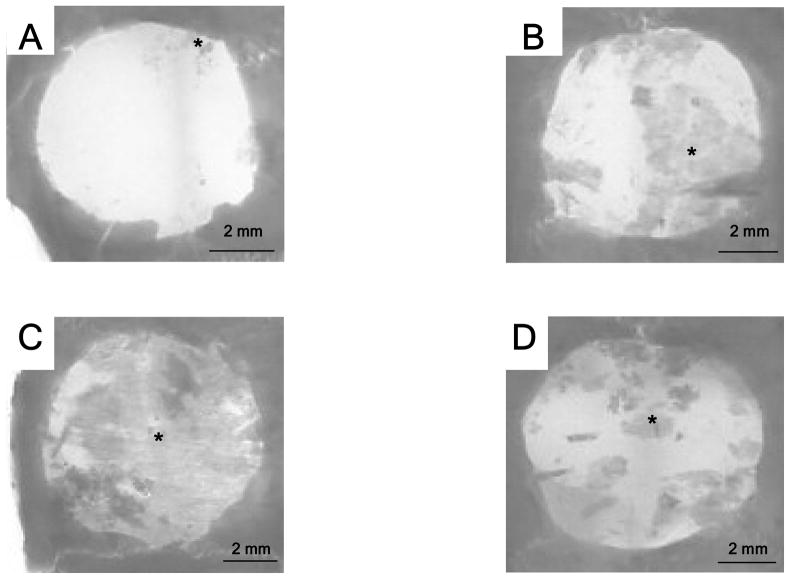
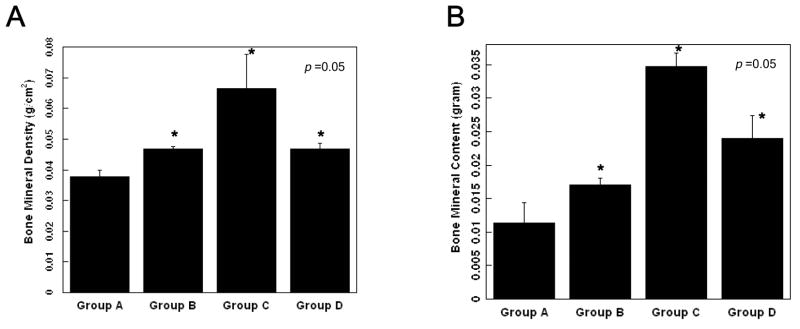
All groups showed favorable tissue response and no significant inflammatory reaction (Fig. 4). In microradiographic images, the control group showed minimal bone formation in the defect, while the angiogenic factor-enriched PRP and rhBMP-2 group showed increased bone healing around the defect margins. The healing of the calvarial defects was improved by angiogenic factor-enriched PRP. This was also confirmed by microCT images. (Fig. 5 & 6) Interestingly, synergistic effects on bone healing with co-administration of PRP and rhBMP-2 were noted. Human angiogenic factor-enriched PRP demonstrated significantly increased BMC (0.017 ± 0.003 g) and BMD (0.46 ± 0.0006 g/cm2) versus control defects (BMC; 0.011 ± 0.003, BMD; 0.037 ± 0.002, p < 0.05, Fig. 7). The rhBMP-2 condition also induced significant bone healing (BMC; 0.024 ± 0.003, BMD; 0.046 ± 0.001), and PRP combined with rhBMP-2 showed the greatest effects. (BMC; 0.034 ± 0.002, BMD; 0.066 ± 0.011)
Fig. 4.
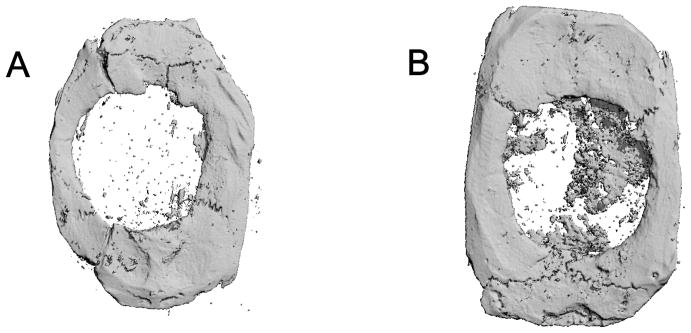
Photomicrographs of histologic sections of critical size (8 mm) rat craniotomy defects following implantation of PGA scaffold, angiogenic factor-enriched PRP and rhBMP-2 (8 weeks). A. blank scaffold only, B. PGA scaffold + PRP, C. PGA scaffold + PRP + rhBMP-2 (200 ng), D. PGA scaffold + rhBMP-2 (200 ng). Compared to the blank scaffold (A), PRP/PGA (B) and rhBMP-2/PGA (D) scaffolds (black arrow) are replaced with more new bone more. In PRP + rhBMP-2/PGA (C) scaffold, new bone is thicker and remains less scaffold remnants (black arrow).
Fig. 5.
Radiographic images of critical size (8 mm) rat craniotomy defects following implantation of fibronectin-coated polyglycolic acid (PGA) non-woven fabric scaffolds with or without angiogenic factors-enriched PRP and rhBMP-2 (8 weeks). A. PGA scaffold only, B. PGA scaffold + PRP, C. PGA scaffold + PRP + rhBMP-2 (200 ng), D. PGA scaffold + rhBMP-2 (200 ng). PRP/PGA (B) and rhBMP-2/PGA (D) scaffolds show more sporadic new bone formations (black asterisk mark) than the blank scaffold (A). In PRP + rhBMP-2/PGA scaffold, new bone formation is distributed in the whole defect.
Fig. 6.
Three dimensional reconstruction of microcomputed tomographic images of critical size (8 mm) rat craniotomy defects (8 weeks). A. blank PGA scaffold only, B. PGA scaffold + PRP
Fig. 7.
Bone mineral density (A) and bone mineral content (B) of the defects following implantation of polyglycolic acid (PGA) non-woven fabric scaffolds, rat angiogenic factors-enriched PRP and/or human recombinant bone morphogenetic protein-2 (rhBMP-2) in 8 mm critical size craniotomy defects of athymic rats (8 weeks). Group A. PGA scaffold only, Group B. PGA scaffold and angiogenic factors-enriched PRP, Group C. PGA scaffold, angiogenic factors-enriched PRP and rhBMP-2 (200 ng), Group D. PGA scaffold and rhBMP-2 (200 ng). Values represent mean and standard deviation. * indicates statistically significant differences, as compared to Group A.
DISCUSSION
These results demonstrate that angiogenic factor-enriched PRP, optimized for early angiogenesis, enhances bone healing in the critical-size rat calvarial defect. More importantly, our findings show that this modified PRP and BMP-2 act synergistically to enhance both bone formation and bone healing. Two factors must likely be considered to overcome the reported inconsistency of the PRP effect in bone healing. One is the short life-span of platelets and their effects, and the other is the insufficiency of the cellular component in some autogenous cortical bone graft and alloplastic materials. Since the life-span of platelets is not more than 1 week, it can hardly be expected that their effect is retained during matrix formation by osteoblasts. In some studies, unsatisfactory effects of bone healing with PRP has been attributed to this short effective time.15 It is well known that angiogenesis plays a critical role in bone formation and healing, and microenviromental alterations stemming from a lack of proper blood supply and ensuing tissue hypoxia may also directly hinder osteoblast differentiation.16 Iliac cancellous marrow contains abundant cellular components that activate angiogenesis and migration of osteoprogenitors, maintain their survival, and promote their differentiation into osteoblasts. In particulate cortical bone or alloplastic bone substitutes, however, proper osteoblastic differentiation or proliferation might be delayed until the hypoxic condition is improved. This may be a reason for the conflicting results with PRP in bone healing. The importance of the cellular component in PRP-induced bone healing was supported by studies using a combined graft of stromal stem cells, PRP and allogenic bone in sheep long bone critical size defects.17
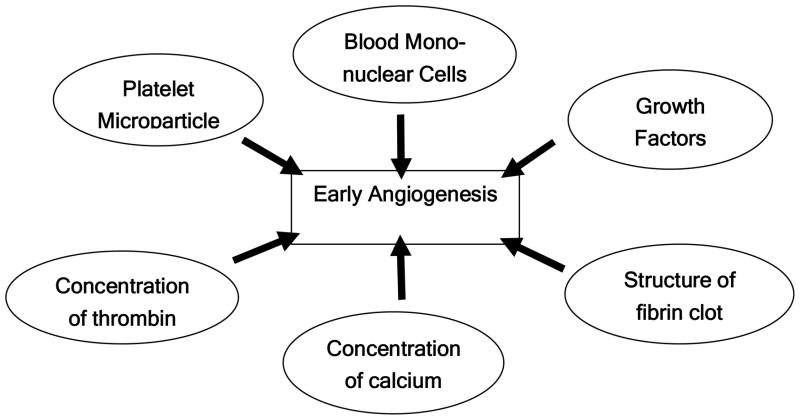
Through literature review, five factors in PRP preparation that likely influence angiogenesis can be identified. They are the concentration of thrombin and calcium, initial release of VEGF, morphology of the fibrin clot, production of platelet microparticles (PMPs), and peripheral blood mononuclear cells (PBMNCs). In a pilot study, we found that a low dose of thrombin (10 U/ml per 1×109 platelets) with 0.2 mg/ml CaCl2 could enhance proliferation of vascular endothelial cells and initial VEGF release (data not shown). These results correspond to previous studies. Borrelli et al. reported that 0.1 U/ml of thrombin is the maximum concentration to increase endothelial cell proliferation done with 4×104 cell number, and Maloney et al. found that over 1 U/ml of thrombin per 3 × 108 platelets approximately decreased the initial release of VEGF.9, 18 Also, thrombin was known to increase the migration of osteogenic cells (up to 1 U/ml).19 One potential limitation, though, is that low amounts of thrombin decrease the activation of platelets, resulting in diminished production of PMPs. Microparticles (0.05–1μm) are small vesicles that are released from all cell types subjected to activation, and their composition reflects the state of the membrane of the originating cell. Although the exact functions of microparticles are uncertain, microparticles generated from activated platelets (PMPs) were known to stimulate vascular endothelial cell proliferation, angiogenesis, and vascular hyperactivity resulting in increased cellular migration, hematopoietic cell proliferation and their engraftment.20 It has been also reported that the effect of 5×105/ml PMPs appears to be at least as potent as that of 50 ng/ml VEGF in vitro. Therefore, sufficient PMP production is another strategy to enhance rapid angiogenesis21. Decreased PMP production could be compensated with application of shear stress and pre-activation with a low dose of collagen (20μg/ml), according to a previous report.22 This type of preparation lid to faster migration of endothelial progenitors through a fibrin clot than conventional PRP (high dose thrombin and calcium), and it was suggested that the fibrin configuration influenced cell behavior in addition to the increased angiogenic factors. In previous studies, it was shown that high thrombin concentrations makes fibrin highly branched and reduces cell migration into bone defects.9 Further studies are needed to clarify the correlation between fibrin morphology and cell migration.
In conventional PRP, the whole leukocyte fraction has been usually excluded because of an adverse inflammatory reaction. But peripheral blood mononuclear cells, one component of the leukocyte cell population, have been suggested to give beneficial effects in angiogenesis. Some of these cells enhance arterial formation and collateral arterial growth,23 and their effects can be augmented by incorporation of platelets. An advantage of inclusion of the peripheral blood mononuclear cell fraction into PRP is that the angiogenic effect can be maintained up to 1 month, and this complements the short term effect of the growth factors in PRP.24 Our study demonstrates that these integrated approaches in PRP preparation improve vascular perfusion around the defect (periosteum) and new vessel formation inside the defect, and result in enhanced bone healing (Fig. 8). The angiogenic factor-enriched PRP improved bone healing and increased bone density.
Fig. 8.
Factors to be considered for preparation of platelet-rich plasma to improve new bone formation through enhanced early angiogenesis.
Another interesting feature of these studies is that PRP and BMP-2 acted synergistically in bone formation to enhance bone density. A major challenge to the successful application of BMP in the clinical situation is the significant amounts of the protein required for bone formation.25 Therefore, combining anabolic agents that have a synergistic effect is an attractive solution to this problem. Although there is some evidence of BMPs in platelets, PRP is known to have a strong mitogenic effect without osteogenic differentiation. Our study shows that angiogenic factor-enriched PRP supports the function of a reduced concentration of BMP. This could be advantageous, as lower BMP doses may diminish adverse reactions to exogenous, genetically modified synthetic proteins and also reduce the cost of the therapy. Our result is supported by previous findings of synergy in bone formation by combined VEGF and BMP-4 application, and co-administration of a low dose of FGF and BMP-2 in vivo.5, 26, 27 But, it is unclear whether this synergistic effect comes from an enhanced osteogenic function of BMP-2 by PRP, or augmented angiogenesis of PRP by the BMP-2. BMP-2 is an important protein in vascular development, and in our study, vascular perfusion and vessel formation were improved with a small amount of BMP-2 (200 ng per animal). However, this finding contradicts previous reports, which indicated that the osteogenic differentiation induced by BMPs can be suppressed by platelet-released supernatant in vitro.28 Such conflicting results may be attributable to the differences between in vivo and in vitro studies. Additional experiments are needed to clarify the exact mechanism, and the optimal ratio between angiogenic factor-enriched PRP and BMP-2.
In summary, the modified PRP used in this study plays a favorable role in bone regeneration, and its effects appear to be mediated by improved angiogenesis. The findings also suggest a reduced effective dose of osteogenic protein (BMP-2) when used with this PRP preparation. Bone healing is a multi-factorial process and PRP contains many components whose interactions may be altered by the preparation method. Therefore, all these factors should be carefully considered and controlled to generate beneficial effects and prevent detrimental reactions.
Acknowledgments
The authors acknowledge generous financial support from Academy of Osseointegration 2005 AO/3i research grant, and the NIH/NIDCR (R37 DE013033) for this work.
Footnotes
This work was presented at the 21st Annual Meeting of Academy of Osseointegration, Seattle, WA, March 2006.
Contributor Information
Eun-Jin Park, Department of Restorative Dentistry and Biomaterials Sciences, Harvard School of Dental Medicine, 188 Longwood Ave. Boston, MA 02115.
Eun-Seok Kim, Department of Oral and Maxillofacial Surgery, College of Medicine, Chungnam National University, 640 Daesa-Dong, Jung-Gu, Daejeon 302-722, South Korea.
Hans-Peter Weber, Department of Restorative Dentistry and Biomaterials Sciences, Harvard School of Dental Medicine, 188 Longwood Ave. Boston, MA 02115.
Robert F. Wright, Department of Restorative Dentistry and Biomaterials Sciences, Harvard School of Dental Medicine, 188 Longwood Ave. Boston, MA 02115.
David J. Mooney, Email: mooneyd@deas.harvard.edu, Division of Engineering and Applied Sciences, Harvard University, Pierce Hall 319, 29 Oxford Street, Cambridge, MA 02138, Phone: 617-384-9624 Fax: 617-495-9837.
References
- 1.Balk SD. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci USA. 1971;68(2):271–5. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazono K, Takaku F. Platelet-derived growth factors. Blood Rev. 1989;3(4):269–76. doi: 10.1016/0268-960x(89)90034-9. [DOI] [PubMed] [Google Scholar]
- 3.Schliephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–84. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 4.Sipe JB, Zhang J, Waits C, Skikne B, Garimella R, Anderson HC. Localization of bone morphogenetic proteins (BMPs)-2, -4, and -6 within megakaryocytes and platelets. Bone. 2004;35(6):1316–22. doi: 10.1016/j.bone.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35(2):562–9. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20(5):848–57. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 7.Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, Safadi FF, Wikesjo UM. Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. J Clin Periodontol. 2005;32(9):966–72. doi: 10.1111/j.1600-051X.2005.00772.x. [DOI] [PubMed] [Google Scholar]
- 8.Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16(3):349–56. doi: 10.1111/j.1600-0501.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 9.Borrelli V, Sterpetti AV, Coluccia P, Randone B, Cavallaro A, Santoro D’Angelo L, Cucina A. Bimodal concentration-dependent effect of thrombin on endothelial cell proliferation and growth factor release in culture. J Surg Res. 2001;100(2):154–60. doi: 10.1006/jsre.2001.6231. [DOI] [PubMed] [Google Scholar]
- 10.Loeb MJ. Altering the fate of stem cells from midgut of Heliothis virescens:the effect of calcium ions. Arch Insect Biochem Physiol. 2005;59(4):202–10. doi: 10.1002/arch.20060. [DOI] [PubMed] [Google Scholar]
- 11.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 12.Glowacki J. Angiogenesis in fracture repair. Clin Orthop. 1998:S82–9. doi: 10.1097/00003086-199810001-00010. [DOI] [PubMed] [Google Scholar]
- 13.Wallace AL, Makki R, Weiss JB, Hughes SP. Measurement of serum angiogenic factor in devascularized experimental tibial fractures. J Orthop Trauma. 1995;9:324–32. doi: 10.1097/00005131-199509040-00009. [DOI] [PubMed] [Google Scholar]
- 14.Osusky KL, Hallahan DE, Fu A, Ye F, Shyr Y, Geng L. The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis. 2004;7(3):225–33. doi: 10.1007/s10456-004-3149-y. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005;63(3):370–6. doi: 10.1016/j.joms.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Salim A, Nacamuli RPP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biolo Chem. 2004;279:40007–16. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- 17.Lucarelli E, Fini M, Becheroni A, Giavaresi G, Bella CD, Aldini NN, Guzzardella G, Martini L, Cenacchi A, Maggio ND, Sangiorgi L, Fornasari PM, Mercuri M, Giardino R, Donati D. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin Orthop Relat Res. 2005;435:62–8. doi: 10.1097/01.blo.0000165736.87628.12. [DOI] [PubMed] [Google Scholar]
- 18.Maloney JP, Silliman CC, Ambruso DR, Wang J, Tuder RM, Voelkel NF. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol. 1998;275(3 Pt 2):H1054–61. doi: 10.1152/ajpheart.1998.275.3.H1054. [DOI] [PubMed] [Google Scholar]
- 19.Karp JM, Tanaka TS, Zohar R, Sodek J, Shoichet MS, Davies JE, Stanford WL. Thrombin mediated migration of osteogenic cells. Bone. 2005;37(3):337–48. doi: 10.1016/j.bone.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Martinez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005;288(3):H1004–9. doi: 10.1152/ajpheart.00842.2004. [DOI] [PubMed] [Google Scholar]
- 21.Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124(3):376–84. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88(9):3456–64. [PubMed] [Google Scholar]
- 23.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002 Dec;283(6):H2411–9. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 24.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106(15):2019–25. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 25.Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, et al. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001;19:332–5. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- 26.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetis protein-4. J Clin Invest. 2002;110:751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, Wakitani S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone. 2005;36:399–407. doi: 10.1016/j.bone.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Gruber R, Kandler B, Fischer MB, Watzek G. Osteogenic differentiation induced by bone morphogenetic proteins can be suppressed by platelet-released supernatant in vitro. Clin Oral Impl Res. 2006;17:188–93. doi: 10.1111/j.1600-0501.2005.01216.x. [DOI] [PubMed] [Google Scholar]