Abstract
Cell attachment and the assembly of cytoskeletal and signaling complexes downstream of integrins are intimately linked and coordinated. Although many intracellular proteins have been implicated in these processes, a new paradigm is emerging from biochemical and genetic studies that implicates integrin-linked kinase (ILK) and its interacting proteins, such as CH-ILKBP (α-parvin), paxillin, and PINCH in coupling integrins to the actin cytoskeleton and signaling complexes. Genetic studies in Drosophila, Caenorhabditis elegans, and mice point to an essential role of ILK as an adaptor protein in mediating integrin-dependent cell attachment and cytoskeletal organization. Here we demonstrate, using several different approaches, that inhibiting ILK kinase activity, or expression, results in the inhibition of cell attachment, cell migration, F-actin organization, and the specific cytoskeletal localization of CH-ILKBP and paxillin in human cells. We also demonstrate that the kinase activity of ILK is elevated in the cytoskeletal fraction and that the interaction of CH-ILKBP with ILK within the cytoskeleton stimulates ILK activity and downstream signaling to PKB/Akt and GSK-3. Interestingly, the interaction of CH-ILKBP with ILK is regulated by the Pi3 kinase pathway, because inhibition of Pi3 kinase activity by pharmacological inhibitors, or by the tumor suppressor PTEN, inhibits this interaction as well as cell attachment and signaling. These data demonstrate that the kinase and adaptor properties of ILK function together, in a Pi3 kinase–dependent manner, to regulate integrin-mediated cell attachment and signal transduction.
INTRODUCTION
The integrin-linked kinase (ILK) is an ankyrin-repeat containing serine/threonine protein kinase that interacts with the cytoplasmic domain of β 1 and β 3 integrins and regulates integrin-dependent functions (Hannigan et al., 1996). ILK couples integrins and growth factors to downstream signaling pathways, leading to the regulation of such diverse processes as cell cycle progression, survival, division, and changes in morphology and spreading (reviewed in Dedhar, 1999, 2000, Wu and Dedhar, 2001). At the molecular level, ILK has been demonstrated to induce the phosphorylation and activation of PKB/Akt (at Ser-473), and the phosphorylation and inhibition of GSK3 (at Ser 21/9; Delcommenne et al., 1998, Persad et al., 2000, 2001). This leads to the activation of cyclin D1 (D'Amico et al., 2000) and several transcription factors, such as AP-1 (Troussard et al., 2000), NFKB (Tan et al., 2002), and the β-catenin T cell/lymphoid enhancer factor 1 (TCF/LEF) complex (Tan et al., 2001, Persad et al., 2000) and likely explains many of ILK's oncogenic properties. ILK activity is Pi3 kinase and phosphoinositide-dependent (Delcommenne et al., 1998; Lynch et al., 1999; Persad et al., 2000); in PTEN-null prostate cancer cells in which PiP3 levels are high, ILK is constitutively active (Persad et al., 2000). PTEN has also been shown to play a role in the regulation of integrin-mediated function by suppressing migration in a variety of cell types and altering focal adhesion formation (Tamura et al., 1998; Liliental et al., 2000; Yamada and Araki, 2002).
The link between ILK and cytoskeletal organization, however, has remained more elusive. It is known that upon integrin-mediated cell adhesion to the extracellular matrix (ECM), a massive reorganization of the actin cytoskeleton occurs, resulting in the formation of focal adhesion plaques (Zamir et al., 1999; Petit and Thiery, 2000). Many proteins, including catalytic proteins such as ILK (Li et al., 1999) and focal adhesion kinase (FAK; Parsons et al., 2000), and structural proteins such as talin, vinculin and paxillin, are recruited to these focal adhesions in response to cell adhesion (Calderwood et al., 2000; Zamir and Geiger, 2001). This leads to morphological changes that contribute to cell spreading, migration, and cell signaling.
Recently, several structural focal adhesion components have been identified that interact with ILK directly. The calponin homology domain-containing ILK binding protein CH-ILKBP (also known as α-parvin and actopaxin) was identified as an interactor with the C-terminus of ILK (Tu et al., 2001). CH-ILKBP localizes to focal adhesions and the cytoskeleton and has been shown to regulate cell adhesion and spreading and the localization of ILK to focal adhesions (Zhang et al., 2002). It has also been demonstrated that ILK, CH-ILKBP, and the LIM protein PINCH form a ternary complex at fibrillar adhesions, and disruption of this complex reduces fibronectin (FN) deposition and cell proliferation in primary mesangial cells (Guo and Wu, 2002). A close homolog of CH-ILKBP, affixin (also known as β-parvin), also interacts with ILK and regulates cell spreading (Yamaji et al., 2001) as well as platelet aggregation (Yamaji et al., 2002). Also, the focal adhesion protein paxillin has been reported to interact with the C-terminal domain of ILK, through the paxillin LD1 motif (Nikolopoulos and Turner, 2001, 2002).
The importance of ILK in regulating integrin-mediated function has been underscored in many recent studies. Epithelial cells that overexpress ILK have increased resistance to anoikis or the suspension-induced apoptosis that occurs when the integrin-ECM interaction is disrupted (Attwell et al., 2000; Wang et al., 2001). This suggests that constitutive ILK activation overrides the need for integrin engagement in cell survival. Recently, it has been reported that the Caenorhabditis elegans pat-4/ILK null mutant shows serious defects at sites of integrin-mediated muscle cell attachments (Mackinnon et al., 2002). Similar findings in Drosophila ILK null mutants suggest that ILK functions as a crucial adaptor protein at sites of integrin muscle cell adhesion (Zervas et al., 2001). However, it was concluded from these studies that the kinase activity of ILK may be unimportant in the regulation of integrin adhesion and that ILK functions mainly as an adaptor protein. This was due to the fact that an ILK “kinase-dead” mutant, which has been shown to have partial loss of kinase activity, was able to rescue the null mutant phenotype. Recently, it has been shown that mice lacking ILK expression die at the peri-implantation stage and that ILK deficient fibroblasts display defects in cell adhesion, spreading, and formation of stress fibers (Sakai et al., 2003). Similarly, this study also questions the importance of ILK kinase activity, because of the fact that PKB/Akt Ser-473 levels remained unchanged, and a partial kinase dead mutant of ILK was able to rescue the phenotype.
Here we show that inhibition of ILK activity results in the inhibition of cell attachment to FN and cell migration as well as the localization of ILK binding partners to the focal adhesions. We also show that ILK is preferentially active in the cytoskeletal fraction and that the interaction of CH-ILKBP with ILK stimulates ILK-mediated signaling in DU145 prostate cancer cells. In PTEN-null prostate cancer cells (PC3), we show that the ILK:CH-ILKBP interaction is dependent on the Pi3 kinase pathway. These data suggest that upon integrin engagement, ILK and CH-ILKBP are recruited to focal adhesions in a Pi3 kinase–dependent manner, resulting in ILK activation. Activated ILK is then involved in downstream “outside in” signaling and also in maintaining the β1 integrin in an activated state by sustaining CH-ILKBP and paxillin localization to focal adhesions. Together, these data demonstrate important cooperative roles for ILK, CH-ILKBP, and PTEN in cytoskeletal organization, integrin-mediated cell attachment and signaling.
MATERIALS AND METHODS
Cell Culture
DU145, PC3, and Hek 293 cells were cultured in DMEM supplemented with 10% fetal bovine serum. Cells were cultured in 35- or 100-mm dishes, as indicated.
Treatment with Inhibitors
Cells were treated with 50 or 100 μM KP-392 (formerly KP-SD-1, Kinetek Pharmaceuticals, Vancouver, BC, Canada), a highly selective inhibitor of ILK activity (Persad et al., 2001), for 16 h, unless otherwise specified. Cells were also treated with 25 or 50 μM LY294002 (Sigma, St. Louis, MO), 100 nM Wortmannin (Sigma), or 50 μM PD98059 (Cell Signaling Technology, Beverly, MA) for 6 h. An equivalent amount of vehicle control (DMSO) was added to all control reactions.
RNA Inhibition
A 21-base pair double-stranded small interfering RNA (siRNA) molecule targeting the PH-like domain of ILK (ILK-H), or a control, nonspecific 21-base pair sequence were made by Qiagen (Santa Clarita, CA; see Troussard et al., 2003, for sequence). Cells were transfected with the siRNA molecules at concentrations of 10, 25, or 50 nM, using lipofectin reagent (Invitrogen, San Diego, CA). Cells were transfected for 16 h and then allowed to recover for 72 h.
Transfections and Western Blots
Cells in 35-mm wells were transfected with empty vector, CH-ILKBP, CH-ILKBPF271D (both provided by Chuanyue Wu), pcDNA3.1, pcDNA3.1-ILKWT:V5, pcDNA3.1 ILKKD:V5, or pEGFP-PTEN to a total of 1 μg of DNA, using Fugene 6 reagent (by Roche Molecular Biochemicals, Indianapolis, IN, for Du145 cells), or Lipofectin reagent (by Invitrogen, for PC3 cells) according to manufacturer's instructions. Forty-eight hours after transfection, cells were serum-starved overnight and then serum-refed for 1 h. Cells were lysed in NP-40 lysis buffer, and Western blots were performed as described (Hannigan et al., 1996), using antiphospho GSK-3 α/β Ser 9/21, anti-GSK-3, antiphospho PKB/Akt Ser 473, anti-PKB/Akt (all from New England Biolabs, Beverly, MA), anti-GFP (Boehringer, Indianapolis, IN), anti-CH-ILKBP, anti-FLAG: HRP (Sigma), anti-ILK (UBI), anti-paxillin (UBI), and anti-vinculin (Chemicon, Temecula, CA). Blots were stripped and reprobed a maximum of one time, using Restore Western Blot Stripping Buffer (Pierce, Rockford, IL).
Adhesion Assay
After transfection or treatment with KP-392, cells were harvested in PBS-EDTA 5 mM, resuspended in DMEM containing 0.5% BSA, and plated for 1 h on FN (wells coated with FN at 10 μg/ml). Unattached cells were removed by three washes with PBS and attached cells were fixed in PBS containing 4% paraformaldehyde, stained with 1% toluidine blue, and lysed in NP-40 lysis buffer. OD at 570 nm was then measured.
Wounding Assay
After transfection or treatment of PC3 cells with KP-392, wounding assays were performed as described in Carrieras et al. (1999). After 24 h, cell migration was recorded using a Nikon Eclipse TE300 microscope (Garden City, NY), and cells that migrated into the wound were counted in five separate fields of vision.
Preparation of Soluble and Insoluble Fractions
Cells were grown on either FN- or poly-HEMA (PH)-coated plates overnight as described previously (Attwell et al., 2000) and treated with either 50 or 100 μm KP-392 or an equivalent concentration of vehicle control DMSO. Cells transfected with siRNA were plated on FN-coated plates overnight in serum-free DMEM. Cells plated on PH were collected by centrifugation, and cells plated on FN were left adherent. Cells were washed with cell solubilization buffer (CSB) containing 10 mM PIPES, 50 mM KCl, 10 mM EGTA, 3 mM MgCl2, and 2 M glycerol. Cells were then washed for exactly 2 min in 37°C CSB containing 1% Triton X-100 (for Western blots) or 0.5% Triton X-100 (for immunostaining) and the protease inhibitors PMSF (1 mM), leupeptin (1 mM), aprotinin (1 mM), NaF (2 mM), and NaVO3 (1 mM). This soluble fraction was then removed, and the remaining cytoskeletal fraction was either fixed for immunostaining or resuspended in extraction buffer containing 20 mM Tris-HCL, 80 mM KCL, 30 mM MgCl2, 1 mM EGTA, 0.25 M NaCl, 1 mM DTT, 1 mM leupeptin, and 0.5 mM PMSF. The cytoskeletal fraction was passed through a 25-gauge syringe three times, and then the samples were sonicated before protein quantification and Western blot analysis.
ILK Kinase Assay
Serum-starved PC3 cells were plated on FN- or PH-coated 100-mm plates for 1 h, and the soluble and cytoskeletal fractions were separated as described above (see Figure 5A). Alternatively, as shown in Figures 5B and 6, whole cells were lysed in NP-40 lysis buffer, as described previously (Delcommenne et al., 1998). Protein (250 μg) was then immunoprecipitated with 4 μg of anti-ILK antibody (UBI), or mouse IgG control, overnight, and then incubated with protein A/G plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. Beads were then washed in lysis buffer five times, and the kinase assay was then performed in 25 μl of buffer containing kinase reaction buffer (Delcommenne et al., 1998), 200 μM ATP, and 2.5 μg of GSK-3 fusion protein (NEB), used as a substrate. Phosphorylation of the substrate was detected by Western blotting, using Rb-anti phospho GSK-3 Ser 21/9 antibody (NEB).
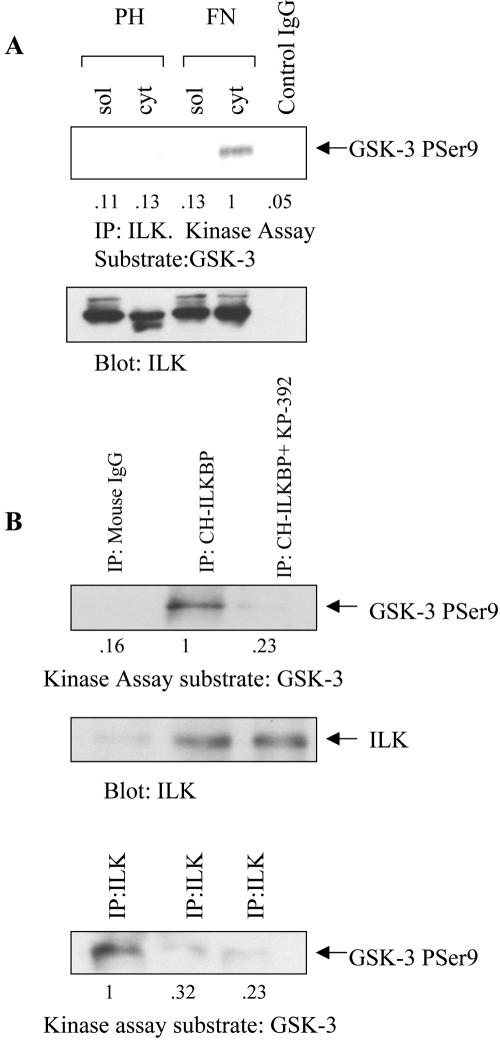
Figure 5.
Active ILK is bound to CH-ILKBP, and is localized to the cytoskeletal fraction when cells are plated on FN. (A) Serum-starved PC3 cells were plated on the substrates poly-HEMA (PH) or fibronectin (FN) for 1 h, and the soluble and cytoskeletal fractions were separated. ILK (or control mouse IgG) was immunoprecipitated from the cytoskeletal fraction, and kinase assays were performed using GSK-3 fusion protein as a substrate. Samples were then Western blotted with antiphospho GSK-3α/β Ser 21/9, and anti-ILK. (B) PC3 cells lysates were immunoprecipitated with anti-mouse IgG, or with anti CH-ILKBP, and kinase assays were performed using GSK-3 fusion protein as a substrate. The blot was stripped and reprobed with anti-ILK, to show immunoprecipitation. The leftover lysates (cleared with anti mouse IgG or anti–CH-ILKBP) were then immunoprecipitated with anti-ILK antibody, and the ILK kinase assay was then performed on the GSK-3 fusion protein substrate. Results are representative of three independent trials.
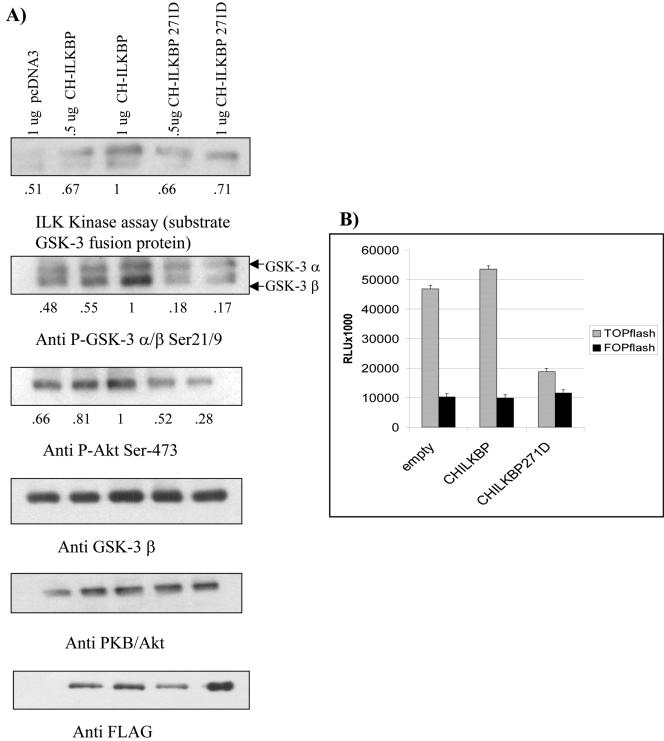
Figure 6.
CH-ILKBP, but not an ILK-binding mutant (CH-ILKBP F271D) stimulates ILK signaling in DU145 prostate cancer cells. (A) Cells were transfected with empty vector, CH-ILKBP, or CH-ILKBP F271D. After 48 h, cells were serum-starved overnight, then refed for 1 h. Samples were Western blotted with anti-phospho GSK-3α/β Ser 21/9, anti-phospho PKB/Akt Ser 473, anti-GSK-3 β, anti-PKB/Akt, and anti-FLAG. Kinase assay was performed using GSK-3 fusion protein as a substrate, and then Western blotting with anti-phospho GSK-3α/β Ser 21/9. (B) HEK-293 cells were transfected with empty vector, CH-ILKBP, or CH-ILKBP F271D, as well as either the TOP or FOP FLASH reporter constructs, and pRenilla. After 48 h, a dual luciferase reporter assay was performed. Results are representative of three independent trials.
Luciferase Assay
Hek-293 cells in 35-mm wells were transfected with 0.5 μg of either empty vector, CH-ILKBP, or CH-ILKBPF271D, 0.5 μg of either TOP or FOP FLASH reporter constructs, and 0.01 μg of pRenilla as a reporter control. After 48 h, the luciferase assay was performed using Promega's (Madison, WI) dual luciferase assay reporter kit, according to manufacturer's instructions.
Coimmunoprecipitation
Du145 cells were plated overnight on either FN or PH and and treated with either KP-392 or equivalent amounts of DMSO (see Figure 3C). Serum-starved PC3 cells in 100-mm wells were treated for 3 h with DMSO, 100 nM wortmannin, or 25 μM LY294002 (see Figure 7). Alternatively, PC3 cells were transfected with GFP, PTEN:GFP, pcDNA3:V5, ILKWT:V5, or ILKR211A:V5 to a total of 9 μg, using Lipofectin (Invitrogen) according to manufacturer's instructions. Forty-eight hours after transfection, cells were serum-starved overnight, and then lysed with NP-40 lysis buffer. The Bradford assay was then performed as in Delcommenne et al. (1998), and samples were each adjusted to contain 250 μg of protein. Lysates were then immunoprecipitated overnight with 4 μg of either Rb-anti ILK (UBI) or mouse-IgG (Invitrogen). Immune complexes were then conjugated to 30 μL protein A/G plus-agarose beads (Santa Cruz Biotechnology) for 1 h. Samples were washed with NP-40 lysis buffer five times, and Western blots were then performed, using mouse anti–CH-ILKBP.
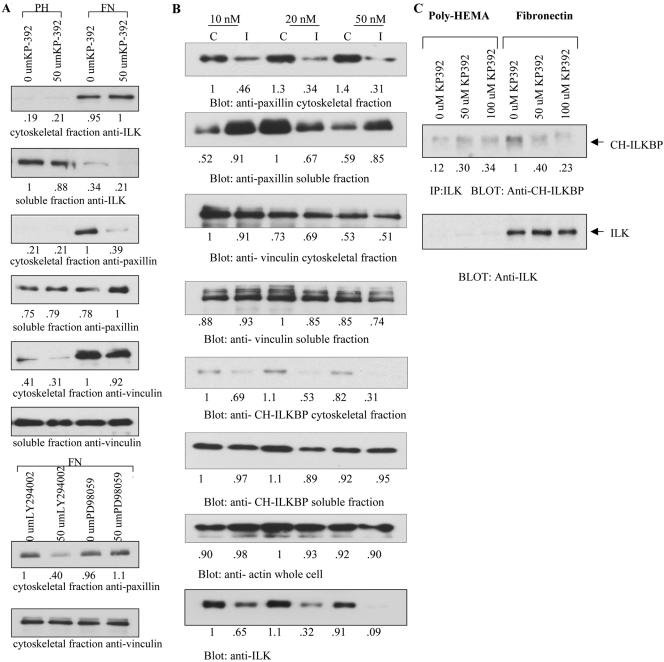
Figure 3.
Inhibition of ILK activity by KP-392 disrupts the localization of paxillin to the Triton-insoluble fraction, and disrupts ILK:CH-ILKBP binding. (A) Inhibition by KP-392. Du145 cells were plated on either poly-HEMA (PH) or fibronectin (FN)-coated plates, and treated with either DMSO or 50 μM KP-392 for 16 h. Soluble and cytoskeletal fractions were then separated, and Western blots performed, with 20 μg of lysate per sample, as described in MATERIALS AND METHODS. (B) PC3 cells were transiently transfected with ILK-H siRNA specific to the PH-like domain of ILK (I), or with control siRNA (C), at concentrations of either 10, 25, or 50 nM. After 72 h, cells were then treated as in A. (C) Du145 cells were plated on poly-HEMA or FN and treated with increasing amounts of KP-392. The cytoskeletal fraction was isolated, and this fraction was immunoprecipitated with anti-ILK antibody. Western blots were then performed as described in MATERIALS AND METHODS. All results are representative of 3 independent trials.
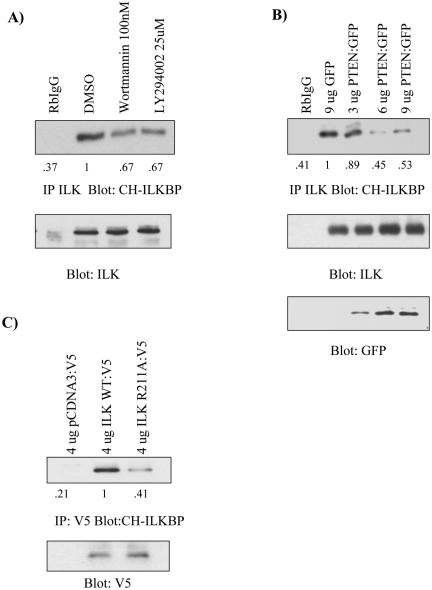
Figure 7.
THE ILK:CH-ILKBP interaction is Pi3 kinase dependent. (A) Chemical inhibitors disrupt the ILK:CH-ILKBP interaction. PC3 cells were treated with either DMSO, wortmannin, or LY294002 for 3 h. Cells were then lysed with NP-40, and immunoprecipitated with anti-ILK. Samples were then Western blotted with anti-CH-ILKBP, and stripped and reprobed with anti-ILK. (B) PTEN reintroduction disrupts the ILK:CH-ILKBP interaction. PC3 cells were transfected with increasing amounts of PTEN. After 48 h, cells were treated as in A, and samples were also stripped and reprobed with anti-GFP as a transfection control. (C) The ILK:CH-ILKBP interaction is reduced when the proposed PiP3 binding domain of ILK is mutated. PC3 cells were transfected with pCDNA3:V5, ILK-WT:V5, or ILK R211A:V5. After 48 h, cells were lysed with NP-40 lysis buffer, immunoprecipitated with anti-V5 antibody, and then treated as in A. Results are representative of three independent trials.
Immunofluorescence Microscopy
Du145 cells were grown on glass coverslips coated with FN (150 μg/ml) in six-well plates. Cells were allowed to attach to the FN and then treated for 16 h with either 50 μM KP-392 or DMSO and fixed for 10 min in 4% paraformaldehyde in PBS, pH 7.4. For cytoskeletal staining, cells were solubilized (as described above) before fixation. Whole cells were permeabilized with 0.2% Triton X-100 before staining. Cells were blocked in PBS containing 5% NGS and 1% BSA. Primary antibodies to ILK (1:100, Upstate Biotechnology, Lake Plaicd, NY), paxillin (1:40, Santa Cruz Biotechnology), vinculin (1:50, Chemicon), and CH-ILKBP (undiluted) were incubated for 1 h at 37°C. Protein was detected with anti-rabbit rhodamine- or anti-mouse fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Santa Cruz Biotechnology). Phalloidin (Sigma) was diluted 1:1000 in PBS, and incubated for 30 min at room temperature.
Densitometric Analysis
Relevant blots were analyzed densitometrically using Bio-Rad's Quantity One program (Cambridge, MA). Values are shown as a fraction of the first, or the most intense band.
RESULTS
Inhibition of ILK Activity Decreases Cell Attachment to FN and Cell Migration
We have previously reported that ILK is constitutively active in the PTEN-null prostate cancer cell line PC3 and that reintroduction of PTEN inhibits this activity (Persad et al., 2000). To determine whether ILK kinase activity is crucial to the regulation of integrin function, we studied the effect of inhibition of ILK activity on integrin function in PC3 cells. This was done by treatment with the small molecule ATP-analog ILK inhibitor KP-392, which has previously been reported to inhibit ILK kinase activity in a highly selective, dose-dependent manner (D'Amico et al., 2000; Persad et al., 2000, 2001; Troussard et al., 2000; Tan et al., 2002, Yoganathan et al., 2002; Mills et al., 2003; Cruet-Hennequart et al., 2003), transfection of a kinase-deficient, dominant negative form of ILK (ILK-DN), which contains a point mutation (E359K) in the kinase domain (Wu et al., 1998; Persad et al., 2001) or reintroduction of PTEN, a negative regulator of ILK activity (Morimoto et al., 2000; Persad et al., 2000). The ILK inhibitor, KP-392, was identified in a high throughput kinase activity screen using active recombinant ILK (Persad et al., 2001).
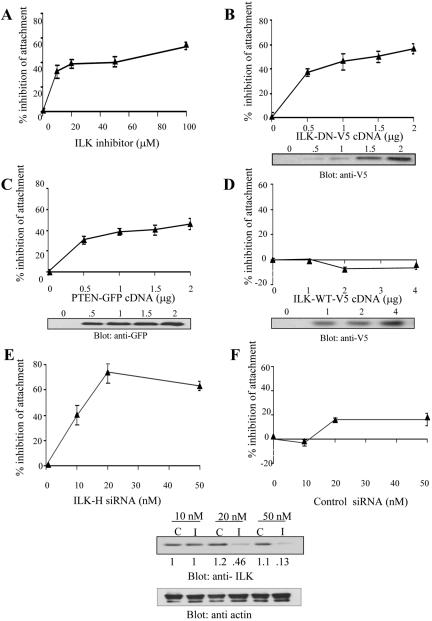
Inhibition of ILK activity with KP-392 showed a dose-dependent inhibition of cell attachment to FN (Figure 1A). Transient transfection of a dominant negative, kinase deficient form of ILK (ILK-DN, Figure 1B), or transient transfection of PTEN-WT (Figure 1C) also decreased attachment of PC3 cells to FN in a dose-dependent manner. Transfection of wild-type ILK, on the other hand, did not affect attachment of PC3 cells to FN (Figure 1D).
Figure 1.
Inhibition of ILK activity decreases cell attachment. PC3 cells were treated with increasing amounts of inhibitor (A) for 24 h, were transiently transfected with ILK-DN:V5 (B), PTEN:GFP (C), or ILK-WT:V5 (D) and left to recover for 48 h, or were transfected with ILK-H siRNA specific to the PH-like domain of ILK (I) (E), or control siRNA (C) (F), and left to recover for 72 h. Attachment assay was performed as described in methods. Each sample was repeated in triplicate, as indicated by error bars, and the experiment was repeated three times. Data are plotted as % increase in the inhibition of attachment vs. control.
To test the effect of loss of total ILK protein, RNA inhibition experiments were also performed. Transfection of an siRNA molecule targeted to the ILK sequence (I) inhibited cell attachment (Figure 1E), whereas a control, nonspecific siRNA (C; Figure 1F) had no effect.
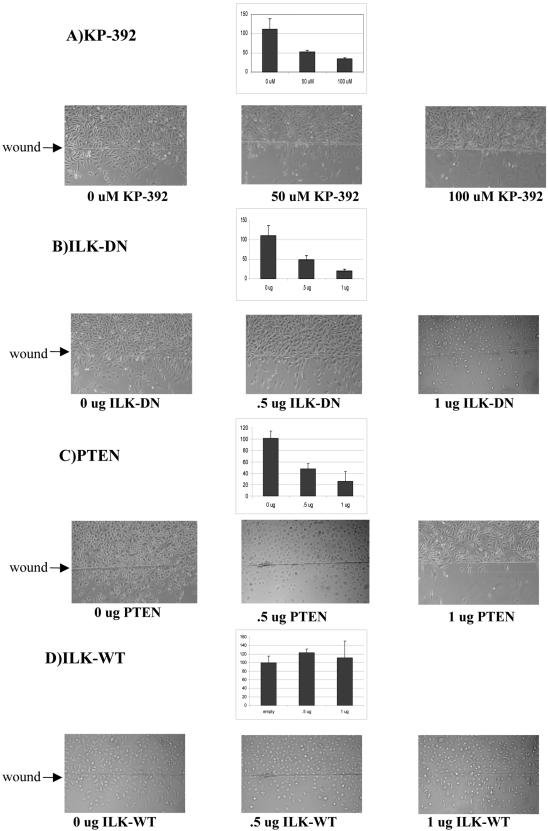
Because integrin function is required for proper cell attachment and migration (Brakebusch et al., 2002), we next examined the effect of the inhibition of ILK activity on cell migration in a wounding assay. As seen in Figure 2, KP-392 (A), dominant-negative ILK (B), and wild-type PTEN (C) all decreased cell migration in a dose-dependent manner. Again, ILK-WT (D) did not affect migration in these cells. Together, these data demonstrate that ILK activity is required for cell attachment and migration.
Figure 2.
Inhibition of ILK activity disrupts cell migration. (A) A wound was introduced to PC3 cells as described in MATERIALS AND METHODS, and cells were then treated with increasing amounts of KP-392 for 24 h. Migrated cells were photographed and counted in five separate fields. Alternatively, cells were transfected with PTEN:GFP (B), ILK-DN:V5 (C), or ILK-WT:V5 (D). Wounding assay was then performed 24 h posttransfection. Results are representative of three independent trials.
Inhibition of ILK Activity Affects the Localization of Paxillin and CH-ILKBP, But Not Vinculin, to the Focal Adhesions
We next examined the effect of inhibition of ILK activity on the proper localization of focal adhesion proteins. PC3 cells were plated on PH or FN, and the localization of several proteins to either the soluble or cytoskeletal fraction was determined. As seen in Figure 3A, ILK localized to the cytoskeletal fraction when cells were plated on FN, and this localization was not affected when ILK activity was inhibited by KP-392. However, the cytoskeletal localization of paxillin, which has been shown to bind ILK directly (Nikolopoulos and Turner, 2001) was significantly inhibited by KP-392, whereas the localization of vinculin, which does not bind ILK directly, was not affected by KP-392. Because we have shown a dependence of ILK activity on the Pi3 kinase pathway (Persad et al., 2000), we also examined the effect of Pi3 kinase inhibition on the localization of paxillin and vinculin. As shown in Figure 3A, inhibition of Pi3 kinase by LY294002 also inhibited paxillin, but not vinculin, localization to the cytoskeletal fraction. The MEK 1 inhibitor compound PD98059 had no effect on the localization of paxillin and vinculin. We next examined the effect of the inhibition of ILK protein expression by RNA inhibition. As shown in Figure 3B, when cells were transiently transfected with ILK-H, an siRNA molecule specific to the PH-like domain of ILK (I), or a control siRNA (C), ILK-depleted cells displayed a dose-dependent loss of the localization of paxillin and CH-ILKBP, but not vinculin, to the cytoskeletal fraction. Inhibition of ILK activity also resulted in the inhibition of the interaction of ILK with its binding partner CH-ILKBP in the cytoskeletal fraction. As shown in Figure 3C, KP-392 inhibits the association of ILK with CH-ILKBP in the cytoskeletal fraction when cells are plated on FN.
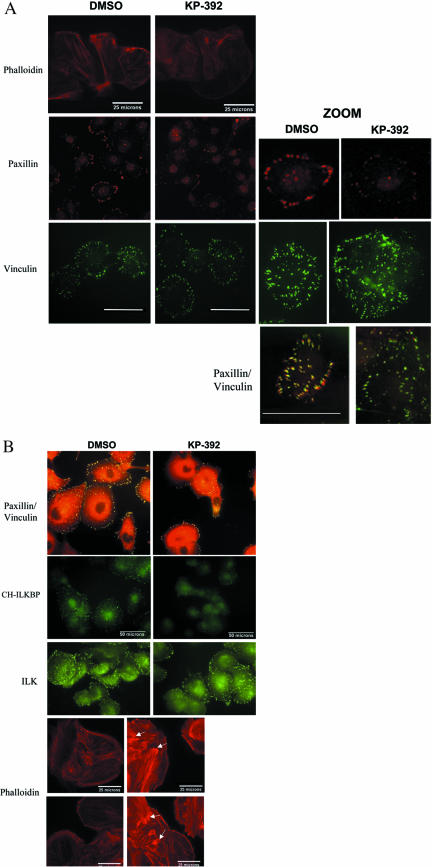
The localization of ILK, paxillin, CH-ILKBP, vinculin, and actin were also examined by immunofluorescent microscopy. Paxillin, vinculin, and actin localization was examined in both whole cells and the cytoskeletal fraction. However, because of lower total levels of ILK and CH-ILKBP in Du145 cells, it was only possible to stain for these proteins in whole cells. As shown in Figure 4A, in the cytoskeletal fraction paxillin and vinculin colocalize to focal adhesion plaques. However, upon treatment with KP-392, paxillin is dramatically reduced from the focal adhesion plaques, whereas vinculin remains unchanged. Paxillin-vinculin costaining was also examined in whole cells (Figure 4B); however, because of much higher background staining levels of paxillin in the whole cells, only a slight change in focal adhesion staining is visible. The focal adhesions do appear reduced in size and number. Actin organization (shown by phalloidin staining) is also altered in the KP-392–treated cells, showing increased formation of stress fibers and accumulation of F-actin. This alteration in actin organization and accumulation is more obvious in the whole cell staining (Figure 4B) where there is a clear increase in stress fibers and loss of peripheral, cortical actin. There are also significant areas of F-actin accumulation (arrows). The whole cell staining also shows a selective loss of paxillin at the focal adhesions upon treatment with KP-392 as well as loss of CH-ILKBP at the focal adhesions. ILK staining, however, remains unchanged. It is important to note that cells were allowed to attach to FN before treatment with KP-392. KP-392 does not cause detachment of attached cells, but will inhibit the rate of attachment with preincubation (as seen in Figure 1).
Figure 4.
Inhibition of ILK activity with KP-392 disrupts the localization of paxillin and CH-ILKBP, but not ILK and vinculin, to focal adhesion plaques. Du145 cells were plated on FN-coated coverslips, with DMSO or 50 μM KP-392, for 16 h. Cells were then either solubilized with a Triton wash (A), or fixed as whole cells (B). Samples were then stained with the appropriate antibodies. (A) Solubilized staining. Rhodamine; bar, 25 μm. Paxillin (rhodamine) and vinculin (FITC), bar represents 50 μm. Paxillin and vinculin zoom, Bar, 39 μm. (B) Whole cell staining. Paxillin (rhodamine) and vinculin (FITC) merge. CH-ILKBP (FITC), ILK (FITC), and phalloidin. Paxillin and CH-ILKBP are specifically dissociated from focal adhesion plaques upon inhibition of ILK activity. Results are representative of three independent trials. Bar, 50 μm.
ILK Activity Is Stimulated in the Cytoskeletal Fraction
To determine if ILK activity is dependent on its subcellular localization, an ILK kinase assay was performed on the soluble and cytoskeletal fractions of PC3 cells plated on the β1 integrin ECM substrate FN, or on PH, a control substrate to which cells cannot bind. As shown in Figure 5A, although roughly equal amounts of ILK are immunoprecipitated in 250 μg of protein in each of the samples assayed, the activity of the ILK found in the cytoskeletal fraction of the cells plated on FN is substantially more active than ILK present in the soluble fraction. This suggests that ILK is preferentially more active in the insoluble focal adhesions that are formed after integrin engagement.
Active ILK Is Bound to CH-ILKBP
As shown in Figure 5B, an immunoprecipitated CH-ILKBP complex is able to phosphorylate GSK-3 fusion protein on serine 9, and this phosphorylation is blocked by the ILK inhibitor KP-392. Stripping and reprobing this blot shows that ILK is present in this complex. When these depleted lysates are immunoprecipitated with anti-ILK antibody, there is very little ILK kinase activity left in the CH-ILKBP–depleted lysate, showing that most of the active ILK is bound to CH-ILKBP.
CH-ILKBP Stimulates ILK Activity and Signaling
Previously, it has been shown that CH-ILKBP is required for the recruitment of ILK to focal adhesions (Zhang et al., 2002). Because we have demonstrated that ILK is preferentially active in the cytoskeletal fraction, we next examined the effect of CH-ILKBP on ILK signaling. We transfected either empty vector, CH-ILKBP, or an ILK-binding defective mutant form of CH-ILKBP (CH-ILKBP F271D; Tu et al., 2001) into PTEN-positive DU145 cells, in which ILK activity is inducible. When the cells were serum-starved and then refed for 1 h, we observed that CH-ILKBP stimulated ILK kinase activity in a dose-dependent manner (Figure 6A). Furthermore, CH-ILKBP stimulated GSK-3 β phosphorylation on Ser 9, and phosphorylation of PKB/Akt on Ser 473, both of which have been shown previously to be regulated by ILK (Delcommenne et al., 1998). CH-ILKBP also slightly increased β catenin TCF/LEF reporter activity, as seen by the TOP/FOP FLASH reporter assay (Figure 6B). In contrast, the ILK-binding defective mutant form of CH-ILKBP (CH-ILKBPF271D), did not appear to stimulate ILK activity or signaling, and indeed, appeared to behave as a dominant-negative mutant, decreasing basal levels of GSK-3 and PKB/Akt phosphorylation and dramatically decreasing β-catenin TCF/LEF reporter activity in these cells.
PTEN and Inhibitors of Pi3 Kinase Disrupts the ILK:CH-ILKBP Interaction
Because ILK activity has been shown previously to be Pi3 kinase dependent, we next tested the effect of disruption of the Pi3 kinase pathway on the ILK:CH-ILKBP interaction in PC3 cells. As shown in Figure 7, A and B, both the pharmacological inhibition of Pi3 kinase and reintroduction of PTEN disrupt the ILK:CH-ILKBP interaction. Trypan blue staining confirmed that PTEN, wortmannin, and LY294002 had no effect on cell viability in the concentrations used (our unpublished results).
To further confirm the role of Pi3 kinase and its product PiP3 in the regulation of the ILK:CH-ILKBP interaction, we used a PiP3-binding domain point mutant of ILK (ILK R211A), which disrupts the ability of ILK to promote PKB/Akt Ser 473 phosphorylation (Persad et al., 2001). As shown in Figure 7C, this mutant is defective in the ability to bind CH-ILKBP, providing further evidence that activation by Pi3 kinase/PiP3 pathway is required for proper ILK/CH-ILKBP interaction and function.
DISCUSSION
Recent studies in Drosophila, C. elegans, and mouse have demonstrated that ILK null mutants display significant inhibition of integrin-related cell adhesion and cytoskeletal organization (Zervas et al., 2001; Mackinnon et al., 2002; Sakai et al., 2003), supporting a crucial role for ILK in regulating cell adhesive functions. In this article, we have used several different methods to inhibit ILK activity: KP-392, a small molecule ILK inhibitor that has previously been shown to inhibit ILK kinase activity in a highly selective manner (Persad et al., 2001); ILK-DN, a kinase-deficient point mutant of ILK that behaves as a dominant negative (Wu et al., 1998); the tumor suppressor PTEN, which when reintroduced into PTEN-deficient PC3 cells decreases the kinase activity of ILK (Morimoto et al., 2000; Persad et al., 2000), and finally, siRNA targeting the ILK protein. Here, we show that reducing ILK kinase activity or downregulating ILK expression by siRNA inhibits cell attachment and cell migration. These results agree with the effects of knocking out ILK in embryonic stem cells and chondrocytes (Grashoff et al., 2003; Sakai et al., 2003; Terpstra et al., 2003), which have shown that ILK knockout results in embryonic lethality and severe defects in cell attachment, migration, proliferation, and F-actin accumulation. However, some of these studies raised questions about the importance of the kinase activity of ILK in regulating attachment and migration, because of a rescue of these phenotypes by a partial kinase deficient mutant of ILK (Sakai et al., 2003). Therefore, the mechanism for the inhibition of cell attachment and spreading in ILK-null cells remains unclear. We hypothesized that the inhibition of cell attachment and migration upon inhibition of ILK activity could be due to a role of ILK in focal adhesion formation and actin organization. To investigate this possibility, we studied the effect of inhibiting ILK activity, and ILK protein expression, on the localization of several focal adhesion proteins to the actin cytoskeleton. Here, we have shown that both inhibition of ILK activity and ILK protein expression decreased the localization of the ILK-binding partners, paxillin (Nikolopoulos and Turner, 2001), and CH-ILKBP to focal adhesions and also decreased the association of ILK with CH-ILKBP, in response to cell adhesion on FN. Interestingly, another component of focal adhesion plaques, vinculin, which does not bind ILK directly, was unaffected by inhibition of ILK activity. In addition, we have found that the inhibition of ILK activity also results in altered F-actin accumulation similar to that observed in ILK knockout fibroblasts (Sakai et al., 2003). This suggests that in the absence of ILK activity, currently unidentified substrates are not phosphorylated, thus preventing proper focal adhesion formation and F-actin organization. This, in turn could lead to defective integrin function and changes in actin cytoskeletal formation. Alternatively, ILK in the inactive conformation may be unable to bind paxillin and CH-ILKBP, thus preventing their recruitment to focal adhesion plaques. To substantiate the evidence that both the kinase activity and adaptor functions of ILK are crucial in regulating integrin function, we have shown three different methods of inhibiting ILK. Although complete loss of ILK protein is generally lethal to most cell types, we have found here that largely downregulating ILK protein levels and activity causes dramatic, dose-dependent changes to integrin and focal adhesion function. Although there are caveats in each of the methods used to inhibit ILK activity and protein levels in this article, we believe that taken together, these data provide strong evidence that ILK plays an integral role, as a kinase as well as an adaptor protein, in regulating cell adhesion and focal adhesion formation.
The kinase activity of ILK is transiently stimulated upon integrin engagement with the ECM (Wu et al., 1998; Dedhar, 2000). It has also been reported that ILK is recruited to focal adhesions upon cell attachment (Li et al., 1999) and that the ILK-interacting proteins CH-ILKBP (Tu et al., 2001, Zhang et al., 2002) and affixin (Yamaji et al., 2001) play crucial roles in this process. Here, we show that the fraction of ILK that is recruited to focal adhesions in response to cell adhesion has higher enzymatic activity and that active ILK is specifically bound to CH-ILKBP. This suggests that ILK is activated once recruited to the focal adhesions or, alternatively, that ILK is recruited specifically in its active conformation.
It has recently been reported that CH-ILKBP is necessary for the proper recruitment of ILK to focal adhesions after cell attachment (Zhang et al., 2002). Given that the activity of ILK depends on its localization, we wanted to determine if CH-ILKBP affected ILK signaling. It was observed that CH-ILKBP transiently increased ILK activity and stimulated its downstream targets, in a dose-dependent manner. A mutant form of CH-ILKBP (CH-ILKBPF271D), which does not bind ILK, however, displayed a dominant-negative effect in downregulating ILK signaling. From these results, we propose that CH-ILKBP is responsible for recruiting ILK, in its active conformation, to focal adhesion complexes, where it then participates in downstream signaling events such as the stimulation of PKB/Akt and GSK-3 phosphorylation. Because the ILK-binding mutant of CH-ILKBP inhibited ILK's downstream signaling, it is possible that this mutant is competing with wild-type CH-ILKBP, thus preventing ILK recruitment and having a negative effect on ILK signaling. Interestingly, knock-down of CH-ILKBP results in the inhibition of PKB/Akt activation and stimulation of apoptosis (Fukuda et al., 2003). The apoptosis was rescued by membrane-targeted PKB/Akt, implicating a direct role for CH-ILKBP in the activation of PKB/Akt. However, in light of the data presented in this article, another explanation could be that in the absence of CH-ILKBP, ILK activity is reduced, resulting in decreased PKB/Akt phosphorylation on Serine-473 and decreased kinase activity. We have recently shown that ILK knockout results in complete inhibition of PKB/Akt phosphorylation on Serine-473 and that ILK is essential for PKB/Akt activation (Troussard et al., 2003).
The tumor suppressor PTEN has previously been shown to inhibit cell migration and proper focal adhesion formation (Yamada et al., 2002). As mentioned previously, PTEN also has a negative effect on ILK kinase activity (Morimoto et al., 2000; Persad et al., 2000), because of its ability to dephosphorylate PiP3, a product of Pi3 kinase that activates ILK (Delcommenne et al., 1998). Thus, in PTEN-negative cells, ILK is constitutively active (Persad et al., 2000). Here, we have shown that in PTEN-negative PC3 cells, the interaction of ILK and CH-ILKBP is constitutive, even under serum-starved conditions and that reintroduction of PTEN disrupts the interaction. Pharmacological inhibition of Pi3 kinase also has the same effect. Thus, the observed effect of PTEN on cell migration and focal adhesion formation may be partially due to the disruption of the ILK:CH-ILKBP interaction. It is unclear exactly how PTEN disrupts the ILK:CH-ILKBP interaction; it is possible that partial activation of ILK by the Pi3 kinase pathway is required for binding and subsequent recruitment by CH-ILKBP. It is also possible that the effect may be mediated by other molecules that are regulated by PTEN and the Pi3 kinase inhibitors. Further evidence that Pi3 kinase activation is required for proper ILK:CH-ILKBP interaction is provided by experiments involving the PiP3-binding mutant of ILK (ILKR211A; Figure 7C). This mutant, which is unable to stimulate PKB/Akt Ser 473 phosphorylation, presumably due to its inability to bind PiP3, is also defective in CH-ILKBP binding. It is important to note that CH-ILKBP has been reported to interact with the C-terminal end of ILK (Tu et al., 2001), so it is unlikely that a point mutation within the PH-like domain of ILK would disrupt binding to CH-ILKBP.
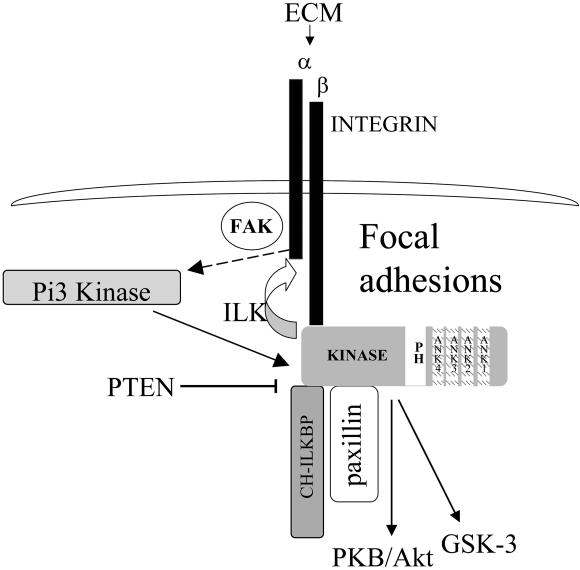
Pi3 kinase is transiently activated upon integrin engagement, probably via integrin aggregation with growth factor receptors (Downward, 1998, Wu, 1999), and activation by focal adhesion kinase (FAK; Parsons et al., 2000). Stimulation of ILK activity is dependent on Pi3 kinase (Lynch et al., 1999; Wang et al., 2001; Yamaji et al., 2002), and ILK activity is inhibited by PTEN (Morimoto et al., 2000; Persad et al., 2000). Here, we have shown that the ILK:CH-ILKBP interaction is also dependent on Pi3 kinase and that PTEN disrupts this interaction. We propose a model where upon integrin engagement with the ECM, Pi3 kinase is activated, resulting in the stimulation of ILK activity and inducing the ILK:CH-ILKBP interaction, causing translocation to focal adhesions. In focal adhesions, ILK is crucial for both proper focal adhesion formation and activation of the β1 integrin, and downstream signaling to both PKB/Akt and GSK-3 (see Figure 8 for summary). In the absence of ILK activation, paxillin and CH-ILKBP are not properly localized to focal adhesion plaques, resulting in alterations in actin organization and accumulation and inhibition of β1 integrin function. These results underscore the importance of the Pi3 kinase pathway in the regulation of the ILK:CH-ILKBP interaction and focal adhesion formation, as well as the importance of ILK activity in focal adhesion formation, cell adhesion, and migration.
Figure 8.
Summary of ILK recruitment and activity at focal adhesions. On integrin engagement with the extracellular matrix (ECM), Pi3 kinase is activated, through FAK, and clustering and coactivation of growth factor receptor tyrosine kinases. ILK is then activated through Pi3 kinase, allowing binding with CH-ILKBP and paxillin, and recruitment to focal adhesion plaques. At the focal adhesion plaques, ILK activity is crucial for maintaining upstream signaling to β1 integrins, and downstream signaling to PKB/Akt and GSK-3.
Acknowledgments
S.A. is supported by the National Science and Engineering Research Council. S.D. is supported by the National Cancer Institute of Canada with funds from the Terry Fox Foundation. C.W. is supported by National Institutes of Health Grants GM65188 and DK54639.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–05–0308. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0308.
References
- Attwell, S., Roskelley, C., and Dedhar, S. (2000). The integrin-liked kinase (ILK) suppresses anoikis. Oncogene 19, 3811–3815. [DOI] [PubMed] [Google Scholar]
- Brakebusch, C., Bouvard, D., Stanchi, F., Sakai, T., and Fassler, R. (2002). Integrins in invasive growth. J. Clin. Invest. 109, 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood, D.A., Shattil, S.J., and Ginsberg, M.H. (2000). Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 275, 22607–22610. [DOI] [PubMed] [Google Scholar]
- Carrieras, F., Rigot, V., Cruet, S., Andre, F., Gauduchon, P., and Marvaldi, J. (1999). Migration properties of the human ovarian adenocarcinoma cell line IGROV 1, Importance of αvβ3 integrins and vitronectin. Int. J. Cancer. 80, 285–94. [DOI] [PubMed] [Google Scholar]
- Cruet-Hennequart, S., Maubant, S., Luis, J., Gauduchon, P., Staedel, C., and Dedhar, S. (2003). αv integrins regulate cell proliferation through integrin-linked kinase (ILK) in ovarian cancer cells. Oncogene 22, 1688–1702. [DOI] [PubMed] [Google Scholar]
- D'Amico, M. et al. (2000). The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and cAMP-responsive element-binding protein-dependent pathways. J. Biol. Chem. 275, 32649–32657. [DOI] [PubMed] [Google Scholar]
- Dedhar, S. (1999). Integrins and signal transduction. Curr. Opin. Hematol. 6, 37–43. [DOI] [PubMed] [Google Scholar]
- Dedhar, S. (2000). Cell-substrate interactions and signaling through ILK. Curr. Opin. Cell Biol. 12, 250–256. [DOI] [PubMed] [Google Scholar]
- Delcommenne, M., Tan, C., Gray, V., Rue, L., Woodgett, J., and Dedhar, S. (1998). Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 95, 11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward, J. (1998). Lipid-regulated kinases: some common themes at last. Science 279, 673–674. [DOI] [PubMed] [Google Scholar]
- Fukuda, T., Guo, L., Shi, X., and Wu, C. (2003). CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J. Cell Biol. 160, 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff, C., Aszodi, A., Sakai, T., Hunziker, E.B., and Fassler, R. (2003). Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 4, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L., and Wu, C. (2002). Regulation of fibronectin matrix deposition and cell proliferation by the PINCH-ILK-CH-ILKBP complex. FASEB J. 16, 1298–1300. [DOI] [PubMed] [Google Scholar]
- Hannigan, G.E., Leung-Hagesteijn, C., Fitz-Gibbon, L., Coppolino, M.G., Radeva, G., Filmus, J., Bell, J.C., and Dedhar, S. (1996). Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379, 91–96. [DOI] [PubMed] [Google Scholar]
- Li, F., Zhang, Y., and Wu, C. (1999). Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J. Cell Sci. 112, 4589–4599. [DOI] [PubMed] [Google Scholar]
- Liliental, J., Moon, S.Y., Lesche, R., Mamillapalli, R., Li, D., Zheng, Y., Sun, H., and Wu, H. (2000). Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10, 401–404. [DOI] [PubMed] [Google Scholar]
- Lynch, D.K., Ellis, C.A., Edwards, P.A., and Hiles, H.D. (1999). Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene 18, 8024–8032. [DOI] [PubMed] [Google Scholar]
- Mackinnon, A.C., Qatoda, H., Norman, K.R., Moerman, D.G., and Williams, B.D. (2002). C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12, 787–797. [DOI] [PubMed] [Google Scholar]
- Mills, J., Digicaylioglu, M., Legg, A.T., Young, C.E., Young, S.E., Barr, A.M., O'Connor, T.P., and Dedhar, S. (2003). Role of integrin-linked kinase in NGF stimulated neurite outgrowth. J. Neurosci. 23, 1638–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, A.M., Tomlinson, M.G., Nakatani, K., Bolen, J.B., Roth, R.A., and Herbst, R. (2000). The MMAC1 tumor suppressor phosphatase inhibits phospholipase C and integrin-linked kinase activity. Oncogene 19, 200–2009. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos, S.N., and Turner, C.E. (2001). Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J. Biol. Chem. 276, 23499–23505. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos, S.N., and Turner, C.E.(2002). Molecular dissection of actopaxin-integrin-linked kinase-paxillin interactions and their role in subcellular localization. J. Biol. Chem. 277, 1568–1575. [DOI] [PubMed] [Google Scholar]
- Parsons, J.T., Martin, K.H., Slack, J.K., Taylor, J.M., and Weed, S.A. (2000). Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19, 5606–5613. [DOI] [PubMed] [Google Scholar]
- Persad, S., Attwell, S., Gray, V., Delcommenne, M., Troussard, A., Sanghera, J., and Dedhar, S. (2000). Inhibition of integrin-linked kinase (ILK) suppresses activation of PKB/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl. Acad. Sci. USA 97, 3207–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad, S., Attwell, S., Gray, V., Mawji, N., Deng, J.T., Leung, D., Yan, J., Sanghera, J., Walsh, M.P., and Dedhar, S. (2001). Regulation of protein kinase B/Akt-Serine 473 phosphorylation by integrin-linked kinase. J. Biol. Chem. 276, 27462–27469. [DOI] [PubMed] [Google Scholar]
- Petit, V., and Thiery, J.P. (2000). Focal adhesions: structure and dynamics. Biol. Cell 92, 477–494. [DOI] [PubMed] [Google Scholar]
- Sakai, T., Li, S., Docheva, D., Grashoff, C., Sakai, K., Kostka, G., Braun, A., Pfeifer, A., Yurcheno, P.D., and Fassler, R. (2003). Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 17, 926–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, M., Gu, J., Matsumoto, K., Aota, S., Parsons, R., and Yamada, K.M. (1998). Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617. [DOI] [PubMed] [Google Scholar]
- Tan, C., Costello, P., Sanghera, J., Dominguez, D., Garcia de Harreros, A., and Dedhar, S. (2001). Inhibition of integrin-linked kinase (ILK) suppresses betacatenin-Lef/Tcf-dependent transcription and expression of the E-cadherin suppressor, snail in APC-/-human colon carcinoma cells. Oncogene 20, 133–140. [DOI] [PubMed] [Google Scholar]
- Tan, C., Mui, A., and Dedhar, S. (2002). Integrin-linked kinase regulates inducible nitric oxide synthase and cyclooxygenase-2 expression in an NF-kappa B-dependent manner. J. Biol. Chem. 277, 3109–3116. [DOI] [PubMed] [Google Scholar]
- Terpstra, L., Prud'homme, J., Arabian, A., Takeda, S., Karsenty, G., Dedhar, S., and St-Arnaud, R. (2003). Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the Integrin-linked kinase (ILK) in chondrocytes. J. Cell Biol. 162, epub ahead of print. [DOI] [PMC free article] [PubMed]
- Troussard, A.A., Costello, P., Yoganathan, T.N., Kumagai, S., Roskelley, C.D., and Dedhar, S. (2000). The integrin-linked kinase induces an invasive phenotype via Ap-1 transcription factor dependent upregulation of matrix metalloproteinase (MMP-9). Oncogene. 19, 5444–5452. [DOI] [PubMed] [Google Scholar]
- Troussard, A.A., Mawji, N.N., Ong, C., Mui, A., St-Arnaud, R., and Dedhar, S. (2003). Conditional knock-out of integrin-linked kinase (ILK) demonstrates an essential role in PKB/Akt activation. J. Biol. Chem. 278, 22374–22378. [DOI] [PubMed] [Google Scholar]
- Tu, Y., Huang, Y., Zhang, Y., Hua, Y., and Wu, C. (2001). A new focal adhesion protein that interacts with Integrin-linked kinase and regulates cell adhesion and spreading. J. Cell Biol. 153, 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.C., Makino, K., Xia, W., Kim, J.S., Im, S.A., Peng, H., Mok, S.C., Singletary, S.E., and Hung, M.C. (2001). DOC-2/hDab-2 inhibits ILK activity and induces anoikis in breast cancer cells through an Akt-independent pathway. Oncogene 20, 6960–6964. [DOI] [PubMed] [Google Scholar]
- Wu, C., Keightly, S.Y., Leung-Hagesteijn, C., Radeva, G., Coppolino, M., Goicoechea, S., McDonald, J.A., and Dedhar, S. (1998). Integrin-linked protein kinase regulates fibronectin matrix assembly, e-cadherin expression, and tumorigenicity. J. Biol. Chem. 273, 528–536. [DOI] [PubMed] [Google Scholar]
- Wu, C. (1999). Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J. Cell Sci. 112, 4485–4489. [DOI] [PubMed] [Google Scholar]
- Wu, C., and Dedhar, S. (2001). Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J. Cell Biol. 155, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, K.M., and Araki, M. (2002). Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J. Cell Sci. 114, 2375–2382. [DOI] [PubMed] [Google Scholar]
- Yamaji, S., Suzuki, A., Sugiyama, Y., Koide, Y., Yoshida, M., Kanamori, H., Mohri, H., Ohno, S., and Ishigatsubo, Y. (2001). A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell substrate interaction. J. Cell Biol. 153, 1251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji, S., Suzuki, A., Kanamori, H., Mishima, W., Takabayashi, M., Fujimaki, K., Tomita, N., Fujisawa, S., Ohno, S., and Ishigetsubo, Y. (2002). Possible role of ILK-affixin complex in integrin-cytoskeleton linkage during platelet aggregation. Biochem. Biophys. Res. Commun. 297, 1324. [DOI] [PubMed] [Google Scholar]
- Yoganathan, N. et al. (2002). Integrin-linked kinase, a promising cancer therapeutic target: biochemical and biological properties. Pharmacol. Ther. 93, 233–242. [DOI] [PubMed] [Google Scholar]
- Zamir, E., Katz, B.Z., Aota, S., Yamada, K.M., Geiger, B., and Kam, Z. (1999). Components of cell-matrix adhesions. J. Cell Biol. 112, 1655–1669. [DOI] [PubMed] [Google Scholar]
- Zamir, E., and Geiger, B. (2001). Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 114, 3577–3579. [DOI] [PubMed] [Google Scholar]
- Zervas, C.G., Gregory, S.L., and Brown, N.H. (2001). Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Chen, K., Tu, Y., Velyvis, A., Yang, Y., Qin, J., and Wu, C. (2002). Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J. Cell Sci. 115, 4777–4786. [DOI] [PubMed] [Google Scholar]