Abstract
Many postmenopausal women question whether to start or continue hormone therapy because of recent clinical trial negative results. However, evidence from other studies of postmenopausal women, and from studies in menopausal monkeys, indicate that estrogen has neurocognitive protective effects, particularly when therapy is initiated close to the time of menopause before neural systems become increasingly compromised with age. In this review, we present studies of menopausal women and female monkeys that support the concept that estrogen therapies protect both cognitive function and neurobiological processes.
Keywords: monkeys, hormones, menopause, neurobiology, memory
Results from the Women’s Health Initiative Memory Study (WHIMS) [Espeland et al., 2004; Rapp et al., 2003a; Shumaker et al., 2003; Shumaker et al., 2004] has caused many postmenopausal women to question whether to start or to continue hormone therapy (HT) to decrease the risk of developing dementia and has caused many others to question the role of estrogen in protecting cognitive function from age-related decline in general. However, several critical factors likely influenced the WHIMS negative results, including the advanced age of the subjects, delayed timing of HT initiation in relation to when menopause occurred, and the presence of pathological states (e.g., diabetes, hypertension, obesity) [Ancelin and Ritchie, 2005; Genazzani et al., 2007; Maki, 2006; Sherwin, 2005]. In conjunction with the positive results of estrogen obtained in randomized clinical trials in younger women [Sherwin and Henry 2008], multiple factors have led to the development of the “critical period hypothesis” in which it is theorized that estrogen exerts protective effects on cognition only when it is initiated closely in time to menopause, before neural systems become increasingly challenged by age-related changes and/or neurons become less sensitive or responsive to HT.
Animal models have proven useful in investigating different parameters of HT neuroprotection in ways that otherwise are not possible in women. Over the last 15 years, our laboratory has used surgically menopausal macaque monkeys as models of postmenopausal women in studies to investigate the neuroprotection by HT of both cognitive and neurobiological parameters. Unlike rodents [Steger and Peluso, 1987], female macaques have 28 day menstrual cycles and patterns of ovarian hormone fluctuations that are similar to those of human females [Goodman et al., 1977; Jewitt and Dukelow, 1972]. These monkeys experience a menopause that closely resembles that of women [Gilardi et al., 1997; Johnson and Kapsalis, 1995], and they also have physiological responses to surgical menopause and estrogen therapy (ET) that are similar to women [Adams et al., 1990; Jayo et al., 1998; Jerome et al., 1994].
In the following sections, we present studies of menopausal women and female monkeys that support the concept that estrogen therapies protect both cognitive function and neurobiological processes.
Neuroprotection of Cognitive Function
Evidence in Postmenopausal Women
Several lines of evidence support the view that HT provides cognitive neuroprotection in postmenopausal women. Randomized clinical trials in surgically menopausal middle-age women provide the most compelling evidence, with beneficial effects observed in younger women with therapy initiated at the time of surgery [Phillips and Sherwin, 1992; Sherwin, 1988; Sherwin and Phillips, 1990], compared to studies where estrogen had no effect in women who were older or did not receive HT until several years following natural or surgical menopause [Almeida et al., 2006; Binder et al., 2001; Janowsky et al., 2000; LeBlanc et al., 2007; Schiff et al., 2005; Wolf et al., 2005]. In addition, two to three years of HT given in the early phase of menopause (1–5 years of amenorrhea) provided protection against general cognitive impairment [Bagger et al., 2005]. Of note are reports of benefits of HT in reducing the risk for cognitive decline only in women with normal cognitive function at baseline [Viscoli et al., 2005] or of greater adverse effects of HT in women with lower cognitive function [Espeland et al., 2004]. Epidemiological studies also indicate that women who receive HT at menopause have a lower risk of developing AD than never-users [Kawas et al., 1997; Tang et al., 1996; Zandi et al., 2002], unlike older women who received therapy [Mulnard et al., 2000; Shumaker et al., 2003]. Indeed, a stratified analysis demonstrated that HT was associated with a significantly decreased risk of AD, but only in younger postmenopausal women [Henderson et al., 2005]. These studies are in line with the “critical hypothesis” view that estrogen’s cognitive effects are strongest when given near the time of menopause before neural systems become challenged by age-related changes and/or disease processes [Genazzani et al., 2007; Maki, 2006; Sherwin and Henry, 2008].
More direct evidence of estrogen’s neurocognitive protection in postmenopausal women comes from two recent pharmacological studies. Pretreatment with estradiol for 3 months attenuated the detrimental effects of the cholinergic (ACh) muscarinic receptor antagonist, scopolamine (SCOP) in tests of attention and psychomotor function [Dumas et al., 2006]. These findings support our own SCOP data in young OVX monkeys [Voytko, 2002] and emphasize the use of the cholinergic system by estrogen to modulate visuospatial attention. In a subsequent study of postmenopausal women, estradiol pretreatment attenuated the effects of SCOP on verbal memory in younger (mean age 55.8 years) postmenopausal women, but impaired performance under SCOP in older (mean age of 74.3 years) postmenopausal women [Dumas et al., 2008]. These studies suggest that estrogen protects cognitive function from the insult of anti-ACh challenges, but that this protection may only occur when estrogen is given near the time of menopause, before neural insults associated with advancing age or neurodisease become increasingly present and severe. Notably, the effects of estrogen in these studies of women were observed only under the pharmacologic challenge and not at baseline. Although cognition was equivalent under normal conditions, under circumstances of a neural insult (SCOP), only women receiving estradiol demonstrated neuroprotection.
Evidence in Menopausal Monkeys
Numerous studies in ovariectomized (OVX) rodents [Daniel, 2006; Gibbs and Gabor, 2003] have demonstrated the cognitive benefits of ET. We and others also have shown that HT is not only beneficial for cognitive function, but it also provides cognitive neuroprotection in monkey models of menopause. In our studies in young adult monkeys, we found that visuospatial attention function was disrupted within one week following OVX and these deficits continued to significantly worsen during the two months following surgery [Voytko, 2002]. However, these attentional impairments were reversed within one month of starting continuous ET and the reversal was maintained through 14 months of therapy [Voytko, 2002]. Unlike attention processes, aspects of learning, memory, and cognitive flexibility were unaffected by almost two years loss of estrogen or by ET in these same monkeys [Voytko, 2000]. Our results have since been confirmed in studies of memory in OVX young adult monkeys in other laboratories [Hao et al., 2007; Lacreuse and Herndon, 2003]. The absence of HT effects on memory in OVX young monkeys at first glance appears to be in contrast to the HT improvements of memory found in some studies of postmenopausal women [reviewed in Zec and Trivedi, 2002], however the majority of these studies found this HT effect primarily for verbal memory [Zec and Trivedi, 2002; Sherwin, 2003]; inconsistent HT effects have been reported for nonverbal memory in postmenopausal women [Zec and Trivedi, 2002].
Pharmacological challenges to directly assess the capability of ET to protect cognitive function in OVX monkeys have been performed only in our laboratory. In a series of investigations, we challenged OVX young monkeys with SCOP and then tested them on a spatial memory task and a visuospatial attention task. Following SCOP challenges, we found comparable levels of accuracy in a delayed response memory task in monkeys receiving placebo and those receiving ET [Voytko, 2002]. In contrast to memory, we found that the presence of estrogen did affect responses to SCOP in an attention task [Voytko, 2002]. Using a visuospatial cued reaction time task, young OVX monkeys treated with placebo were considerably less sensitive to SCOP challenges than monkeys receiving ET, who performed similar to intact monkeys challenged with SCOP [Davidson et al. 1999; Voytko, 2002]. These findings on attention function suggest that 1) loss of ovarian hormones significantly compromised the ACh system so that it no longer responded normally to challenges with SCOP, and 2) ET sufficiently protected the ACh system so that it could respond to SCOP challenges normally. Importantly, our pharmacologic studies in young OVX monkeys also suggest that the neuroprotective effects of ET may depend on the cognitive domain examined. Our observations in OVX monkeys [Voytko, 2002] concur with those made in postmenopausal women receiving SCOP [Dumas et al., 2006] where ET interacted with the cholinergic system to affect attention, but not memory. Although the specific manner in which estrogen interacted with the cholinergic system to protect attention function varied across female human and nonhuman primates, collectively, these studies emphasize the use of the ACh system by estrogen to modulate visuospatial attention.
With a desire to expand our studies to a closer model of the middle-aged woman experiencing menopause, we began to investigate the effects of OVX and HT in middle-age female monkeys. Our rationale was that the effects of loss and treatment with HT may affect cognitive function differently than what we and others were observing in young OVX monkeys because of the interactions with the complexities associated with advancing age. Using the visual recognition memory task of delayed matching-to-sample, we found that treatment with either ET or the combination of estrogen plus progesterone (E+P) preserved visual memory in older OVX monkeys compared to older OVX monkeys receiving placebo [Voytko et al., 2008]. Similar improvements in memory were found when ET was given within six months of OVX in middle-age monkeys who were tested on a variant of the task that we used that is called the delayed nonmatching-to-sample task [Rapp et al., 2003b]; however ET given many years after OVX in older monkeys had no influence on performance in this recognition memory task [Lacreuse et al., 2002]. These HT benefits however did not extend to other aspects of memory that have been examined in older OVX monkeys [Lacreuse et al., 2002; Rapp et al., 2003b; Voytko et al., 2008].
Neuroprotection of Neurobiological Function
Evidence in Postmenopausal Women
Brain imaging studies indicate that HT may be neuroprotective for postmenopausal women. Specifically, magnetic resonance imaging studies show that HT may prevent age-related brain atrophy in regions that are vulnerable to alterations with age. In cross-sectional studies, women using HT had larger hippocampi compared to nonusers [Eberling et al., 2003; Erickson et al., 2005; Lord et al., 2008], greater cortical grey matter volumes [Erickson et al., 2005; Erickson et al., 2007; Ghidoni et al., 2006], greater white matter volume [Erickson et al., 2005; Ha et al., 2007], and reduced numbers of white matter hyperintensities [Cook et al., 2002; Schmidt et al., 1996]. Moreover, the effects of HT on brain tissue were greater with increasing age suggesting that HT may protect the brain against age-related challenges to brain integrity [Erickson et al., 2005]. Longitudinal imaging studies also support a neuroprotective effect of HT in the brain. Shrinkage of cortical tissue over five years was less in women on HT than in controls [Raz et al., 2004]. After two years, greater increases in regional cerebral blood flow were found in frontal or temporal cortex and the hippocampus of women receiving HT than nonusers, suggesting that HT modulation of cerebral blood flow may protect against Alzheimer’s disease [Maki and Resnick, 2000].
Recent studies in women also demonstrate that the ACh, dopaminergic, and serotonergic systems respond to HT. Single photon emission tomography studies revealed that ACh nerve terminal concentration increased with increasing duration of HT in several cortical regions including in the prefrontal and temporal cortex [Smith et al., 2001], and ACh muscarinic receptor density increased in prefrontal cortex and hippocampus in women taking estrogen compared to never-users [Norbury et al., 2007]. Estrogen increased the dopamine transporter in postmenopausal women [Gardiner et al., 2004] and long-term use of estrogen enhanced central dopaminergic responsivity in postmenopausal women [Craig et al., 2004]. Moreover, estrogen increased serotonin 5HT2 receptors in cerebral cortex in postmenopausal women [Kugaya et al., 2003; Moses-Kolko et al., 2003] and augmented the responses of cortisol and prolactin to a serotonergic agonist [Halbreich et al., 1995].
Evidence in Menopausal Monkeys
Estrogen has demonstrated neuroprotective effects against many insults in vitro, including challenges with amyloid β peptide, glutamate, hydrogen peroxide, iron, and mitochondria toxins [Behl, 2002; Green and Simpkins, 2000]. Estrogen also provides neuroprotection in vivo when animals are challenged with acute cerebral ischemia [McCullough and Hurn, 2003; Wise et al., 2005], neurotrauma [Horvath et al., 2002; Rabbani et al., 1997], or neurotoxins [Morissette et al., 2008]; progesterone also exerts neuroprotection after many of these same types of brain injury [Gibson et al., 2008; Kipp et al., 2006; Stein, 2008]. Estrogen’s greatest neuroprotection appears when therapy is initiated pre-challenge in cell cultures or in vivo, at the time of OVX, or at least 24 hours prior to a neurological insult [Chen et al., 2006; Dubal et al., 1998; McCullough and Hurn, 2003; Suzuki et al., 2007]. Importantly, the middle-aged brain is equally responsive to estrogen’s neuroprotective effects [Wise, 2006].
Synaptic Modulation
Although studies in monkeys are more limited than those in rodents, increasing evidence is accumulating from OVX monkeys to indicate that HT also provides neurobiological neuroprotection in brain regions directly involved in cognitive function. One month of ET that was initiated within three months of OVX prevented dendritic spine loss in the CA1 region of the hippocampus in young monkeys [Leranth et al., 2002; Hao et al., 2003] and old monkeys [Hao et al., 2003]. The prefrontal cortex also is critically involved in cognitive functions and ET initiated within six months of OVX in young monkeys increased the number of dendritic spines in the dorsolateral prefrontal cortex (area 46) following only one month of therapy [Tang et al., 2004] or several years of therapy [Hao et al., 2007]. Longer durations of ET (2–3 years) also increased spine densities in the dorsolateral prefrontal cortex of older OVX monkeys compared to OVX monkeys receiving placebo [Hao et al., 2006]; indeed, the spine density in older ET monkeys was nearly equivalent to young OVX monkeys treated with placebo [Hao et al., 2007]. Finally, ET provided for one month within six months of OVX, increased both pre- and post-synaptic proteins in the CA1 hippocampal region of OVX monkeys, although the combination of E+P did not have this same effect [Choi et al., 2003].
Cholinergic System
Besides the synaptic modulation by ET in the hippocampus and prefrontal cortex of OVX monkey, ET also influences many different neurochemical systems in the primate brain. The ACh system has been the focus of much research in both rodents and more recently in monkeys. In rodents, ET increases binding properties of ACh receptors [Dohanich et al., 1982; Olson et al., 1988; Paradiso et al., 2001; Rainbow et al., 1980] and other ACh markers also respond favorably to ET or E+P in rodents, although not all ACh markers are influenced by HT [reviewed in Gibbs, 2000; Gibbs and Gabor, 2003]. The few studies conducted thus far in OVX monkeys suggest that HT may modulate only particular parameters of the primate ACh system. Two years of ET, initiated at the time of OVX, preserved the ACh fiber density in the prefrontal cortex of young and middle-aged monkeys [Tinkler et al., 2004a; Williams et al., 2004] and short-term ET or E+P initiated within 3–7 months following OVX in monkeys preserved or restored ACh fibers in several cortical regions, including prefrontal cortex [Kompoliti et al., 2004; Kritzer and Kohama, 1999]. However, ACh fiber density was unaffected in the parietal cortex [Tinkler et al., 2004a]. Activity of choline acetyltransferase or acetylcholinesterase in OVX monkeys also was unaffected by long-term ET or E+P in several brains regions [Gibbs et al., 2002]. Two years of OVX or ET did not alter the number or size of ACh neurons in the nucleus basalis of Meynert of young monkeys [Tinkler et al., 2004a], however, ET increased the number of ACh neurons in specific regions of the nucleus basalis in middle-aged monkeys [Browne et al., 2009]. In contrast, ACh neurons in the medial septal/diagonal band nuclei of either young or middle-age monkeys are unaffected by long term ET [Browne et al., 2009]. In concert, observations made in OVX monkeys indicate that HT can modulate the ACh system in primates, but the effects may be specific to age, brain region, and ACh marker.
Dopaminergic System
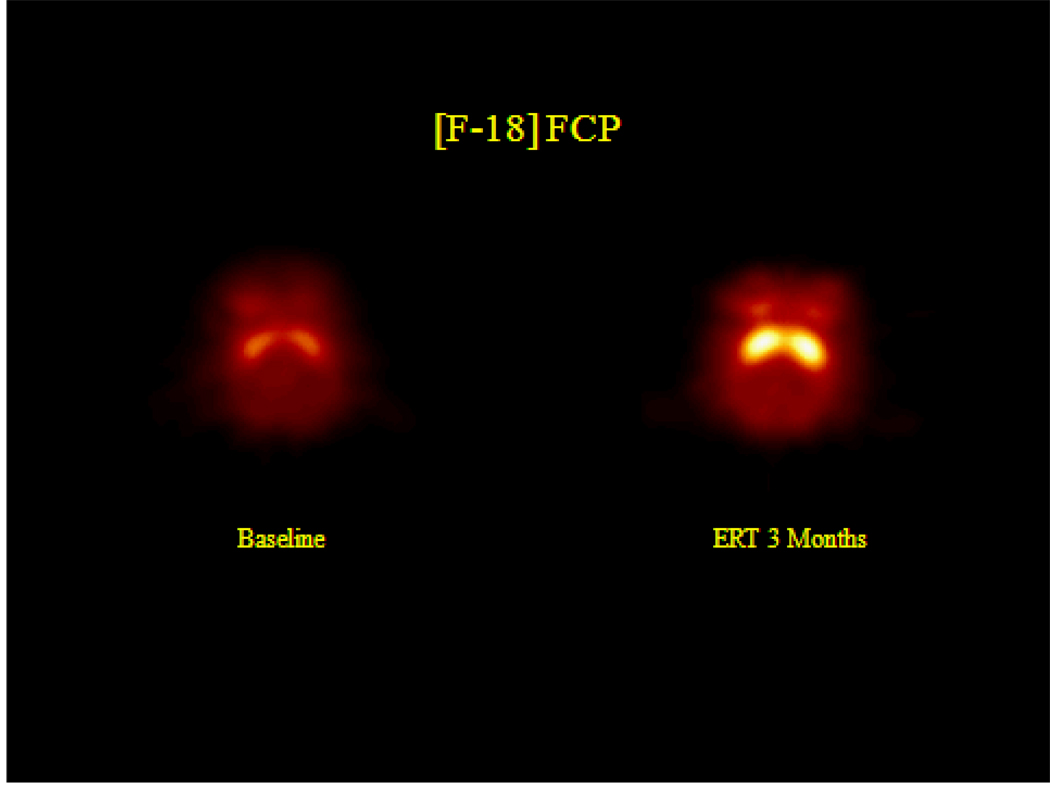
Estrogen modulates many different aspects of DA function, including release, turnover, uptake, and receptors in OVX rodents [e.g., Di Paolo, 1994; Disshon et al., 1998; Hruska and Nowak, 1988; Lee and Mouradian, 1999; Morissette et al., 1992]. In OVX monkeys, short-term ET has beneficial effects in the DA mesostriatal system. Density of tyrosine hydroxylase fibers in the striatum were increased following ET or E+P that was initiated within six months of OVX [ Kritzer et al., 2003] and numbers of mesencephalic DA neurons in monkeys were preserved with ET, but only when ET was initiated within ten days of OVX [Leranth et al., 2000]. In a preliminary study, we used positron emission tomography and the radiotracer, [18F]-Fluoroclebopride ([18F]FCP), to visualize DA D2 receptors in the brain of a female rhesus OVX monkey prior to receiving ET and following three months of therapy. In comparison to images obtained pretreatment, there was a significant increase in D2 receptor binding in the basal ganglia following three months of ET (Fig. 1). The effects of longer durations of OVX or HT in the primate DA mesostriatal system is unknown. The primate DA mesocortical system is not as consistent in responding to HT. One month of ET partially restored decreased tyrosine hydroxylase fiber density in prefrontal cortex of OVX monkeys [Kritzer and Kohama, 1998], however in studies of our own, we did not find that DA fiber density in the prefrontal cortex of monkeys was sensitive to two years of either OVX or ET [Tinkler et al. 2004b]. In addition, two years of OVX or ET in monkeys did not affect levels of DA or its metabolite dihyroxyphenylacetic acid in several brain regions of OVX monkeys [Gibbs et al., 2006], or the numbers or size of DA neurons in the midbrain areas that project to cortex [Tinkler et al., 2003]. Collectively, these findings in the primate DA mesocortical system suggest that the effects of OVX and ET in this DA system may depend on the timing or duration of the manipulations, or that the mesocortical system may be less sensitive to these manipulations in comparison to the mesostriatal DA system.
Figure 1.
[18F]Fluoroclebopride (FCP) binding in the striatum of an ovariectomized female monkey at baseline (before receiving estrogen therapy) and following three months of estrogen therapy. Binding of FCP is notably greater following estrogen therapy.
Serotonergic System
Ovarian hormones modulate many facets of the serotonergic system in monkeys. Serotonin production, turnover, and neural responses are influenced by HT [Bethea et al., 2002; Lu and Bethea, 2002; Lu et al., 2003]. Additionally, HT regulates genes and pathways involved in the caspase pathways to affect serotonin neuronal survival [Bethea and Reddy, 2008; Tokuyama et al., 2008]. While ovariectomy increased serotonin fibers in prefrontal cortex, HT lowered fiber density in this region [Kritzer and Kohama, 1999]. Two years of therapy with conjugated equine estrogens plus a progestagen decreased levels of serotonin and 5-hydroxyindole acetic acid (5-HIAA) in the dorsal raphe and frontal cortex of primates, although estrogen alone had no effect [Gibbs et al., 2006].
CONCLUSIONS
Aging and age-associated neurodegenerative disease in particular provide progressively greater challenges and neural insults to the brain. These challenges will increasingly compromise cognitive processes and result in cognitive decline. Thus it is important that therapeutic interventions are identified that can protect cognitive and neurobiological functions during the aging process. The previous sections provide evidence that ET, and possibly E+P, are neuroprotective for both cognitive and neurobiological function in both menopausal women and female monkeys, especially under the condition in which these therapies are administered prior to a challenge or insult. While evidence is accumulating, it is clear that many questions remain unanswered regarding the factors that play a role in the cognitive neuroprotection afforded by HT. For example, how long is the critical window of opportunity for which HT is neuroprotective, if HT is discontinued does its neuroprotective effects continue and for what duration, and do the benefits of early therapy with HT continue to be realized with long durations of usage? Because of their similarity to women in endocrine and reproductive profiles, female macaque monkeys will continue to be critical models to investigate these questions to inform and guide the health care community in the treatment of women as they experience aging and menopause.
ACKNOWLEDGEMENTS
The research reported by the authors was supported by grant AG13204 from the National Institute on Aging (MLV). This research complied with the ASP Principles for the Ethical Treatment of Animals and the requirements of the National Institutes of Aging.
References
- Adams MR, Kaplan JR, Manuck SB, Kroritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys: Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27:141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Ancelin ML, Ritchie K. Lifelong endocrine fluctuations and related cognitive disorders. Curr Pharm Des. 2005;11:4229–4252. doi: 10.2174/138161205774913228. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C PERF Study Group. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Behl C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Effect of ovarian hormones on survival genes in laser captured serotonin neurons from macaques. J Neurochem. 2008;105:1129–1143. doi: 10.1111/j.1471-4159.2008.05213.x. [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38:137–146. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- Browne C, Tobin JR, Voytko ML. Effects of two years of conjugated equine estrogens on cholinergic neurons in young and middle-aged ovariectomized monkeys. Br Res. 2009 doi: 10.1016/j.brainres.2009.01.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Nilsen J, Brinton RD. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: therapeutic implications. Endocrinology. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwak Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144:4734–4738. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- Cook IA, Morgan ML, Dunkin JJ, David S, Witte E, Lufkin R, Abrams M, Rosenberg S, Leuchter AF. Estrogen replacement therapy is associated with less progression of subclinical structural brain disease in normal elderly women: a pilot study. Int J Geriatr Psychiatry. 2002;17:610–618. doi: 10.1002/gps.644. [DOI] [PubMed] [Google Scholar]
- Craig MC, Cutter WJ, Wickham H, van Amelsvoort TA, Rymer J, Whitehead M, Murphy DG. Effect of long-term estrogen therapy on dopaminergic responsivity in post-menopausal women--a preliminary study. Psychoneuroendocrinology. 2004;29:1309–1316. doi: 10.1016/j.psyneuen.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Cutrell EB, Marrocco R. Scopolamine slows the orienting of attention in primates to cued visual targets. Psychopharmacology. 1999;142:1–8. doi: 10.1007/s002130050855. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol. 1998;345:207–211. doi: 10.1016/s0014-2999(98)00008-9. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Witcher JA, Weaver DR, Clemens LG. Alteration of muscarinic binding in specific brain areas following estrogen treatment. Brain Res. 1982;241:347–350. doi: 10.1016/0006-8993(82)91075-7. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology. 2006;31:2065–2078. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging. 2003;24:725–732. doi: 10.1016/s0197-4580(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28:179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, Cohen NJ, McAuley E, Kramer AF. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol Aging. 2005;26:1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J Women's Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Gardiner SA, Morrison MF, Mozley PD, Mozley LH, Brensinger C, Bilker W, Newberg A, Battistini M. Pilot study on the effect of estrogen replacement therapy on brain dopamine transporter availability in healthy, postmenopausal women. Am J Geriatr Psychiatry. 2004;12:621–630. doi: 10.1176/appi.ajgp.12.6.621. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Pluchino N, Luisi S, Luisi M. Estrogen, cognition and female ageing. Hum Reprod Update. 2007;13:175–187. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- Ghidoni R, Boccardi M, Benussi L, Testa C, Villa A, Pievani M, Gigola L, Sabattoli F, Barbiero L, Frisoni GB, Binetti G. Effects of estrogens on cognition and brain morphology: involvement of the cerebellum. Maturitas. 2006;54:222–228. doi: 10.1016/j.maturitas.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Oestrogen and the cholinergic hypothesis: implications for oestrogen replacement therapy in postmenopausal women. Novartis Found Symp. 2000;230:94–111. doi: 10.1002/0470870818.ch8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Edwards D, Lazar N, Nelson D, Talameh J. Effects of long-term hormone treatment and of tibolone on monoamines and monoamine metabolites in the brains of ovariectomised, cynomologous monkeys. J Neuroendocrinol. 2006;18:643–654. doi: 10.1111/j.1365-2826.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologus monkeys. Neuroscience. 2002;113:907–914. doi: 10.1016/s0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biology of Reproduction. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Descalzi CD, Johnson DK, Hodgen GD. Composite pattern of circulating LH, FSH, estradiol, and progesterone during the menstrual cycle in cynomolgus monkeys. Proc Soc Exp Biol Med. 1977;155:479–481. doi: 10.3181/00379727-155-39834. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Ha DM, Xu J, Janowsky JS. Preliminary evidence that long-term estrogen use reduces white matter loss in aging. Neurobiol Aging. 2007;28:1936–1940. doi: 10.1016/j.neurobiolaging.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estrogen augments serotonergic activity in postmenopausal women. Biol Psychiatry. 1995;37:434–441. doi: 10.1016/0006-3223(94)00181-2. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath KM, Hårtig W, Van der Veen R, Keijser JN, Mulder J, Ziegert M, Van der Zee EA, Harkany T, Luiten PG. 17beta-estradiol enhances cortical cholinergic innervation and preserves synaptic density following excitotoxic lesions to the rat nucleus basalis magnocellularis. Neuroscience. 2002;110:489–504. doi: 10.1016/s0306-4522(01)00560-7. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Nowak MW. Estrogen treatment increases the density of D1 dopamine receptors in the rat striatum. Brain Res. 1988;442:349–350. doi: 10.1016/0006-8993(88)91523-5. [DOI] [PubMed] [Google Scholar]
- Janowsky SJ, Chavez B, Orwoll F. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Jayo MJ, Register TC, Carlson CS. Effects on bone of oral hormone replacement therapy initiated 2 years after ovariectomy in young adult monkeys. Bone. 1998;23:361–366. doi: 10.1016/s8756-3282(98)00106-9. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Kapsalis E. Ageing, infecundity, and reproductive senescence in free-ranging female rhesus monkeys. Journal of Reproduction and Fertility. 1995;105:271–278. doi: 10.1530/jrf.0.1050271. [DOI] [PubMed] [Google Scholar]
- Jerome CP, Carlson CS, Register TC, Bain FT, Jayo MJ, Weaver DS, Adams MR. Bone functional changes in intact, ovariectomized, hormone-supplemented adult cynomolgus monkeys (Macaca fascicularis) evaluated by serum markers and dynamic histomorphometry. J Bone Mineral Res. 1994;9:527–540. doi: 10.1002/jbmr.5650090413. [DOI] [PubMed] [Google Scholar]
- Jewitt DA, Dukelow WR. Cyclicity and gestation length of Macaca fascicularis. Primates. 1972;13:327–330. [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Kipp M, Karakaya S, Pawlak J, Araujo-Wright G, Arnold S, Beyer C. Estrogen and the development and protection of nigrostriatal dopaminergic neurons: concerted action of a multitude of signals, protective molecules, and growth factors. Front Neuroendocrinol. 2006;27:376–390. doi: 10.1016/j.yfrne.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Chu Y, Polish A, Roberts J, McKay H, Mufson EJ, Leurgans S, Morrison JH, Kordower JH. Effects of estrogen replacement therapy on cholinergic basal forebrain neurons and cortical cholinergic innervation in young and aged ovariectomized rhesus monkeys. J Comp Neurol. 2004;472:193–207. doi: 10.1002/cne.20050. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Bethea CL. Ovarian hormone influences on the density of immunoreactivity for tyrosine hydroxylase and serotonin in the primate corpus striatum. Neuroscience. 2003;122:757–772. doi: 10.1016/s0306-4522(03)00548-7. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine beta- hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160:1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG. Estradiol selectively affects processing of conspecifics' faces in female rhesus monkeys. Psychoneuroendocrinology. 2003;28:885–905. doi: 10.1016/s0306-4530(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause. 2007;14:191–202. doi: 10.1097/01.gme.0000230347.28616.1c. [DOI] [PubMed] [Google Scholar]
- Lee SH, Mouradian MM. Up-regulation of D1A dopamine receptor gene transcription by estrogen. Mol Cell Endocrinol. 1999;156:151–157. doi: 10.1016/s0303-7207(99)00133-1. [DOI] [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE. Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution and function in female macaques. Mol Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- McCollough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Morissette M, Lévesque D, Di Paolo T. Effect of chronic estradiol treatment on brain dopamine receptor reappearance after irreversible blockade: an autoradiographic study. Mol Pharmacol. 1992;42:480–488. [PubMed] [Google Scholar]
- Morissette M, Sweidi SA, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson's disease. Mol Cell Endocrinol. 2008;290:60–99. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Berga SL, Greer PJ, Smith G, Cidis Meltzer C, Drevets WC. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80:554–559. doi: 10.1016/s0015-0282(03)00973-7. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer's Disease Cooperative Study. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, Murphy DG. Estrogen therapy and brain muscarinic receptor density in healthy females: a SPET study. Horm Behav. 2007;51:249–257. doi: 10.1016/j.yhbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Olson KL, Edwards E, Schechter N, Whalen RE. Muscarinic receptors in preoptic area and hypothalamus: effects of cyclicity, sex and estrogen treatment. Brain Res. 1988;448:223–229. doi: 10.1016/0006-8993(88)91259-0. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH. The C terminus of the human nicotinic alpha4beta2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21:6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Rabbani O, Panickar KS, Rajakumar G, King MA, Bodor N, Meyer EM, Simpkins JW. 17 beta-estradiol attenuates fimbrial lesion-induced decline of ChAT-immunoreactive neurons in the rat medial septum. Exp Neurol. 1997;146:179–186. doi: 10.1006/exnr.1997.6516. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Degroff V, Luine VN, McEwen BS. Estradiol 17 beta increases the number of muscarinic receptors in hypothalamic nuclei. Brain Res. 1980;198:239–243. doi: 10.1016/0006-8993(80)90362-5. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003a;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003b;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Hormone replacement therapy and age-related brain shrinkage: regional effects. Neuroreport. 2004;15:2531–2534. doi: 10.1097/00001756-200411150-00020. [DOI] [PubMed] [Google Scholar]
- Schiff R, Bulpitt CJ, Wesnes KA, Rajkumar C A randomised placebo controlled pilot cross-over study. Short-term transdermal estradiol therapy, cognition and depressive symptoms in healthy older women. Psychoneuroendocrinology. 2005;30:309–315. doi: 10.1016/j.psyneuen.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacher H, Eber B, Schumacher M, Freidl W. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J Am Geriatr Soc. 1996;44:1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci. 2005;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Phillips S. Estrogen and cognitive functioning in surgically menopausal women. Ann NY Acad Sci. 1990;592:474–475. [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Smith YR, Giordani B, Lajiness-O'Neill R, Zubieta JK. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertil Steril. 2001;76:1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- Steger RW, Peluso JJ. Sex hormones in the aging female. Endocrinology and Metabolism Clinics. 1987;16:1027–1043. [PubMed] [Google Scholar]
- Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Dopaminergic neurons in the ventral tegmental area of intact and surgically menopausal monkeys. Soc Neuroscience. 2003:17. Abstr Program No. 843. [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol. 2004a;469:507–521. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Dopaminergic fibers in the prefrontal cortex following two years of estrogen loss or replacement in young adult surgically menopausal monkeys. Soc Neurosci. 2004b:10. Abstr Program No. 72. [Google Scholar]
- Tokuyama Y, Reddy AP, Bethea CL. Neuroprotective actions of ovarian hormones without insult in the raphe region of rhesus macaques. Neuroscience. 2008;154:720–731. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. Estrogen therapy and risk of cognitive decline: results from the Women's Estrogen for Stroke Trial (WEST) Am J Obstet Gynecol. 2005;192:387–393. doi: 10.1016/j.ajog.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Voytko ML. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys. Behav Neurosci. 2000;114:1078–1087. [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys. Behav Neurosci. 2002;116:187–197. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience. 2008;154:1205–1217. doi: 10.1016/j.neuroscience.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PN, Tinkler GP, Tobin JR, Voytko ML. Cholinergic fiber density in area 46 of the prefrontal cortex of middle-aged surgically menopausal monkeys. Soc Neurosci. 2004:2. Abstr 30, Program No. 72. [Google Scholar]
- Wise PM. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–835. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women's health initiative. Endocr Rev. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Heinrich AB, Hanstein B, Kirschbaum C. Estradiol or estradiol/progesterone treatment in older women: no strong effects on cognition. Neurobiol Aging. 2005;26:1029–1033. doi: 10.1016/j.neurobiolaging.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zec RF, Trivedi MA. The effects of estrogen replacement therapy on neuropsychological functioning in postmenopausal women with and without dementia: a critical and theoretical review. Neuropsychol Rev. 2002;12:65–109. doi: 10.1023/a:1016880127635. [DOI] [PubMed] [Google Scholar]