SUMMARY
Deer tick virus is related to Powassan virus, a tickborne encephalitis virus. A 62-year-old man presented with a meningoencephalitis syndrome and eventually died. Analyses of tissue samples obtained during surgery and at autopsy revealed a widespread necrotizing meningoencephalitis. Nucleic acid was extracted from formalin-fixed tissue, and the presence of deer tick virus was verified on a flavivirus-specific polymerase-chain-reaction (PCR) assay, followed by sequence confirmation. Immunohistochemical analysis with antisera specific for deer tick virus identified numerous immunoreactive neurons, with prominent involvement of large neurons in the brain stem, cerebellum, basal ganglia, thalamus, and spinal cord. This case demonstrates that deer tick virus can be a cause of fatal encephalitis.
Deer tick virus is a member of the tickborne encephalitis group of flaviviruses and is closely related to Powassan virus. Deer tick virus was first isolated from Ixodes scapularis ticks in 1997 in North America.1 The complete sequence of the deer tick virus has been determined.2 The viral genome is 10.8 kb in length and shares 84% nucleotide sequence identity and 94% amino acid sequence identity with the Powassan virus genome. The two viruses are antigenically related,3 and it has been suggested that they share a common origin and represent two viral lineages related to Powassan virus in North America.2 Ebel et al.4 refer to deer tick virus as Powassan virus lineage II, and in this report we use the same terminology.
Several members of the tickborne encephalitis group of flaviviruses, including tickborne encephalitis virus and Powassan virus, cause encephalitis in humans and animals, with tickborne encephalitis virus causing the most serious outbreaks. These viruses are closely related antigenically and are found predominantly in the northern hemisphere. In Europe, tickborne encephalitis occurs mainly in eastern and central regions and affects approximately 50 to 199 persons per 100,000 inhabitants annually.5 The seroprevalence of antibodies to Powassan virus is estimated to be 0.5 to 4.0% in areas in which the disease is endemic.6
Infection with tickborne encephalitis virus can be mild or asymptomatic, or it can result in meningitis and encephalitis. Powassan virus can be pathogenic in human beings and can cause severe encephalitis with a fatality rate of up to 60% and long-term neurologic sequelae in survivors.7 In contrast, Central European encephalitis that is caused by tick bites typically produces mild or silent infection. Other disease-causing flaviviruses include West Nile virus, St. Louis encephalitis virus, dengue virus, and yellow fever virus.8 These viruses are transmitted by mosquitoes and cause a spectrum of diseases including meningitis, encephalitis, dengue fever, and yellow fever.
In certain locations of the northeastern and north central United States, the prevalence of deer tick virus in adult deer ticks is high,9,10 but human infection has not been reported previously. This could indicate that the virus does not easily infect humans or that it is not particularly pathogenic. Diagnostic testing for Powassan virus is not routinely performed for patients with symptoms of encephalitis. Human incidence may thus be currently underestimated.
CASE REPORT
In late spring, a 62-year-old man was admitted to a local New York State hospital with a 4-day history of fatigue, fever, bilateral maculopapular palmar rash, and an onset of diplopia, dysarthria, and weakness in the right arm and leg. He was a native of New York State and had no history of recent travel. He owned horses and spent time outdoors in a wooded area. Reports of Lyme disease were common in his county of residence, indicating tick activity in the area. His medical history included chronic lymphocytic leukemia–small lymphocytic lymphoma (CLL–SLL), which had been diagnosed 4 years earlier and had initially been treated with fludarabine. He was not taking corticosteroids. On admission, he was given nonsteroidal antiinflammatory medication and an oral antibiotic (amoxicillin–clavulanate), which had been prescribed by his primary care physician for a recent exacerbation of chronic sinusitis that had been recurrent for more than a year. His baseline white-cell count was 15,000 cells per cubic millimeter and had increased to 70,000 cells per cubic millimeter during the past 6 to 8 months. He was started on broad-spectrum antibiotics and acyclovir (700 mg administered intravenously every 8 hours) for presumed infection of the central nervous system. The differential diagnosis included cerebral ischemia, possibly related to leukostasis, infection (viral, bacterial, or fungal), and lymphoma.
Initial laboratory results were notable for a markedly elevated peripheral-blood white-cell count (144,200 cells per cubic millimeter) and cerebrospinal fluid with normal glucose, minimally elevated protein, no white cells, and a negative Gram’s stain (Table 1). The erythrocyte sedimentation rate was 4, blood cultures were sterile, and antibody titers were negative for Borrelia burgdorferi and Anaplasma phagocytophilum. The neurologic symptoms progressed, and after 2 days he was transferred to another hospital. At the time of transfer, the peripheral-blood white-cell count was 174,800 per cubic millimeter (with 4% neutrophils and 94% lymphocytes) (Table 1).
Table 1.
Results of Analysis of Cerebrospinal Fluid and Blood of the Patient.*
| Variable | First Hospitalization | Day 1 after Transfer to Second Hospital | Normal Range |
|---|---|---|---|
|
Cerebrospinal fluid | |||
| Glucose level (mg/dl) | 59 | 47 | 40–70 |
| Protein level (mg/dl) | 64 | 192 | 15–45 |
| White-cell count (cells/mm3) | 0 | 891 | 0–5 |
| Neutrophils (%) | 1 | 0 | |
| Lymphocytes (%) | 93 | 70 | |
|
Complete blood count | |||
| White-cell count (cells/mm3) | 144,200 | 174,800 | 3500–9100 |
| Neutrophils (%) | 2 | 4 | 38–80 |
| Lymphocytes (%) | 98 | 94 | 15–40 |
To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Findings on flow cytometry were characteristic of CLL–SLL. Bacterial and fungal blood cultures were sterile. Sputum cultures for tuberculosis and legionella species were negative. No serum antibodies to Bartonella henselae or leptospira or brucella species were detected. One day after admission, a repeat spinal tap showed an elevated protein level of 192 mg per deciliter, lymphocytic pleocytosis with 891 cells per cubic millimeter (with 1% neutrophils and 93% lymphocytes), and a normal glucose level (Table 1). Flow cytometry of the cerebrospinal fluid demonstrated a predominantly reactive T-cell population (98% of CD45+ cells were CD3+/CD5+ small T cells), with no evidence of CLL–SLL. Bacterial culture and Gram’s staining of the cerebrospinal fluid were negative. India-ink staining, cryptococcus antigen test, and PCR analyses for herpes simplex virus types 1 and 2 and JC–BK virus were negative in cerebrospinal fluid.
Magnetic resonance imaging (MRI) performed after transfer (hospital day 1) revealed abnormal T2-weighted and fluid-attenuated inversion recovery (FLAIR) images, with hyperintensities most prominent in the superior cerebellum, left pons, and bilateral basal ganglia (Fig. 1A, 1B, and 1C). An axial diffusion-weighted image and apparent-diffusion-coefficient sequences revealed restricted diffusion in the superior cerebellum, suggesting an ischemic process (Fig. 1D). The patient remained febrile (maximum temperature, 104.5°F [40.3°C]), and antimicrobial coverage was broadened to include an antifungal agent. His neurologic function deteriorated, which necessitated intubation, and his function did not improve despite maximal medical therapy.
Figure 1. Magnetic Resonance Imaging (MRI) of the Brain on Hospital Admission.
MRI scanning that was performed on hospital day 1 revealed abnormal T2-weighted signaling in the superior cerebellum (Panel A, arrow) and abnormal T2-weighted fluid-attenuated inversion recovery images with hyperintensities in the cerebellum and left pons (Panel B, arrows) and in the bilateral basal ganglia (Panel C). The superior cerebellum was bright on diffusion-weighted imaging (Panel D) and dark on apparent-diffusion-coefficient sequences, which suggested an ischemic process.
On hospital day 4, his fever abated, and computed tomographic imaging revealed a mild obstructive hydrocephalus, leading to placement of an external ventricular drain. On hospital day 5, repeat MRI revealed worsening of signal abnormalities and markedly increased hydrocephalus. He was taken urgently to the operating room for decompression with a suboccipital craniotomy, at which time cerebellar biopsy was performed. Analysis of the biopsy specimen revealed severe meningoencephalitis with a dense meningeal lymphoid infiltrate containing mainly reactive CD4+ T cells, lymphocytic venous invasion and destruction, widespread loss of cerebellar Purkinje cells, occasional microglial nodules, and marked Bergmann gliosis (Fig. 1A to 1H in the Supplementary Appendix, available with the full text of this article at NEJM.org). The parenchyma was infiltrated by activated microglia–macrophages and predominantly CD8+ T cells (Fig. 1I and 1J in the Supplementary Appendix). All biopsy cultures were negative, and staining of biopsy tissue was negative for bacterial, fungal, and mycobacterial organisms and viral antigens (including herpes simplex virus 1 and 2, varicella–zoster virus, cytomegalovirus, influenza A, parainfluenza 3, adenovirus, and parvovirus).
MRI of the brain on hospital day 7 revealed progression of signal abnormalities, new lesions in the right thalamus and bilateral cerebral hemispheres, and persistent hydrocephalus (Fig. 2 in the Supplementary Appendix). By hospital day 11, there was no improvement in his status. Life support was withdrawn, and he died 17 days after the onset of symptoms. An autopsy was performed.
METHODS
CLINICAL SPECIMENS
A surgical biopsy of the cerebellum was fixed in formalin and embedded in paraffin. After autopsy, the brain was formalin-fixed for 2 weeks, and standard tissue blocks were paraffin-embedded. Unembedded, formalin-fixed brain tissue from the midbrain, cerebellum, pons, and spinal cord was submitted for PCR testing. (For details on viruses and control samples that were used, see the Methods section in the Supplementary Appendix.)
REVERSE-TRANSCRIPTASE–PCR AND SEQUENCE ANALYSIS
Nucleic acid was extracted from formalin-fixed tissue with the use of the WaxFree DNA extraction kit (TrimGen). This kit coextracts RNA. Ten microliters of extracted nucleic acid was reverse-transcribed to complementary DNA (cDNA) with the use of the iScript cDNA synthesis kit (Bio-Rad). Heminested reverse-transcriptase PCR (RT-PCR) for the detection of flavivirus with the use of universal primers was performed as described previously.11,12 (In the Supplementary Appendix, additional information on the PCR primers is listed in Table A, and details regarding the PCR methods, sequence, and histologic and immunohistochemical analyses are listed in the Methods section.)
RESULTS
The general autopsy revealed diffuse lymphadenopathy and splenomegaly and infiltration of liver and kidney by CLL–SLL. The brain weight was 1810 g (normal range in adults, 1300 to 1350), consistent with marked edema. On sectioning, there was marked softening and grayish discoloration throughout the brain stem and cerebellum.
Histologic examination of the brain revealed widespread meningopolioencephalitis and meningopoliomyelitis; there was no evidence of infiltration by CLL–SLL. A mild meningeal lymphocytic infiltrate persisted, and dense perivascular infiltrates were still identified in the parenchyma (Fig. 3C to 3K in the Supplementary Appendix). Throughout the brain, multinodular to patchy mononuclear infiltrates and confluent areas of necrosis were identified, along with microglial nodules and neuronophagia. This was most accentuated in large motor neurons of the brain stem (including cranial nerve nuclei), spinal anterior horns, cerebellum, basal ganglia, and thalamus (Fig. 2, and Fig. 3 in the Supplementary Appendix). Microglia–macrophage infiltration was greatest in gray-matter regions but also involved white-matter tracts to a lesser degree (Fig. 3A in the Supplementary Appendix).
Figure 2. Histologic Findings at Autopsy.
In Panel A, microglial nodules and lymphocytic infiltrates in the pons are visible in basal pontine nuclei (arrowheads), with less prominent involvement of descending fiber tracts (arrow) and pontocerebellar fibers. In Panel B, confluent foci of parenchymal necrosis can be seen in pontine basal nuclei. In Panel C, CD8+ immunostaining of the basis pontis shows a cytotoxic T-cell infiltrate and a close association with surviving neurons (arrows). In Panel D, nearly complete neuronal loss is seen in the substantia nigra with rare surviving neurons (arrows); in the inset, an eosinophilic dying neuron and remaining neuromelanin pigment are engulfed in macrophages or free in the parenchyma (arrowheads). In Panel E, phosphoglucomutase 1 immunostaining of lumbar spinal cord shows marked infiltration by microglia–macrophages and in the anterior horn and focal microglial nodules in the lateral corticospinal tract (arrow) and posterior column (arrowhead). In Panels A, B, and D, paraffin sections were stained with hematoxylin and eosin.
As in the surgical biopsy, lymphocytic infiltrates in leptomeninges and perivascular spaces contained predominantly CD4+ helper T cells, whereas those in the parenchyma were predominantly CD8+ cytotoxic T cells (Fig. 4 in the Supplementary Appendix). CD8+ T cells were also more frequently identified in close apposition to surviving neurons (Fig. 2C, and Fig. 4A, 4B, and in the Supplementary Appendix).
On the extracted nucleic acid from the formalin-fixed brain tissue, the following analyses were performed: a PCR panel including real-time PCR assays for the detection of herpes simplex viruses 1 and 2, Epstein–Barr virus, cytomegalovirus, human herpesvirus type 6, varicella–zoster virus, and adenovirus; real-time RT-PCR assays for the detection of West Nile virus and eastern equine encephalitis virus; a real-time PCR assay using a cDNA template for the detection of enterovirus; a group-specific RT-PCR assay for the detection of alphaviruses13; and conventional PCR assays using a cDNA template for the detection of St. Louis encephalitis, California serogroup, and Cache Valley viruses. PCR assays for the detection of borrelia species, including B. burgdorferi, and of A. phagocytophilum were performed on DNA extracts from the cerebellum and spinal cord. All results were negative. A group-specific RT-PCR assay for the detection of flaviviruses gave PCR products of the expected size for both the first-round PCR and the nested PCR.11 The PCR products of approximately 250 bp and 220 bp were purified from the gel and sequenced. A search with the use of the nucleotide Basic Local Alignment Search Tool (BLAST) algorithm posted on the Web site of the National Center for Biotechnology Information identified a 220-bp sequence sharing 97% of the sequence of deer tick virus strains CTB30 (accession number, AF311056.1), and IPS001 (accession number, AF310947.1) and Powassan virus strain R59266 (accession number, AF310948.1). To confirm the lineage of the virus, sequencing was performed with the use of previously published and newly designed primer sets from the envelope coding region, NS5, and sequences in the 3′ untranslated region1,4 (Table A in the Supplementary Appendix).
With a total of 23 primer sets used, two regions of the virus were sequenced: 2748 bp, spanning part of the RNA polymerase coding sequence and the 3′ untranslated region of the virus, and 1180 bp of the envelope coding sequence. Phylogenetic analyses of these fragments indicated that the virus, named DT-NY-07, was most closely related to the deer tick virus (Fig. 3).14–16
Figure 3. Phylogenetic Tree Showing the Relationship between the Virus (DT-NY-07) Detected in Tissue Sections from the Brain of the Patient and Other Powassan Viruses.
This phylogenetic tree was constructed from 2304 nucleotide sequences of the NS5 region. GenBank accession numbers are in parentheses. The evolutionary history was inferred with the use of the neighbor-joining method.14 The optimal tree with the sum of branch length equaling 0.60849794 is shown. The percentage of replicate trees in which the associated taxa are clustered together in the bootstrap test (1000 replicates) is shown next to each branch. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to construct the phylogenetic tree. To root the dendrogram, louping ill virus was used as the outgroup. The evolutionary distances were computed with the use of the maximum-composite-likelihood method15 and are expressed in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the data set. Phylogenetic analyses were conducted with the use of Molecular Evolutionary Genetics Analysis (MEGA) software, version 4.0.16
To confirm that deer tick virus antigens were detectable in brain tissue from the patient, two polyclonal mouse antibody reagents were generated against whole deer tick virus and recombinant deer tick virus E protein (rEDTV). Both antiserum samples showed similar immunohistochemical specificity in both the cerebellar biopsy and autopsy specimens, although generally a larger number of neurons and viral antigens in macrophages were labeled with the whole-virus serum (Fig. 4, and Fig. 5 in the Supplementary Appendix). The whole-virus antiserum labeled neuronal-cell bodies, dendrites, and axons. The rEDTV serum and rarely the whole-virus serum also labeled rounded, granular-to-tubular profiles within the neuronal cytoplasm of large motor neurons, with a cellular distribution highly reminiscent of the Golgi apparatus in some neurons (Fig. 4A, and Fig. 6 in the Supplementary Appendix). Alternatively, the structures may represent viral particles within the lysosomal–endosomal system. A segmental distribution of labeled neurons was prominent in the hippocampus (Fig. 4B). In isocortical regions, occasional labeled neurons and a focus of infected cells consistent with oligodendrocytes were also identified (Fig. 4D).
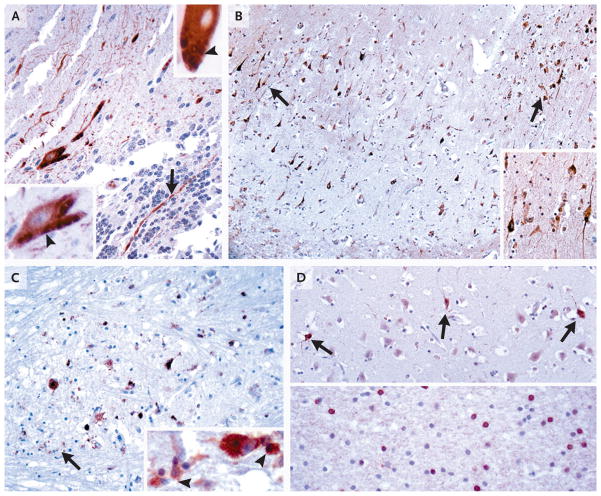
Figure 4. Immunohistochemical Analysis with Deer Tick Virus Antiserum Samples.
Paraffin sections of cerebellar samples obtained from the patient on biopsy (Panel A) and samples from the hippocampus (Panel B), pons (Panel C), and temporal cortex (Panel D) obtained at autopsy were stained either with antibody against whole deer tick virus (Panel A, upper inset; and Panels B and C) or with antibody against recombinant deer tick virus E protein (rEDTV) (Panel A; Panel A, lower inset; and Panel D). In Panel A, in the cerebellar-biopsy sample, both types of antiserum recognized surviving Purkinje cells, with prominent filling of their dendrites in the molecular layer and occasional identification of axons in the granule-cell layer (arrow); in the insets, several Purkinje cells were identified with immunoreactive granular-to-tubular profiles (arrowheads). In Panel B, many hippocampal pyramidal neurons were immunolabeled in a segmental distribution (in area surrounding arrows), with prominent decoration of apical and basal processes (inset). In Panel C, many surviving immunolabeled neurons in the basis pontis are visible. The whole deer tick virus antibody also recognized viral antigens engulfed in macrophages (arrow; inset, arrowheads), whereas the rEDTV antibody did not have such recognition. In Panel D, in temporal cortex, immunoreactive neurons that were not associated with inflammatory reaction were occasionally identified (upper panel, arrows). In the temporal white matter, a focus of labeled cells consistent with oligodendrocytes was seen (lower panel). (For more details, see Fig. 5 and 6 in the Supplementary Appendix.)
DISCUSSION
Strains of Powassan virus lineages I and II are distinct and are maintained in separate enzootic cycles because of differences in transmission vectors and geographic distribution. Lineage I strains are transmitted by ticks and have been reported in North America (mainly in New York State and Canada) and in eastern Russia, whereas lineage II strains have been isolated in the Atlantic Coast of the United States and in Wisconsin.4 Lineage I strains appear to be associated with I. cookei and groundhogs (Marmota monax), whereas lineage II strains are associated with deer ticks and white-footed mice (Peromyscus leucopus).7 In addition, lineage II strains have not previously been associated with human disease, whereas a number of infections in humans associated with lineage I strains have been documented.17–21 From these reports, it appears that lineage I Powassan encephalitis is characterized by respiratory distress, fever, vomiting, convulsions, and occasionally paralysis.17,19 Studies in the northern Ontario region of Canada show an antibody prevalence rate of as much as 3.2%, indicating that infection does not always cause severe disease.22 In a phylogenetic study of Powassan-related viruses of North America, a lineage II strain (ON97) was reportedly isolated from human brain tissue.2 However, no other information regarding the case was provided.
Confirmation of infection with a lineage I strain of Powassan virus has been made principally by serologic methods. Because of serologic cross-reactivity, these methods do not necessarily distinguish lineage I from lineage II strains. Neutralization assays are required for confirmation; molecular detection and sequence determination, as performed in our investigation, allowed for definitive classification of the virus.
In this study, we detected deer tick virus by both molecular and immunohistochemical methods in the central nervous system of a patient with encephalitis. The neurotropism seen in this case, with involvement of both gray and white matter, matches the pattern of central nervous system infection for arboviruses, which may be highly neuroinvasive.23
The patient was known to have frequented wooded areas, although no specific contact with ticks had been reported. He presented in late spring, which suggested that transmission was probably from nymphal deer ticks, which are most active during spring and summer months. In addition, since nymphal deer ticks are small in size (1.5 mm in diameter), it is not uncommon for their bites to remain undetected. It is possible that the patient’s underlying condition (CLL–SLL) predisposed him to particularly serious disease. Reports of elderly and immunocompromised patients being at a greater risk for severe encephalitis caused by West Nile virus are well documented.24,25
Our immunohistochemical studies with newly generated deer tick virus antibodies demonstrated prominent labeling of neuronal-cell bodies and their processes; a focus of apparent oligodendroglial infection was also identified (Fig. 4). In addition, some neurons contained rounded granular-to-tubular profiles. A segmental distribution of immunolabeling was evident in the hippocampus, as was seen in cerebellum infected by central European tickborne encephalitis virus, as described previously.26 The parenchymal lymphocytic infiltrates in this case and in previous pathological studies of tickborne encephalitis virus26,27 were predominantly CD8+ cytotoxic T cells, which were also seen in close apposition to surviving neurons, further indicating that immunologic mechanisms may have contributed to nerve-cell destruction in tickborne encephalitides.
Diagnostic testing for Powassan virus is not routinely performed in patients with encephalitis. More extensive testing for arboviruses, including Powassan virus, might reveal that arboviral infections are more widespread than previously reported. For Powassan virus, testing is especially important during the summer months and in regions where infected ticks are prevalent. Deer ticks transmit several tickborne diseases, including Lyme disease, human babesiosis, and human granulocytic anaplasmosis.28 This report of deer tick virus resulting in a fatal case of encephalitis emphasizes the significance of deer ticks in transmitting a variety of infections. There are limited data on the prevalence of infection with deer tick virus among adult deer ticks, although a rate of 0.6 to 1.3% in limited geographic areas in the United States has been reported.9 Because no specific antiviral therapy is available for Powassan infection, the best strategy remains prevention (i.e., avoidance of contact with the arthropod vector). Studies to elucidate the prevalence and relative pathogenic features of Powassan lineages I and II are warranted.
Supplementary Material
Acknowledgments
Supported in part by the National Institute of Allergy and Infectious Diseases, by a contract (N01-A1-25490) and a grant (AI067380) from the National Institutes of Health, and by a cooperative agreement (U01-CI000311) with the Centers for Disease Control and Prevention (CDC).
We thank Dr. Laura Kramer and the Arbovirus Laboratory staff at Wadsworth Center for cultures of Powassan virus, Bryce Chackerian for assistance with mouse immunization, Dr. Gino Battaliogli for assistance in nucleic acid extraction from formalin-fixed tissues, the Bacteriology Laboratory at the Wadsworth Center for performing PCR assays on B. burgdorferi and A. phagocytophilum, and the Molecular Genetics Core at the Wadsworth Center for performing the sequencing reactions.
Footnotes
No potential conflict of interest relevant to this article was reported.
The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the CDC.
References
- 1.Telford SR, III, Armstrong PM, Katavolos P, et al. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997;3:165–70. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuno G, Artsob H, Karabatos N, Tsuchiya KR, Chang GJJ. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am J Trop Med Hyg. 2001;65:671–6. doi: 10.4269/ajtmh.2001.65.671. [DOI] [PubMed] [Google Scholar]
- 3.Beasley DWC, Suderman MT, Holbrook MR, Barrett ADT. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res. 2001;79:81–9. doi: 10.1016/s0168-1702(01)00330-6. [DOI] [PubMed] [Google Scholar]
- 4.Ebel GD, Spielman A, Telford SR., III Phylogeny of North American Powassan virus. J Gen Virol. 2001;82:1657–65. doi: 10.1099/0022-1317-82-7-1657. [DOI] [PubMed] [Google Scholar]
- 5.Charrel RN, Attoui H, Butenko AM, et al. Tick-borne virus diseases of human interest in Europe. Clin Microbiol Infect. 2004;10:1040–55. doi: 10.1111/j.1469-0691.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- 6.Artsob H. Powassan encephalitis. In: Monath TP, editor. The arboviruses: epidemiology and ecology. Boca Raton, FL: CRC Press; 1988. pp. 29–49. [Google Scholar]
- 7.Gritsun TS, Nuttall PA, Gould EA. Tick-borne flaviviruses. Adv Virus Res. 2003;61:317–71. doi: 10.1016/s0065-3527(03)61008-0. [DOI] [PubMed] [Google Scholar]
- 8.Burke DS, Monath TP. Flaviviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, editors. Fields virology. 4. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1043–126. [Google Scholar]
- 9.Ebel GD, Campbell EN, Goethert HK, Spielman A, Telford SR., III Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg. 2000;63:36–42. doi: 10.4269/ajtmh.2000.63.36. [DOI] [PubMed] [Google Scholar]
- 10.Ebel GD, Foppa I, Spielman A, Telford SR., III A focus of deer tick virus transmission in the northcentral United States. Emerg Infect Dis. 1999;5:570–4. doi: 10.3201/eid0504.990423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaramozzino N, Crance J-M, Jouan A, DeBriel DA, Stoll F, Garin D. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flavivirus targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol. 2001;39:1922–7. doi: 10.1128/JCM.39.5.1922-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavakoli NP, Tobin EH, Wong SJ, et al. Identification of dengue virus in respiratory specimens from a patient who had recently traveled from a region where dengue virus is endemic. J Clin Microbiol. 2007;45:1523–7. doi: 10.1128/JCM.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeffer M, Proebster B, Kinney RM, Kaaden O-R. Genus-specific detection of alphaviruses by a semi-nested reverse transcription-polymerase chain reaction. Am J Trop Med Hyg. 1997;57:709–18. doi: 10.4269/ajtmh.1997.57.709. [DOI] [PubMed] [Google Scholar]
- 14.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A. 2004;101:11030–5. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 17.Gholam BA, Puksa S, Provias JP. Powassan encephalitis: a case report with neuropathology and literature review. CMAJ. 1999;161:1419–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Embil JA, Camfield P, Artsob H, Chase DP. Powassan virus encephalitis resembling herpes simplex encephalitis. Arch Intern Med. 1983;143:341–3. [PubMed] [Google Scholar]
- 19.Wilson MS, Wherrett BA, Mahday MS. Powassan virus meningoencephalitis: a case report. Can Med Assoc J. 1979;121:320–3. [PMC free article] [PubMed] [Google Scholar]
- 20.Goldfield M, Austin SM, Black HC, Taylor BF, Altman R. A non-fatal human case of Powassan virus encephalitis. Am J Trop Med Hyg. 1973;22:78–81. doi: 10.4269/ajtmh.1973.22.78. [DOI] [PubMed] [Google Scholar]
- 21.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708–11. [PMC free article] [PubMed] [Google Scholar]
- 22.McLean DM, McQueen EJ, Petite HE, MacPherson LW, Scholten TH, Ronald K. Powassan virus: field investigations in northern Ontario, 1959 to 1961. CMAJ. 1962;86:971–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Love S, Wiley CA. Viral infections. In: Love S, Louis DW, Ellison DW, editors. Green-field’s neuropathology. 8. London: Hodder Arnold; 2008. pp. 1323–33. [Google Scholar]
- 24.Penn RG, Guarner J, Sejvar JJ, et al. Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis. 2006;42:680–3. doi: 10.1086/500216. [DOI] [PubMed] [Google Scholar]
- 25.Ravindra KV, Freifeld AG, Kalil AC, et al. West Nile virus-associated encephalitis in recipients of renal and pancreas transplants: case series and literature review. Clin Infect Dis. 2004;38:1257–60. doi: 10.1086/383325. [DOI] [PubMed] [Google Scholar]
- 26.Gelpi E, Preusser M, Garzuly F, Holzmann H, Heinz FX, Budka H. Visualization of Central European tick-borne encephalitis infection in fatal human cases. J Neuropathol Exp Neurol. 2005;64:506–12. doi: 10.1093/jnen/64.6.506. [DOI] [PubMed] [Google Scholar]
- 27.Gelpi E, Preusser M, Laggner U, et al. Inflammatory response in human tick-borne encephalitis: analysis of postmortem brain tissue. J Neurovirol. 2006;12:322–7. doi: 10.1080/13550280600848746. [DOI] [PubMed] [Google Scholar]
- 28.Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001;33:676–85. doi: 10.1086/322681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.