Abstract
Prostate-specific membrane antigen (PSMA) is a transmembrane protein expressed at high levels in prostate cancer and in tumor-associated neovasculature. In this study, we report that PSMA is internalized via a clathrin-dependent endocytic mechanism and that internalization of PSMA is mediated by the five N-terminal amino acids (MWNLL) present in its cytoplasmic tail. Deletion of the cytoplasmic tail abolished PSMA internalization. Mutagenesis of N-terminal amino acid residues at position 2, 3, or 4 to alanine did not affect internalization of PSMA, whereas mutation of amino acid residues 1 or 5 to alanine strongly inhibited internalization. Using a chimeric protein composed of Tac antigen, the α-chain of interleukin 2-receptor, fused to the first five amino acids of PSMA (Tac-MWNLL), we found that this sequence is sufficient for PSMA internalization. In addition, inclusion of additional alanines into the MWNLL sequence either in the Tac chimera or the full-length PSMA strongly inhibited internalization. From these results, we suggest that a novel MXXXL motif in the cytoplasmic tail mediates PSMA internalization. We also show that dominant negative μ2 of the adaptor protein (AP)-2 complex strongly inhibits the internalization of PSMA, indicating that AP-2 is involved in the internalization of PSMA mediated by the MXXXL motif.
INTRODUCTION
Prostate-specific membrane antigen (PSMA) was originally identified by the monoclonal antibody (mAb) 7E11-C5 raised against the human prostate cancer cell line LNCaP (Horoszewicz et al., 1987). Subsequently, the PSMA gene was cloned (Israeli et al., 1993) and mapped to chromosome 11q (Rinker-Schaeffer et al., 1995). PSMA is a type II membrane protein with a short cytoplasmic N-terminal region (19 amino acids), a transmembrane domain (24 amino acids), and a large extracellular C-terminal portion (707 amino acids) (Israeli et al., 1993) with several potential N-glycosylation sites. Recently, it has been shown that PSMA is homologous to glutamate carboxypeptidase II (85% at nucleic acid level) isolated from rat brain (Coyle, 1997) and has folate hydrolase activity (Pinto et al., 1996; Halsted et al., 1998), and N-acetylated α-linked acidic dipeptidase (NAALDase) activity (Carter et al., 1996, 1998). The extracellular domain of PSMA shows homology (26% identity at the amino acid level) to the transferrin receptor I (Israeli et al., 1993) and to a recently cloned transferrin receptor II (Kawabata et al., 1999). The functional significance of homology between PSMA and transferrin receptor is not known.
PSMA has been the subject of increasing interest in cancer research due to its potential as a diagnostic and therapeutic target for human prostate cancer (Chang et al., 1999a). PSMA is abundantly expressed in prostate cancer cells. Its expression is further increased in higher-grade cancers, metastatic disease, and hormone-refractory prostate carcinoma (Wright et al., 1996; Silver et al., 1997). In addition, PSMA has become the focus of even more intense interest due to the recent findings that it is selectively expressed in the neovasculature of nearly all types of solid tumors, but not in the vasculature of normal tissue (Liu et al., 1997; Silver et al., 1997; Chang et al., 1999b,c). The function of PSMA with respect to vascular endothelial cell biology and the direct correlation between its expression and increasing tumor aggressiveness in prostate cancer remain intriguing and unclear. Although a significant amount of research is being carried out using antibodies against PSMA for immunotherapy of prostate cancer (McDevitt et al., 2001; Smith-Jones et al., 2003), very little is known about the mechanism of internalization of this protein.
In general, the endocytic pathway includes internalization of the receptor-ligand complex via clathrin-coated pits and accumulation in the endosomes. The receptor–ligand complex then dissociates in the endosomes and the dissociated molecules are either recycled back to the cell surface or targeted to lysosomes for degradation (Pastan and Willingham, 1981; Mellman, 1996). Targeting of most receptors to coated pits and their traffic through endocytic compartments are generally mediated by endocytic signals located in the cytoplasmic domain of proteins (reviewed in Trowbridge et al., 1993; Kirchhausen et al., 1997; Mukherjee et al., 1997; Bonifacino and Traub, 2003). These signals fall into two major categories such as tyrosine-based and di-leucine based signals.
The tyrosine-based signals are represented by NPXY and YXXΦ consensus motifs (Bonifacino and Traub, 2003) with the Y residue being critical for their function (Lazarovits and Roth, 1988). NPXY signals mediate internalization of several type-1 membrane proteins such as LDL receptor, epidermal growth factor receptor, insulin receptor, and others (Bonifacino and Traub, 2003). The YXXΦ (Φ, bulky hydrophobic side chain) signals mediate internalization and lysosomal targeting of several type I and type II membrane proteins such as transferrin receptor, mannose-6-phosphate receptor, asialoglycoprotein receptor, polymeric immunoglobulin receptor, and others (reviewed in Trowbridge et al., 1993; Marks et al., 1997; Bonifacino and Dell'Angelica, 1999; Bonifacino and Traub, 2003).
The di-leucine based signals require two consecutive leucines or a leucine-isoleucine pair for their function (Letourneur and Klausner, 1992; Sandoval and Bakke, 1994). Recent studies have identified two distinct classes of dileucine based signals represented by [DE]XXXL[LI] and DXXLL consensus sequences. Both these signals are involved in internalization and lysosomal targeting of several membrane proteins. Proteins such as CD3-γ, LIMP-II, tyrosinase CD4, and GLUT4 have a [DE]XXXL type signal, whereas a DXXLL signal has been characterized in mannose 6-phosphate/insulin-like growth factor-II receptor, the cation-dependent mannose-6-phosphate receptor, LDL-receptor related proteins, β-secretase, and others (Bonifacino and Traub, 2003).
We have shown that PSMA undergoes internalization via clathrin-coated pits in LNCaP cells (Liu et al., 1998), a human prostate cancer cell line that abundantly expresses endogenous PSMA. To further understand the mechanism of PSMA internalization, we searched for possible internalization motifs in the cytoplasmic domain of PSMA. In this study we demonstrate that the cytoplasmic tail N-terminal five amino acid residues, including the start codon methionine and the fifth amino acid residue leucine form a novel MXXXL motif that mediates PSMA internalization. By transferring this MXXXL motif to a noninternalized protein, the α-chain of interleukin 2-receptor (Tac), we further show that this motif is sufficient for PSMA internalization. Furthermore, using an inducible dominant-negative μ2 subunit of the adaptor protein (AP)-2 adaptor complex, we provide evidence that internalization of this clinically important protein requires the function of AP-2.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
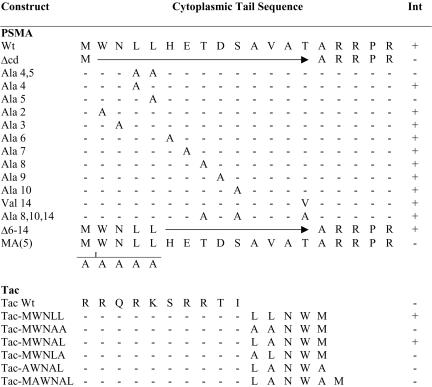
Cloning and characterization of full-length cDNA of PSMA was described previously (Israeli et al., 1993). The alanine scan mutagenesis approach was used to mutate each of the cytoplasmic tail amino acids in the cytoplasmic tail of PSMA. Alanine scan mutagenesis was essentially carried out by polymerase chain reaction (PCR) by using sense primers carrying respective mutation of the cytoplasmic tail amino acid (positions 2–15) to alanine. A Kozak consensus sequence (GCCACC) and a translation start site (ATG) were incorporated at the N-terminus of the sense primers. A cytoplasmic tail deletion mutant of PSMA was created by deleting the N-terminal 15 amino acids by using PCR. An alanine and three arginine residues proximal to the transmembrane domain were retained because these three arginine residues may be necessary to maintain the type II orientation of the protein (von Heijne, 1988). Also, PSMA constructs in which the cytoplasmic tail amino acids 6–14 were deleted or all the three putative phosphorylation sites were mutated (PSMA-T8A/S10A/T14A) and a PSMA construct containing five alanines inserted after the start codon [PSMA-MA(5)] were generated using PCR. Tac-PSMA chimera were also generated using PCR. Full-length Tac (gift from Dr. Bonifacino, National Institutes of Health, Bethesda, MD) was described previously (Leonard et al., 1984). Tac cytoplasmic tail chimera containing the di-leucine–like motif of PSMA (Tac-MWNLL), di-leucine motif mutated to alanine (Tac-MWNAA), leucine at position 5 mutated to alanine (Tac-MWNLA), leucine at position 4 mutated to alanine (Tac-MWNAL), methionine at first position mutated to alanine in Tac with leucine at position 4 mutated to alanine (Tac-AWNAL), and with an extraalanine (Tac-MAWNAL) were generated. Because Tac is a type I membrane protein, to have the N-terminal methionine free as in PSMA, we used primers encoding the respective amino acids in the reverse orientation. Full-length PSMA (designated as wild-type PSMA [PS-MAwt]), cytoplasmic tail mutants of PSMA, and Tac-PSMA chimeras were inserted into eukaryotic expression vector pCDNA3. The mutations were verified by DNA sequencing. Constructs used in this study are shown in Figure 1.
Figure 1.
Schematic representation of PSMA cytoplasmic tail mutants and Tac-PSMA chimera used in this study. Deletions are shown by horizontal arrows, and insertion of additional alanines is indicated. Amino acids converted to alanine or valine are indicated as A and V, respectively. Internalization (INT) positive (+) or negative (–) for the respective constructs is indicated.
Cell Culture and Transfection
COS-7 cells (ATCC CRL 1651) were grown in DMEM supplemented with 10% fetal bovine serum containing streptomycin and penicillin at 5% CO2 in a water-saturated atmosphere. Cells grown on glass coverslips were transiently transfected by the calcium phosphate method as described previously (Rajasekaran et al., 1994). After transfection (48 h), the cells were tested for the uptake of antibodies as described below. HeLa cells expressing hemagglutinin-tagged D176A/W421A mutant μ2 constructs under the control of a tetracycline-repressible promoter have been described previously (Nesterov et al., 1999). The cells were grown in DMEM supplemented with 10% fetal bovine serum containing streptomycin and penicillin, 400 μg/ml G418, 200 ng/ml puromycin, and 10 ng/ml doxycycline at 5% CO2 in a water-saturated atmosphere. Cells plated on glass coverslips were used for transient transfection by the calcium phosphate method. Twelve hours after transfection, expression of the mutant μ2 protein was induced by replacing the culture medium with doxycyline-free medium. Eight hours before the planned experiments, sodium butyrate was added to the culture medium to ensure high expression levels of the mutant μ2 protein to replace the endogenous wild-type μ2 in AP complexes. Transfected cells were used 60 h after transfection.
Antibody Uptake and Immunofluorescence Analysis
Antibody uptake was carried out as described previously (Liu et al., 1998). In brief, the cells were washed with DMEM containing 0.5% fatty acid-free bovine serum albumin and incubated at 37°C for 2 h with mAb J591 (5 μg/ml). Cells were then fixed, permeabilized, and incubated with Texas Red-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). To visualize PSMA localization in endosomes, cells were coincubated with fluorescein isothiocyanate (FITC)-conjugated transferrin (Jackson ImmunoResearch Laboratories) during J591 incubation. To monitor the internalization of Tac-PSMA chimera, mAb against the extracellular domain of Tac, 7G7 (Rubin et al., 1985) was used. For kinetic analysis of PSMA uptake the cells were incubated with J591 and FITC-conjugated transferrin for 1 h at 4°C, washed three times, and then incubated in DMEM at 37°C, 5% CO2 to allow for uptake. The cells were fixed at the indicated time points and incubated with Texas Red-conjugated secondary antibody. Uptake of antibodies (mAbs J591 and 7G7) and transferrin were visualized and quantitated by confocal microscopy (see below). To visualize surface expression of PSMA and Tac-PSMA chimeras, COS cells transfected with the respective plasmid were fixed and stained with mAb J591 and 7G7, respectively, under nonpermeabilized conditions.
Confocal Microscopy
Codistribution of internalized mAbs J591or 7G7 and transferrin were examined using a Fluoview laser scanning confocal microscope (Olympus America, Melville, NY). To detect simultaneously FITC- and Texas Red-labeled antigens, samples were excited at 488 and 568 nm with argon and krypton lasers, respectively, and the light emitted between 525 and 540 nm was recorded for FITC and >630 nm for Texas Red. Images were generated using Fluoview software (version 2.1.39). Transfected cells (30–40) were examined for each transfection done in duplicate and the representative data are shown.
Quantification of internalization in COS cells expressing PSMAwt and PSMA harboring mutation of the fourth leucine (PSMA-L4A) or fourth and fifth leucine (PSMA-L4A/L5A) was done using image analysis software (Fluoview, version 2.1.39). Average pixel intensities of internalized transferrin (green), and mAb J591 (red) from optical sections of 30–40 cells were determined. Because the transferrin uptake was more or less uniform PSMA internalization was normalized to transferrin uptake. An analysis of variance was used to compare the PSMA/transferrin ratios as a function of time between PSMAwt and PSMA-L4A. A logarithmic transform was used to stabilize variance and for computing 95% confidence intervals for the geometric mean of PSMA-L4A mutant ratios as a percentage of PSMAwt ratios.
NAALDase Activity
NAALDase activity was determined as described by Sekiguchi et al. (1989). COS cells were transfected with PSMAwt on 60-mm culture dishes. After 48 h of transfection, cells were incubated with 1 μCi/ml [3H]NAAG (PerkinElmer Life Sciences, Boston, MA) in Krebs-Ringer bicarbonate medium or in Dulbecco's modified Eagle's medium for 1 h. The medium was removed and the cells were washed three times with their respective incubation medium. Cells were then lysed in 1% Triton X-100, and the radioactivity was determined using a scintillation counter (Beckman LS 6500). Counts were normalized to protein. Protein concentrations of the cell lysates were determined using the Bio-Rad DC reagent (Bio-Rad, Hercules, CA) according to manufacturer's instructions.
RESULTS
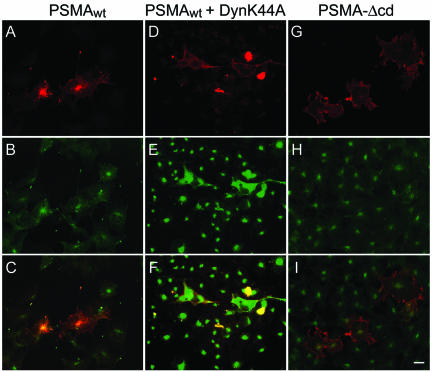
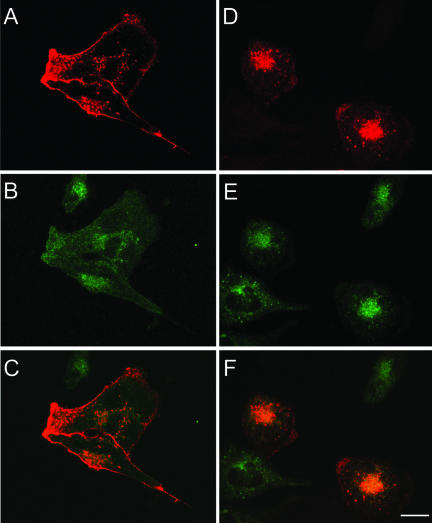
To study the internalization of PSMA, COS cells were transiently transfected with PSMAwt cDNA (Figure 1) and uptake of mAb J591 was monitored by immunofluorescence and confocal microscopy. The internalized antibody showed a distinct spot-like staining pattern at the perinuclear region (Figure 2A). This spot-like staining is reminiscent of the recycling endosomal compartment and internalized transferrin, a marker for this compartment, colocalized with endocytosed PSMA (Figure 2, A–C), indicating that PSMA is localized to the recycling endosome. We have shown earlier that PSMA is internalized via clathrin-coated vesicles in LNCaP cells (Liu et al., 1998). To further confirm that PSMA is internalized via a clathrin-dependent endocytic mechanism in COS cells, we tested whether PSMA is internalized in cells expressing a GTPase-deficient dynamin mutant (K44A), which is known to inhibit clathrin-dependent endocytosis in cultured cells (Herskovits et al., 1993; van der Bliek et al., 1993). In these cells internalization of PSMA was not detected (Figure 2, D–F) further confirming that PSMA is internalized via a clathrin-dependent endocytic pathway.
Figure 2.
PSMA internalization in COS-7 cells expressing wild-type PSMA (PSMAwt) and the cytoplasmic tail deletion mutant (PSMA-Δcd). (A–C) Internalization of PSMAwt and FITC-transferrin. COS cells transiently transfected with PSMAwt were simultaneously incubated with mAb J591 (A) and FITC-transferrin (B) for 2 h, washed, fixed in cold methanol, and stained with Texas Red-conjugated anti-mouse antibody. Representative medial optical sections are shown. (C) Merged image. The yellow color indicates the codistribution of FITC-transferrin and internalized PSMA. (D–F) COS cells expressing Dynamin K44A and PSMAwt cDNA were incubated with mAb J591 for 2 h, washed fixed, and stained with FITC-conjugated anti-mouse antibody to detect PSMA (D) and with polyclonal anti-dynamin antibody and Texas Red-conjugated anti-rabbit antibody to detect cells expressing the dynamin mutant (E). (F) Merged image. Note that in cells expressing DynaminK44A, PSMA was not internalized. (G–I) PSMA-Δcd–expressing cells were incubated with mAb J591 (G) and FITC-transferrin (H) as described above. PSMA-Δcd does not internalize and therefore, does not colocalize with internalized transferring (I). Bar, 5μm.
To test whether the cytoplasmic tail of PSMA contains a signal that mediates its internalization the PSMA cytoplasmic tail was deleted and the mutant (PSMA-Δcd) was expressed in COS cells. PSMA-Δcd was clearly expressed on the cell surface as revealed by immunofluorescence staining under nonpermeabilized condition (our unpublished data). Incubation of these cells with mAb J591 did not show uptake or localization to the endosomes and internalized transferrin did not reveal a codistribution with PSMA (Figure 2, G–I), indicating that the cytoplasmic tail of PSMA contains a signal that mediates its internalization.
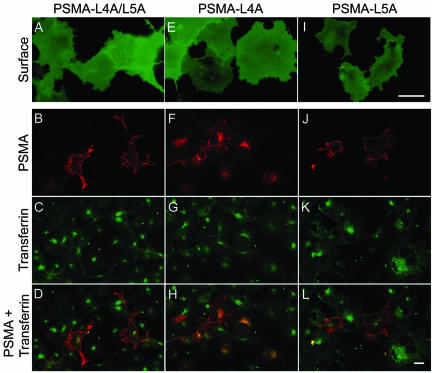
The cytoplasmic tail of PSMA contains two consecutive leucines as reported for di-leucine-like motifs (Figure 1). To examine whether this motif functions as an internalization signal for PSMA, the di-leucine pair was converted to dialanine (PSMA-L4A/L5A), the mutant protein was expressed in COS cells and uptake of mAb J591 was monitored. The di-alanine mutant of PSMA was clearly expressed on the cell surface as revealed by staining with mAb J591 under nonpermeabilized condition (Figure 3A). Our internalization assay revealed that mAb J591 was not internalized in cells expressing the di-alanine mutant of PSMA (Figure 3B) and did not show codistribution with the internalized FITC-transferrin (Figure 3, C and D), indicating that mutation of the di-leucine pair in the cytoplasmic tail of PSMA abrogates its internalization. We then examined whether both these leucines are essential for the internalization of PSMA. For this purpose single leucine residues at positions 4 (PSMA-L4A) and 5 (PSMA-L5A) were mutated to alanine and the uptake of mAb J591 was studied. Both these mutants were expressed on the cell surface as revealed by staining with mAb J591 under nonpermeabilized condition (Figure 3, E and I). J591 was clearly internalized in cells expressing PSMA-L4A (Figure 3F) and internalized transferrin (Figure 3G) codistributed with the internalized mAb J591 (Figure 3H). By contrast, in cells expressing PSMA-L5A, mAb J591 was not internalized (Figure 3J) and the antibody primarily stained the plasma membrane similar to cells expressing PSMA-L4/L5 (Figure 3B). In these cells, colocalization of PSMA and internalized FITC-transferrin was not detected (Figure 3, K and L). These results indicated that the fifth leucine in the cytoplasmic tail of PSMA is crucial for its internalization.
Figure 3.
Internalization of the cytoplasmic tail di-leucine mutants of PSMA. (A, E, and I) Surface expression of PSMA in COS-7 cells expressing PSMA-L4A/L5A, PSMA-L4A, and PSMA-L5A mutants, respectively. Forty-eight hours after transfection, the cells were fixed in paraformaldehyde under nonpermeabilized conditions and labeled with mAb J591 followed by FITC-conjugated anti-mouse antibody and visualized by epifluorescence microscopy. (B, F, and J) Internalization of PSMA mutants. (C, G, and K) FITC-transferrin uptake. (D, H, and L) Merged images of PSMA and FITC-transferrin. Representative medial optical sections are shown. Yellow color in H indicates the codistribution of FITC-transferrin and internalized PSMA. Bars, 10 μm (A, E, and I) and 5 μm (B, C, D, F, G, H, K, and L).
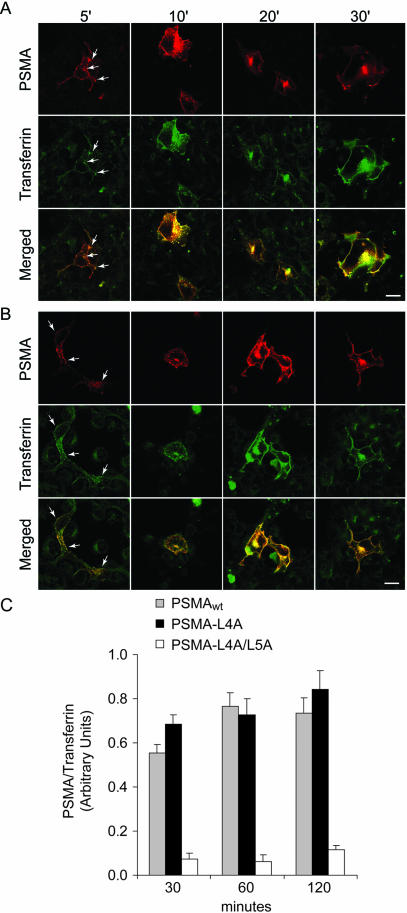
Because PSMA-L4A was internalized similar to PSMAwt we determined the kinetics of PSMA uptake in cells expressing these constructs. Our efforts to obtain quantitative data by using iodinated mAb J591 were not successful because mAb J591 bound to the cell surface was not quantitatively released after acid wash procedures commonly used to release bound antibody on the cell surface. Therefore, we used immunofluorescence and confocal microscopy approaches to determine the kinetics of mAb J591 uptake in cells expressing PSMAwt and PSMA-L4A. As shown in Figure 4, both PSMAwt and PSMA-L4A expressing cells internalized PSMA rapidly. After 5-min incubation both PSMA and transferrin showed predominant plasma membrane localization with small amounts localized to peripheral vesicles (arrow). Similarly, after 10 min PSMA and transferrin codistributed in more peripheral vesicles, whereas after 20 min it accumulated in the recycling endosomal compartment. Cells expressing PSMA-L4A (Figure 4B) showed a similar internalization pattern. These results indicated that mutation of the fourth leucine in the cytoplasmic tail of PSMA has a minimal effect on the internalization of PSMA in COS cells. To obtain quantitative data we determined the average pixel intensity represented by internalized PSMA and transferrin by using image analysis software (Fluoview, version 2.1.39). Because quantification of internalized PSMA and transferrin was more reliable after 30 min, we quantified internalized PSMA in cells expressing PSMAwt and PSMA-L4A at 30, 60, and 120 min. We used internalized transferrin as an internal control for defining the area representing the internalized PSMA. Comparison of the internalization kinetics of PS-MAwt and PSMA-L4A revealed that PSMA-L4A is internalized with kinetics similar to PSMAwt (Figure 4C). An analysis of the variance demonstrated that internalization increased with time (p = 0.04), but there was no statistical difference between the internalization profiles for PSMAwt and PSMA-L4A mutants (p > 0.2). The 95% confidence intervals for PSMA-L4A mutant internalization (as percentage of PSMAwt) were 100–148% at 30 min, 72–116% at 60 min, and 89–143% at 120 min, indicating that mutation of the fourth leucine does not alter the internalization properties of PSMA.
Figure 4.
Kinetic analysis of internalization of PSMAwt and PSMA-L4A in COS cells. (A and B) Time course of PSMAwt (A) and PSMA-L4A (B) internalization. Transiently transfected COS cells were incubated with mAb J591 and FITC-transferrin for the indicated time points, as described under EXPERIMENTAL PROCEDURES and stained with Texas Red-conjugated anti-mouse antibody. Representative medial optical sections are shown. Arrows indicate peripheral vesicles containing PSMA and transferrin. Bar, 5 μm. (C) COS cells expressing PSMAwt, PSMA-L4A, or PSMA-L4A/L5A were incubated with J591 and FITC-conjugated transferrin for 1 h at 4°C, washed, and incubated at 37°C to allow for uptake. The cells were fixed after 30, 60, and 120 min and incubated with Texas Red-conjugated secondary antibody. Uptake of mAbs J591 and transferrin were visualized and quantitated by confocal microscopy as described under EXPERIMENTAL PROCEDURES. PSMA internalization was normalized to transferring uptake. The bars indicate SE of 30–40 cells analyzed for each condition.
To further test whether amino acid residues other than the fifth leucine are essential for the internalization of mAb J591 we systematically mutated each of the cytoplasmic tail amino acids into alanine. These point mutations did not affect the internalization of mAb J591 (our unpublished data). Moreover, the construct in which amino acids 6–14 were deleted (PSMA-Δ6–14) internalized mAb J591 when expressed in COS cells. These results demonstrated that the N-terminal first five amino acids in the cytoplasmic tail of PSMA are sufficient to mediate PSMA internalization and the fifth amino acid leucine is crucial for its internalization activity.
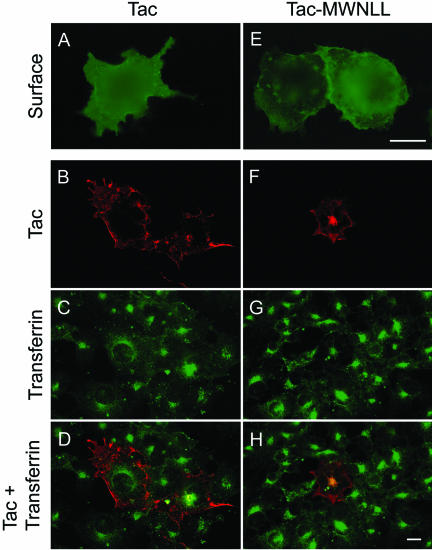
To further confirm that this five amino acid motif of PSMA is sufficient for internalization, we transferred the five N-terminal amino acids of PSMA to the noninternalized protein Tac, a type I membrane protein (Letourneur and Klausner, 1992). Internalization of Tac was monitored by uptake of mAb 7G7 raised against the extracellular domain of Tac (Rubin et al., 1985). In nonpermeabilized COS cells wild-type Tac (Tacwt) showed a distinct plasma membrane localization (Figure 5A), indicating that this protein is targeted to the plasma membrane but incubation with mAb 7G7 did not result in the internalization of this antibody, confirming that Tacwt is not internalized in COS cells (Figure 5B) as reported previously (Letourneur and Klausner, 1992). Codistribution of mAb 7G7 staining and internalized transferrin was not detected in these cells (Figure 5, C and D). By contrast, incorporation of the amino acids MWNLL into the Tac cytoplasmic tail (Tac-MWNLL) resulted in the internalization of mAb 7G7 (Figure 5F). The internalized antibody clearly colocalized with internalized FITC-transferrin (Figure 5, G and H) indicating that the N-terminal five amino acids in the cytoplasmic tail of PSMA are transferable and are sufficient to confer internalization properties to a noninternalized protein.
Figure 5.
Internalization of Tac and Tac-PSMA chimera. (A and E) Surface expression of Tac. Forty-eight hours after transfection the cells were fixed in paraformaldehyde under nonpermeabilized condition, labeled with mAb 7G7 followed by FITC-conjugated antimouse antibody, and visualized by epifluorescence microscopy. (B–D, F–H) Internalization of Tac and FITC-transferrin. The cells were incubated with mAb 7G7 and FITC-transferrin for 2 h, washed, fixed in cold methanol, and stained with Texas Red-conjugated anti-mouse antibody. Representative medial optical sections are shown. (B and F) Internalization of Tac antibody. (C and G) Uptake of FITC-transferrin. (D and H) Merged images. Yellow color in H indicates the codistribution of FITC-transferrin and internalized Tac. Bars, 10 μm (A and E) and 5 μm (B, C, D, F, G, and H).
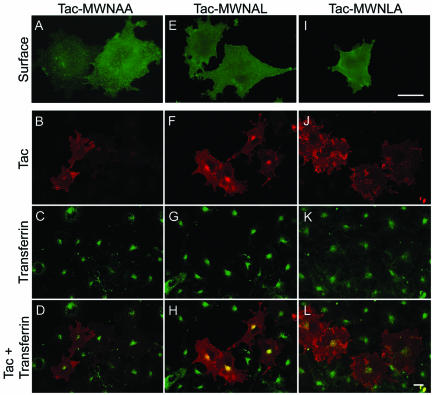
Furthermore, in cells expressing Tac-MWNAA where the two consecutive leucines are mutated to alanines, the mAb 7G7 was not internalized (Figure 6B), although this protein was clearly localized to the plasma membrane (Figure 6A). This mutant did not codistribute with internalized FITC-transferrin (Figure 6, C and D). Internalization of mAb 7G7 was maintained in cells expressing the construct where the fourth leucine is mutated to alanine (Tac-MWNAL) (Figure 6, F–H), whereas in cells expressing Tac-MWNLA, where the leucine at position 5 is mutated, the uptake of 7G7 was not detected (Figure 6, J–L). Both these mutants were clearly expressed on the plasma membrane as revealed by nonpermeablized staining by using mAb 7G7 (Figure 6, E and I). Together, these data demonstrate that the leucine at the fifth position is critical for PSMA internalization.
Figure 6.
Internalization of Tac-PSMA chimeras harboring mutations in the di-leucine signal. Surface expression as well as internalization of PSMA was performed as described in figure legend 5. (A, E, and I) Surface expression of Tac in COS-7 cells expressing Tac-MWNAA, Tac-MWNAL, and Tac-MWNLA chimeras, respectively. (B, F, and J) Internalization of Tac chimera mutants. (C, G, and K) Uptake of FITC-transferrin. (D, H, and L) Merged images. Representative medial optical sections are shown. Yellow color in H indicates the codistribution of FITC-transferrin and internalized PSMA. Bars, 10 μm (A, E, and I) and 5 μm (B, C, D, F, G, H, J, K, and L).
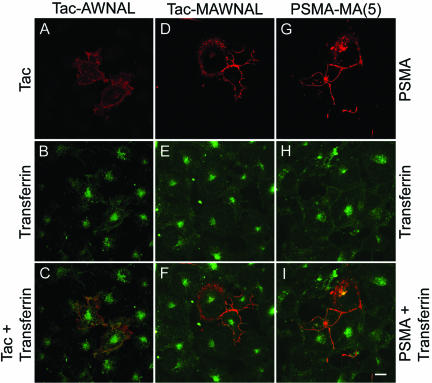
Although it is possible that a single leucine in the cytoplasmic tail of PSMA might play a crucial role in its internalization, it is unlikely that it can function as an internalization motif. Therefore, we decided to test for other potential amino acid residues in the five amino acid motif that might be involved in the internalization of PSMA. We have evidence that mutation of amino acids at position 2 and 3 (our unpublished data) and 4 (Figure 3) of the cytoplasmic tail of PSMA did not affect internalization, whereas mutation of leucine at position 5 abolished its internalization. The only amino acid that remained to be tested was the first amino acid methionine. Therefore, we mutated methionine in the internalizing Tac-MWNAL chimera to generate Tac-AWNAL. Although Tac-AWNAL was expressed on the cell surface (our unpublished data), a drastic reduction in the internalization of mAb 7G7 was noticed in cells expressing this chimera (Figure 7A). Although in these cells FITC-transferrin was clearly internalized (Figure 7B), there was little colocalization of internalized transferrin with Tac-AWNAL (Figure 7C). Small amounts of internalized mAb J591 were seen in peripheral vesicles, in contrast to the intense fluorescence of internalized transferrin seen at the cell center. This result indicated that in addition to the fifth leucine the methionine is also required and that the N-terminal five amino acids, MWNLL, form a motif to mediate the internalization of PSMA. To test whether the length of this motif is involved in PSMA internalization, we inserted an additional alanine between tryptophan and methionine (Tac-MAWNAL) and monitored the internalization of this chimera. In COS cells, Tac-MAWNAL was clearly expressed on the cell surface as revealed by nonpermeabilized staining (our unpublished data). However, internalization of mAb7G7 was highly reduced (Figure 7D), and there was less colocalization of the chimera with internalized FITC-transferrin (Figure 7, E and F). We then tested whether incorporation of alanine into the MWNLL motif of PSMA itself affects internalization. Whereas insertion of one or two amino acids did not affect internalization, insertion of five alanines [PSMA-MA(5)] drastically reduced the internalization of PSMA (Figure 7G).
Figure 7.
Internalization of Tac-PSMA chimeras Tac-AWNAL and Tac-MAWNAL, and of PSMA-MA(5). (A and D) Internalization of Tac chimera mutants and (G) mAb J591 in transiently transfected COS cells. (B, E, and H) Internalization of FITC-transferrin. (C, F, and I) Merged images. Note the lack of codistribution of Tac-chimera mutants or PSMA-MA(5) and FITC-transferrin. Bar, 5 μm.
The endocytic motif of membrane receptors binds to AP complexes, which are heterotetramers and mediate the internalization of membrane receptors (Hirst and Robinson, 1998; Kirchhausen, 1999). The adaptor complex AP-2 has been shown to associate with both tyrosine (Ohno et al., 1995; Honing et al., 1996; Boll et al., 2002) and di-leucine–based signals (Hofmann et al., 1999). To obtain insights into whether AP-2 is involved in the internalization of PSMA, we monitored internalization of PSMA in a HeLa cell line that expresses a dominant negative mutant μ2 of the AP-2 complex under the control of a tetracycline-off system (Nesterov et al., 1999). Strikingly, mutant μ2 drastically reduced the internalization of PSMA (Figure 8A) and transferrin (Figure 8B), and transferrin showed a more diffused localization pattern that codistributed with PSMA (Figure 8C). In noninduced cells that only express wild-type μ2 PSMA as well as transferrin were clearly internalized (Figure 8, D–F), indicating that the μ2-subunit of AP-2 is involved in the internalization mediated by the MWNLL motif of PSMA.
Figure 8.
Internalization of PSMAwt in HeLa cells expressing dominant-negative AP-2 complexes. PSMAwt cDNA was transiently transfected into HeLa cells expressing a tetracycline-repressible dominant-negative mutant of μ2. mAb J591 internalization was monitored in mutant μ2-induced cells (A) and in noninduced cells (D). (B and E) Internalization of FITC-transferrin. (C and F) Merged images. Bar, 5 μm.
DISCUSSION
In this study, we demonstrate that the cytoplasmic tail five N-terminal amino acids MWNLL are sufficient to mediate the internalization of PSMA. Methionine at the first position and leucine at the fifth position are essential, whereas amino acids 2, 3, and 4 are dispensable for the internalization of PSMA. Incorporation of alanine(s) into Tac-chimera (Tac-MAWNAL) and into PSMA [PSMA-MA(5)] drastically reduced the internalization, indicating that the length of this sequence is also important for its internalization function. We also present evidence that the adaptor complex AP-2 is involved in the internalization of PSMA. Based on these results, we suggest that the N-terminal five amino acid residues of PSMA form a novel autonomous methionineleucine based internalization motif (MXXXL). To our knowledge, this is the first study describing a N-terminal amino acid (translation start site) as part of an internalization motif.
Although the presence of two consecutive leucines at position four and five suggested that the cytoplasmic tail of PSMA may contain a di-leucine–like motif, our results indicate that this might not represent a typical di-leucine motif as observed in other membrane proteins. The di-leucine–based signals of the [DE]XXXL[LI] and DXXLL types have an acidic residue at –4 from the first leucine (Pond et al., 1995; Sandoval et al., 2000), which is absent in PSMA and is replaced by an essential methionine. In the [DE]XXXL[LI] type the first leucine is generally indispensable and substitution with other amino acids decreases the efficacy of the signal (Letourneur and Klausner, 1992), whereas in the DXXLL type both the leucines are essential and mutation of any of these residues to alanine inactivates the signal (Johnson and Kornfeld, 1992; Chen et al., 1997). In PSMA, mutation of the first leucine did not change significantly the internalization kinetics. Moreover, in polarized epithelial cells, proteins with di-leucine motif are targeted to the basolateral plasma membrane (Sheikh and Isacke, 1996; El Nemer et al., 1999; Bello et al., 2001). By contrast, PSMA is targeted to the apical plasma membrane in Madin-Darby canine kidney cells (Christiansen et al., 2003) and swapping the cytoplasmic tail of PSMA with the cytoplasmic tail of a di-leucine motif containing protein redirected PSMA to the basolateral plasma membrane (our unpublished data). The absence of tyrosine residues in the cytoplasmic tail of PSMA clearly indicates that this protein does not contain a tyrosinebased signal. Together, these results strongly indicate that the MXXXL motif of PSMA is a novel methionine-leucine–based internalization motif.
PSMA is localized to the recycling endosomal compartment as revealed by its colocalization with internalized transferrin. Colocalization of Tac-MWNLL with transferrin further indicated that the MWNLL sequence is sufficient for the localization of PSMA to the recycling endosomal compartment. We have recently shown that the cytoplasmic tail of PSMA associates with the actin cross linking protein filamin (Anilkumar et al., 2003) and that this association is involved in the localization of PSMA to the recycling endosomal compartment. Future studies will determine whether the MWNLL motif of PSMA associates with filamin and functions as a recycling endosome targeting signal.
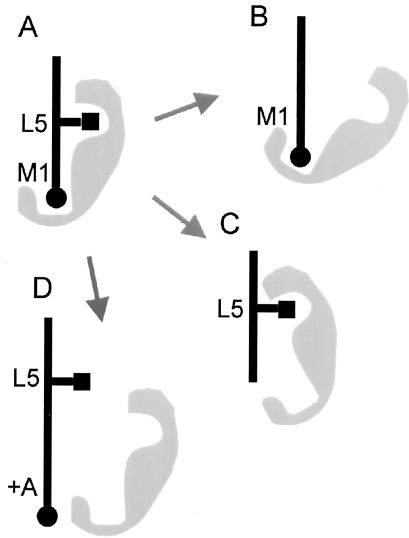
We have shown that dominant negative μ2 of the AP-2 complex reduces the internalization of PSMA, indicating that the AP-2 complex is involved in the internalization mediated by the MXXXL motif of PSMA. Recent structural studies suggested that the YxxΦ endocytic determinant might associate with the μ2 adaptin as a two-pinned plug into a socket with the Y and the Φ residues (the pins) fitting into sterically and chemically complementary pockets of the μ2 surface (Owen and Evans, 1998; Owen and Luzio, 2000). Requirement of the specific length of the MXXXL motif tempts us to speculate that the first amino acid methionine and the fifth leucine of the PSMA endocytic determinant might function as two pins fitting into a complementary pocket of μ2 (Figure 9). Future studies will establish whether μ2 directly associates with the MXXXL motif and whether this motif can compete with the YxxΦ or [DE]XXXL[LI] motifs, which are known to associate with μ2.
Figure 9.
Schematic model of binding of the PSMA internalization motif to μ2 of the AP-2 complex. (A) The endocytic determinant of PSMA might form two pins (methionine at position 1 [black circle] and leucine at position 5 [black square]) that fit into a complementary pocket of a μ2 (gray) associating with the cytoplasmic tail of PSMA. Loss of the side chains of leucine-5 (B) or methionine-1 (C) of the internalization motif might result in an altered association of the adaptor preventing the internalization of PSMA. Similarly, extension of the length of the internalization motif with an additional alanine (D) might prevent the binding of the adaptor protein to the cytoplasmic tail of PSMA and therefore inhibit internalization of the protein.
The catalytic site for glutamate carboxypeptidase/NAALDase activity of PSMA resides in its extracellular domain (Speno et al., 1999). Millimolar concentrations of phosphate used in the culture medium almost completely inhibited the NAALDase activity in COS cells (our unpublished data; Slusher et al., 1999). Because our internalization assays were performed in culture medium that inhibits NAALDase activity, this enzymatic activity seems not to be necessary for the internalization of PSMA. Moreover, in LNCaP cells, incubation with the NAAG substrate for NAALDase did not increase the internalization of PSMA (our unpublished data), whereas incubation with mAb J591 or the Fab fragments of this antibody increased the internalization rate of PSMA (Liu et al., 1998). The antibody and the antibody fragments might mimic a putative ligand for PSMA. These results indicate that the internalization of PSMA might be an independent function from its glutamate carboxypeptidase/NAALDase activity. It is possible that PSMA might function as a receptor mediating the internalization of a putative ligand. Identification of a PSMA ligand combined with the knowledge on PSMA internalization should provide more insights into the function of this protein and, in consequence, provide valuable information for therapeutic applications of PSMA.
Acknowledgments
We thank Dr. Alexander Sorkin for generously providing HeLa cells expressing a dominant negative mutant of μ2 and Dr. Joseph Coyle for the PSMA-T14V mutant plasmid construct. We thank Dr. Esteban Dell'Angelica for critical reading of the manuscript. This work was primarily supported by the Department of Defense grants DAMD17-98-1-8567 and DAMD17-02-1-0661 and in part by a CaP CURE award to A.K.R. S.A.R. was supported by U.S. Department of Health and Human Services (Institutional National Research Service Award T32CA09056. E.O. was partially supported by a Fellowship from Jonsson Comprehensive Cancer Center. N.H.B. is a paid consultant of BZL, Inc. (Framingham, MA) and has developed mAB J591 used in this study, which was patented by the Cornell Research Foundation and licensed to BZL.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–11–0731. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-11-0731.
Abbreviations used: NAALDase, N-acetylated α-linked acidic dipeptidase; PSMA, prostate-specific membrane antigen.
References
- Anilkumar, G., Rajasekaran, S.A., Wang, S., Hankinson, O., Bander, N.H., and Rajasekaran, A.K. (2003). Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer Res. 63, 2645–2648. [PubMed] [Google Scholar]
- Bello, V., Goding, J.W., Greengrass, V., Sali, A., Dubljevic, V., Lenoir, C., Trugnan, G., and Maurice, M. (2001). Characterization of a di-leucine-based signal in the cytoplasmic tail of the nucleotide-pyrophosphatase NPP1 that mediates basolateral targeting but not endocytosis. Mol. Biol. Cell 12, 3004–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll, W., Rapoport, I., Brunner, C., Modis, Y., Prehn, S., and Kirchhausen, T. (2002). The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppO sorting signals at distinct sites. Traffic 3, 590–600. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and Dell'Angelica, E.C. (1999). Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145, 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447. [DOI] [PubMed] [Google Scholar]
- Carter, R.E., Feldman, A.R., and Coyle, J.T. (1996). Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc. Natl. Acad. Sci. USA 93, 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.S., Bander, N.H., and Heston, W.D. (1999a). Monoclonal antibodies: will they become an integral part of the evaluation and treatment of prostate cancer–focus on prostate-specific membrane antigen? Curr. Opin. Urol. 9, 391–395. [DOI] [PubMed] [Google Scholar]
- Chang, S.S., O'Keefe, D.S., Bacich, D.J., Reuter, V.E., Heston, W.D., and Gaudin, P.B. (1999b). Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 5, 2674–2681. [PubMed] [Google Scholar]
- Chang, S.S., Reuter, V.E., Heston, W.D., Bander, N.H., Grauer, L.S., and Gaudin, P.B. (1999c). Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 59, 3192–3198. [PubMed] [Google Scholar]
- Chen, H.J., Yuan, J., and Lobel, P. (1997). Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J. Biol. Chem. 272, 7003–7012. [DOI] [PubMed] [Google Scholar]
- Christiansen, J.J., Rajasekaran, S.A., Moy, P., Butch, A., Goodglick, L., Gu, Z., Reiter, R.E., Bander, N.H., and Rajasekaran, A.K. (2003). Polarity of prostate specific membrane antigen, prostate stem cell antigen, and prostate specific antigen in prostate tissue and in a cultured epithelial cell line. Prostate 55, 9–19. [DOI] [PubMed] [Google Scholar]
- Coyle, J.T. (1997). The nagging question of the function of N-acetylaspartylglutamate. Neurobiol. Dis. 4, 231–238. [DOI] [PubMed] [Google Scholar]
- El Nemer, W., Colin, Y., Bauvy, C., Codogno, P., Fraser, R.H., Cartron, J.P., and Le Van Kim, C.L. (1999). Isoforms of the Lutheran/basal cell adhesion molecule glycoprotein are differentially delivered in polarized epithelial cells. Mapping of the basolateral sorting signal to a cytoplasmic di-leucine motif. J. Biol. Chem. 274, 31903–31908. [DOI] [PubMed] [Google Scholar]
- Halsted, C.H., Ling, E.H., Luthi-Carter, R., Villanueva, J.A., Gardner, J.M., and Coyle, J.T. (1998). Folylpoly-gamma-glutamate carboxypeptidase from pig jejunum. Molecular characterization and relation to glutamate carboxypeptidase II. J. Biol. Chem. 273, 20417–20424. [DOI] [PubMed] [Google Scholar]
- Herskovits, J.S., Burgess, C.C., Obar, R.A., and Vallee, R.B. (1993). Effects of mutant rat dynamin on endocytosis. J. Cell Biol. 122, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst, J., and Robinson, M.S. (1998). Clathrin and adaptors. Biochim. Biophys. Acta 1404, 173–193. [DOI] [PubMed] [Google Scholar]
- Hofmann, M.W., Honing, S., Rodionov, D., Dobberstein, B., von Figura, K., and Bakke, O. (1999). The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J. Biol. Chem. 274, 36153–36158. [DOI] [PubMed] [Google Scholar]
- Honing, S., Griffith, J., Geuze, H.J., and Hunziker, W. (1996). The tyrosinebased lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 15, 5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Horoszewicz, J.S., Kawinski, E., and Murphy, G.P. (1987). Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 7, 927–935. [PubMed] [Google Scholar]
- Israeli, R.S., Powell, C.T., Fair, W.R., and Heston, W.D. (1993). Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 53, 227–230. [PubMed] [Google Scholar]
- Johnson, K.F., and Kornfeld, S. (1992). The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J. Cell Biol. 119, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata, H., Yang, R., Hirama, T., Vuong, P.T., Kawano, S., Gombart, A.F., and Koeffler, H.P. (1999). Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J. Biol. Chem. 274, 20826–20832. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T. (1999). Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T., Bonifacino, J.S., and Riezman, H. (1997). Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 9, 488–495. [DOI] [PubMed] [Google Scholar]
- Lazarovits, J., and Roth, M. (1988). A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell 53, 743–752. [DOI] [PubMed] [Google Scholar]
- Leonard, et al. (1984). Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature 311, 626–631. [DOI] [PubMed] [Google Scholar]
- Letourneur, F., and Klausner, R.D. (1992). A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell 69, 1143–1157. [DOI] [PubMed] [Google Scholar]
- Liu, H., Moy, P., Kim, S., Xia, Y., Rajasekaran, A., Navarro, V., Knudsen, B., and Bander, N.H. (1997). Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 57, 3629–3634. [PubMed] [Google Scholar]
- Liu, H., Rajasekaran, A.K., Moy, P., Xia, Y., Kim, S., Navarro, V., Rahmati, R., and Bander, N.H. (1998). Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 58, 4055–4060. [PubMed] [Google Scholar]
- Luthi-Carter, R., Barczak, A.K., Speno, H., and Coyle, J.T. (1998). Molecular characterization of human brain N-acetylated alpha-linked acidic dipeptidase (NAALADase). J. Pharmacol. Exp. Ther. 286, 1020–1025. [PubMed] [Google Scholar]
- Marks, M.S., Ohno, H., Kirchhausen, T., and Bonifacino, J.S. (1997). Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 7, 124–128. [DOI] [PubMed] [Google Scholar]
- McDevitt, M.R., et al. (2001). Tumor therapy with targeted atomic nanogenerators. Science 294, 1537–1540. [DOI] [PubMed] [Google Scholar]
- Mellman, I. (1996). Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625. [DOI] [PubMed] [Google Scholar]
- Mukherjee, S., Ghosh, R.N., and Maxfield, F.R. (1997). Endocytosis. Physiol. Rev. 77, 759–803. [DOI] [PubMed] [Google Scholar]
- Nesterov, A., Carter, R.E., Sorkina, T., Gill, G.N., and Sorkin, A. (1999). Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 18, 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, H., Stewart, J., Fournier, M.C., Bosshart, H., Rhee, I., Miyatake, S., Saito, T., Gallusser, A., Kirchhausen, T., and Bonifacino, J.S. (1995). Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872–1875. [DOI] [PubMed] [Google Scholar]
- Owen, D.J., and Evans, P.R. (1998). A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, D.J., and Luzio, J.P. (2000). Structural insights into clathrin-mediated endocytosis. Curr. Opin. Cell Biol. 12, 467–474. [DOI] [PubMed] [Google Scholar]
- Pastan, I.H., and Willingham, M.C. (1981). Receptor-mediated endocytosis of hormones in cultured cells. Annu. Rev. Physiol. 43, 239–250. [DOI] [PubMed] [Google Scholar]
- Pinto, J.T., Suffoletto, B.P., Berzin, T.M., Qiao, C.H., Lin, S., Tong, W.P., May, F., Mukherjee, B., and Heston, W.D. (1996). Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res. 2, 1445–1451. [PubMed] [Google Scholar]
- Pond, L., Kuhn, L.A., Teyton, L., Schutze, M.P., Tainer, J.A., Jackson, M.R., and Peterson, P.A. (1995). A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J. Biol. Chem. 270, 19989–19997. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, A.K., Humphrey, J.S., Wagner, M., Miesenbock, G., Le Bivic, A., Bonifacino, J.S., and Rodriguez-Boulan, E. (1994). TGN38 recycles basolaterally in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 5, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker-Schaeffer, C.W., Hawkins, A.L., Su, S.L., Israeli, R.S., Griffin, C.A., Isaacs, J.T., and Heston, W.D. (1995). Localization and physical mapping of the prostate-specific membrane antigen (PSM) gene to human chromosome 11. Genomics 30, 105–108. [DOI] [PubMed] [Google Scholar]
- Rubin, L.A., Kurman, C.C., Biddison, W.E., Goldman, N.D., and Nelson, D.L. (1985). A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma 4, 91–102. [DOI] [PubMed] [Google Scholar]
- Sandoval, I.V., and Bakke, O. (1994). Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 4, 292–297. [DOI] [PubMed] [Google Scholar]
- Sandoval, I.V., Martinez-Arca, S., Valdueza, J., Palacios, S., and Holman, G.D. (2000). Distinct reading of different structural determinants modulates the dileucine-mediated transport steps of the lysosomal membrane protein LIM-PII and the insulin-sensitive glucose transporter GLUT4. J. Biol. Chem. 275, 39874–39885. [DOI] [PubMed] [Google Scholar]
- Sekiguchi, M., Okamoto, K., and Sakai, Y. (1989). Release of endogenous N-acetylaspartylglutamate (NAAG) and uptake of [3H]NAAG in guinea pig cerebellar slices. Brain Res. 482, 78–86. [DOI] [PubMed] [Google Scholar]
- Sheikh, H., and Isacke, C.M. (1996). A di-hydrophobic Leu-Val motif regulates the basolateral localization of CD44 in polarized Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 271, 12185–12190. [DOI] [PubMed] [Google Scholar]
- Silver, D.A., Pellicer, I., Fair, W.R., Heston, W.D., and Cordon-Cardo, C. (1997). Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 3, 81–85. [PubMed] [Google Scholar]
- Slusher, B.S., et al. (1999). Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat. Med. 5, 1396–1402. [DOI] [PubMed] [Google Scholar]
- Smith-Jones, P.M., Vallabhajosula, S., Navarro, V., Bastidas, D., Goldsmith, S.J., and Bander, N.H. (2003). Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J. Nucl. Med. 44, 610–617. [PubMed] [Google Scholar]
- Speno, H.S., Luthi-Carter, R., Macias, W.L., Valentine, S.L., Joshi, A.R., and Coyle, J.T. (1999). Site-directed mutagenesis of predicted active site residues in glutamate carboxypeptidase II. Mol. Pharmacol. 55, 179–185. [DOI] [PubMed] [Google Scholar]
- Trowbridge, I.S., Collawn, J.F., and Hopkins, C.R. (1993). Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 9, 129–161. [DOI] [PubMed] [Google Scholar]
- van der Bliek, A.M., Redelmeier, T.E., Damke, H., Tisdale, E.J., Meyerowitz, E.M., and Schmid, S.L. (1993). Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne, G. (1988). Transcending the impenetrable: how proteins come to terms with membranes. Biochim. Biophys. Acta 947, 307–333. [DOI] [PubMed] [Google Scholar]
- Wright, G.L., Jr., Grob, B.M., Haley, C., Grossman, K., Newhall, K., Petrylak, D., Troyer, J., Konchuba, A., Schellhammer, P.F., and Moriarty, R. (1996). Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 48, 326–334. [DOI] [PubMed] [Google Scholar]