Abstract
Although we carry out most daily tasks nearly automatically, it is occasionally necessary to change a routine if something changes in our environment and the behavior becomes inappropriate. Such behavioral switching can occur either retroactively based on error feedback or proactively by detecting a contextual cue. Recent imaging and electrophysiological data in humans and monkeys have suggested that the frontal cortical areas play executive roles in behavioral switching. The anterior cingulate cortex acts retroactively and the pre-supplementary motor area acts proactively to enable behavioral switching. The lateral prefrontal cortex reconfigures cognitive processes constituting the switched behavior. The subthalamic nucleus and the striatum in the basal ganglia mediate these cortical signals to achieve behavioral switching. We discuss how breaking a routine to allow more adaptive behavior requires a fine-tuned recruitment of the frontal cortical-basal ganglia neural network.
Breaking a routine: difficult but crucial
Driving to one’s workplace is an easy task: a task that most of us do on a daily basis for several years. One sees the same houses, the same trees, and the same traffic lights. One may not even be aware how the car accelerates and slows down, despite being the driver. If on one day there is unexpected congestion in the main road ahead, one may make up one’s mind quickly and turn into a side-road, successfully avoiding the traffic jam. But if the decision is late even by only a second, the chance to turn and avoid the congestion may be missed. This example illustrates that most daily behaviors are composed of well-learned routines–only occasionally, an important decision is made to switch from a routine behavior to an alternative, more appropriate given the context, behavior.
Naturally, behavioral switching has been an important question in experimental psychology 1, and there has been a recent surge of interest among neuroscientists in this issue. Several recent studies with human subjects using neuroimaging methods and transcranial magnetic stimulation as well as with patients with prefrontal lesions suggest that several regions in the frontal cortex play different roles in behavioral switching 2–5.
However, how the brain actually executes behavioral switching is not fully understood from the human data alone. The switching-associated reconfiguration of cognitive processes suggested by the psychological studies is likely composed of serial and parallel neuronal activity changes which occur within a short period before the decision to switch. However, the spatiotemporal resolution of the imaging data may not be sufficient for elucidating such fast changes in neuronal activity. To this end, single unit recording experiments using trained animals provide important complementary data.
In this article, we synthesize the insights provided by human neuroimaging data and animal single neuron data and put forward a framework that specifies the neural circuits involved in the execution of behavioral switching.
Two modes of behavioral switching
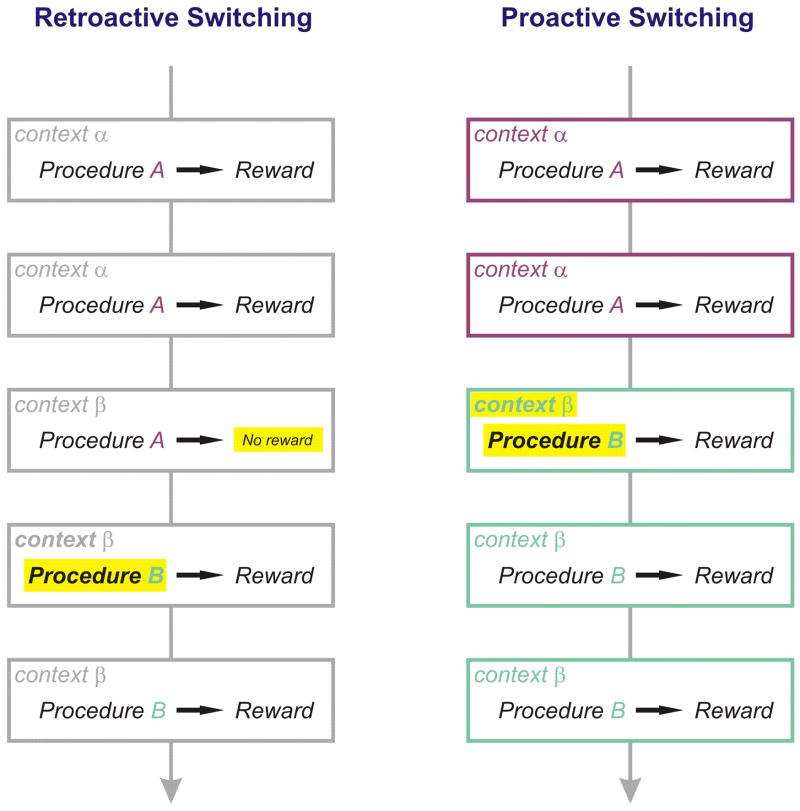
In order to understand the neural mechanisms of behavioral switching, it is important to understand what triggers such switching. Let us consider a situation in which procedure A is the appropriate behavior in order to obtain a reward in context α, while procedure B is the appropriate behavior in context β (Fig. 1), and a motivated subject has already learned these associations. Suppose the context changes from α to β. If the subject is unaware that the context has changed, s/he will perform procedure A and will therefore receive no or little reward (Fig. 1, left). This negative feedback signal triggers behavioral switching on the next trial. On the other hand, if the subject is aware of the context change, s/he will perform procedure B instead of A and will obtain a reward (Fig. 1, right). We term these two modes of switching ‘retroactive switching’ and ‘proactive switching’ respectively.
Figure 1. Retroactive and proactive switching.
Retroactive switching (left) is triggered by a failure (decreased reward value or an error). In this case the context cue is either absent or unknown to the animal (indicated by gray rectangles). Proactive switching (right) is triggered by a cue signaling a context change so that the subject will not experience the failure. This is possible, however, only after the subject has learned the meaning of the cue (indicated by purple and green rectangles). Highlighted in yellow are triggers of behavioral switching and switched procedures.
Note that this classification is different from the proposal by Braver and colleagues on proactive and reactive control of cognitive function 6. Proactive control in their framework refers to a sustained process before the onset of an imperative stimulus, whereas reactive control refers to a transient process after the onset of an imperative stimulus. There is no particular emphasis in their hypothesis on how behavior may switch when the context changes. Instead, our main goal is to understand the switching process where, we believe, the retroactive-proactive distinction is useful.
In retroactive switching (Fig. 1, left), the subject’s behavior is bound to fail on switch trials. This is costly in a dangerous world where one-time failure could be fatal. However, a change in context may be indicated in advance by a change in sensory inputs, which is often called a cue. Detecting the cue enables proactive switching in which the behavior may continue to be optimal even on switch trials. In a social context, the cue may be a change in facial expression or gaze direction of one’s partner or manager 7. It should be emphasized that the subject has to discover the cue from his/her experience. The discovery depends on learning, specifically learning of statistical relationships between the cues and the outcomes (e.g., rewarded or punishing).
A main proposal in this article is that retroactive switching and proactive switching are controlled by different regions in the medial frontal cortex, anterior cingulate cortex (ACC) and the pre-supplementary motor area (pre-SMA).
The anterior cingulate cortex and retroactive switching
The brain region that enables retroactive switching needs to be sensitive to negative feedback (e.g., reduced reward or punishment). It also needs to have access to the brain regions that implement alternative learned procedures. The ACC seems to fulfill both of these requirements.
First, many neurons in the monkey ACC are excited by negative feedback. In experiments using monkeys, the monkeys are trained to perform a task in order to obtain a certain amount of reward. If the reward is absent (e.g., due to poor performance) or reduced in amount experimentally, some ACC neurons are excited 8–11,12. Task-selectivity of ACC neurons is strongest after switching and declines thereafter, consistent with their role in retroactive switching 13. Neuronal activity in the ACC after negative feedback may continue if and until the monkey switches procedures 9 (Box 1). Further, switching is impaired by inactivation of the ACC 9.
Box. 1. Retroactive switching by ACC neurons.
There is empirical evidence that errors result in adjustments of behavior in several ways. First, subjects can correct their action slips resulting from premature responses immediately after they have committed an error 62. Second, subjects slow down on subsequent trials after errors, a phenomenon known as post-error slowing 62. As long as the correct action remains unchanged, such cautious responding is adaptive to attain the intended goal on the next trial. The ACC is implicated in both error detection 79, 80 and post-error adjustments 81. Third, once subjects realize on the basis of feedback (such as reduced reward) that the previously correct action becomes no longer valid, they switch behavior or learn a correct action (retroactive switching).
In a pioneering study designed to explore the role of the ACC in retroactive switching 9, monkeys were trained to perform one of two different arm movements, either pushing or turning a handle, in response to a movement trigger signal. Choosing a correct movement was rewarded and the correct movement remained unchanged in a block of trials, so that monkeys kept selecting the same movement. After a variable number of constant-reward trials, the amount of the reward decreased by 30% for each subsequent correct trial. At this stage monkeys were free to switch to the alternate movement. Once they did, the alternate movement was defined as the correct movement, and the reward reverted to the full amount. Thus, monkeys voluntarily selected one of the two movements based on the reduced amount of reward. An analysis of ACC neurons revealed that neuronal activity increased during the interval between the receipt of reduced reward and the switch to the alternate movement (Figure 1, middle). Notably, no such activation was observed when the monkey was given the full amount of reward in constant-reward trials (Figure 1, top) or when the reward was reduced but the monkey failed to switch to the alternate movement (Figure 1, bottom). Most importantly, chemical inactivation of the ACC impaired switching of movements based on the reduced amount of reward. These data indicate a crucial role for the ACC in retroactive behavioral switching. Similar activity properties were later found in the human ACC 82.
Figure for Box. 1. Retroactive switching by ACC neurons.
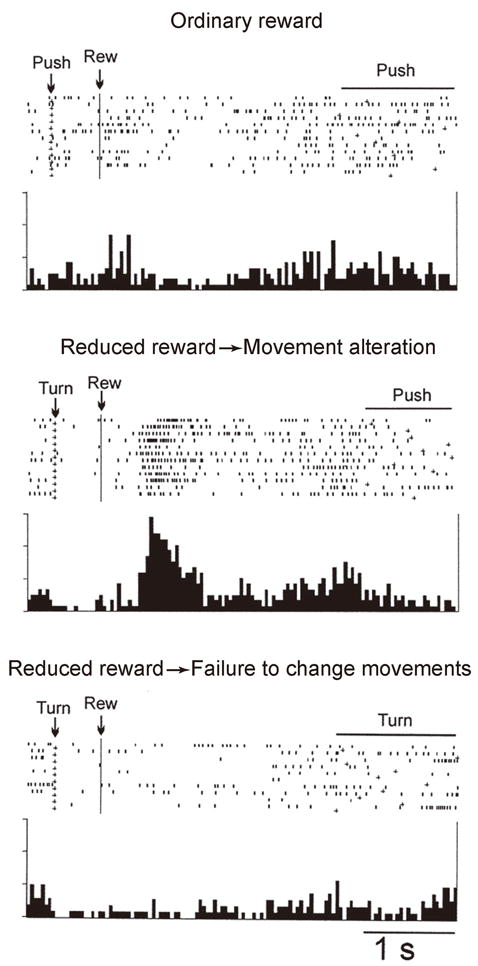
Activity of a representative ACC neuron recorded while the monkey selected one of two movements (pushing or turning a handle) based on reduced reward. Top: The neuron was not very active after ordinary reward and the monkey continued to select the same movement. Middle: The same neuron increased discharges after the receipt of reduced reward and before the initiation of the alternate movement. Bottom: The neuron remained inactive when the monkey did not switch to the alternate movement despite a reduction of reward.
Second, the switching function of the ACC may be mediated by its connections to the lateral prefrontal cortex (LPFC) 14, 15, which is thought to play an executive role in procedure implementation. An alternative pathway may be the connections to the striatum 16, which is equipped with mechanisms for behavioral selection 17. The role of the ACC-striatum connection is perhaps supported by the finding that striatal neurons show rapid changes in activity after retroactive switching in associative learning 18.
Neuroimaging studies with human subjects support the above conclusion. fMRI studies have indicated that the ACC is activated when the subject fails to perform a trial correctly (e.g., by failing to stop a button press) 19–24. Human EEG studies have revealed error-related potentials just after the erroneous motor response or after the error feedback, which are thought to be generated in the ACC 2. Similar error-related potentials are generated within the monkey ACC 25–27, which may be associated with the error-induced burst firing of ACC neurons described above.
The sensitivity of ACC neurons to negative feedback suggests that it may be related to motivational decision-making in general. In fact, some ACC neurons are excited by positive feedback (i.e., reward), but only when the reward is unexpected (i.e., immediately after the correct choice is discovered) 12, 28. These results suggest that the ACC enhances cognitive processes not only before switching (based on an unexpected error) but also after switching (based on an unexpected reward). Indeed, lesions of the ACC may cause general impairments in decision-making based on the history of actions and outcomes 29, 30.
The pre-SMA and proactive switching
A conflict in information processing characteristically occurs in proactive switching. The subject’s performance on switch trials is much worse (high error rate and longer reaction time) than when the same context is repeated (non-switch trial), a phenomenon called ‘switch cost’ 1. This is thought to occur because multiple cognitive operations are executed in response to the switch cue, which may include (1) suppression of the old procedure and (2) facilitation of the new procedure. The switch cost is particularly high if the old procedure has been repeated and therefore has become habitual or automatic.
Various lines of research suggest that the pre-SMA 31 is essential for proactive switching. Functional MRI studies have shown that the pre-SMA is consistently activated when human subjects switch between two tasks proactively in response to a cue 32, 33. Repetitive transcranial magnetic stimulation over the pre-SMA disrupts subjects’ performance in switch trials, but not in non-switch trials 33.
Such pre-SMA activation may be related to the cognitive operations described above. First, the pre-SMA seems to have a powerful mechanism to suppress body movements. For example, electrical stimulation of the pre-SMA suppresses ongoing or impending body movements in humans 34 and monkeys 35. The pre-SMA is activated consistently when the human subject tries to stop an impending movement 36, 19, 37. Such inhibitory control is impaired in patients with lesions including the pre-SMA 38 and in normal subjects when transcranial magnetic stimulation is applied over the pre-SMA 39. Second, the human pre-SMA is activated when two procedures compete with each other 19, 22, 37. Thus, the conflict associated with proactive switching (i.e., conflict between the old and new procedures) is likely to be processed in the pre-SMA 40, 41.
The fact that transcranial magnetic stimulation over the pre-SMA disrupts performance only on switch trials 33 suggests that the pre-SMA generates switch-related signals transiently at the time of switching. This is in contrast to the ACC, where neural processing continues after an erroneous choice 42 and even after a correct choice 12, 28. The hypothesized difference is supported by a recent finding that the pre-SMA and the ACC show transient and sustained responses, respectively, to incentive cues 43.
Another indication that the pre-SMA may be related to behavioral switching comes from studies with trained monkeys. Many pre-SMA neurons are activated before the monkey switches button presses from one target to the other in response to a sensory cue 44. They are also active when the monkey switches from one learned sequential procedure to another learned procedure, but only on the first trial 45. However, it is unclear from these experiments whether the pre-SMA can act rapidly enough to enable proactive switching under the time constraint described above. It is also unclear how the pre-SMA might enable switching.
In a recent study using an oculomotor switching task Isoda & Hikosaka presented evidence that the pre-SMA competes with automatic processes to enable behavioral switching (Box 2) 46. Confirming the above prediction, switch-related pre-SMA neurons are activated transiently at the time of switching. It was also shown that pre-SMA neurons, as a population, perform the two operations hypothesized above: (1) suppression of the old procedure and (2) facilitation of the new procedure. Switching is successful if the activation of pre-SMA neurons precedes the initiation of the automatic process; switching fails if the initiation of the automatic process precedes the activation of pre-SMA neurons.
Box. 2. Electrophysiological evidence for the role of the pre-SMA in proactive switching.
To study the neural mechanisms of proactive switching, Isoda and Hikosaka devised an oculomotor switching task 46. The task can be viewed as a change-signal task in which, just before the subject is about to perform the prepotent response based on the previous cue, a different cue is presented 44. Unlike most of the change-signal tasks, the prepotency is created internally by repeating the same response. This is the hallmark of automaticity or habit formation. Further, there is no special cue for switching.
In the oculomotor switching task the monkeys developed automaticity and showed a clear switch cost which was expressed as an increased rate of errors and increased reaction times 46. On switch trials they tended to make a prepotent but wrong saccade especially when the saccade occurred earlier than a latency which we called ‘behavioral differentiation time’. Many pre-SMA neurons were activated on switch trials, but not non-switch trials. Importantly, the onset of the switch-selective activity preceded the behavioral differentiation time when switching occurred correctly. When the monkey failed to switch, the pre-SMA neurons did become active, but after the wrong saccade. When the pre-SMA neuronal activity was boosted with electrical stimulation before the behavioral differentiation time, the success rate of switching increased.
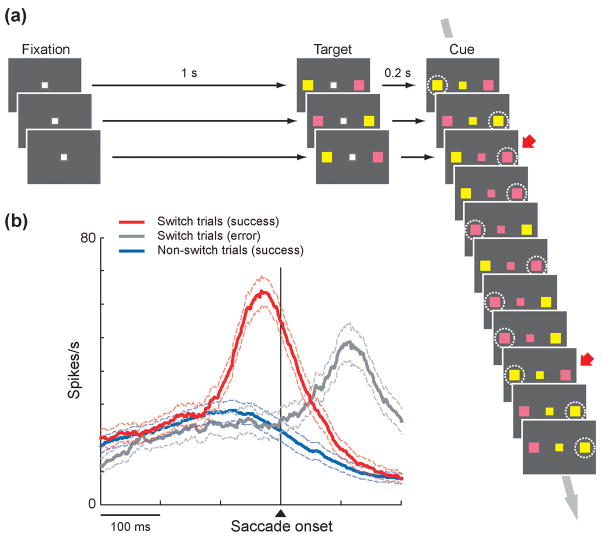
Figure for Box. 2. Proactive switching by pre-SMA neurons.
(a) Oculomotor switching task 46. Each trial began with the onset of a white fixation point followed by the presentation of two stimuli on each side of the fixation point in two different colors. The positions of the pink and yellow stimuli were randomized out of two possible locations. After a short delay, the fixation point became either pink or yellow as a cue, instructing the monkey to make a saccade to the stimulus whose color was the same as the central cue. The central cue color remained unchanged in a block of 1–10 consecutive trials and then was switched in the next block. For simplicity, display panels demonstrating the onset of fixation point (Fixation) and two peripheral stimuli (Target) are illustrated only for the first three trials. White dotted circles, which were not shown to the monkeys during the actual experiments, indicate the correct saccade target. Red arrows indicate switch trials. (b) The population activity of switch-selective pre-SMA neurons for successful switch trials (red), erroneous switch trials (gray) and successful non-switch trials (blue).
The lateral prefrontal cortex and rule implementation
Another cortical area that is thought to be essential for behavioral switching is the LPFC 47. Patients with prefrontal lesions show impairments in switching behaviors 48–50 or in inhibiting prepotent responses 51, 52. Similarly to the pre-SMA, the LPFC is activated when response inhibition is required 36, 53. Other studies suggest that the LPFC is predominantly active when relevant rules are retrieved, maintained, and implemented 13, 54. Strong activation of the LPFC occurs when the rules are complex and require changes in stimulus-response relationships in multiple dimensions, as typically seen in the Wisconsin Card Sorting Task (WCST) 55, 56. Rule-selective activity is also found in single neurons in the monkey LPFC 57. Switching between complex tasks requires reconfiguration of cognitive processes, and this may be done by changes in functional connectivity among frontal cortical areas 58, 59, 5.
The task rules, which are presumably represented in different regions in the LPFC, need to be executed as motor outputs. Each sub-region in the LPFC may select a correct motor response by inhibiting an incorrect response, since neurons specialized for a particular dimension (e.g., color), which are clustered in the LPFC, respond to WCST stimuli selectively when no-go responses are required 60. Part of the LPFC is characterized as a negative motor area (i.e., a cortical area stimulation of which suppresses voluntary movements), the other one around the pre-SMA 34. Thus, it is possible that the LPFC has a mechanism to inhibit motor behavior, but in a selective manner to choose the right behavior. The selection-related inhibition may constitute the LPFC activation during response inhibition described above. Connections to the striatum might mediate such selective inhibitions as well as disinhibitions 61.
Cortico-basal ganglia mechanisms and behavioral switching
The outcome of behavioral switching is a change in motor behavior. A crucial aspect of behavioral switching, as we have suggested above, is the suppression of prepotent body movements. This is particularly clear for proactive switching, but is also true for retroactive switching in which performance often becomes slower after an erroneous trial 62, 63.
One possibility may be that the switch-related cortical signals are mediated by an area that has a powerful capacity to inhibit motor areas. A candidate is the basal ganglia whose final outputs are exclusively inhibitory and are directed to a wide variety of motor structures including the cerebral cortex through the thalamus 64. The basal ganglia contain parallel circuits which are capable of removing inhibition (direct pathway) or enhancing inhibition (indirect and hyperdirect pathway) 65. Most cortical areas, including the pre-SMA, ACC, and LPFC, project to the striatum and STN, both being input zones of the basal ganglia 66. These anatomical features suggest that the basal ganglia are instrumental for selecting appropriate motor behaviors 17.
The function of the basal ganglia is heavily dependent on dopamine, as evidenced in Parkinson’s disease. It has been shown that patients with Parkinson’s disease have difficulty in changing motor or cognitive behaviors 67 and that dopaminergic medication remediates impairments in switching between tasks 68. The contribution of the basal ganglia in behavioral switching is also shown in human subjects without dopamine deficits. Subjects performing switching tasks show activations in the striatum 69–71 and the STN 36, 72, or both 73. There is a tendency for switching that is based on abstract rules to be associated with striatal activations, whereas switching relying on suppression of a prepotent response is associated with STN activations 73. Using a stop-signal task, Aron and colleagues found that stopping a prepotent motor response activated the inferior frontal cortex (IFC), pre-SMA, and STN 36, which were shown to be connected with each other using diffusion-weighted imaging tractography 72. Recent studies by Li and colleagues suggest that the IFC is involved in orienting attention to a salient event (i.e., stop process), whereas the pre-SMA is more specialized for mediating response inhibition via the STN and caudate nucleus 74, 75.
When monkeys perform the oculomotor switching task, a group of STN neurons show a switch-selective activity change (mostly an increase in activity) 76. The activity is similar to that seen in pre-SMA neurons, but occurs slightly later, consistent with the hypothesis that STN neurons receive the switch-related signal from the pre-SMA. The monkeys’ actions, assessed with the go-nogo task, are usually suppressive, suggesting that the STN works mainly to suppress the old no-longer-valid procedure. This conclusion is consistent with a study on patients with Parkinson’s disease. Electrical stimulation of the STN in these patients improved their motor symptoms, but the stimulation interfered with the normal ability to slow down when faced with decision conflict 77.
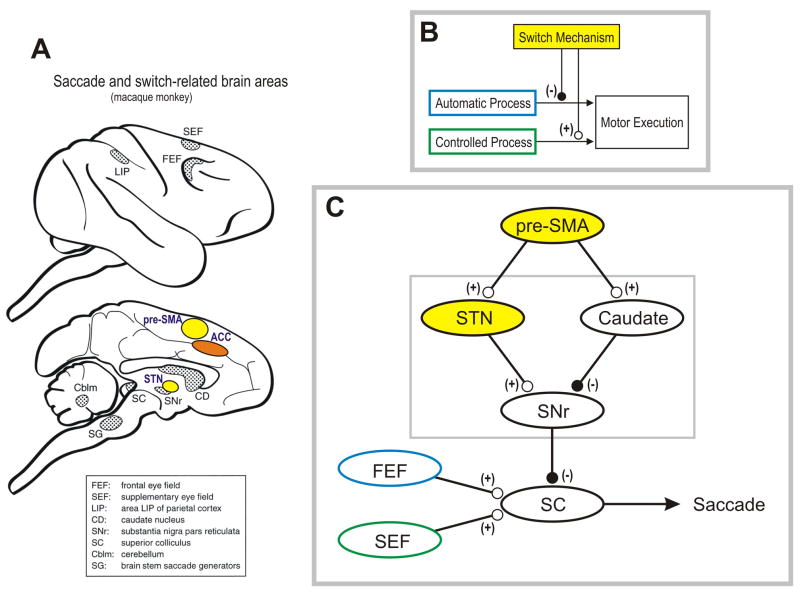
Since the STN has excitatory connections to the final output neurons in the basal ganglia located in the substantia nigra pars reticulata (SNr) or the globus pallidus internal segment (GPi) (Fig. 2) 65, their phasic activation will lead to a phasic inhibition of motor-related neurons in the basal ganglia-recipient thalamus and subcortical motor-related neurons including those in the superior colliculus (SC). Since signal transmission through the hyperdirect pathway is fast 65, the activity of pre-SMA neurons will be translated into an actual stopping action rapidly. These features fulfill one of the two mechanisms requisite to proactive switching: suppression of the old procedure.
Figure 2. Neural mechanism of proactive switching in oculomotor behavior.
A neural mechanism of behavioral switching must be able to (1) detect a change in the context, (2) suppress the prepotent, automatic process, and (3) facilitate the alternative, controlled process (conceptual scheme). The suppression must occur quickly because the automatic process emits a motor signal quickly; the facilitation can occur thereafter because the controlled process is slow. Recent studies have suggested that the pre-SMA, together with other frontal cortical areas, acts as a switch mechanism and the basal ganglia may mediate the switch-related signal from the cortical areas. In our study using saccadic eye movement, many neurons in the pre-SMA became active selectively and proactively on switch trials (Box 2). It was also shown, using a go-nogo task, that some pre-SMA neurons suppress the prepotent saccade, others facilitate the alternative saccade, and the rest have both functions. The suppressive pre-SMA neurons tended to be active earlier than the facilitatory pre-SMA neurons, consistent with the conceptual scheme. In the basal ganglia, the STN may serve to suppress the automatic saccade by enhancing the inhibitory output of the basal ganglia (SNr) on the SC or the thalamo-cortical network. The caudate nucleus might serve to facilitate the controlled saccade by disinhibiting the target of the basal ganglia. We speculate that the signals for the automatic and controlled saccades are carried mainly by the frontal eye field (FEF) and the supplementary eye field (SEF) respectively. In the possible neural network, excitatory and inhibitory connections are indicated by (+) and (−) respectively.
On the other hand, the striatum (caudate or putamen) may also be involved in the execution of behavioral switching. Its output via the direct pathway may be used for the facilitation (disinhibition) of the new procedure (Fig. 2). This could serve as the other mechanism for proactive switching, facilitation of the new procedure, such as the saccade to a different colored target 46 or the antisaccade 71, 78. On the contrary, the output of the striatum via the indirect pathway may be used for the suppression of the old procedure or the task rule-related inhibition of motor outputs.
Concluding remarks
When the circumstances necessitate it, we make the important decision to change our behavior by breaking a routine. Recent studies with human and non-human primate subjects have begun to elucidate the neural mechanisms underlying such behavioral switching. These studies suggest that different areas in the medial and lateral frontal cortices play executive roles in behavioral switching and do so using different algorithms.
What triggers behavioral switching represents one aspect of the switching algorithm. Switching may occur retroactively based on error feedback indicating that the current behavior is no longer appropriate. A critical structure for this retroactive switching is the ACC. In many cases, however, there is a sensory cue that predicts a change in the context. The subject can use the cue to switch behaviors proactively so that failure can be avoided. Such proactive switching is mainly governed by the pre-SMA. Importantly, the subject may not be aware of the presence of the cue initially, but may learn the meaning of the cue with experience.
Another aspect of the switching algorithm arises if the task rule changes before and after switching. In this case, cognitive processes need to be reconfigured to accommodate the rule change. Such cognitive reconfiguration seems to be performed by changes in functional connectivity among frontal cortical areas, including the LPFC. Even if the ACC or pre-SMA sends signals for switching, the switching would not be accomplished if the new rule has not been implemented (e.g., due to malfunction of the LPFC).
However, it is debatable whether each of the ACC, pre-SMA, and LPFC performs an exclusive function described above and is thus requisite for a certain type of behavioral switching. In fact, a lesion in each area may not lead to an impairment in switching. Instead, these prefrontal cortical regions may constitute a large network in which different switching algorithms are computed differentially but in an overlapping manner.
These switching algorithms need to be executed by selecting an appropriate motor behavior. The basal ganglia are considered to be a major mediator of the switch execution signals. In particular, the STN receives the switch-related signal from the pre-SMA and suppresses the ongoing but no-longer-valid behavior so that the new behavior can be executed. The striatum may also contribute to switching based on its input from the frontal cortical areas. Underlying these neural operations may be parallel neural circuits in the basal ganglia (direct, indirect, and hyperdirect pathways) by which a valid behavior can be selected while invalid behaviors can be suppressed.
However, behavioral switching is only part of what animals would do to adapt to changing worlds. Changing behavior gradually, based on reward outcome, is another important type of behavioral adaptation. It is still unclear, however, whether rapid adaptation (i.e., switching) and slow adaptation (i.e., reward-based changes) are controlled by the same or different brain networks (see also Box 3).
Box. 3. Outstanding questions.
Is the ACC necessary for retroactive switching?
We have proposed that the ACC is essential for retroactive switching. However, unlike a reversible inactivation study 9, recent lesion studies indicate that retroactive switching per se is impaired neither by ACC lesions 29 nor by lesions in different parts of the LPFC or the orbitofrontal cortex 30. This raises the possibility that, although the ACC is necessary for retroactive switching in the intact animal, other brain areas take over after ACC lesion and enable switching.
What are the roles of neuromodulators in behavioral switching?
The brain areas related to behavioral switching, especially the ACC and pre-SMA, receive substantial dopaminergic inputs from the ventral tegmental area and the substantia nigra 83. Since some dopamine neurons carry reward-related value signals 84, 85, it is plausible that dopamine in the medial frontal cortex is essential for behavioral switching 2. Experimental evidence in support of this hypothesis is currently lacking, however. These medial frontal cortical areas are also mutually connected with the locus coeruleus, which is a major source of noradrenergic signals. Since the locus coeruleus is thought to regulate the balance between exploration and exploitation 86, it may also be related to behavioral switching.
How is behavioral switching related to reward-based learning?
A dominant theory proposes that reward-based learning is based on plasticity in corticostriatal synapses which is conditioned by dopaminergic inputs 87. However, as the animal experiences two alternating task conditions repeatedly, reward-based changes in behavior tend to become faster 88. It is thus likely that any reward-based change in behavior involves both striatum-based plasticity and medial frontal cortex-based switching. The transition of the dopamine neuron’s response from the reward outcome to a predictive cue 84 might be related to the hypothetical transition from retroactive switching to proactive switching.
How important is behavioral switching in social contexts?
Behavioral switching may be particularly important in social contexts. An animal (or human) is surrounded by many animals (or humans) which have different behavioral traits. It is then crucial to switch behaviors in anticipation of (rather than in response to) the other individual’s behavior. Facial expressions, gestures, vocalization, and gaze direction can provide many cues for switching, which the animal may need to learn in order to enable proactive switching 7.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 2.Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 3.Rushworth MF, et al. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Botvinick MM, et al. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- 6.Braver TS, et al. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway A, et al., editors. Variation in working memory. Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- 7.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 8.Niki H, Watanabe M. Cingulate unit activity and delayed response. Brain Res. 1976;110:381–386. doi: 10.1016/0006-8993(76)90412-1. [DOI] [PubMed] [Google Scholar]
- 9.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 10.Ito S, et al. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 11.Amiez C, et al. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quilodran R, et al. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57:314–325. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Johnston K, et al. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Pandya DN, et al. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 15.Morecraft RJ, Van Hoesen GW. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. J Comp Neurol. 1993;337:669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- 16.Haber SN, et al. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hikosaka O, et al. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 18.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 19.Garavan H, et al. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 20.Li CS, et al. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience. 2008;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon V, et al. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- 23.Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci. 2008;28:14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gemba H, et al. ‘Error’ potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neurosci Lett. 1986;70:223–227. doi: 10.1016/0304-3940(86)90467-2. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, et al. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emeric EE, et al. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto M, et al. Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci. 2007;10:647–656. doi: 10.1038/nn1890. [DOI] [PubMed] [Google Scholar]
- 29.Kennerley SW, et al. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 30.Buckley MJ, et al. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- 31.Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res. 1994;19:251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 32.Dove A, et al. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- 33.Rushworth MF, et al. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 34.Luders HO, et al. Cortical electrical stimulation in humans. The negative motor areas. Adv Neurol. 1995;67:115–129. [PubMed] [Google Scholar]
- 35.Isoda M. Context-dependent stimulation effects on saccade initiation in the presupplementary motor area of the monkey. J Neurophysiol. 2005;93:3016–3022. doi: 10.1152/jn.01176.2004. [DOI] [PubMed] [Google Scholar]
- 36.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nachev P, et al. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- 39.Chen CY, et al. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2009;44:537–545. doi: 10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Ridderinkhof KR, et al. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PC, et al. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. J Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung N, et al. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 43.Kouneiher F, et al. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaka Y, Tanji J. Changing directions of forthcoming arm movements: Neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex. Journal of Neurophysiology. 1996;76:2327–2342. doi: 10.1152/jn.1996.76.4.2327. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura K, et al. Neuronal activity in medial frontal cortex during learning of sequential procedures. Journal of Neurophysiology. 1998;80:2671–2687. doi: 10.1152/jn.1998.80.5.2671. [DOI] [PubMed] [Google Scholar]
- 46.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 47.Brass M, et al. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Rogers RD, et al. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121 ( Pt 5):815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 49.Aron AR, et al. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- 50.Rowe JB, et al. Is the prefrontal cortex necessary for establishing cognitive sets? J Neurosci. 2007;27:13303–13310. doi: 10.1523/JNEUROSCI.2349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aron AR, et al. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 52.Chambers CD, et al. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 53.Chikazoe J, et al. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- 54.Crone EA, et al. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- 55.Nakahara K, et al. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- 56.Mansouri FA, et al. Prefrontal cell activities related to monkeys’ success and failure in adapting to rule changes in a Wisconsin Card Sorting Test analog. J Neurosci. 2006;26:2745–2756. doi: 10.1523/JNEUROSCI.5238-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallis JD, et al. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- 58.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;245:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 59.Koechlin E, et al. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 60.Sakagami M, et al. A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. Journal of Neuroscience. 2001;21:4801–4808. doi: 10.1523/JNEUROSCI.21-13-04801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferry AT, et al. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 62.Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- 63.Botvinick MM, et al. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 64.Hikosaka O. GABAergic output of the basal ganglia. Prog Brain Res. 2007;160:209–226. doi: 10.1016/S0079-6123(06)60012-5. [DOI] [PubMed] [Google Scholar]
- 65.Nambu A, et al. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 66.Takada M, et al. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci. 2001;14:1633–1650. doi: 10.1046/j.0953-816x.2001.01789.x. [DOI] [PubMed] [Google Scholar]
- 67.Cools AR, et al. Cognitive and motor shifting aptitude disorder in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1984;47:443–453. doi: 10.1136/jnnp.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cools R, et al. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- 69.Cools R, et al. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casey BJ, et al. Early development of subcortical regions involved in non-cued attention switching. Dev Sci. 2004;7:534–542. doi: 10.1111/j.1467-7687.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- 71.Cameron IG, et al. Role of the basal ganglia in switching a planned response. Eur J Neurosci. 2009;29:2413–2425. doi: 10.1111/j.1460-9568.2009.06776.x. [DOI] [PubMed] [Google Scholar]
- 72.Aron AR, et al. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monchi O, et al. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59:257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- 74.Chao HH, et al. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time--an intra-subject analysis. BMC Neurosci. 2009;10:75. doi: 10.1186/1471-2202-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duann JR, et al. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frank MJ, et al. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 78.Ford KA, Everling S. Neural activity in primate caudate nucleus associated with pro- and antisaccades. J Neurophysiol. 2009;102:2334–2341. doi: 10.1152/jn.00125.2009. [DOI] [PubMed] [Google Scholar]
- 79.Falkenstein M, et al. Effects of errors in choice reaction time tasks on the ERP under focused and divided attention. In: Brunia CHM, et al., editors. Psychophysiological Brain Research. Tilburg University Press; 1990. pp. 192–195. [Google Scholar]
- 80.Gehring WJ, et al. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- 81.Klein TA, et al. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 82.Williams ZM, et al. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 83.Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- 84.Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 85.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 87.Wickens JR, et al. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe K, Hikosaka O. Immediate changes in anticipatory activity of caudate neurons associated with reversal of position-reward contingency. J Neurophysiol. 2005;94:1879–1887. doi: 10.1152/jn.00012.2005. [DOI] [PubMed] [Google Scholar]