Abstract
Bone marrow (BM)-resident hematopoietic stem and progenitor cells (HSPCs) are known to enter the blood and to home back to the BM. However, whether these migratory HSPCs also follow extramedullary traffic routes is unknown. Our group has recently shown that mouse thoracic duct (TD) lymph contains clonogenic HSPCs that possess short- and long-term multilineage reconstitution capacity in primary and secondary transplantation assays. Using BM transplantation, homing experiments, and parabiotic mice, we established that TD HSPCs originate in the BM and traffic constitutively to multiple extramedullary tissues, where they reside for several days until entering draining lymphatics to return to the blood. While these migratory properties of HSPCs resemble those of lymphocytes, circulating HSPCs access different target tissues because, unlike lymphocytes, they do not require secondary lymphoid organs to recirculate. The egress of HSPCs from extramedullary tissues into lymph depends on sphingosine-1-phosphate (S1P) receptors, particularly S1P1. We have shown that under physiological conditions migratory HSPCs contribute to the continuous restoration of specialized hematopoietic cells that reside in peripheral tissues. Upon exposure to toll-like receptor (TLR) agonists, migratory HSPCs proliferate locally within extramedullary tissues and generate innate immune effector cells. Thus, HSPCs can survey peripheral organs to replenish tissue-resident hematopoietic cells and act as a source of mature leukocytes during host defense against pathogens.
Keywords: hematopoietic stem cell, sphingosine 1-phosphate, cell migration, innate immune response
Introduction
Blood cell homeostasis depends on a rare population of precursor cells, the hematopoietic stem and progenitor cells (HSPCs), which possess the capacity for self-renewal and multilineage differentiation. HSPCs are thought to reside mostly in specialized niches in bone marrow (BM) cavities. Nonetheless, a considerable number of HSPCs constitutively migrate out of the BM and enter the blood. For example, in adult mice, the blood contains a small, but relatively stable population of several hundred HSPCs, which upon transplantation to irradiated recipients are capable of long-term reconstitution (LTR) of hematopoietic activity.1 Since the blood residence time of individual HSPCs is on the order of minutes, the daily turnover of HSPCs that enter and leave the bloodstream is considered to be high.2
It is commonly assumed that the BM is the exclusive physiological source and destination of blood-borne HSPCs. However, our sparse knowledge of HSPC trafficking does not rule out that circulating HSPCs migrate also to extramedullary sites. A working example is lymphocytes, another stable blood leukocyte population with a short circulation half-life.3 Lymphocytes extravasate continuously into multiple lymphoid and nonlymphoid tissues, including the BM. Many tissue-resident lymphocytes eventually return to the blood via lymphatics that drain into the thoracic duct (TD) or other large lymph conduits. Our group recently examined whether blood-borne HSPCs might follow similar extramedullary traffic patterns as lymphocytes (Fig. 1).
Figure 1.
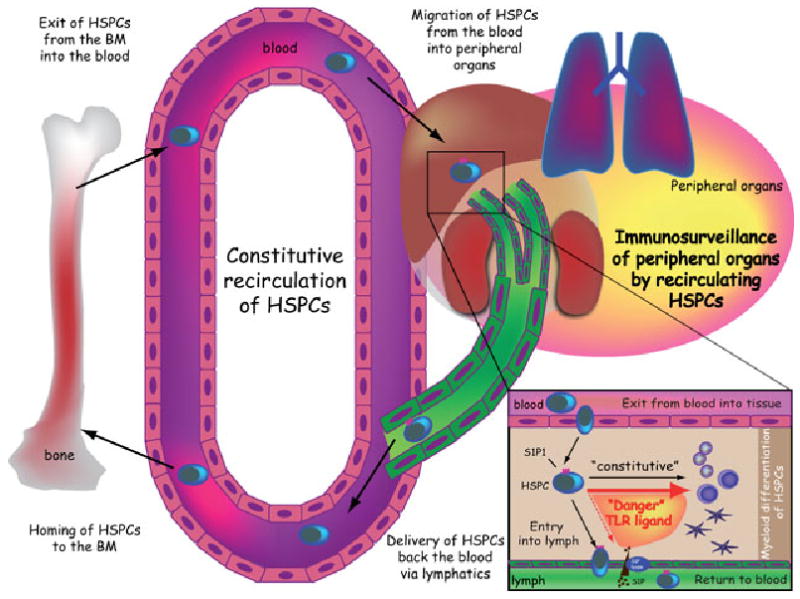
Scheme illustrating the migration of HSCPs. Schematic model illustrating the trafficking of migratory HSPCs under physiological conditions and during inflammation (for details see text).
Hematopoietic Stem and Progenitor Cells Travel in the Lymph of Adult Mice
Initially, we surmised that if HSPCs recirculate through extramyeloid tissues in a way similar to mature leukocytes (including lymphocytes and dendritic cells), then they, too, might become lymph borne. Indeed, we found that murine lymph collected from the TD contained up to 4% Lin−CD45+ mononuclear cells (MNCs).4 This population included rare cells with a primitive HSPC phenotype (Lin−sca-1+c-kithi [LSK]). Methylcellulose-based colony-forming unit culture (CFU-C) assays with MNCs from blood and TD confirmed that both compartments contain functional clonogenic HSPCs. Sorting approaches revealed that clonogenic HSPCs resided almost exclusively in the Lin−c-kit+ fraction of lymph-borne MNCs. These lymph-borne HSPCs were BM derived, since virtually all colonies expressed green fluorescent protein (GFP) when we collected and plated lymph-borne MNCs from lethally irradiated wild-type mice transplanted with BM from GFP-transgenic donors. Based on the frequency of lymph-borne clonogenic cells and the calculated lymph flow (approx. 1.6 mL/h), we estimate that approximately 200 clonogenic HSPCs pass through the lymph of a mouse every day.
In a next step, we addressed whether lymph-borne HSPCs (like blood-derived HSPCs) would home to the BM.2 Fluorescently tagged, HSPC-enriched TD cells were injected i.v. into recipient mice. Notably, donor HSPCs were readily detectable in recipient BM and spleens 24 h later by flow cytometry. Multiphoton intravital microscopy of skull BM5 in anesthetized recipients confirmed that TD-derived, HSPC-enriched cells migrate into BM cavities within 12–24 h after adoptive transfer and were positioned adjacent to the endosteum, which is thought to provide supportive niches for primitive HSPCs.6–9
Having established that lymph-borne HSPCs possess BM-tropism akin to their blood-borne counterparts, we set out to explore their functional engraftment capacity. We transplanted TD-MNC from CD45.2+ donors into sublethally irradiated CD45.1+ congenic recipients. After 6 weeks, about 15% of Gr-1+ peripheral blood granulocytes were donor derived, indicating that lymph-borne HSPCs in fact possess myeloid differentiation potential.2,10 In addition, we found that lymph-derived HSPCs are also able to give rise to T cells after transplantation. Correspondingly, TD-MNC from athymic B6nude/nude mice were able to reconstitute a near-normal T-cell repertoire 6 weeks after transplantation into lethally irradiated Rag2−/−γc−/− mice (which possess a functional thymic anlage but are genetically incapable of generating lymphocytes11,12). This indicates that murine lymph contains multipotent progenitor cells that are able to give rise to both myeloid and lymphoid cells. Notably, lymph contains not only hematopoietic progenitor cells but also some long-term multilineage repopulating HSPCs that meet the functional criteria of true hematopoietic stem cells, as we showed by secondary transplantation assays.
HSPCs Constitutively Travel to Various Nonlymphoid Tissues
Based on the above observations that HSPCs apparently recirculate between blood and lymph similar to naive lymphocytes, it seemed reasonable to speculate that both populations might share the peripheral transit routes guiding them from blood into lymphatics. Naive lymphocytes have to home to lymph nodes or Peyer's patches before they gain access to lymph vessels. Consequently, the number of mature lymphocytes is dramatically reduced in lymph of lymphotoxin (LT)-α-deficient mice, which lack these lymphoid tissues.13 However, we found that unlike mature lymphocytes, HSPCs are still detectable in the TD lymph of LT-α-deficient mice. Thus, HSPCs, in contrast to lymphocytes, do not require secondary lymphoid organs to recirculate.
Next, we employed several strategies to ask where and how HSPCs might leave the blood and pass through extramedullary tissues into the lymph. First, we surveyed cell suspensions of different murine tissues for the presence of HSPCs using CFU-C assays. Clonogenic progenitors were readily detectable in many tissues, including the lung, liver, small intestine, and kidneys. Virtually all clonogenic tissue HSPCs were BM derived, since colonies uniformly expressed GFP in chimeric wild-type recipients of GFP+ BM. Based on the numbers of clonogenic cells recovered from various tissues, we estimate that the total number of HSPCs resident in extramedullary nonlymphoid tissue is at least twice as large as the number of HSPCs estimated to circulate in murine blood.2
To examine whether tissue HSPCs arise from blood-borne migratory HSPCs, we generated pairs of parabiotic mice, in which one partner expressed ubiquitous transgenic GFP. Cross-circulation was established by day 3 after surgical joining, and the host : partner (i.e., GFP+ : GFP−) ratio both of mature leukocytes, but also of HSPCs in blood, plateaued at about 3:2 (corresponding to 40% chimerism) between days 10 and 14.2 In extramedullary tissues of parabiotics, the highest level of HSPC chimerism was seen in the spleen, followed by the lungs, liver, and kidneys. This indicates that tissue HSPCs are constitutively replenished by the pool of HSPCs that circulate in the blood. At least some of these blood-derived HSPCs are not permanently retained in extramedullary tissues, but recirculate freely via lymph and blood compartments, because we also detected marked HSPC chimerism in the lymph (and blood, see above) of parabiotic mice. Again using parabiotics, we established that the mean dwell time of HSPCs that have homed to peripheral tissues is approximately 48 h. Together, these findings suggest that many nonlymphoid organs (in addition to the BM, which lacks lymphatic drainage) are sites of active HSPC trafficking from blood to tissue to lymph.
HSPCs Constitutively Travel to Various Nonlymphoid Tissues
Next, we wanted to address the mechanisms that regulate HSPC recirculation. Interestingly, we found that treatment of mice with pertussis toxin (PTX) for only 6 h led to a nearly complete disappearance of HSPCs from lymph. This implied an essential role for a Gαi-coupled signal that directs tissue-resident HSPCs into draining lymphatics. Recent studies have shown that tissue-resident lymphocyte egress into lymphatics is controlled by S1P1, a Gαi-coupled receptor for sphingosine 1-phosphate (S1P).14–16 Using RT-PCR, we found that murine HSPCs, like resting lymphocytes and human HSPCs,17 express S1P1 mRNA, and migrate toward an S1P gradient in vitro. Thus, we asked whether S1P signaling might influence HSPC recirculation. Remarkably, treatment of mice with FTY720 (which targets the S1P receptors S1P1, 3, 4, and 5) or with SEW2871 (which selectively targets S1P1) nearly completely abolished the appearance of lymph-borne HSPCs. At the same time, treatment of mice with FTY720 resulted in a significant increase in the number of HSPCs residing within extramedullary tissues. Together, our observations clearly suggested that S1P receptors, particularly S1P1, regulate the egress of HSPCs from tissue into the draining lymphatic vasculature.
HSPCs Give Rise to Myeloid Cell in Peripheral Tissues
All of the above findings clearly suggested that there is a constitutive recirculation of HSPCs between BM, blood, extramedullary tissues, and lymph compartment. But what is the purpose? To address this important question, we next asked whether HSPCs might give rise to mature hematopoietic cells once they had entered extramedullary tissues. To find this out, we isolated different populations of HSPC-enriched GFP+ cells from the lymph or BM and transplanted them under the kidney capsule of recipient mice. The contralateral kidney was treated with medium without cells and was used as a control. Notably, a sizeable number of GFP+ progeny were detected 6 days later in the kidney that was injected with GFP+ HSPCs, while only few GFP+ cells were observed in the medium-injected, contralateral control kidney. Virtually all GFP+ cells in injected kidneys co-expressed the pan-hematopoietic marker CD45, indicating that they had retained their hematopoietic origin, and more than 50% of the GFP+ cells co-expressed myeloid lineage markers. This shows that, after deposition within tissues, HSPCs have the capacity to differentiate locally into myeloid hematopoietic lineages.
Migratory HSPCs might, therefore, help to constitutively replenish specialized tissue-resident myeloid cells under steady-state conditions. However, in a next set of experiments we revealed that the migratory pool of HSPCs might play an even more important role during tissue infection. Seminal work by Paul Kincade's lab had previously established that HSPCs express toll-like receptors (TLRs) and their coreceptors, including TLR4, MD-2, and CD14, required for recognition of bacterial lipopolysaccharide (LPS).18 His group reported that TLR signaling drives differentiation of HSPCs into myeloid lineages in vitro. Based on these observations, we speculated that migratory HSPCs that encounter TLR ligands within extramedullary tissues might act as a highly versatile local source to rapidly replenish innate immune cells during infection. To test this hypothesis, we injected both kidneys with LPS and then implanted distinct subsets of TD- or BM-derived, HSPC-enriched GFP+ cell populations underneath the one kidney capsule, whereas medium without cells was injected into the contralateral control kidney capsule. Six days later, large numbers of GFP+ cells were found in the LPS-treated kidney injected with HSPCs, while comparably low numbers of GFP+ cells were found in the contralateral control kidney, treated with LPS without cells. The vast majority of the GFP+ cells expressed myeloid lineage markers. Notably, the numbers of all GFP+ cells and of myeloid GFP+ cells were significantly higher after implantation of HSPCs together with LPS compared to kidneys injected with the same subset of GFP+ HSPCs without LPS. This already strongly suggested that implanted HSPCs had proliferated locally within the tissue after TLR ligand engagement. Correspondingly, the majority of the cells of each of the GFP+ clusters found within the kidneys that were treated with LPS and HSPCs incorporated 5-bromo-2-deoxyuridine (BrDU) into their nucleus and stained positive for Ki-67, implying that in the presence of TLR ligands HSPCs indeed proliferate locally.
Notably, LPS not only amplified local proliferation of HSPCs in extramedullary tissues, but also prevented the exit of clonogenic HSPCs out of peripheral tissues. This was, at least in part, due to interference with S1P-S1P1 signaling in recirculating HSPCs. Correspondingly, incubation of HSPCs with LPS was associated with a down-regulation of S1P1, paralleled by failure of LPS-treated HSPCs to chemotax toward a gradient of S1P. Hence, LPS recognition not only triggers local proliferation and differentiation (see above), but also disrupts the normal S1P-dependent signaling cascade and leads to retention of HSPCs within extramedullary tissues.
Discussion
Our results establish a previously unknown migratory route of HSPCs. We show that HSPCs traffic constitutively to multiple extramedullary tissues, where they remain for approximately 2 days until they enter the draining lymphatics to return to the blood. HSPCs express multiple traffic molecules, including PSGL-1, VLA-4 (CD49d/CD29), LFA-1 (CD11a/CD18), CD44, and the chemokine receptor CXCR4, which are likely to direct HSPCs into nonlymphoid tissues.19 However, the exact recruitment mechanism(s) responsible for HSPC trafficking from blood into extramyeloid tissues will have to be elucidated in future.
Our present findings show that the signaling lipid S1P plays a pivotal role during the subsequent departure of tissue-resident HSPCs into lymphatics. It has been shown previously that steep S1P gradients exist between interstitial fluid and lymph in various tissues, which is established and maintained by the enzyme S1P lyase.20,21 This S1P gradient regulates the egress of mature lymphocytes from the thymus, spleen, and lymph nodes.20,21 Our data now suggest that HSPCs also sense this gradient through their S1P receptors, particularly S1P1, which guides them out of nonlymphoid tissues into efferent lymph vessels. Interestingly, S1P gradients are also required for the migration of bilateral heart progenitors in zebrafish,22,23 indicating that guidance signals provided by extracellular lipids, such as S1P, may represent a conserved navigation mechanism for migrating stem and progenitor cell populations.
All of these findings suggested that there is a constitutive migration of BM-derived HSPCs across blood, nonlymphoid tissues, and lymph. The recirculation of HSPCs between the BM and blood is believed to be important to maintain hematopoietic homeostasis in dispersed BM cavities.2 But what is the purpose of HSPC migration across extramedullary tissues? Recently, hematopoietic stem cells and lineage-specified progenitors have been shown to express functional TLRs,18 which recognize foreign molecules such as the bacterial outer membrane component LPS. Binding of LPS to TLRs promotes entry of quiescent HSPCs into the cell cycle and triggers myeloid differentiation.18 Initially, it was proposed, that HSPCs that reside in the BM could be stimulated by circulating pathogen products. Our experiments now indicate that HSPCs can also address the threat of infection at the pint of entry within peripheral tissues. Once HSPCs enter peripheral tissues and sense microbial danger signals through TLRs, they start to proliferate vigorously and boost the local supply of innate effector cells. Hence, migratory HSPCs possess the ability to survey peripheral tissues and respond rapidly to situations, such as tissue damage and infections, that require the prompt influx of large numbers of innate immune cells. However, we found that even in the absence of inflammation, recirculating HSPCs divide locally in peripheral tissues and differentiate into mature myeloid cells. Under steady-state conditions, homed HSPCs may therefore help to constitutively replenish the diverse population of tissue-resident leukocytes that perform multiple essential functions in peripheral organs, including removal of dead cells and debris.
In conclusion, we show here that HSPCs constitutively survey extramedullary nonlymphoid tissues. Our results raise the possibility that in the absence of infection migratory HSPCs contribute to the continuous restoration of specialized hematopoietic cells that reside in peripheral tissues. During tissue infection, the migratory pool of HSPCs might act as an immediate and highly adaptive source of progenitor cells that proliferate locally and generate innate immune effector cells. It will be important to determine how HSPC traffic within peripheral tissues is influenced by and may modulate the course of infectious diseases and of other acute or chronic inflammatory conditions. Hence, hematopoietic stem cells are actually a part of the immune system, rather than just giving rise to it.
Acknowledgments
Supported by the National Institutes of Health (AI061663, AR42689, and HL56949 to U.H.v.A) and the German Research Foundation DFG (Ma2186/4-1 to S.M.). S.M. is a Heisenberg Scholar of the German Research Foundation DFG.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright DE, et al. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 3.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 4.Massberg S, et al. Physiological recirculation of hematopoietic stem and progenitor cells through blood, lymph and extramedullary tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazo IB, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 9.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 11.Cao X, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 12.Goldman JP, et al. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103:335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]
- 13.De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 14.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 15.Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate–modifiers of lymphocyte migration. N Engl J Med. 2006;355:1088–1091. doi: 10.1056/NEJMp068159. [DOI] [PubMed] [Google Scholar]
- 16.Ledgerwood LG, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 17.Kimura T, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 18.Nagai Y, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright DE, et al. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 21.Pappu R, et al. Promotion of Lymphocyte Egress into Blood and Lymph by Distinct Sources of Sphingosine-1-Phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 22.Kupperman E, et al. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara A, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]


