Abstract
A surge of luteinizing hormone (LH) from the pituitary gland triggers ovulation, oocyte maturation, and luteinization for successful reproduction in mammals. Because the signaling molecules RAS and ERK1/2 (extracellular signal–regulated kinases 1 and 2) are activated by an LH surge in granulosa cells of preovulatory follicles, we disrupted Erk1/2 in mouse granulosa cells and provide in vivo evidence that these kinases are necessary for LH-induced oocyte resumption of meiosis, ovulation, and luteinization. In addition, biochemical analyses and selected disruption of the Cebpb gene in granulosa cells demonstrate that C/EBPβ (CCAAT/Enhancer-binding protein–β) is a critical downstream mediator of ERK1/2 activation. Thus, ERK1/2 and C/EBPβ constitute an in vivo LH-regulated signaling pathway that controls ovulation- and luteinization-related events.
In the mammalian ovary, the female germ cells (oocytes) reside within the ovarian follicles and are surrounded by somatic cell–derived granulosa cells (GCs) and cumulus cells that have endocrine functions and control oocyte maturation. Female reproductive success depends on the growth of ovarian follicles and differentiation of GCs as well as oocyte maturation and ovulation (1, 2). Although LH plays a critical role in the initiation of ovulation and in the terminal differentiation of GCs to luteal cells that compose the corpora lutea (CLs) and produce progesterone, the precise molecular targets in these processes remain ill-defined. Cyclic adenosine 3´,5´-monophosphate (cAMP) is a well-known mediator of LH action, but LH also induces expression of the epidermal growth factor (EGF)–like factors that, via activation of the EGF receptor, RAS, and extracellular signal–regulated kinases 1 and 2 [ERK1/2, also known as mitogen-activated protein kinases 3 and 1 (MAPK3/1)], may act as the intrafollicular mediators to stimulate the cumulus cell-oocyte complex (COC) expansion and oocyte maturation (3–5). However, specific role(s) of the EGF network and, more specifically, of ERK1/2 in regulating ovulation, oocyte maturation, and the global reprogramming of GCs to luteal cells have not yet been analyzed or defined clearly in vivo.
ERK1 and ERK2 are coexpressed in all mammalian tissues and implicated as key regulators of cell proliferation and differentiation as well as oocyte maturation in culture (6, 7). Mutant mouse models have shown that Erk1-null mice are viable and fertile (8), but mutation of the Erk2 gene is embryonic lethal in mice (9). To analyze the specific ovarian functions of ERK1 and ERK2 in vivo, we crossed Erk2fl/fl mice (10) with the Cyp19-Cre transgenic mice (11), and the resultant Erk2fl/fl;Cyp19-Cre mice were further crossed into the Erk1−/− background (8), yielding Erk1−/−;Erk2fl/fl;Cyp19-Cre (Erk1/2gc−/−) mice. Efficient depletion of ERK1 and/or ERK2 in the mutant GCs and cumulus cells, as well as the lack of phosphorylation of ERK1/2 in the mutant GCs in vivo, was demonstrated (fig. S1, A to C). Loss of ERK1/2 did not impair LH-mediated activation of known upstream regulators of ERK1/2 or other LH-regulated signaling molecules (fig. S1, D and E) but did block phosphorylation of the ERK1/2 target RPS6KA2 (P90RSK) (fig. S1D).
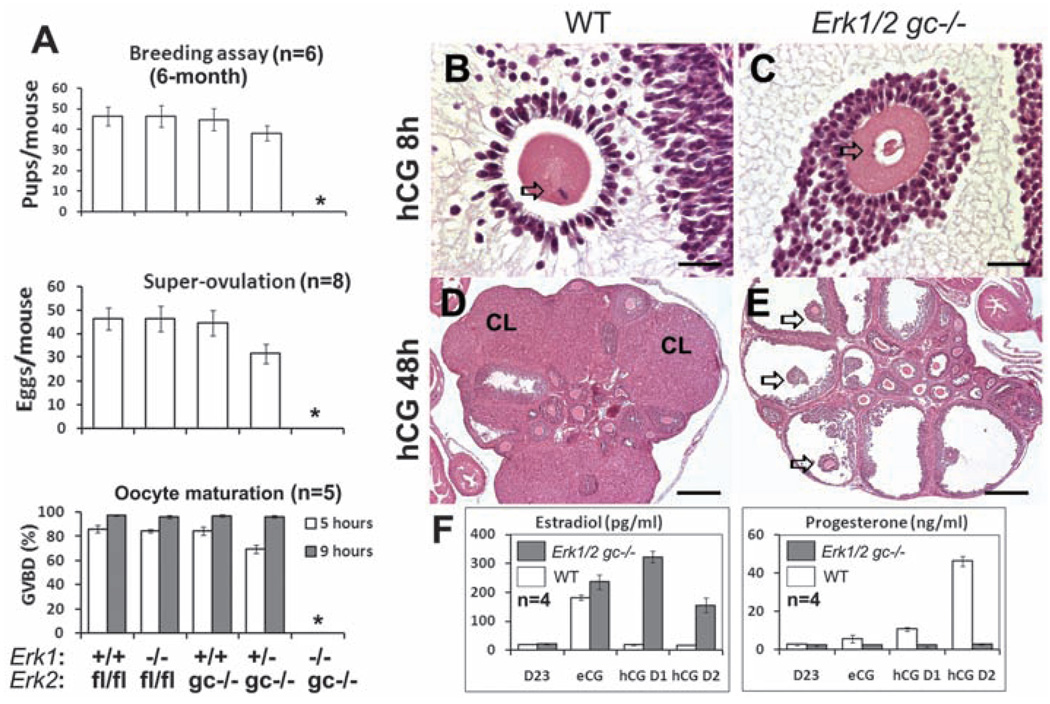
The Erk1−/− and Erk2gc−/− females were fertile; however, the Erk1/2gc−/− females failed to ovulate and were completely infertile (Fig. 1A). Ovaries of adult Erk1/2gc−/− females contained preovulatory follicles but no CLs (fig. S2A). Concentrations of estradiol in serum were elevated (fig. S2B), causing constant vaginal estrus (fig. S2C). Even in immature Erk1/2gc−/− females treated with exogenous hormones, events associated with ovulation did not occur: No oocytes matured, as indicated by the complete lack of germinal vesicle breakdown (GVBD) (Fig. 1A); no COCs expanded (Fig. 1, B and C, and fig. S2D); no follicles ruptured to form CLs (Fig. 1, D and E); and the concentration of estradiol in serum was elevated but that of progesterone was not (Fig. 1F), indicating profound endocrine changes in the mutant mouse ovaries. GCs in the equine chorionic gonadotropin (eCG)-primed Erk1/2gc−/− females were viable but exhibited signs of apoptosis when luteinization failed (fig. S2E).
Fig. 1.
ERK1/2 mediate LH-induced oocyte maturation, ovulation, and luteinization. (A) Breeding assays, superovulation assays, and oocyte maturation (germinal vesicle breakdown; GVBD) rate in the different mouse genotypes. An asterisk (*) indicates that the data point is zero. Error bars denote SD. (B to E) Hematoxylin and eosin (H&E) staining of ovaries from immature WT and Erk1/2gc−/− mice treated with hCG for 8 hours (B and C) or 48 hours (D and E). CL: corpus luteum. In (B) and (C), arrows indicate the nuclear configuration of the oocyte nucleus. Scale bars, 31.25 µm. In (D) and (E), arrows indicate nonovulated COCs in preovulatory follicles. Scale bars, 250 µm. (F) Changes in estradiol and progesterone concentrations in serum after hCG treatment of eCG-primed 3-week-old WT and Erk1/2gc−/− females. D23: postnatal day 23; eCG: 23-day-old female mice treated with eCG for 48 hours; hCG D1 and hCG D2: 23-day-old females treated with eCG for 48 hours followed by hCG for 24 hours (D1) or 48 hours (D2).
Specific analyses of oocyte functions showed that oocytes isolated from the COCs of Erk1/2gc−/− mutant mice spontaneously underwent GVBD in culture and progressed to metaphase II stage, as did controls (fig. S3A). By contrast, when spontaneous GVBD was blocked by hypothanxine (HX), the EGF-like factor amphiregulin (AREG) stimulated GVBD in wild-type (WT) but not Erk1/2gc−/− COCs (fig. S3B). In addition, AREG stimulated expansion of COCs isolated from WT, but not the Erk1/2gc−/− mice (fig. S3C). These results indicate that the oocytes that retain ERK1/2 in the Erk1/2gc−/− mice are competent for meiotic maturation, but the cumulus cells lacking ERK1/2 (fig. S1B) fail to respond to EGF-like factors.
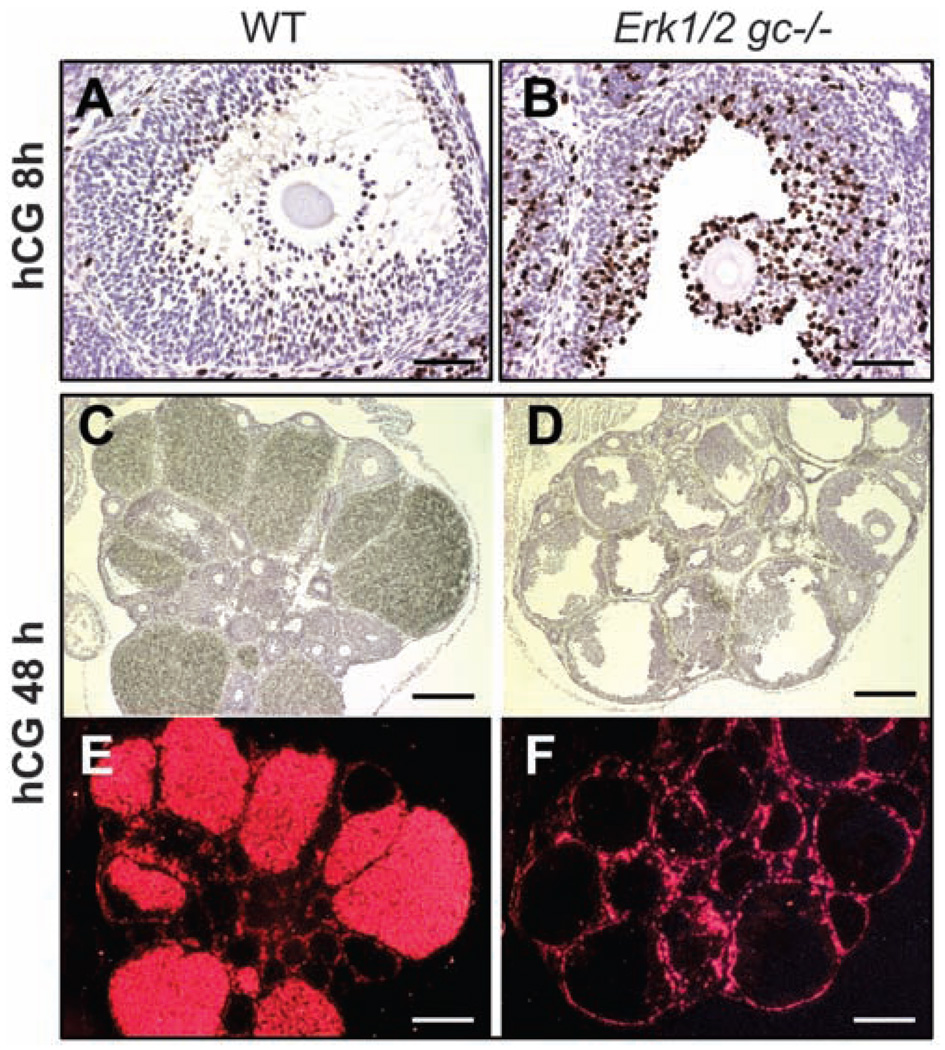
Furthermore, the ability of LH/hCG (human chorionic gonadotropin) to terminate GC proliferation was impaired in the Erk1/2gc−/− mice, as indicated by elevated incorporation of bromodeoxyuridine (BrdU) (Fig. 2, A and B, and fig. S4, A and B) and expression of positive cell cycle–regulatory molecules CCND2, CCNA, and E2F1 and reduced expression of CDKN1B and CDKN1A (fig. S4, C and D) in the mutant ovaries compared to controls. Thus, ERK1/2 control GC fate decisions at this critical stage of their differentiation.
Fig. 2.
ERK1/2 are required for the terminal differentiation of GCs during ovulation. (A and B) BrdU staining of WT and Erk1/2gc−/− ovary sections at 8 hours after hCG treatment. Scale bars, 62.5 µm. (C to F) In situ hybridization shows the expression of Cyp11a1 mRNA in ovaries of WT and Erk1/2gc−/− mice at 48 hours after hCG treatment. Histology of the ovaries is shown by hematoxylin staining (C and D); localization of Cyp11a1 mRNA is shown by dark-field images (E and F). Scale bars, 250 µm.
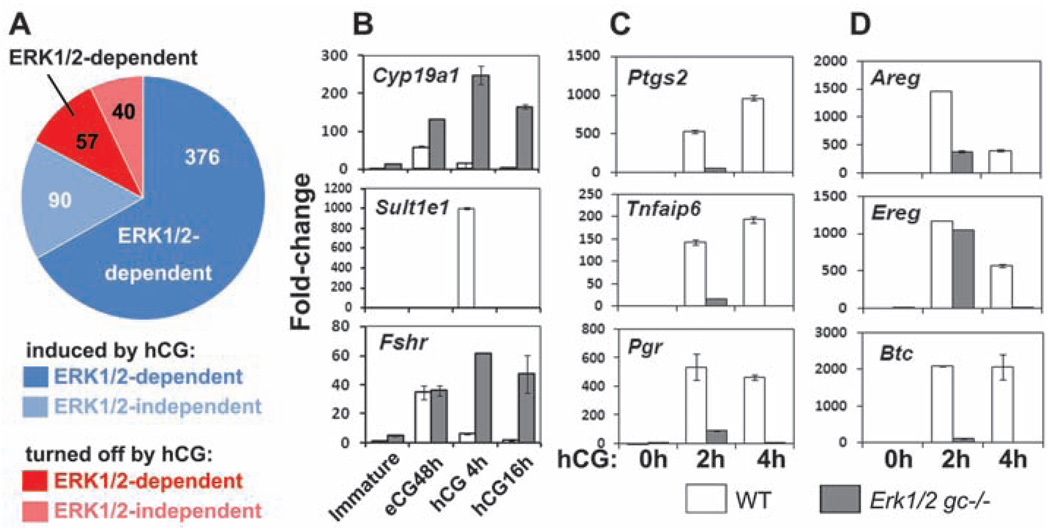
To identify ERK1/2 target genes in preovulatory follicles, we analyzed gene expression profiles using RNA prepared from GCs obtained from WT and Erk1/2gc−/− mice treated with eCG for 48 hours and at 0, 2.5, and 4 hours after hCG injection because the activation of ERK1/2 peaks at 2 hours after hCG treatment (11, 12) and many genes that affect ovulation are maximally expressed by 4 hours (5). Among the 563 highly regulated LH target genes (more than fourfold, hCG at 4 hours versus hCG at 0 hours; 466 with increased and 97 with decreased expression, tables S1 and S2), 77% (376 with increased and 57 with decreased expression) lost their response to LH/hCG in the Erk1/2gc−/− cells (Fig. 3A). Two identified ERK1/2 target genes that regulate estradiol biosynthesis and activity are Cyp19a1 (aromatase), which converts testosterone to estradiol, and Sult1e1, which deactivates estradiol (13). Whereas hCG turns off Cyp19a1 expression in WT mice, Cyp19a1 was markedly increased in the Erk1/2gc−/− cells. By contrast, hCG transiently induced Sult1e1 in WT but not Erk1/2−/− GCs (Fig. 3B and tables S1 and S2), leading to inappropriately high estradiol concentrations (Fig. 1F and fig. S2B) that may adversely affect the ovary and other tissues as well (13). Conversely, two luteinization markers, Star and Cyp11a1, were substantially reduced in the Erk1/2gc−/− ovaries, consistent with the lack of CLs in the mutant mice (Fig. 2, C to F, and fig. S5, A and B).
Fig. 3.
ERK1/2 extensively regulate the expression of FSH/LH-target genes during ovulation. (A) Summary of gene expression profiling data. Genes regulated more than fourfold between 0 and 4 hours after hCG treatment were defined as LH-target genes; those that failed to respond to hCG in the Erk1/2gc−/− cells were defined as ERK1/2-dependent genes. (B to D) qRT-PCR shows the expression of selected FSH/eCG and LH/hCG target genes in GCs of WT and Erk1/2gc−/− mice after hCG treatment. Error bars denote SD.
The expression of many genes associated with COC expansion and ovulation (Ptgs2, Tnfaip6, Has2, Ptx3, and Pgr) was abolished in the Erk1/2gc−/− ovaries, as indicated by the microarray data (table S1), quantitative reverse transcription–polymerase chain reaction (qRT-PCR) (Fig. 3C and fig. S5A), and immunofluorescence (fig. S5D), whereas genes encoding the two EGF-like factors Areg and Ereg, presumed mediators of LH action, were induced rapidly to normal (Ereg) or reduced (Areg) levels compared to controls but these levels were not sustained. By contrast, the induction of betacellulin (Btc) was blocked completely in Erk1/2gc−/− ovaries (Fig. 3D). Thus, the initial induction of Areg and Ereg by LH/hCG is largely independent of ERK1/2 activation. However, the induction of Btc and the secondary maintenance of Areg and Ereg expression is likely dependent on ERK1/2 induction of Ptgs2, leading to prostaglandin E2 (PGE2) production and activation of the EP2 receptor (5).
By contrast, genes in addition to Cyp19a1 that are expressed and/or induced by follicle-stimulating hormone (FSH) and equine chorionic gonadotropin (eCG) in GCs during preovulatory follicle development (Fshr, Lhcgr, and Nr5a2) (fig. S6) and normally down-regulated during the early stages of LH-induced luteinization (14) were expressed at elevated levels in ovaries of the hormone-treated Erk1/2gc−/− mice (Fig. 3B and fig. S5C). Thus, ERK1/2 is required for suppressing the expression of FSH target genes.
Mice null for the transcription factor CCAAT/Enhancer-binding protein–β (Cebpb) are one of a only few knockout mouse models that show an Erk1/2gc−/−-like ovarian phenotype (15). C/EBPβ protein is increased rapidly by hCG in GCs in vivo (fig. S7, A and B), and in cultured cell lines. C/EBPβ is the substrate of ERK1/2 and/or RPS6KA2 (P90RSK) (16), making C/EBPβ a potential mediator of ERK1/2 in GCs. Indeed, when undifferentiated GCs were infected with an adenoviral vector encoding C/EBPβ, C/EBPβ was expressed, phosphorylated in response to AREG at a known ERK1/2 site and by a MEK1/2-dependent mechanism (C/EBPβ-T188; Fig. 4A). Only in C/EBPβ-expressing cells could AREG induce ERK1/2-dependent (U0126 sensitive) expression of target genes (Ptgs2, Tnfaip6, Pgr, and Star) (Fig. 4A and fig. S8A). AREG and C/EBPβ induced the expression of LH/ERK1/2 target genes in Erk1/2gc−/− GCs only when an ERK2 expression vector restored kinase activity (Fig. 4A). The synergistic actions of AREG and C/EBPβ were compromised dramatically by an ERK1/2 site, but not P90RSK site, point mutation (fig. S8A), indicating that C/EBPβ is a direct target of ERK1/2 and both constitute a critical signaling network in preovulatory GCs.
Fig. 4.
C/EBPβ is a key mediator of ERK1/2 activity in preovulatory GCs. (A) Immature WT or Erk1/2gc−/− mice were primed with eCG for 24 hours. GCs were collected and cultured overnight, and some Erk1/2gc−/− GCs were transfected with an ERK2 expression plasmid. The cells were then infected with an adenoviral vector encoding C/EBPβ for 4 hours and further treated with AREG with (+) or without (−) U0126 for another 4 hours. Total RNA or protein was extracted. LH, hCG, and AREG downstream genes were quantified by qRT-PCR. Western blots document AREG-induced phosphorylation of ERK1/2 and C/EBPβ and overexpression of C/EBPβ. Error bars denote SD. (B) H&E staining shows the absence of CLs in the ovary of adult Cebpbgc−/− mice. Follicles are indicated by arrows. Scale bar, 250 µm. (C) The number of ovulated COCs decreased at 16 hours after hCG injection in Cebpbgc−/− mice. (D) Summary. LH, cAMP, and protein kinase A (PKA) induce AREG and EREG, which activate RAS and ERK1/2. Activated ERK1/2 is required to (i) induce and activate CEBPβ and genes essential for oocyte maturation, ovulation, and luteinization; (ii) maintain AREG, EREG, and BTC by inducing Ptgs2/PGE and activation of EP2, cAMP, and PKA; and (iii) silence the FSH-regulated program. LH may also transactivate RAS and ERK1/2 directly and regulate C/EBPβ expression by other pathways. FSH and LH pathways are shown in green and red, respectively.
Because the GC-specific functions of C/EBPβ have not been studied in vivo, Cebpbfl/fl mice (17) were crossed with the Cyp19-Cre mice, generating the Cebpbfl/fl;Cyp19-Cre (Cebpbgc−/−) mouse strain with C/EBPβ depleted in GCs (fig. S7, B to D). As in the Cebpb-null mice (15), the adult Cebpbgc−/− mice were subfertile (fig. S7E). CLs were absent in 70% of the adult Cebpbgc−/− mice (n = 10, Fig. 4B); the number of ovulated COCs was reduced (Fig. 4C); and the expression of Ptgs2, Star, and Cyp11a1 was decreased, consistent with impaired CL formation (fig. S7, F to G). However, hCG-induced phosphorylation of MEK1/2 and ERK1/2 was normal in ovaries of Cebpbgc−/− mice (fig. S7D). Expression of C/EBPβ was decreased but not totally blocked in the Erk1/2gc−/− mice (fig. S8, B and C), indicating that C/EBPβ expression is regulated by additional pathways and that ERK1/2-mediated phosphorylation and activation of C/EBPβ is critical. Thus, C/EBPβ is a downstream effector of ERK1/2 in GCs during ovulation and luteinization. The lower penetrance of ovulation and gene expression defects in Cebpbgc−/− mice, compared with the Erk1/2gc−/− mice, suggests that C/EBPβ is one, but not the only, critical transcription factor regulated by ERK1/2 in vivo.
The Erk1/2gc−/− mouse model illustrates that disruption of Erk1/2 in GCs in vivo completely derails the ability of LH to induce genes controlling ovulation, COC expansion, oocyte maturation, and luteinization without altering genes that regulate normal follicular development to the preovulatory stage (summarized in Fig. 4D). As a consequence of ERK1/2 depletion in GCs, the FSH program is extended rather than being abruptly terminated by LH/hCG. Thus, our results demonstrate in animals that the critical roles of ERK1/2 in GCs are highly selective and cell context–specific, confirming findings of in vitro studies (6, 18). Moreover, ERK1/2 are activated for a relatively short period of time (from 0.5 to 2 hours) in GCs of the preovulatory follicles exposed to LH/hCG (11, 12, 19), and this brief window of activation is necessary and sufficient to reprogram preovulatory GCs to cease dividing and terminally differentiate.
The effect of ERK1/2 activation in GCs of preovulatory follicles but not in follicles at earlier stages of growth indicates that activation of these kinases is controlled tightly by specific mechanisms. Indeed, inappropriate activation of ERK1/2 in GCs of small growing follicles might disrupt normal follicular development because mice in which ovarian GCs express a constitutively active K-RAS mutant suffer impaired follicle development and premature ovarian failure (11). Thus, further understanding of the molecular mechanisms by which ERK1/2 regulate ovarian cell functions will help unravel some of the causes of ovarian pathologies and cancer, as well as lead to therapies for female infertility.
Supplementary Material
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/324/5929/938/DC1
Materials and Methods
Figs. S1 to S8
Tables S1 and S2
References
References and Notes
- 1.Hunzicker-Dunn M, Maizels ET. Cell. Signal. 2006;18:1351. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Science. 2002;296:2178. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 3.Park JY, et al. Science. 2004;303:682. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh M, et al. Mol. Cell. Biol. 2007;27:1914. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Mol. Endocrinol. 2006;20:1352. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 6.Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Endocrinology. 2002;143:2221. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, et al. Chem. Rev. 2001;101:2449. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 8.Pages G, et al. Science. 1999;286:1374. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 9.Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Biochimie. 2006;88:1091. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. Immunity. 2005;23:431. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Fan HY, et al. Development. 2008;135:2127. doi: 10.1242/dev.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panigone S, Hsieh M, Fu M, Persani L, Conti M. Mol. Endocrinol. 2008;22:924. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershon E, Hourvitz A, Reikhav S, Maman E, Dekel N. FASEB J. 2007;21:1893. doi: 10.1096/fj.06-7688com. [DOI] [PubMed] [Google Scholar]
- 14.Richards JS. Endocrinology. 2001;142:2184. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- 15.Sterneck E, Tessarollo L, Johnson PF. Genes Dev. 1997;11:2153. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bück M, Poli V, vander Geer P, Chojkier M, Hunter T. Mol. Cell. 1999;4:1087. doi: 10.1016/s1097-2765(00)80237-3. [DOI] [PubMed] [Google Scholar]
- 17.Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC. Oncogene. 2006;25:1272. doi: 10.1038/sj.onc.1209144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Endocrinology. 2005;146:1236. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- 19.Maizels ET, Cottom J, Jones JC, Hunzicker-Dunn M. Endocrinology. 1998;139:3353. doi: 10.1210/endo.139.7.6188. [DOI] [PubMed] [Google Scholar]
- 20.We thank J. Pouyssegur and J. Shao for providing Erk1−/− mice and adenoviral vector of C/EBPβ, respectively. This work was supported by NIH grants NIH-HD16229, NIH-HD07495, Project II (J.S.R.), Grant-in-Aid for Scientific Research No. 18688016 from the Japan Society for the Promotion of Science (M.S.), Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research (P.F.J. and E.S.), and NIH Postdoctoral Training Grant NIH-HD07165 (H.-Y.F.). The Gene Expression Omnibus accession number for microarray data is GSE15135.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.