Abstract
Unlike individuals with mild stroke, individuals with severe stroke are constrained to stereotypical movement patterns attributed to abnormal coupling of shoulder abductors with elbow flexors, and shoulder adductors with elbow extensors. Whether abnormal muscle coactivation and associated joint torque patterns can be changed in this population is important to determine given that it bears on the development of effective rehabilitation interventions. Eight subjects participated in a protocol that was designed to reduce abnormal elbow/shoulder joint torque coupling by training them to generate combinations of isometric elbow and shoulder joint torques away from the constraining patterns. After training, subjects demonstrated a significant reduction in abnormal torque coupling and a subsequent significant increase in ability to generate torque patterns away from the abnormal pattern. We suggest the rapid time-course of these changes reflects a residual capacity of the central nervous system to adapt to a novel behavioral training environment.
Keywords: arm, rehabilitation, strength, stroke, synergy
Motor recovery following stroke is characterized by a progressive reduction in sensorimotor impairment.6,31 However, weakness, changes in stretch reflex excitability, and constraints on available joint torque patterns due to abnormal muscle synergies persist in severely affected individuals.1,3,10,12 An important question is whether severe stroke results in a permanent reduction in available muscle coactivation and associated joint torque patterns. Modifiability of these impairments would support continued effort and likelihood of success in developing effective rehabilitation interventions. To answer this question, a protocol was devised to alter and preferably reduce abnormal joint torque coupling by training stroke survivors to generate joint torque patterns away from the abnormal patterns.
We previously demonstrated12 that, during shoulder abduction, individuals with moderate to severe chronic stroke spontaneously generate near-maximal elbow-flexion torque, with lesser torques in shoulder extension and external rotation. This abnormal torque-coupling pattern reflected coactivation of shoulder abductors (deltoid) with elbow flexors (biceps brachii, brachialis, and brachioradialis).12 Subsequent studies have described the detrimental impact of joint torque coupling on volitional isometric3 and dynamic1,2 tasks in the hemiparetic upper limb. All subjects exhibited a reduced capacity to generate isometric elbow-extension torque concurrently with shoulder abduction, with the most deleterious effects measured at maximal abduction levels and in subjects with the greatest overall impairment.13
In this study, we tested the hypothesis that abnormal elbow/shoulder joint torque coupling is modifiable in severely affected individuals at >1 year after stroke. It was postulated that alterations and preferably a reduction in abnormal torque coupling would be evident in a reduced amount of spontaneous isometric elbow flexion measured during maximal isometric shoulder abduction and that, after the 8-week intervention, subjects would demonstrate increases in strength in single-DOF (degree-of-freedom) torque directions and in combined multi-DOF tasks away from the abnormal torque pattern.
MATERIALS AND METHODS
Subjects
Six men and 2 women, ages 41– 80 years, whose stroke had occurred 14 – 66 months earlier, participated in the study. All subjects were screened for the study by the primary investigator and granted medical consent by their physician. Exclusion criteria were difficulty with sitting for long durations, recent changes in the medical management of hypertension, any acute or chronic painful condition in the upper limbs or spine, and greater than minimal sensory loss in the affected upper limb. The arm-motor portion of the Fugl–Meyer Motor Assessment14 was administered as an initial screening measure to qualitatively determine the presence and extent of flexion synergy and categorize the level of impairment severity. The inclusion criteria for the research study required severe to moderate impairment equivalent to scoring within the range of 15– 40 out of a maximum of 66 points. Our subjects scored from 18 to 37, fitting a normal distribution. All subjects were able to support the upper limb against gravity while generating concurrent active elbow extension. Each subject’s passive range of motion of the affected upper limb was measured using methods described elsewhere.26 Passive range of motion to at least 90° of shoulder flexion, abduction, and neutral internal/external rotation was required to participate in the study. Overpressure at the end of the range of motion was used as a medical screening measure to verify the absence of an inflammatory condition at the shoulder, elbow, wrist, and fingers.16 After screening, the subjects provided written consent to participate in the study, which was approved by our institutional review board.
Experimental Arrangement
Subjects were seated in a Biodex chair with shoulder and waist strapping to restrain trunk and shoulder girdle movement during testing. Two different arm configurations were studied involving elbow angles of 70° and 90° (straight arm = 180°). For both configurations the forearm and hand rested in a horizontal plane on a support plate with the shoulder in 75° of abduction and the distal point of the third digit aligned to the midline of the torso. Pre- and posttraining measurements of maximum voluntary torques (MVTs) were performed for both limb configurations, and the 70° configuration was used during training. The forearm, wrist, and hand were fixed to a 6-DOF load cell (Model 45E15A; JR3, Inc., Woodland, CA) using fiberglass casting and a Delrin ring mounted at the wrist (Fig. 1). Orthogonal forces and moments measured by the load cell were filtered and converted on-line to torques at the elbow and shoulder via methods described elsewhere.3 Real-time visual feedback was provided to the subject, via computer monitor, of the torque produced at the shoulder or elbow joint.
FIGURE 1.
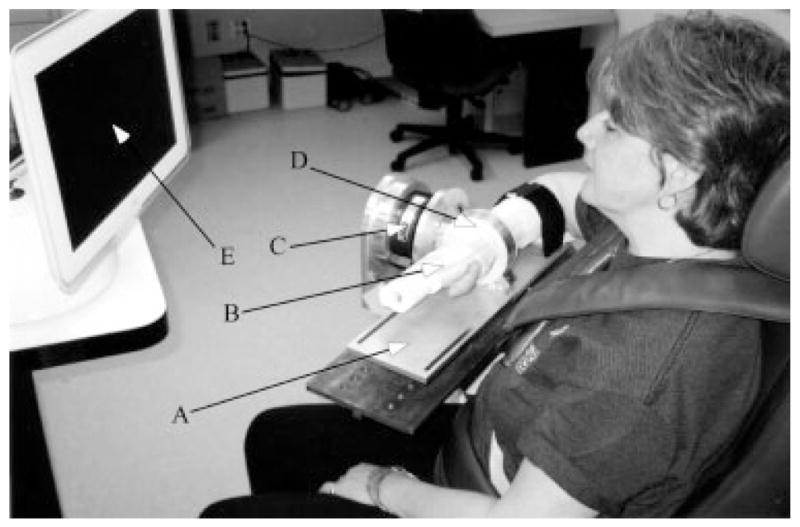
Image of the experimental arrangement: A—forearm interface plate mounted directly to load cell; B—fiberglass cast; C— 6-DOF load cell; D—Delrin cast interface ring; E—video feedback monitor.
During pre- and posttesting of MVTs, surface electromyography (EMG) signals were recorded for the brachioradialis; biceps brachii; lateral and long heads of triceps brachii; anterior, intermediate, and posterior deltoid; and vertical fibers of pectoralis major. Correct electrode placements were verified by examination of EMG activity. Active differential electrodes (16-channel Bagnoli EMG System; Delsys, Boston, MA) with a 1-cm interelectrode distance were used to record surface EMG from the upper-limb muscles. The Delsys EMG system also provided preamplification (gain: 1000) and single-pole high-pass filtering (cutoff frequency: 6 Hz). All EMG signals were filtered at 500 Hz (8-pole Butterworth; Model 9016, Frequency Devices, Haverhill, MA) to prevent aliasing and amplified in a second stage prior to data collection. The force/torque and EMG signals were collected at a sampling rate of 1000 Hz via an analog/digital converter and stored on a computer for future analysis.
Maximum Voluntary Torque Protocol
MVTs were measured at the shoulder and elbow before and after the 8-week experimental intervention and served as the primary outcome for measuring changes in abnormal joint torque coupling. Three random blocks consisting of shoulder abduction/adduction, flexion/extension, and elbow flexion/extension were completed for both the 70° and 90° limb configurations, using methods reported previously.10 Joint torques were concurrently measured at both the shoulder and elbow while the subject attempted to maximize the torque in the primary direction, which was shown in real time on a computer monitor. The best representation of MVT was ensured by mandating that the three largest trials per torque direction were within 10% of magnitude from each other and that the last trial recorded was not the greatest.
Multi-DOF Isometric Protocol
The training protocol spanned 8 weeks, during which subjects attended sessions three times per week for approximately 1.5 hours per session. Within each training session, subjects performed three sets of each of three multi-DOF isometric tasks, ordered randomly and containing 12 repetitions per set. Each repetition was 5 seconds in duration and sampled at 1000 Hz for all DOFs. The training position (70° elbow angle) was identical to the limb configuration used during pre-and posttesting for MVTs. The three tasks were designed to train the subject to create joint torque combinations with the affected upper limb in patterns away from the dominant abnormal torque-coupling pattern (shoulder abduction/shoulder extension/elbow flexion). The three training tasks were as follows. In task 1, subjects attempted to maintain a fixed level of shoulder abduction while maximizing elbow extension and shoulder flexion; for task 2, subjects attempted to maintain a fixed level of shoulder abduction while maximizing elbow extension and blinded to the shoulder flexion/extension DOF; and for task 3 they attempted to maximize shoulder flexion and elbow extension while blinded to the shoulder abduction/adduction DOF.
During each task the subject viewed a computer monitor that displayed, in real time, the magnitude of isometric joint torques that they were generating for the specific task (Fig. 2). Shoulder abduction was represented by counterclockwise rotation of the needle within the circular cursor, whereas vertical and horizontal movement of the circular cursor represented shoulder flexion and elbow extension, respectively. A pie-piece shape within the cursor represented the target window for shoulder abduction torque; targeted levels of elbow extension and shoulder flexion were represented by a square (Fig. 2).
FIGURE 2.
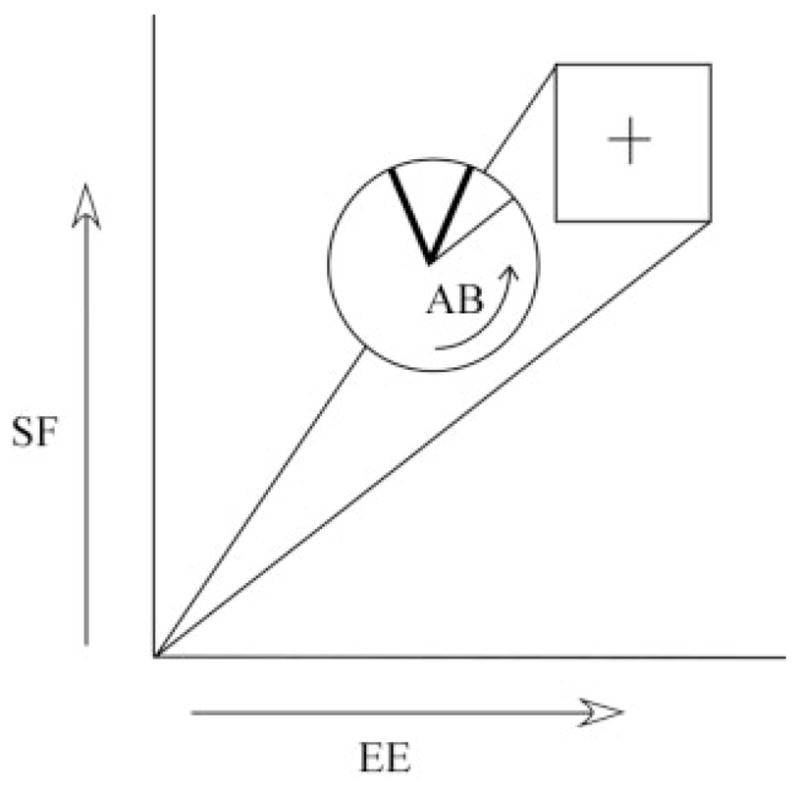
Isometric shoulder flexion (SF), shoulder abduction (AB), and elbow extension (EE) torque indicated by arrows superimposed on the visual feedback display viewed by the right-handed subject.
The subject was required to develop isometric shoulder and elbow torques that would move the cursor toward and into the target square while the needle was maintained in the pie-piece portion of the cursor. Constant verbal encouragement to maintain the needle in place was given during tasks 1 and 2. Task 3 did not include shoulder abduction and task 2 did not include shoulder flexion. In these tasks, the feedback was modified so that the excluded components would not be displayed by the visual feedback.
The priority of the intervention was to enhance the capacity to generate elbow extension and shoulder flexion torques while lifting the upper limb (abduction component) against gravity. Past work has demonstrated that many moderately to severely impaired subjects cannot generate significant elbow extension during abduction against gravity.3 Accordingly, at the beginning of training the pie-piece shape within the cursor was set to represent 50 ± 5% of the abduction torque required to support the arm against gravity, as calculated from anthropometric measurements of the arm.34 The abduction level was modified in increments corresponding to 25% of limb weight over the course of training as performance of tasks 1 and 2 improved, with a maximum abduction equivalent to 200% limb weight to avoid overtraining and to maintain the functional relevance of the reaching-based exercise. On every fourth training session, MVTs for abduction, shoulder flexion, and elbow extension were measured. Visual feedback parameters were maintained at 50% MVT for shoulder flexion and elbow extension throughout the training. Although these parameters were submaximal, volitional generation of a combination of torques in two or more directions resulted in target levels that were always just out of reach for each subject. This insured adequate visual feedback to motivate subjects to maximize their performance.
Finally, subjects were monitored for signs of overuse injury at the shoulder and elbow joints due to prior non-use of the affected limb and to the intensity of the training regimen. They were also instructed to maintain their daily routines and avoid starting any new exercise programs targeting the affected upper limb.
Data Analysis
Analysis of Isometric Single-DOF Data
Pre- and posttraining MVTs for each of the six torque directions were determined using custom software written within the Matlab environment (The MathWorks, Natick, MA). For each 5-s trial and in each torque direction, the peak torque was determined by identifying the 250-ms window with the largest average torque magnitude. MVT for each direction was taken as the maximum peak torque across all trials. The elbow-flexion torque generated concurrently during the 250-ms window of peak abduction torque was used to quantify the magnitude of abnormal joint torque coupling10 (described previously as flexion synergy) before and after the training protocol.
The percentage change in MVT from pre- to posttraining was calculated for each subject for all torque directions and both limb configurations. The normality of the data was confirmed using the Shapiro–Wilks test. Two-tailed paired t-tests were calculated for all torque directions to determine whether the percent changes in torque were different from zero. Wilcoxon signed rank tests were used when data were not normally distributed.
A repeated-measures single-factor analysis of variance was completed to test the effect of session on the secondary elbow-flexion torque generated during abduction MVTs for each limb configuration. Elbow-flexion joint torque was normalized to the MVT occurring within the testing session for comparison of abnormal torque coupling between subjects.
EMG Analysis
Individual muscle activation during abduction MVT was chosen to identify abnormal muscle coactivation patterns before and after training. EMG signals were rectified and averaged during a 250-ms window that was temporally offset by 30 ms to the corresponding peak torque time window to deal with the electromechanical delay of the human skeletal muscle.4,8,20 Averaged EMG was normalized by the maximum EMG measured within the same testing session to facilitate comparison between sessions. All EMG data were confirmed to be normally distributed by the Shapiro–Wilks test. Group mean EMG and standard error were calculated for each muscle (n = 8) during each session and at each limb configuration. A repeated-measures single-factor analysis of variance was completed to test the effect of session on muscle activation for each muscle during abduction MVT for both limb configurations.
Analysis of Isometric Multi-DOF Protocol Data
Torque data were obtained for all repetitions of each task on the first and last (24th) session of the 8-week training period to measure the voluntary capacity of each subject in generating multi-DOF isometric torque patterns away from the abnormal torque-coupling pattern. Comparison of changes in voluntary torque patterns was used to identify whether the effects of abnormal torque coupling were amenable to training. First and last training-session torque data were averaged across the entire 5 s of each repetition (3 sets of 12 repetitions = 36 repetitions) for all relevant torque directions and normalized to pre-and posttraining MVTs, respectively, to facilitate comparison of torque patterns without the influence of individual torque direction-strengthening effects. Normalized joint torque generated during each repetition was averaged across all subjects on the first and last training session. Standard errors were calculated across subject means for each of the torques generated per session. A repeated-measures single-factor analysis of variance was used to test the effect of session on joint torque for each torque direction in each task. All statistical analyses were performed using the 0.05 level of significance.
RESULTS
Generation of Torque Patterns Away from the Abnormal Patterns
The ability of each subject to generate torque patterns away from the abnormal shoulder-abduction/extension/elbow-flexion pattern was assessed on the first and last training sessions to determine whether a positive training effect had occurred. The patterns assessed were: abduction/shoulder flexion/elbow extension (task 1); abduction/elbow extension (task 2); and shoulder flexion/elbow extension (task 3). Mean normalized joint torques and standard errors for the first and last training sessions of each task are presented in Figure 3A–C. Joint torques in the first and last session for shoulder flexion and elbow extension were normalized to the respective MVTs from pre- and posttesting, whereas abduction was normalized to the torque required to support the limb against gravity. The reason for normalizing shoulder abduction to limb weight was to emphasize the detrimental effect of lifting the limb against gravity on generating concurrent shoulder flexion and elbow extension (away from the constrained torque pattern). Normalizing shoulder flexion and elbow extension in the first session to pretraining MVT and last session to posttraining MVT was done to remove any potential single-DOF strengthening effect that would otherwise confound the comparison of pre- and posttraining torque patterns.
FIGURE 3.
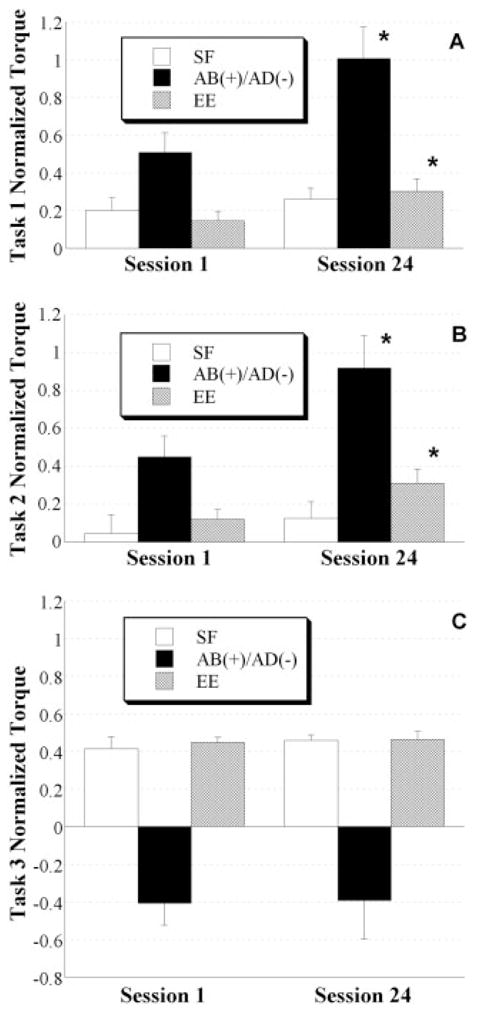
Mean normalized joint torque patterns and standard errors on the first (1) and last (24) training sessions are illustrated for each isometric multi-DOF task. In task 1 (A), the subject attempted to maximize shoulder flexion (SF), shoulder abduction (AB), and elbow extension (EE) torque directions. In task 2 (B), the subject attempted to maximize AB and EE and was blinded to the shoulder flexion/extension DOF. In task 3 (C), the subject attempted to maximize SF and EE and was blinded to the shoulder abduction/adduction (AB/AD) DOF. Significant pre- vs. post-training improvements are indicated, supporting the ability of subjects to generate greater concurrent joint torques away from the abnormal pattern. Asterisk denotes difference between sessions at the 0.05 level of significance.
Subjects were able to generate shoulder-flexion/elbow-extension torques while abducting at the shoulder during tasks 1 and 2 in both the first and last training sessions. However, a positive training effect was evident in the increased magnitude of the torque patterns, specifically elbow extension at limb-weight abduction levels, measured in the last session. For task 1, both abduction and elbow extension increased from 51 ± 28% (mean ± SD) to 101 ± 44% of limb weight and 15 ± 13% to 30 ± 17% of maximum elbow extension, respectively. For task 2, both abduction and elbow extension increased from 45 ± 29% to 92 ± 45% of limb weight and 12 ± 14% to 31 ± 20% of maximum elbow extension, respectively. For task 3, no training effect was found for any of the torque directions.
MVTs before and after the Multi-DOF Protocol
Maximum voluntary torques were measured in all six torque directions before and after training to detect changes in maximum torque. These torques were measured in both the training limb configuration (70° elbow angle) and a second limb configuration (90° elbow angle) to evaluate the specificity of training effects. The percent changes in absolute MVTs for all torque directions from pre- to posttraining are presented in Figure 4A and B for both limb configurations. Two-tailed t-tests were used to determine whether there was a significant difference between the percent improvement and zero for each torque direction and limb configuration. The Shapiro–Wilks test indicated that the data deviated from a normal distribution for shoulder and elbow extension in the 70° limb configuration and elbow extension in the 90° limb configuration. For these cases, the Wilcoxon signed rank test was used to test for a significant difference.
FIGURE 4.
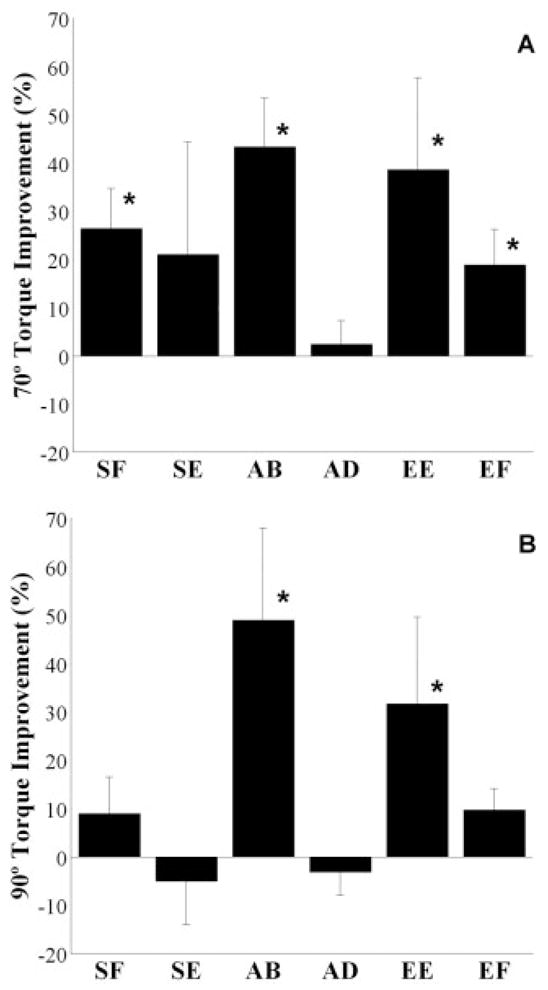
Mean percent improvement and standard error in single-DOF torque directions of shoulder flexion (SF), extension (SE), abduction (AB), adduction (AD), and elbow extension (EE) and flexion (EF) in both the 70° (A) and 90° limb (B) configurations. Asterisk denotes significant difference from zero.
There was a positive training effect measured for the torque directions composing the training tasks (abduction, shoulder flexion, and elbow extension). On a group basis, shoulder flexion increased after training by 26 ± 8%, corresponding to 8.3 ± 9.1 Nm, at the 70° configuration, but was not significantly different at the 90° configuration. Shoulder abduction increased 43 ± 10% or 8.0 ± 5.4 Nm at the 70° and 49 ± 19% or 6.0 ± 4.3 Nm at the 90° configurations. Elbow extension increased 39 ± 19% or 8.0 ± 6.9 Nm at the 70° and 32 ± 18% or 6.0 ± 5.3 Nm at the 90° configurations.
In general, differences between pre- and post-training MVTs in untrained torque directions were not significant. Adduction and shoulder extension in the 70° and 90° configurations and elbow flexion in the 90° configuration did not change (Fig. 4). However, elbow flexion in the 70° configuration increased by 19 ± 7% or 4.4 ± 1.7 Nm.
Abnormal Abduction/Elbow-Flexion Torque Coupling before and after Training
Spontaneous coupling of elbow flexion during abduction MVT was measured before and after training to elucidate mechanisms supporting changes measured in the ability of subjects to generate torque patterns away from the constrained pattern. Secondary or coupled elbow flexion was normalized to elbow-flexion MVT that was measured within the same session. This normalization procedure eliminated the effect of strengthening that may have occurred in elbow flexion, allowing for an unbiased comparison of torque coupling between pre- and posttraining (Fig. 5). Spontaneous elbow-flexion coupling was reduced after training in both the 70° and 90° limb configurations from 70 ± 17% to 45 ± 28% and 83 ± 13% to 70 ± 16%, respectively. Specifically, subject 3 demonstrated a torque direction reversal or coupling with elbow extension in the 70° configuration after training. On a group basis, abnormal torque coupling was greater in the 90° configuration.
FIGURE 5.
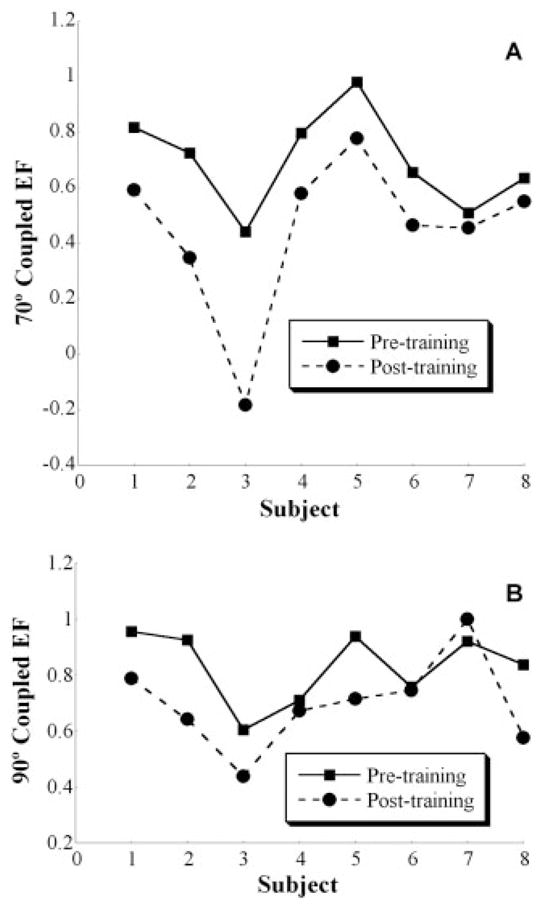
Individual-subject (subjects 1– 8) pre- and posttraining spontaneous elbow-flexion (EF) torque (normalized to maximum within session) during maximal shoulder abduction (AB) at 70° (A) and 90° (B) limb configurations. The graphs illustrate the overall reduction in abnormal joint torque coupling in both limb positions after training.
We compared elbow flexion and extension MVTs at both limb positions for both pre- and posttraining sessions to rule out the possibility of agonist/antagonist strength imbalances corrupting the measurement of abnormal abduction/elbow-flexion torque coupling.24 There was no difference between elbow-flexion and -extension MVTs (mean ± SEM) at the pretraining session for both the 70° (elbow flexion, 24.2 ± 3.3 Nm; extension, 26.1 ± 5.7 Nm) and 90° (elbow flexion, 30.5 ± 4.9 Nm; extension, 22.8 ± 4.8 Nm) configurations. Similarly, there was no difference between elbow flexion and extension at the posttraining session for both the 70° (elbow flexion, 28.6 ± 4.1 Nm; extension, 34.1 ± 6.4 Nm) and 90° (elbow flexion, 32.7 ± 4.6 Nm; extension, 28.7 ± 5.4 Nm) limb configurations. The lack of difference between elbow-flexion and -extension MVT eliminated the possibility that agonist/antagonist strength imbalances contributed to the abnormal torque coupling.
Mean and standard errors of normalized EMG during abduction MVT were calculated. There was no difference between sessions at both limb positions for any muscle except brachioradialis, which decreased from 53 ± 14% to 40 ± 11% in the 70° configuration.
DISCUSSION
This study has demonstrated the modifiability of spontaneous or abnormal coupling of elbow flexion during the generation of maximal shoulder abduction in severely impaired individuals at >1 year after a stroke. There was a strengthening effect in the single-DOF torque directions used within the training tasks and a concomitant improvement in the generation of multi-DOF joint torque combinations away from the constrained patterns. These results may be attributed to neural adaptation of the motor system.
Effects of Single-DOF and Multi-DOF Strengthening
The current consensus is that the response to resistance training in adults is specific to both movement patterns and force–velocity characteristics,21 and that stroke survivors show a reduction of musculo-skeletal impairment after progressive resistance training.25 We postulated that a multi-DOF progressive resistance protocol would increase MVTs for shoulder and elbow muscle groups and enhance the ability of subjects to generate multi-DOF joint torque patterns away from constraining poststroke patterns. Our subjects demonstrated an increase in MVTs after the multi-DOF training protocol (Fig. 4). On a group basis, significant increases were seen only in the torque directions composing the multi-DOF tasks, with the exception of a small increase in elbow flexion in the 70° limb configuration. Although the lack of significant torque increases for nontrained shoulder directions (adduction and extension) was expected, we observed considerable variability across subjects for the shoulder extension torque direction. The large variability in percent improvement of shoulder extension may reflect the greater difficulty reported by subjects for this than other torque directions.
These improvements are consistent with previous reports of a positive strengthening effect of progressive strength training in stroke survivors.25 Furthermore, our subjects also improved in strength at a joint angle greater than the training angle, similar to the reported strengthening effect of isometric training in the general adult population.19 Kitai and Sale found an isometric strengthening effect at the training joint angle and at ±5°. Increases in strength at more distant joint angles were less. Similarly, our stroke subjects demonstrated strength increases away from the training joint angle.
Our subjects substantially improved their ability to generate multi-DOF patterns away from the constraining flexion pattern despite a short total training duration. Short-term strengthening studies in adults have shown that the most profound strengthening occurs during the first 4 – 8 weeks17 of training, with “untrained” individuals demonstrating the largest gains.21 Furthermore, early and rapid strength increases due to resistance training are likely due to neural adaptations as opposed to increases in muscle cross-sectional area, regardless of age and gender.27,28 Among middle-aged and elderly subjects, such as those we studied, increases in strength, even over longer durations of resistance training, have also been attributed to neural adaptation rather than muscle hypertrophy.15 Results for our subjects were congruent in the rapid time-course of response to progressive resistance training. The congruency between both populations may relate to a similar underlying neural mechanism being responsible for short-term strength changes.
The specific multi-DOF gains by our subjects were for the two tasks that included shoulder abduction, identifying this torque direction as a potential key component of the training protocol. In the third task, subjects were not given feedback of the torque generated in the abduction/adduction DOF, and they tended to generate a large amount of adduction. That is, subjects generated torques within the abnormal pattern of shoulder adduction with shoulder flexion and elbow extension.10 The inclusion of task 3 may have been counterproductive due to its facilitation of a second abnormal torque pattern.
Reduction of Abnormal Torque Coupling
A reduction in abnormal torque coupling may explain the improvement of all subjects in generating torque patterns away from the constraining abduction/elbow-flexion pattern. We measured a reduction in spontaneous elbow-flexion coupling during maximal descending drive to the spinal cord (abduction MVT) when the greatest joint torque coupling was expected.10 Our results suggest that couplings between shoulder/elbow muscles and associated joint torques are not hard-wired in severely impaired individuals at >1 year after stroke and can be modified by a multi-DOF progressive resistance protocol.
Relative Weakness and Neural Constraint
Strength imbalances about the elbow joint, specifically relative weakness of the elbow extensors24 or flexors,9 may contribute to the measurement of abnormal elbow-flexion coupling during maximum shoulder abduction after stroke. We compared absolute elbow-flexion and -extension joint torques across subjects in both limb configurations and during both testing sessions and found no difference between them. Therefore, our measurement of abduction/elbow-flexion coupling reflects a true neural constraint in available muscle coactivation and associated joint torque patterns.
The positive response of the elderly population to resistance training has been attributed to a reduction in muscle cocontraction about a single joint.15 There was cocontraction at the shoulder (primary DOF agonists: anterior, intermediate, and posterior deltoid; antagonist: pectoralis major) and the elbow (secondary DOF agonists: brachioradialis and biceps brachii; antagonists: lateral and long head of triceps brachii) during abduction MVTs. However, a symmetrical change in cocontraction at the elbow in both limb positions, defined as a change in the ratio of activation of elbow flexors and extensors, was not evident.
A training-induced change in the underlying muscle coactivation pattern of shoulder abductors with elbow flexors may explain the subsequent reduction of abnormal torque coupling and represent a neural adaptation after training. Brachioradialis EMG was less in the 70° limb configuration, suggesting a training-induced alteration in muscle coactivation patterns. The reduction in elbow-flexion torque coupling in both limb configurations may also be attributed to the reduction in coactivation of another elbow flexor that was not measured. The brachialis muscle is known to contribute heavily to abnormal elbow-flexion coupling,12 but could not be reliably recorded with surface electrodes due to its anatomical position. Reduction in brachialis coactivation is a likely possibility considering its synergistic relationship with the brachioradialis muscle.7
Asymmetrical changes in MVT about the elbow may be related to the reduction of elbow-flexion torque coupling in the 90° configuration. Elbow-extension MVTs increased to a greater degree than elbow-flexion MVTs in the 90° configuration. Without monitoring the brachialis, it cannot be ruled out that the net reduction in elbow-flexion torque coupling during abduction MVTs was partially due to increased extensor muscle strength. However, the consistency in EMG and torque data for the 70° training configuration supports the explanation of an altered muscle coactivation pattern and a position specificity of the training response.
Neural Mechanism Underlying Reduction in Abnormal Shoulder-Abduction/Elbow-Flexion Coupling
Neuroimaging data were not collected, but the observed reduction of joint torque coupling may be related to training-induced reorganization18,22 of the motor cortex and spinal cord. A decrease in overlapping of cortical areas responsible for joint motion33 or adaptive changes in segmental reflex function11 may have contributed to the observed reductions in abnormal joint torque coupling. Currently, our laboratory is pursuing neuroimaging and neuropharmacological experiments to investigate the contributions of these potential mechanisms.
Clinical Implications
Joint torque coupling may be a result of postlesional reorganization30 and a potential target for therapeutic intervention. The present study was not designed to test a therapeutic intervention but instead sought to demonstrate the mutability of joint torque coupling. The isometric nature of the experimental protocol eliminated the influence of spasticity and directly targeted the abnormal torque coupling impairment. Our results suggest that individuals with severe stroke may be amenable to future therapeutic interventions. Dynamic movement protocols are currently being undertaken in our laboratory that incorporate a similar framework of reducing abnormal torque coupling and are expected to show a positive therapeutic effect or carryover into activities of daily living. The therapeutic benefit of physical intervention has not been well documented in severely impaired stroke survivors. An individual with severe impairment failed to show improvement after an intensive physical intervention5 that was beneficial in individuals with less impairment and attributed to training-induced cortical reorganization.29,32 Modifiability of muscle activation patterns has been demonstrated,23 but no underlying mechanisms for these changes could be distilled because of the complexity of the intervention employed by the investigators. Interventions that encourage patients to generate appropriate joint torque patterns are likely to be successful in enhancing functional reaching recovery. Such interventions will more directly target the underlying neural mechanisms responsible for the torque-coupling impairment.
Acknowledgments
This work was supported by a National Institute of Disability and Rehabilitation Research Field Initiated Research Grant (H133G980063) and, in part, by a National Institutes of Health RO1 Grant (HD39343).
Abbreviations
- DOF
degree of freedom
- EMG
electromyography
- MVT
maximum voluntary torque
References
- 1.Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- 2.Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- 3.Beer RF, Gven JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 1999;80:766–772. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- 4.Bober T, Kornecki S, Lehr RP, Jr, Zawadzki J. Biomechanical analysis of human arm stabilization during force production. J Biomech. 1982;15:825–830. doi: 10.1016/0021-9290(82)90047-1. [DOI] [PubMed] [Google Scholar]
- 5.Bonifer N, Anderson KM. Application of constraint-induced movement therapy for an individual with severe chronic upper-extremity hemiplegia. Phys Ther. 2003;83:384–398. [PubMed] [Google Scholar]
- 6.Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. New York: Harper & Row; 1970. p. 192. [Google Scholar]
- 7.Buchanan TS, Rovai GP, Rymer WZ. Strategies for muscle activation during isometric torque generation at the human elbow. J Neurophysiol. 1989;62:1201–1212. doi: 10.1152/jn.1989.62.6.1201. [DOI] [PubMed] [Google Scholar]
- 8.Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- 9.Colebatch JG, Gandevia SC, Spira PJ. Voluntary muscle strength in hemiparesis: distribution of weakness at the elbow. J Neurol Neurosurg Psychiatry. 1986;49:1019–1024. doi: 10.1136/jnnp.49.9.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Dewald JP, Beer RF, Given JD, McGuire JR, Rymer WZ. Reorganization of flexion reflexes in the upper extremity of hemiparetic subjects. Muscle Nerve. 1999;22:1209–1221. doi: 10.1002/(sici)1097-4598(199909)22:9<1209::aid-mus7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 13.Dewald JP, Sheshadri V, Dawson ML, Beer RF. Upper-limb discoordination in hemiparetic stroke: implications for neurorehabilitation. Top Stroke Rehabil. 2001;8:1–12. doi: 10.1310/WA7K-NGDF-NHKK-JAGD. [DOI] [PubMed] [Google Scholar]
- 14.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 15.Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Malkia E, et al. Changes in agonist–antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol. 1998;84:1341–1349. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- 16.Hertling D, Kessler RM. Management of common musculo-skeletal disorders: physical therapy principles and methods. Philadelphia: Lippincott; 1996. p. 795. [Google Scholar]
- 17.Hickson RC, Hidaka K, Foster C. Skeletal muscle fiber type, resistance training, and strength-related performance. Med Sci Sports Exerc. 1994;26:593–598. [PubMed] [Google Scholar]
- 18.Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Plastic changes of motor network after constraint-induced movement therapy. Yonsei Med J. 2004;45:241–246. doi: 10.3349/ymj.2004.45.2.241. [DOI] [PubMed] [Google Scholar]
- 19.Kitai TA, Sale DG. Specificity of joint angle in isometric training. Eur J Appl Physiol Occup Physiol. 1989;58:744–748. doi: 10.1007/BF00637386. [DOI] [PubMed] [Google Scholar]
- 20.Kornecki S, Zschorlich V. The nature of the stabilizing functions of skeletal muscles. J Biomech. 1994;27:215–225. doi: 10.1016/0021-9290(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 21.Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 23.Lum PS, Burgar CG, Shor PC. Evidence for improved muscle activation patterns after retraining of reaching movements with the MIME robotic system in subjects with post-stroke hemiparesis. IEEE Trans Neural Syst Rehabil Eng. 2004;12:186–194. doi: 10.1109/TNSRE.2004.827225. [DOI] [PubMed] [Google Scholar]
- 24.Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve. 2003;27:211–221. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- 25.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil. 2004;18:27–39. doi: 10.1191/0269215504cr699oa. [DOI] [PubMed] [Google Scholar]
- 26.Norkin CC, White DJ. Measurement of joint motion: a guide to goniometry. Philadelphia: F.A. Davis; 2003. p. 404. [Google Scholar]
- 27.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20(suppl):S135–S145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- 28.Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, et al. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol. 1994;76:1247–1255. doi: 10.1152/jappl.1994.76.3.1247. [DOI] [PubMed] [Google Scholar]
- 29.Taub E, Uswatte G, Morris DM. Improved motor recovery after stroke and massive cortical reorganization following constraint-induced movement therapy. Phys Med Rehabil Clin N Am. 2003;14(suppl):S77–S91. doi: 10.1016/s1047-9651(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 30.Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 31.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 32.Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist. 2002;8:325–338. doi: 10.1097/01.nrl.0000031014.85777.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao J, Dewald JP. Cortical activity related to joint discoordination in chronic stroke: a functional brain imaging study incorporating quantitative motor performance measures. Soc Neurosci Abstr. 2004:878.5. (on-line) [Google Scholar]
- 34.Zatsiorsky V, Seluyanov V. Estimation of the mass and inertia characteristics of the human body by means of the predictive regression equations. In: Winter DA, Norman RW, Wells RP, Hayes KC, Patla AE, editors. Biomechanics IX-B. 5B. Chicago: Human Kinetics; 1985. pp. 233–239. [Google Scholar]


