Abstract
Background K+ channels of the TASK family are believed to participate in sensory transduction by chemoreceptor (glomus) cells of the carotid body (CB). However, studies on the systemic CB-mediated ventilatory response to hypoxia and hypercapnia in TASK1- and/or TASK3-deficient mice have yielded conflicting results. We have characterized the glomus cell phenotype of TASK-null mice and studied the responses of individual cells to hypoxia and other chemical stimuli. CB morphology and glomus cell size were normal in wild-type as well as in TASK1−/− or double TASK1/3−/− mice. Patch-clamped TASK1/3-null glomus cells had significantly higher membrane resistance and less hyperpolarized resting potential than their wild-type counterpart. These electrical parameters were practically normal in TASK1−/− cells. Sensitivity of background currents to changes of extracellular pH was drastically diminished in TASK1/3-null cells. In contrast with these observations, responsiveness to hypoxia or hypercapnia of either TASK1−/− or double TASK1/3−/− cells, as estimated by the amperometric measurement of catecholamine release, was apparently normal. TASK1/3 knockout cells showed an enhanced secretory rate in basal (normoxic) conditions compatible with their increased excitability. Responsiveness to hypoxia of TASK1/3-null cells was maintained after pharmacological blockade of maxi-K+ channels. These data in the TASK-null mouse model indicate that TASK3 channels contribute to the background K+ current in glomus cells and to their sensitivity to external pH. They also suggest that, although TASK1 channels might be dispensable for O2/CO2 sensing in mouse CB cells, TASK3 channels (or TASK1/3 heteromers) could mediate hypoxic depolarization of normal glomus cells. The ability of TASK1/3−/− glomus cells to maintain a powerful response to hypoxia even after blockade of maxi-K+ channels, suggests the existence of multiple sensor and/or effector mechanisms, which could confer upon the cells a high adaptability to maintain their chemosensory function.
INTRODUCTION
Oxygen-regulated K+ channels, initially described in the rabbit carotid body (CB) glomus cell (López-Barneo et al., 1988; Ganfornina and López-Barneo, 1991), are believed to play a fundamental role in chemosensory transduction. It is broadly accepted that reduction of glomus cell K+ conductance in hypoxemia is the major event leading to depolarization and Ca2+ channel opening, rise of cytosolic [Ca2+], and transmitter release. These transmitters stimulate afferent nerve fibers acting on brainstem respiratory neurons to evoke hyperventilation (López-Barneo et al., 1993; Buckler and Vaughan-Jones, 1994; Ureña et al., 1994; Montoro et al., 1996; for recent reviews see Prabhakar, 2006; López-Barneo et al., 2008). Different functional subtypes of O2-regulated K+ channels have been reported in glomus cells from several mammalian species (Peers, 1990; Stea and Nurse, 1991; Ganfornina and López-Barneo, 1992; Wyatt and Peers, 1995; Buckler, 1997; Pérez-García et al., 2004) as well as in other neurosecretory cell classes acutely responding to hypoxia (for review see López-Barneo et al., 2001; Nurse et al., 2006).
Although the understanding of the cellular bases of CB chemotransduction has advanced considerably, the precise molecular nature of the O2 sensor(s) and the effector K+ channel(s) is unknown (see Kemp, 2006). Progress in this field is hampered by methodological limitations derived from the gaseous nature of the stimulus and the delicacy of the O2-sensing apparatus, which can be altered during cell dissociation (Ortega-Sáenz et al., 2007). Additionally, the small size of the CB has precluded large-scale biochemical analyses. These limitations can be partially overcome by the use of genetically modified mice, in which the functional consequences of targeted molecular ablation can be unambiguously demonstrated (e.g., Ortega-Sáenz et al., 2006; Mulkey et al., 2007). To this end, we developed the mouse CB thin slice preparation, where reproducible responses of glomus cells to chemosensory stimuli can be routinely obtained (Piruat et al., 2004; Ortega-Sáenz et al., 2007).
Here, we have evaluated the chemosensitivity of CB glomus cells from mice deficient of TASK channels. These belong to the tandem pore domain (K2P) family of channels and contribute to the leak or background K+ conductance in a broad variety of cells. TASK1 (Kcnk3 or K2P3.1) and TASK3 (Kcnk9 or K2P9.1), the relevant members of the TASK channel class (Duprat et al., 1997; Kim et al., 2000; Rajan et al., 2000), can form heteromers (Czirják and Enyedi, 2002) and have been proposed to be involved in peripheral and central chemoreception (Bayliss et al., 2001; Feldman et al., 2003; Mulkey et al., 2004). Recombinant TASK1 channel activity is reduced upon exposure to low O2 tension (Kemp et al., 2004; Lee et al., 2006; however, for contrasting results see Johnson et al., 2004), and these channels appear to mediate the hypoxic depolarization of cerebellar granule cells (Plant et al., 2002). In rat CB glomus cells, an O2-sensitive TASK-like standing K+ current with weak outward rectification (in physiological asymmetrical K+) and blocked by extracellular Ba2+ but resistant to the classical K+ channel blockers TEA and 4-aminopyridyne (4-AP), has been reported (Buckler, 1997). The CB standing K+ current shares other pharmacological properties, such as activation by the volatile anesthetic halothane and inhibition by anandamide, with currents mediated by TASK1 channels (Buckler et al., 2000). Moreover, background single K+ channel current activity in glomus cells shows flickering kinetics and slope conductance compatible with those reported for recombinant, heterologously expressed TASK1 channels (Williams and Buckler, 2004). Therefore, a popular view is that TASK1 channels may be fundamental for CB O2 sensing (Duprat et al., 2007). However, a detailed recent study by Kim et al. (2009) has shown that heteromeric TASK1/TASK3 are the major O2-sensitive background K+ channels in rat CB glomus cells.
In recent years, TASK1- and/or TASK3-deficient mice have been independently generated in two laboratories that reported the animals to be healthy and with normal lifespan (Aller et al., 2005; Brickley et al., 2007; Mulkey et al., 2007). The absence of TASK1 and/or TASK3 results only in a minor phenotype in central neurons, despite the loss of acid sensitivity in some neuronal groups and the complete disappearance of halothane effect on membrane currents or conductance. In some mice strains, invalidation of TASK1 channels disrupts adrenal gland zonation and produces hyperaldosteronism (Heitzmann et al., 2008). However, the impact of TASK deficiency on peripheral chemoreception is a subject of controversy. Although one group has reported normal ventilatory responses to hypoxia and hypercapnia in the double TASK1 and TASK3 (TASK1/3−/−) knockout animals (Mulkey et al., 2007), another laboratory has observed in TASK1, but not in TASK3, knockout mice decreased ventilation and afferent sinus nerve discharges in response to hypoxia and hypercapnia (Trapp et al., 2008). Herein, we report the basic electrophysiological properties and intrinsic chemosensory activity of individual glomus cells from TASK-deficient animals. We show that glomus cells from TASK1-null animals appear to be normal, but TASK1/3 knockout cells exhibit characteristic electrophysiological alterations and decreased sensitivity to external pH. Nonetheless, the secretory responses of TASK1/3−/− cells to hypoxia as well as other chemosensory stimuli remain essentially unaltered. The implications of these findings for CB O2 sensing are discussed.
MATERIALS AND METHODS
Animals
For the experiments, we used young adult (2–6-mo-old) TASK-null mice (either TASK1−/−, TASK3−/−, or double TASK1/3−/−) and the corresponding wild-type littermates provided by M. Aller (Instituto de Neurociencias, Alicante, Spain). The nonfunctional alleles were generated as described in detail previously (Aller et al., 2005; Brickley et al., 2007). Mice were genotyped as described previously (Aller et al., 2005; Brickley et al., 2007). Animal care and experimentation were performed according to the institutional animal care committee guidelines.
RNA analysis
Four animals for every genotype were killed by sodium pentobarbital overdose (intraperitoneally [i.p.]), and the CBs were dissected, pooled, and stored in liquid nitrogen. mRNA was extracted using Dynabeads mRNA DIRECT micro kit (Invitrogen). First-strand cDNA was synthesized from total mRNA extraction using the Superscript first-strand synthesis system for reverse transcription (RT)-PCR (Invitrogen). PCR amplifications of TASK1, TASK3, and GAPDH mRNAs were performed using the following primers: TASK1 (Kcnk3; 515 bp): 5′-CACCGTCATCACCACAATCG-3′ and 5′-TGCTCTGCATCACGCTTCTC-3′; TASK3 (Kcnk9; 413 bp), 5′-ATGAGATGCGCGAGGAGGAGAAAC-3′ and 5′-ACGAGGCCCATGCAAGAAAAGAAG-3′; and GAPDH (255 bp): 5′-CAAAATGGTGAAGGTCGGTGTG-3′ and 5′-TTTGATGTTAGTGGGGTCTCGC-3′. For quantitative RT-PCR analysis, four groups of three young adult double TASK1/3−/− or control mice were killed by pentobarbital overdose (i.p.), and the CBs were processed as described above. Real-time PCR was performed in an ABI Prism 7500 Sequence Detection System (Applied Biosystems) using SYBR Green PCR Master mix (Applied Biosystems) and the thermocycler conditions recommended by the manufacturer. Each sample was analyzed for cyclophilin to normalize for RNA input amounts and to perform relative quantifications. To normalize mRNA levels in knockout mice to those in control samples, we calculated an average cycle threshold of the control samples and processed all the samples in the experiment relative to this average cycle threshold. Primers were designed using the computer program Primer Express (Applied Biosystems). The following primers were used: maxi-K+ channel α subunit (Kcnma; 76 bp): 5′-CATGGCTTTCAACGTGTTCTTC-3′ and 5′-GCCAGAACCACAGCTTATCATTG-3′; TASK5 (Kcnk15; 53 bp): 5′-GCCTACTACTACTGCTTCATCACTCTCA-3′ and 5′-ACGAAGTCGCCGAAGCCT-3′; and cyclophilin A (Ppia; 75 bp): 5′-GCACTGGTGGCAAGTCCAT-3′ and 5′-GCCAGGACCTGTATGCTTCAG-3′. Melting curve analysis showed a single sharp peak with the expected Tm for all samples.
Immunocytochemistry and morphological studies
For every genotype, three animals were killed by sodium pentobarbital overdose (i.p.), and the carotid bifurcations were dissected, washed with PBS, fixed 2 h at 4°C in 4% paraformaldehyde, and equilibrated for 12 h in a 30% sucrose solution. Bifurcations were included in OCT (optimal cutting temperature; Tissue Tek; Sakura) and snap-frozen by quenching in dry ice. 10-µm thick slices were cut with a cryostat. Sections were stained with the anti–tyrosine hydroxylase (TH) polyclonal antibody (1:1,000; Pel-Freez Biologicals). The Envision+ kit (Dako) was used for immunohistochemistry according to the manufacturer’s recommended protocol. The signal was developed with DAB (Dako). Images were acquired under a microscope (BX-61; Olympus). Estimation of CB volume was performed across the entire CB parenchyma using the CAST Grid System. A Cavalieri size of 912.7 µm2 was used.
Patch clamp recordings
Macroscopic currents were recorded from dispersed mouse glomus cells using either the perforated patch or the whole cell configurations of the patch clamp technique as adapted to our laboratory (Muñoz-Cabello et al., 2005; García-Fernández et al., 2007). Preparation of dispersed mouse CB cells was performed as described previously (Piruat et al., 2004; Ortega-Sáenz et al., 2006). Patch electrodes (1.5–2.5 MΩ) were pulled from capillary glass tubes (1.5–1.6 mm OD; Kimax; Kimble Products), fire polished on a microforge MF-830 (Narishige), and coated with silicone elastomer (Sylgard 184; Corning) to decrease capacitance. Voltage clamp recordings were obtained with an EPC-8 patch clamp amplifier (HEKA) using standard voltage clamp protocols designed with Pulse software (HEKA). Unless otherwise noted, holding potential was −80 mV. Data were filtered at 10 kHz, digitized at a sampling interval of 20 µs with an ITC-16 A/D converter (HEKA), and stored on a Macintosh computer. Offline analysis of data was performed using custom software and Pulse Fit (HEKA). All experiments were conducted at room temperature, 23–26°C. Experiments designed to estimate the cell’s resting potential and input resistance, as well as the pH dependence of the background K+ currents, were made using perforated patches with amphotericin B in the pipette solution. This solution also contained (in mM): 70 K2SO4, 30 KCl, 2 MgCl2, 1 EGTA, and 10 HEPES, pH 7.2. The standard bath solution contained (in mM): 140 NaCl, 2.5 KCl, 10 HEPES, 10 glucose, 2.5 CaCl2, and 4 MgCl2, pH 7.4. For the pH experiments, the external solutions contained (in mM): 3 KCl, 118 NaCl, 1 MgCl2, 1.5 CaCl2, 25 HEPES, and 10 glucose, with the pH adjusted to the desired level using NaOH or HCl. We also added 10 mM TEA and 5 mM 4-AP to the solution to block voltage-dependent K+ channels. Estimated values of resting potential are given after correction for junction potentials. Macroscopic Ca2+, Na+, and K+ currents were recorded in dialyzed glomus cells. The solutions used for the recording of whole cell Na+ and Ca2+ currents contained (in mM): external: 140 NaCl, 9 BaCl2, 1 CaCl2, 10 HEPES, and 10 glucose; pH 7.4 and osmolality 300 mOsm/kg; and internal: 110 CsCl, 30 CsF, 10 EGTA, 10 HEPES, and 4 ATP-Mg; pH 7.2 and osmolality 285 mOsm/kg. The solutions used for the recording of whole cell K+ currents contained (in mM): external: 140 NaCl, 2.5 KCl, 10 HEPES, 10 glucose, 2.5 CaCl2, and 4 MgCl2, pH 7.4); and internal: 80 potassium glutamate, 50 KCl, 1 MgCl2, 10 HEPES, 4 MgATP, and 5 EGTA, pH 7.2.
Amperometric recording of single-cell catecholamine secretion in slices
CB slices were used because the most reproducible single–glomus cell responses to hypoxia are obtained in this preparation (Pardal et al., 2000). Mice CB dissection, slicing, and culture, as well as the measurement of catecholamine secretion, were performed following the same procedures described previously (Ortega-Sáenz et al., 2003, 2006). CBs were resected, cleaned of connective tissue, and included in agarose. After mounting the piece on the stage of a vibratome, 150-µm thick slices were cut, placed in a Petri dish with culture medium (Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 1% penicillin/streptomycin, 1% l-glutamine, and 84 U of insulin per ml), and maintained at 37°C in a 5% CO2 incubator for 24–48 h. Slices were transferred to a recording chamber and continuously perfused with a solution containing (in mM): 117 NaCl, 4.5 KCl, 23 NaHCO3, 1 MgCl2, 2.5 CaCl2, 5 glucose, and 5 sucrose. The osmolality of the solution was ∼280 mOsm/kg. The “normoxic” solution was bubbled with a gas mixture of 5% CO2, 20% O2, and 75% N2 (O2 tension, ∼145 mm Hg). The “hypoxic” solution was bubbled with 5% CO2 and 95% N2 to reach an O2 tension in the chamber of ∼15 mm Hg. To perform dose–response curves, the solutions were also bubbled with 12 and 6% O2, keeping CO2 at 5%. When these solutions were used, the approximate values of O2 tension in the chamber were, respectively, 90 and 50 mm Hg. Experiments of secretion induced by hypercapnia were done with the same control solution bubbled with 10% CO2, 20% O2, and 70% N2 or 20% CO2, 20% O2, and 60% N2 (10 or 20% hypercapnia, respectively). In these conditions, extracellular pH decreased from ∼7.3 in 5% CO2 to 7.1 in 10% CO2 or 6.8 in 20% CO2. All the experiments were made at a temperature in the chamber of ∼36°C. Secretory events were recorded with a polarized (+750 mV) 10-µm carbon fiber electrode positioned near a cell under visual control and connected to the current-to-voltage converter of an EPC-8 patch clamp amplifier. Amperometric currents were filtered at 100 Hz and stored on a computer. The cumulative secretion signal was obtained by the sum of the time integral of successive amperometric events (Pardal and López-Barneo, 2002). Secretion rate (in either femto- or picocoulombs/min) was calculated as the amount of charge transferred to the recording electrode during a given time period.
Statistical analysis
Unless otherwise specified, data are expressed as mean ± SE, with the number (n) of experiments indicated. Statistical analysis was performed by unpaired Student’s t test. A value of P < 0.05 was considered as statistically significant.
Online supplemental material
Confocal fluorimetric recordings of intracellular Ca2+ concentration in dispersed mouse glomus cells are shown in Fig. S1. The data indicate that in both wild-type and TASK1/3−/− glomus cells, hypoxia induces an increase of intracellular Ca2+ concentration. In Fig. S2, we show in dispersed chromaffin cells the inhibition of Ca2+-dependent macroscopic K+ currents by paxilline, a selective blocker of maxi-K+ channels. Figs. S1 and S2 are available at http://www.jgp.org/cgi/content/full/jgp.200910302/DC1.
RESULTS
Morphology of TASK-null carotid bodies
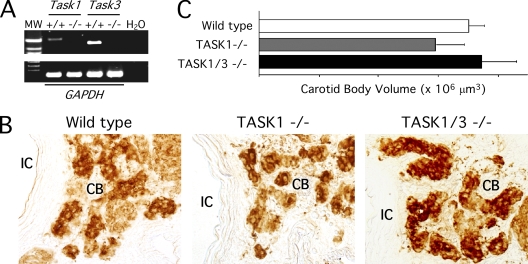
In wild-type animals, we confirmed by PCR the expression of TASK1 and TASK3 mRNAs in the CB and their complete disappearance in TASK-deficient animals (Fig. 1 A). Immunocytochemical analyses with anti-TH antibodies demonstrated in all the animal types that the CBs were normal and appeared organized in the characteristic clusters (glomeruli) of TH-positive glomus cells (Pardal et al., 2007) (Fig. 1 B). The volume occupied by the CB parenchyma was similar in TASK1 or TASK1 and TASK3 double (TASK1/3) knockout mice compared with wild-type littermates (Fig. 1 C). The size of individual CB glomus cells, as indicated by the value of total capacitance measured in perforated patch-clamped cells, was also similar in the three animal strains (see Table I).
Figure 1.
Molecular and histological characterization of TASK1- and double TASK1/3–deficient mice. (A) RT-PCR analysis showing the absence of Task1 or Task3 mRNA expression in the CB of TASK1- or TASK1/3-null mice. (B) CB anatomy in young TASK1- or TASK1/3-deficient animals. Representative sections of control (wild type; left), TASK1−/− (middle), and TASK1/3−/− (right) carotid bodies. CB glomus cells are stained with an antibody against TH. IC, internal carotid artery. (C) Graph representing the CB volume occupied by TH-positive cells. Data are n = 3 for each experimental condition.
Table I.
Electrophysiological parameters of wild-type and TASK-null mouse glomus cells
| Parameter | Wild type | Task1−/− | Task1/3−/− |
| Cell capacitance (pF) | 2.9 ± 0.1 (25) | 2.7 ± 0.1 (29) | 2.9 ± 0.1 (28) |
| Input resistance (GOhm) | 7.8 ± 0.9 (25) | 8.4 ± 0.8 (29) | 10.8 ± 0.9* (31) |
| Membrane potential (mV) | −57.0 ± 1.2 (24) | −54.5 ± 1.8 (29) | −51.5 ± 0.8* (33) |
| Current density (−20 mV, pH 8.2, pA/pF) | 7.9 ± 2.7 (13) | — | 1.6 ± 0.5* (14) |
| Peak K+ current (+30 mV, pA/pF) | 362 ± 17 (13) | 390 ± 21 (11) | 262 ± 17** (21) |
| Peak Na+ current (+10 mV, pA/pF) | −26.2 ± 5.0 (11) | −24.5 ± 5.2 (12) | −27.5 ± 3.4 (22) |
| Peak Ca2+ current (+20 mV, pA/pF) | −7.5 ± 0.9 (21) | −9.1 ± 1.9 (12) | −3.3 ± 0.4** (33) |
Recordings were performed using perforated patches with the exception of those done to measure macroscopic voltage-dependent K+, Na+, and Ca2+ current density, in which we used whole cell (dialyzed) patch-clamped cells. Values are given as mean ± SE, with the number of experiments in parentheses. Asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01) with respect to corresponding values in wild-type cells.
Electrophysiological parameters and pH sensitivity of TASK-deficient glomus cells
TASK1 and TASK3 channels are expressed in numerous neural and non-neural tissues, as well as in the CB. These channels are open over a broad range of membrane voltages and contribute to set the cell’s resting potential and membrane resistance. Hence, we sought to see whether the absence of TASK channels resulted in modifications in the electrophysiological parameters of glomus cells. For the sake of simplicity, some of these experiments were performed on glomus cells from the TASK1/3 double knockout, although, when necessary, experiments were also done on single TASK1-null cells because these last channels are those proposed to participate more specifically in CB O2 sensing (see Duprat et al., 2007; Trapp et al., 2008). In our experimental conditions, the estimated resting membrane potential of dispersed wild-type cells recorded with the perforated patch technique was −57 ± 1.2 mV (n = 24); this value was only slightly changed in TASK1-deficient cells (−54.5 ± 1.8 mV; n = 29) but decreased significantly (to −51.5 ± 0.8 mV; n = 33; P < 0.05) in TASK1/3-null cells. Glomus cells from TASK1/3 knockout mice also had a statistically significant increase of input resistance compared with controls (Table I).
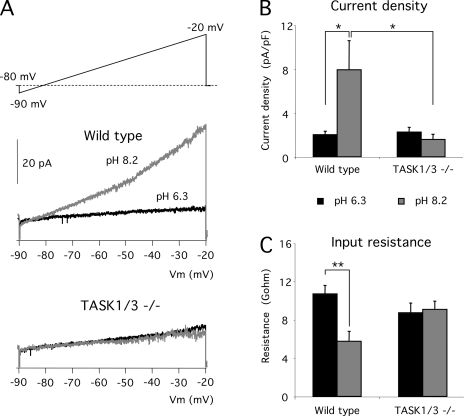
Recombinant TASK1 and TASK3 channels are highly sensitive to extracellular pH due to a proton-sensing histidine residue located at the external mouth of the channels (Rajan et al., 2000). Based on pharmacological experiments, it has been proposed that TASK1 channels contribute to pH sensing in glomus cells (Buckler et al., 2000). In cells bathed in external solution containing TEA and 4-AP to minimize the ion fluxes through voltage-dependent K+ channels, currents evoked by depolarizing ramps were increased by pH alcalinization (switching from pH 6.3 to 8.2) and, as expected, this effect was small or even negligible in TASK1/3-null cells (Fig. 2, A and B). Upon exposure to pH 8.2, outward K+ current density at −20 mV in wild-type cells was on average fivefold larger than in cells from TASK1/3-deficient animals (Fig. 2 B and Table I). In parallel with the effect on K+ current density, ablation of the TASK1/3 channel genes also abolished the decrease of membrane resistance induced by alcalinization (Fig. 2 C). Collectively, these data suggest that the lack of TASK channels in CB glomus cells (particularly TASK3) does indeed result in consistent alterations of their electrophysiological parameters, as well as the responsiveness to changes in extracellular pH.
Figure 2.
Sensitivity to external pH of background potassium currents from wild-type and TASK-null mouse glomus cells. (A; top) Voltage ramp protocol applied to dispersed glomus cells studied with the perforated patch technique. 10 mM TEA and 5 mM 4-AP were added to the external solution to block voltage-dependent K+ channels. (Middle) Currents recorded in wild-type glomus cells exposed to external solutions with pH 6.3 and 8.2. (Bottom) Currents recorded in double TASK1/3–null glomus cells exposed to the same external pH shift. (B) Quantitative analysis of the potassium current density (−20 mV) at different pH of wild-type (n = 13) and TASK1/3−/−-deficient (n = 14) cells. *, P < 0.05. (C) Quantitative analysis of the effect of pH on input cell resistance of wild-type (n = 13) and TASK 1/3−/− (n = 14) cells. **, P < 0.01.
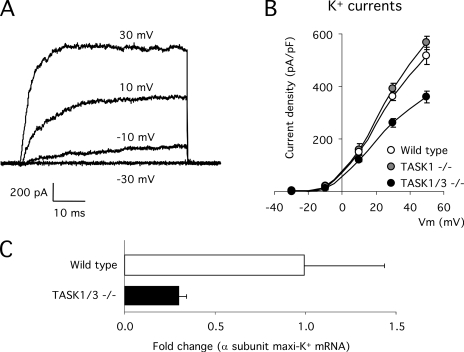
To further investigate the TASK-null CB phenotype, we measured the density of voltage-dependent K+, Na+, and Ca2+ currents in wild-type and TASK1 or TASK1/3 knockout glomus cells. Large voltage-dependent outward K+ currents were recorded in the three glomus cell types (Fig. 3 A). The amplitude of the current was unchanged in TASK1−/− cells, but a clear reduction of K+ current density (∼30%) was observed in TASK1/3−/− preparations (Fig. 3 B and Table I). We have tested by quantitative RT-PCR whether the mRNA expression of other K+ channel genes is altered in TASK1/3−/− CB cells. TASK5 channels, the closest relative to TASK1 and TASK3 within the TASK family (Duprat et al., 2007), were not significantly expressed in the CB tissue. The maxi-K+ channel α subunit, functionally expressed in mouse CB (Yamaguchi et al., 2004; Ortega-Sáenz et al., 2006), appeared to be down-regulated in TASK1/3−/− animals (Fig. 3 C).
Figure 3.
Voltage-dependent K+ currents in mouse glomus cells recorded with the whole cell configuration of the patch clamp technique. (A) Representative family of K+ currents recorded at various membrane potentials. The holding potential was −80 mV, and the depolarization voltages (mV) are indicated near each trace. (B) Potassium current–voltage relationship obtained from wild-type (n = 13), TASK1−/− (n = 11), and TASK1/3−/− (n = 21) glomus cells. (C) Level of expression of the mRNA of maxi-K+ channel α subunit estimated by quantitative PCR from CB tissue of wild-type and TASK1/3-null animals. Data are scaled with respect to values in wild-type animals (n = 4 experiments).
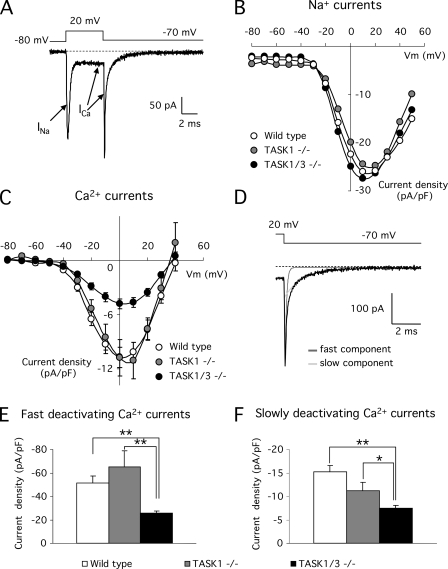
Dispersed mouse glomus cells exhibited relatively large Na+ and/or Ca2+ inward currents (Fig. 4 A). The current–voltage relation of the Na+ current was unaltered in TASK-deficient cells, and TASK1−/− cells also had Ca2+ currents of normal amplitude (Table I and Fig. 4, B and C). However, in TASK1/3−/− cells, the peak Ca2+ current density decreased to ∼50% of control values, although the voltage dependence of the current remained unchanged (Table I and Fig. 4 C). As their rabbit counterparts (Ureña et al., 1989), mouse glomus cells have two well-represented distinct populations of Ca2+ channels that were easily separated by their deactivations kinetics (at −70 mV: τ fast = 0.123 ± 0.009 ms and τ slow = 1.60 ± 0.12 ms; n = 25; Fig. 4 D). These correspond to high threshold, or fast deactivating, and low threshold, or slowly deactivating, Ca2+ channels, which in neurosecretory cells can contribute to transmitter release (see Carabelli et al., 2007; Levitsky and López-Barneo, 2009). The deactivation time constants of these channels types were also indistinguishable between wild-type and TASK-null cells (TASK1−/−: τ fast = 0.093 ± 0.006 ms and τ slow = 1.47 ± 0.09 ms; n = 12; TASK1/3−/−: τ fast = 0.106 ± 0.005 ms and τ slow = 1.66 ± 0.09 ms; n = 34). The decrease of Ca2+ current density observed in TASK1/3−/− glomus cells was mainly due to decrease of the high voltage–activated current, the most predominant component in glomus cells (Fig. 4 E). Nevertheless, the density of the low voltage–activated current was also significantly reduced in the TASK1/3 knockout cells (Fig. 4 F).
Figure 4.
Voltage-dependent Na+ and Ca2+ currents in mouse glomus cells recorded with the whole cell configuration of the patch clamp technique. (A) Representative macroscopic sodium (INa) and calcium (ICa) currents recorded in a cell during a depolarization to +20 mV from a holding potential of −80 mV. The decay of the tail current generated on repolarization to −70 mV reflects the closing time course of the channels open during the pulse. (B) Peak sodium current–voltage relationship for wild-type (n = 11), TASK1−/− (n = 12), and TASK1/3−/− (n = 22) glomus cells. (C) Peak calcium current–voltage relationship for wild-type (n = 18), TASK1−/− (n = 12), and TASK1/3−/− (n = 24) glomus cells. (D) Single-exponential functions fitted to the Ca2+ tail current (dark gray, fast deactivating component; light gray, slowly deactivating component). The fast and slow time constant values in this example are, respectively, 0.11 and 1.21 ms. (E and F) Quantitative analysis of the current density of fast and slowly deactivating components of Ca2+ tail currents from wild-type (n = 25), TASK1−/− (n = 12), and TASK1/3−/− (n = 34) glomus cells. *, P < 0.05; **, P < 0.01.
Chemosensory responses to hypoxia of glomus cells from TASK-null mice
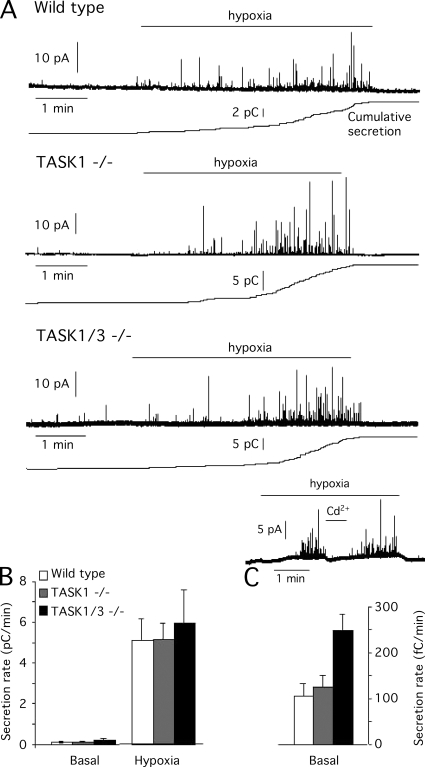
Responsiveness of CB glomus cells to hypoxia in TASK-deficient mice was studied using thin CB slices, where the intrinsic O2 sensitivity of intact glomus cells can be evaluated separately from the other steps along the chemosensory pathway involved in the hypoxic ventilatory responses (Piruat et al., 2004; Ortega-Sáenz et al., 2006). Representative recordings of catecholamine release from individual glomus cells subjected to low O2 tension (PO2, ∼15 mm Hg) are illustrated in Fig. 5 A, and a quantitative summary of the secretion rate in normoxic and hypoxic conditions is shown in Fig. 5 (B and C). Secretion rate induced by hypoxia in wild-type glomus cells (5,133 ± 1,010 fC/min; n = 7) was similar to the values observed in cells deficient of either TASK1 (5,175 ± 719 fC/min; n = 12) or TASK1/3 (5,939 ± 1,595 fC/min; n = 5) channels (Fig. 5 B). Basal secretion in normoxic conditions was, however, over twofold higher in TASK1/3-null cells than in the two other glomus cell types (Fig. 5 C). As the Ca2+ channel density was reduced in TASK1/3−/− cells, we also tested that, similar to other rodent glomus cells (Pardal et al., 2000; Piruat et al., 2004), the secretory response to hypoxia was abolished by blockade of Ca2+ channels with Cd2+ (Fig. 5 A, inset, bottom), thus suggesting that it was triggered by transmembrane Ca2+ influx. Preliminary experiments performed on dispersed glomus cells also indicate that hypoxia induces similar rises of cytosolic [Ca2+] in wild-type and TASK1/3-null cells (Fig. S1).
Figure 5.
Responsiveness to acute hypoxia of CB glomus cells from wild-type, TASK1-, and double TASK1/3–null mice. (A) Amperometric recordings and corresponding cumulative secretion signals (in pC) of catecholamine release induced by hypoxia (O2 tension, ∼15 mm Hg) in CB glomus cells from the different mice strains studied. The inset at the bottom of the panel shows that the secretory response induced by hypoxia in TASK1/3-null glomus cells was inhibited by blockade of Ca2+ channels with 0.2 mM Cd2+. (B) Quantification of the increase in secretion rate (pC/min) induced by hypoxia. Statistical significance (P < 0.05) with respect to basal values in normoxia (O2 tension, 145 mm Hg; n = 7, 12, and 5 for wild-type, TASK1−/−, and TASK1/3−/− cells, respectively). (C) Basal secretion rate of glomus cells from the various mice studied in normoxic conditions. Basal secretion rate (in fC/min) in TASK1/3-null cells was significantly different (P < 0.05) with respect to values in wild-type cells.
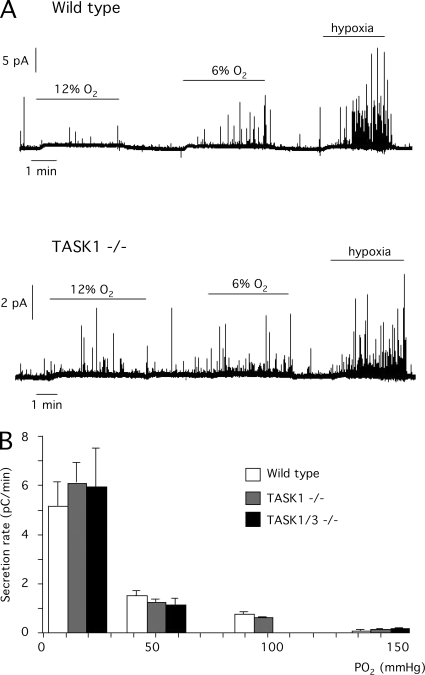
Sensitivity to hypoxia of TASK-null glomus cells was further studied by dose–response experiments in which mouse CB slices were exposed to various PO2 levels. Glomus cells showed a graded response upon exposure to progressively higher hypoxia with a characteristic hyperbolic correlation that was practically similar in all the animal strains studied (wild type, TASK1 null, and TASK1/3 null). These dose–response relationships at the cellular level are almost similar to the correlation between arterial PO2 and the afferent discharges of the CB sinus nerve recorded in vivo or in the explanted CB in vitro (Fig. 6, A and B).
Figure 6.
Responsiveness of CB glomus cells to different levels of hypoxia in wild-type, TASK1-, and double TASK1/3–null mice. (A) Amperometric recordings of catecholamine secretion induced by different levels of O2 tension in CB glomus cells from the indicated mouse strains (control, 21% O2, 145 mm Hg; 12% O2, 90 mm Hg; 6% O2, 50 mm Hg; hypoxia, 15 mm Hg). (B) Dose–response curves estimated from glomus cells of the various mouse strains exposed to solutions equilibrated with various levels of O2 (150, 90, 50, and 15 mm Hg). Data are from three to seven experiments. The average secretion rate values in wild-type, TASK1−/−, and TASK1/3−/− glomus cells at each O2 tension were not significantly different (P > 0.05).
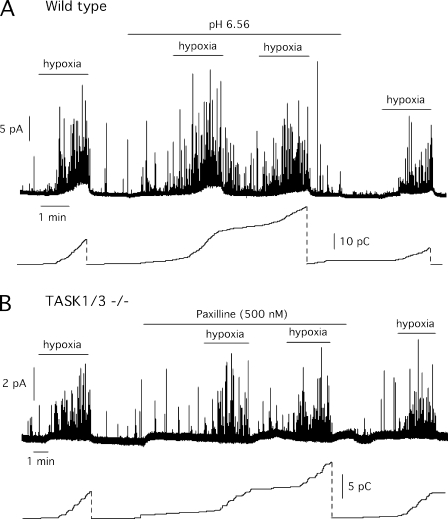
To gain further insight into the role of TASK channels in CB O2 sensing, we performed amperometric experiments in normal (wild-type) mouse glomus cells exposed to acidic pH to minimize the activity of pH-sensitive background channels. Low pH, which presumably inhibits TASK channels (see Fig. 2), induced secretory activity in glomus cells but did not prevent a full response upon subsequent exposure to hypoxia. Indeed, low pH appeared to potentiate the effect of hypoxia (Fig. 7 A). Although TASK1/3−/− glomus cells showed down-regulation of both macroscopic K+ current amplitude (Fig. 3 B) and maxi-K+ channel mRNA expression (Fig. 3 C), we studied whether in the mutant animals the O2-sensitive maxi-K+ current was necessary to compensate for the lack of TASK channels. In these experiments, maxi-K+ channels were blocked with paxilline, a broadly used selective antagonist (Gribkoff et al., 1996; Sheehan et al., 2009) that in our experimental conditions fully inhibited maxi-K+ channels (Fig. S2). In the presence of high concentrations of paxilline, the secretory response to hypoxia of TASK1/3-deficient cells remained unaltered (Fig. 7 B).
Figure 7.
Maintenance of the secretory response to hypoxia in wild-type and TASK1/3−/− glomus cells after blockade of different potassium channels. (A) Amperometric recording and corresponding cumulative secretion signal (in pC) of catecholamine release induced by hypoxia (15 mm Hg) in wild-type CB glomus cells before and after blockade of TASK channels with acidic pH. The composition of the control solution (pH 7.4) is indicated in Materials and methods and contained 23 mM HCO3−. In the acidic (pH 6.5) solution, this was replaced with 10 mM HCO3− plus 12 mM NaH2PO4 and 1 mM Na2HPO4. Secretion rate during hypoxia and acidic hypoxia were 3,694 and 5,888 fC/min, respectively. (B) Secretory response to hypoxia and corresponding cumulative secretion signal (in pC) in TASK1/3-null mice glomus cells in the presence of the maxi-K+ channel blocker paxilline (500 nM). Secretion rate during hypoxia was 3,193 fC/min and changed to 4,095 fC/min in the presence of paxilline.
Chemosensory responses to hypercapnia and hypoglycemia of glomus cells from TASK-null mice
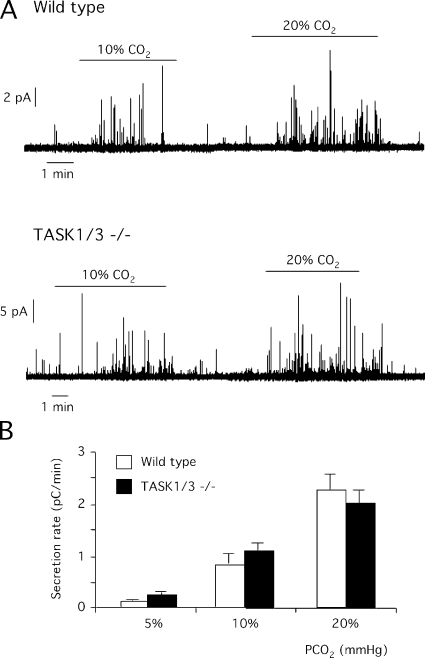
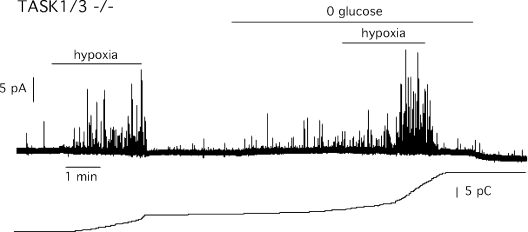
The CB is a polimodal chemosensory receptor that besides hypoxia or pH is also activated by hypercapnia and hypoglycemia (Pardal and López-Barneo, 2002; García-Fernández et al., 2007; Zhang et al., 2007; Fitzgerald et al., 2009). The responsiveness of wild-type and TASK1/3 double knockout glomus cells to hypercapnia was analyzed keeping constant O2 tension and changing CO2 concentration from 5 to 10 and 20%. Exposure to high CO2 tension evoked a surge of transmitter release from glomus cells that, although weaker than the response to hypoxia, was also concentration dependent (Fig. 8 A). As noticed in a previous set of experiments, basal secretion rate was higher in TASK1/3-deficient than in control glomus cells; however, sensitivity to hypercapnia (either 10 or 20%) was unaltered by ablation of the TASK genes (Fig. 8 B). TASK1/3-deficient glomus cells exhibited a secretory response to removal of glucose qualitatively similar to that described previously in the rat CB (Pardal and López-Barneo, 2002), which was also potentiated upon concomitant exposure to hypoxia (Fig. 9).
Figure 8.
Responsiveness of CB glomus cells to hypercapnia in wild-type and double TASK1/3–null mice. (A) Amperometric recordings of catecholamine secretion induced by different levels of CO2 (10 and 20% CO2) tension. (B) Dose–response curves estimated from glomus cells of wild-type and TASK 1/3−/−-deficient mice exposed to solutions equilibrated with various levels of CO2 (10 and 20% CO2). Data are from 8–12 experiments. The average secretion rate values in wild-type and TASK1/3−/− glomus cells at each CO2 tension were not significantly different (P > 0.05).
Figure 9.
Secretory responses of glomus cells exposed concomitantly to hypoxia and hypoglycemia. Representative amperometric recording and corresponding cumulative secretion signal (in pC) of catecholamine release induced by hypoxia (15 mm Hg) and hypoglycemia in CB glomus cells from TASK1/3-null mice. Secretion rate during hypoxia, hypoglycemia, and hypoxia plus hypoglycemia were 8,092, 1,255, and 14,242 fC/min, respectively. Data are from two experiments.
DISCUSSION
Morphological and electrophysiological properties of TASK1- and TASK1/3-null glomus cells
In this paper, we show that TASK1- or TASK1/3-null mice have morphologically normal CB, with TH-positive glomus cells arranged in clusters (glomeruli) typical of the CB parenchyma (Pardal et al., 2007). TASK1-deficient glomus cells had normal passive electrophysiological parameters. In contrast, glomus cells lacking both TASK1 and TASK3 channels showed a clear electrophysiological phenotype characterized by an increase of membrane resistance and cell depolarization. These observations suggest that TASK3 channels (or heteromers of TASK1 and TASK3 channels) contribute to set the resting potential of normal mouse glomus cells (see Kim et al., 2009).
Background K+ currents recorded from TASK1/3-null glomus cells were less sensitive to changes in extracellular pH than those in control cells. This finding fits well with the proposal that TASK-like channels participate in extracellular acid sensing in the CB (Buckler et al., 2000; for discussion on CB acid sensing see López-López and Pérez-García, 2007). Our data also agree with previous studies reporting that the absence of TASK1 in cerebellar granule neurons has no effect on any of the parameters (membrane potential and resistance, as well as density of voltage-dependent channels) analyzed (Aller et al., 2005; Mulkey et al., 2007). Ablation of the TASK1 gene does not seem to induce up-regulation of other TASK channels; however, some central neurons lose their sensitivity to pH or halothane, thus suggesting that other K+ channels (or replacement of TASK1/TASK3 heteromers by TASK3 homomers) compensate for the lack of TASK1 (Aller et al., 2005; Mulkey et al., 2007). In contrast, neurons without TASK3 (or TASK1/3) channels exhibit increased excitability and alteration in their firing frequency due to Na+ channel inactivation (Brickley et al., 2007). These results, in accord with the electrophysiological phenotype observed in TASK1/3-null glomus cells, suggest that in some neurons, TASK3 channels (that have larger conductance than TASK1) are absolutely required for the maintenance of their normal resting potential. In the case of mouse glomus cells, this could be of critical importance because they have a significant population of low threshold T-type Ca2+ channels that, as described in neonatal or hypoxic adult chromaffin cells (Carabelli et al., 2007; Levitsky and López-Barneo, 2009), could be activated by small membrane depolarizations to induce exocytosis. Indeed, we have observed an increased basal secretion rate in TASK1/3-null cells (see below), which is compatible with the sustained depolarization and increased excitability of this cell type.
Voltage-dependent (Na+, K+, and Ca2+) current densities were normal in TASK1 knockout glomus cells; however, K+ and Ca2+ currents were markedly reduced in TASK1/3-deficient cells. These observations further support the view that although TASK1 channels might be dispensable, TASK3 channels (either as homomers or TASK1/TASK3 heteromers) are absolutely required for the maintenance of the physiological phenotype of glomus cells. The decrease of Ca2+ and K+ current density in TASK1/3 knockout cells could be the result of an electrophysiological remodeling induced by the persistent depolarization of these cells. In this regard, it is known that L-type Ca2+ channel α subunit is down-regulated by chronic depolarization in PC12 and smooth muscle cells (Feron and Godfraind, 1995). On the other hand, maintained Ca2+ influx also seems to inhibit the expression of T-type Ca2+ channels in PC12 cells (Del Toro et al., 2003). Although the amplitude of the whole cell K+ current decreased in TASK1/3-null glomus cells, it is possible that other specific O2-sensitive K+ channel subtypes were up-regulated to compensate for the lack of TASK1 and TASK3. A detailed study of the expression of the various K+ channel α subunits in TASK1/3 knockout glomus cells was outside the scope of this work. However, we have found that TASK5, the closest relative to TASK1 and TASK3, was not significantly expressed in CB tissue from either wild-type or TASK1/3-null animals. The mRNA expression of the maxi-K+ α subunit, another O2-regulated K+ channel type expressed in mouse glomus cells (Yamaguchi et al., 2004; Ortega-Sáenz et al., 2006), was down-regulated in TASK1/3-null animals.
Secretory activity and responsiveness to hypoxia/hypercapnia of TASK-null glomus cells
TASK1 deficiency did not cause any appreciable alteration in the secretory activity of glomus cells. However, we systematically observed an increase in the basal secretion rate of TASK1/3-null glomus cells, which is compatible with the enhanced excitability characteristic of cells from this animal strain. Sensitivity of either TASK1- or TASK1/3-deficient glomus cells to modifications of environmental O2 or CO2 tension was indistinguishable with respect to wild-type glomus cells. In fact, O2 or CO2 dose–response curves were quite similar among the CB cells of the various genetically modified animals studied. Moreover, after the exposure of wild-type glomus cells to low pH, which blocked TASK1 and TASK3 channels, responsiveness to hypoxia was maintained or even enhanced. Potentiation of hypoxia by hypoglycemia, a response characteristic of glomus cells (Pardal and López-Barneo, 2002; García-Fernández et al., 2007), was also maintained in TASK1/3-null carotid bodies (see also Guyon et al., 2009).
The observations summarized above are consistent with the lack of electrophysiological phenotype exhibited by TASK1-null cells and further suggest that TASK1 channels, although they could contribute to the formation of TASK1/3 heteromers in normal animals, might not be absolutely required for the maintenance of chemosensory function in mouse glomus cells. However, it is surprising that TASK1/3-deficient cells showed normal secretory responses to hypoxia and hypercapnia, despite the fact that they have major electrophysiological alterations. The most likely explanation is that the increase of TASK1/3 knockout cell excitability (membrane depolarization, increase of input resistance, and decrease of voltage-gated K+ currents) compensates for the reduction of Ca2+ current density, thus resulting in cells with apparently normal chemosensory function. These experimental data obtained from the TASK-deficient model are compatible with the existence in normal mouse glomus cells of O2-sensitive, TASK3-mediated currents similar to those described in the rat (Kim et al., 2009). We have shown here that this current regulates the cell’s resting potential, and therefore its inhibition by hypoxia should contribute to the initiation of the chemosensory response. In cells lacking TASK3 channels (due to genetic ablation or blockade by low pH), responsiveness to hypoxia must be necessarily mediated by other O2-sensitive currents, although the depolarization and enhanced excitability resulting after TASK3 inhibition is possibly what makes the cells exhibit quantitatively normal chemosensory responses.
The behavior of TASK1- or TASK1/3-deficient cells observed in CB slices are in accord with the in vivo study by Mulkey et al. (2007), who did not see any change in the ventilatory response to hypoxia or hypercapnia in TASK1/3-null animals. However, the authors contradict (at least partially) a paper by Trapp et al. (2008), in which a blunted ventilatory response to hypoxia and hypercapnia in TASK1 knockout, but not in TASK3 knockout, animals was described. These authors also reported a decrease of carotid sinus nerve–afferent discharges in in vitro TASK1-deficient CBs exposed to hypoxia or hypercapnia with respect to controls. Interestingly, the TASK1/3-null animals analyzed by Trapp et al. (2008) showed similar phenotype as TASK1 knockout but a higher baseline level in terms of frequency of spikes in carotid sinus nerve activity, which is compatible with the increased glomus cell basal transmitter release observed in our experiments on the same animal model.
The discrepancies between our results obtained from CB slices and those of Trapp et al. (2008) using the in vitro whole CB sinus nerve preparation could derive, at least in part, from functional differences in the cellular elements monitored with each experimental procedure. The dose–response relation obtained with the amperometric recordings from cells in CB slices almost perfectly matches the characteristic hyperbolic correlation between arterial O2 tension and afferent discharges of the CB sinus nerve (see Weir et al., 2005). Nevertheless, chemoreceptor nerve excitation may not be strictly proportional to catecholamine release from glomus cells, as the firing of afferent fibers also depends on postsynaptic mechanisms (e.g., the type and density of ion channels involved in repetitive action potential generation and propagation; e.g., see Faustino and Donnelly, 2006). Thus, it could be that the lack of TASK1 channels, although leaving the glomus cells’ O2- and CO2-sensing mechanisms little affected, alters signal transmission at the chemosensory synapse (see Ortega-Sáenz et al., 2007), either by itself or through the induction of other ion channel types. This last possibility is plausible, as the reverse phenomenon, recruitment of TASK channels in neurons devoid of GABA A receptors, has been reported (Millar et al., 2000; Brickley et al., 2001).
Implications of findings in TASK-null cells for CB O2 and CO2 sensing
The fact that hypoxia responsiveness (in terms of catecholamine release) remains practically intact in TASK-null glomus cells has, in our view, important implications for the mechanisms of CB O2 (and CO2) sensing. We have shown that TASK1/3-null cells manage to show a quantitatively “normal” secretory response to hypoxia, even though they have clear electrophysiological alterations resulting from the absence of TASK3 channels. Moreover, responsiveness to hypoxia was maintained in TASK1/3-deficient cells in which maxi-K+ currents were pharmacologically blocked, an observation that confirms previous data showing that blockade of maxi-K+ channel with iberiotoxin does not prevent a powerful hypoxic secretory response in mouse and rat glomus cells (Ortega-Sáenz et al., 2006). In fact, maxi-K+ channel α subunit mRNA appeared to be down-regulated in TASK1/3-deficient cells. Therefore, in the TASK1/3-null mouse model, suppression of two well-established effectors of hypoxia (TASK and maxi-K+ channels) is compensated by other mechanisms to minimize the impact that abolishment of these channels might have on the functional responses of glomus cells. These observations suggest that glomus cells have a high adaptive capability to maintain their chemosensory function, possible due to multiplicity of sensor and/or effector mechanisms.
It has traditionally been proposed that, as in other sensory systems, CB glomus cells have specific O2 sensor molecule(s) selectively associated with some K+ channel type(s) to regulate glomus cell resting potential and/or action potential firing frequency (López-López et al., 1989; López-Barneo, 1994; Kemp, 2006). However, molecular selectivity in hypoxic chemotransduction is difficult to reconcile with the experimental observations, as O2 sensitivity is a property shared by various subtypes of K+, as well as Na+ and Ca2+, channels broadly distributed in numerous cell types and animal species (for detailed review see López-Barneo et al., 2001; for recent commentary see Wyatt and Peers, 2009). The results of our current study further suggest that whatever the nature of the O2 sensor mechanism(s) may be, it must promiscuously interact with the various subtypes of K+ (and possibly other) channels expressed in glomus cells to redundantly modulate Ca2+ entry and transmitter release. Good candidates for broad O2 modulation of membrane conductance are redox mediators, which could be produced in glomus cells upon exposure to decreased O2 tension and thereby alter the redox state of several K+ channels, thus leading to cell depolarization. Although there is some experimental evidence against a role for redox in glomus cell O2 sensing (for review and discussion see López-Barneo, 2003), redox-based O2 sensing has been proposed for hypoxic pulmonary vasoconstriction (see Weir et al., 2005). Recently, hydrogen sulphide, a broadly distributed cytosolic gaseous reductant (Wang, 2002), has been suggested to participate in CB O2 sensing through redox modulation of maxi-K+ channels in glomus cells (Li et al., 2010). Interestingly, internal application of reduced glutathione produces in single O2-sensitive rabbit K+ channels biophysical alterations that resemble those induced by hypoxia (López-Barneo et al., 1998). As it occurs with O2 sensing, CO2 chemotransduction could depend on the change of internal pH and the redundant decrease of K+ conductance resulting from the protonation of residues in various subtypes of K+ channels.
Regardless of the nature of the mechanisms involved in O2/CO2 sensing, whose molecular characterization obviously needs further research, it is likely that what makes CB cells suitable for arterial chemoreception is their unique morphological and biophysical design. Glomus cells are only ∼10 µm in diameter and have severalfold smaller surface areas and higher input resistances than other neural crest–derived cells, such as chromaffin cells or sympathetic neurons. Glomus cells contain several subtypes of background K+ channels that, combined with the expression of a standing Na+ current (Carpenter and Peers, 2001; García-Fernández et al., 2007), set their resting membrane potential to values (approximately −55 mV) near the activation threshold of voltage-dependent Na+ and Ca2+ channels. Hence, subtle changes in transmembrane current (that could be functionally negligible in other cell types with smaller resistances and more hyperpolarized resting potentials) might result in a depolarization of glomus cells of sufficient amplitude to activate Na+ and/or Ca2+ channels and trigger exocytosis.
Acknowledgments
We wish to express our gratitude to Dr. M. Isabel Aller and Prof. William Wisden (Imperial College, London, UK) for the gift of TASK1 and TASK3 knockout mice.
Research was supported by the Spanish Ministry of Science and Health, the Marcelino Botin Foundation, and the Andalusian Government.
Lawrence G. Palmer served as editor.
Footnotes
Abbreviations used in this paper:
- 4-AP
- 4-aminopyridyne
- CB
- carotid body
- RT
- reverse transcription
- TH
- tyrosine hydroxylase
References
- Aller M.I., Veale E.L., Linden A.M., Sandu C., Schwaninger M., Evans L.J., Korpi E.R., Mathie A., Wisden W., Brickley S.G. 2005. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J. Neurosci. 25:11455–11467 10.1523/JNEUROSCI.3153-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss D.A., Talley E.M., Sirois J.E., Lei Q. 2001. TASK-1 is a highly modulated pH-sensitive ‘leak’ K(+) channel expressed in brainstem respiratory neurons. Respir. Physiol. 129:159–174 10.1016/S0034-5687(01)00288-2 [DOI] [PubMed] [Google Scholar]
- Brickley S.G., Revilla V., Cull-Candy S.G., Wisden W., Farrant M. 2001. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 409:88–92 10.1038/35051086 [DOI] [PubMed] [Google Scholar]
- Brickley S.G., Aller M.I., Sandu C., Veale E.L., Alder F.G., Sambi H., Mathie A., Wisden W. 2007. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J. Neurosci. 27:9329–9340 10.1523/JNEUROSCI.1427-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K.J. 1997. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J. Physiol. 498:649–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K.J., Vaughan-Jones R.D. 1994. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J. Physiol. 476:423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K.J., Williams B.A., Honore E. 2000. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol. 525:135–142 10.1111/j.1469-7793.2000.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V., Marcantoni A., Comunanza V., de Luca A., Díaz J., Borges R., Carbone E. 2007. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J. Physiol. 584:149–165 10.1113/jphysiol.2007.132274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter E., Peers C. 2001. A standing Na+ conductance in rat carotid body type I cells. Neuroreport. 12:1421–1425 10.1097/00001756-200105250-00025 [DOI] [PubMed] [Google Scholar]
- Czirják G., Enyedi P. 2002. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J. Biol. Chem. 277:5426–5432 10.1074/jbc.M107138200 [DOI] [PubMed] [Google Scholar]
- Del Toro R., Levitsky K.L., López-Barneo J., Chiara M.D. 2003. Induction of T-type calcium channel gene expression by chronic hypoxia. J. Biol. Chem. 278:22316–22324 10.1074/jbc.M212576200 [DOI] [PubMed] [Google Scholar]
- Duprat F., Lesage F., Fink M., Reyes R., Heurteaux C., Lazdunski M. 1997. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 16:5464–5471 10.1093/emboj/16.17.5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F., Lauritzen I., Patel A., Honoré E. 2007. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 30:573–580 10.1016/j.tins.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Faustino E.V., Donnelly D.F. 2006. An important functional role of persistent Na+ current in carotid body hypoxia transduction. J. Appl. Physiol. 101:1076–1084 10.1152/japplphysiol.00090.2006 [DOI] [PubMed] [Google Scholar]
- Feldman J.L., Mitchell G.S., Nattie E.E. 2003. Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 26:239–266 10.1146/annurev.neuro.26.041002.131103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O., Godfraind T. 1995. Regulation of the L-type calcium channel alpha-1 subunit by chronic depolarization in the neuron-like PC12 and aortic smooth muscle A7r5 cell lines. Pflugers Arch. 430:323–332 10.1007/BF00373906 [DOI] [PubMed] [Google Scholar]
- Fitzgerald R.S., Shirahata M., Chang I., Kostuk E. 2009. The impact of hypoxia and low glucose on the release of acetylcholine and ATP from the incubated cat carotid body. Brain Res. 1270:39–44 10.1016/j.brainres.2009.02.078 [DOI] [PubMed] [Google Scholar]
- Ganfornina M.D., López-Barneo J. 1991. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc. Natl. Acad. Sci. USA. 88:2927–2930 10.1073/pnas.88.7.2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M.D., López-Barneo J. 1992. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxygen. J. Gen. Physiol. 100:401–426 10.1085/jgp.100.3.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fernández M., Ortega-Sáenz P., Castellano A., López-Barneo J. 2007. Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes. 56:2893–2900 10.2337/db07-0122 [DOI] [PubMed] [Google Scholar]
- Gribkoff V.K., Lum-Ragan J.T., Boissard C.G., Post-Munson D.J., Meanwell N.A., Starrett J.E., Jr., Kozlowski E.S., Romine J.L., Trojnacki J.T., Mckay M.C., et al. 1996. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol. Pharmacol. 50:206–217 [PubMed] [Google Scholar]
- Guyon A., Tardy M.P., Rovère C., Nahon J.L., Barhanin J., Lesage F. 2009. Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J. Neurosci. 29:2528–2533 10.1523/JNEUROSCI.5764-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann D., Derand R., Jungbauer S., Bandulik S., Sterner C., Schweda F., El Wakil A., Lalli E., Guy N., Mengual R., et al. 2008. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J. 27:179–187 10.1038/sj.emboj.7601934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.P., O’Kelly I.M., Fearon I.M. 2004. System-specific O2 sensitivity of the tandem pore domain K+ channel TASK-1. Am. J. Physiol. Cell Physiol. 286:C391–C397 10.1152/ajpcell.00401.2003 [DOI] [PubMed] [Google Scholar]
- Kemp P.J. 2006. Detecting acute changes in oxygen: will the real sensor please stand up? Exp. Physiol. 91:829–834 10.1113/expphysiol.2006.034587 [DOI] [PubMed] [Google Scholar]
- Kemp P.J., Peers C., Lewis A., Miller P. 2004. Regulation of recombinant human brain tandem P domain K+ channels by hypoxia: a role for O2 in the control of neuronal excitability? J. Cell. Mol. Med. 8:38–44 10.1111/j.1582-4934.2004.tb00258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Cavanaugh E.J., Kim I., Carroll J.L. 2009. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J. Physiol. 587:2963–2975 10.1113/jphysiol.2009.171181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Bang H., Kim D. 2000. TASK-3, a new member of the tandem pore K(+) channel family. J. Biol. Chem. 275:9340–9347 10.1074/jbc.275.13.9340 [DOI] [PubMed] [Google Scholar]
- Lee Y.M., Kim B.J., Chun Y.S., So I., Choi H., Kim M.S., Park J.W. 2006. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell. Signal. 18:499–507 10.1016/j.cellsig.2005.05.025 [DOI] [PubMed] [Google Scholar]
- Levitsky K.L., López-Barneo J. 2009. Developmental change of T-type Ca2+ channel expression and its role in rat chromaffin cell responsiveness to acute hypoxia. J. Physiol. 587:1917–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Sun B., Wang X., Jin Z., Zhou Y., Dong L., Jiang L.H., Rong W. 2010. A crucial role for hydrogen sulphide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid. Redox Signal . In press. [DOI] [PubMed] [Google Scholar]
- López-Barneo J. 1994. Oxygen-sensitive ion channels: how ubiquitous are they? Trends Neurosci. 17:133–135 10.1016/0166-2236(94)90084-1 [DOI] [PubMed] [Google Scholar]
- López-Barneo J. 2003. Oxygen and glucose sensing by carotid body glomus cells. Curr. Opin. Neurobiol. 13:493–499 10.1016/S0959-4388(03)00093-X [DOI] [PubMed] [Google Scholar]
- López-Barneo J., López-López J.R., Ureña J., González C. 1988. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 241:580–582 10.1126/science.2456613 [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Benot A.R., Ureña J. 1993. Oxygen sensing and the electrophysiology of arterial chemoreceptor cell. News Physiol. Sci. 8:191–195 [Google Scholar]
- López-Barneo J., Montoro R., Ortega-Sáenz P., Ureña J. 1998. Oxygen-regulated ion channels: functional roles and mechanisms. Oxygen Regulation of Ion Channels and Gene Expression. López-Barneo J., Weir E.K., Futura Publishing Company, Inc., Armonk, NY: 127–144 [Google Scholar]
- López-Barneo J., Pardal R., Ortega-Sáenz P. 2001. Cellular mechanism of oxygen sensing. Annu. Rev. Physiol. 63:259–287 10.1146/annurev.physiol.63.1.259 [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Ortega-Sáenz P., Pardal R., Pascual A., Piruat J.I. 2008. Carotid body oxygen sensing. Eur. Respir. J. 32:1386–1398 10.1183/09031936.00056408 [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. 1989. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J. Gen. Physiol. 93:1001–1015 10.1085/jgp.93.5.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J.R., Pérez-García M.T. 2007. An ASIC channel for acid chemotransduction. Circ. Res. 101:965–967 10.1161/CIRCRESAHA.107.164442 [DOI] [PubMed] [Google Scholar]
- Millar J.A., Barratt L., Southan A.P., Page K.M., Fyffe R.E., Robertson B., Mathie A. 2000. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA. 97:3614–3618 10.1073/pnas.050012597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro R.J., Ureña J., Fernández-Chacón R., Alvarez de Toledo G., López-Barneo J. 1996. Oxygen sensing by ion channels and chemotransduction in single glomus cells. J. Gen. Physiol. 107:133–143 10.1085/jgp.107.1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey D.K., Stornetta R.L., Weston M.C., Simmons J.R., Parker A., Bayliss D.A., Guyenet P.G. 2004. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 7:1360–1369 10.1038/nn1357 [DOI] [PubMed] [Google Scholar]
- Mulkey D.K., Talley E.M., Stornetta R.L., Siegel A.R., West G.H., Chen X., Sen N., Mistry A.M., Guyenet P.G., Bayliss D.A. 2007. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J. Neurosci. 27:14049–14058 10.1523/JNEUROSCI.4254-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Cabello A.M., Toledo-Aral J.J., López-Barneo J., Echevarría M. 2005. Rat adrenal chromaffin cells are neonatal CO2 sensors. J. Neurosci. 25:6631–6640 10.1523/JNEUROSCI.1139-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse C.A., Buttigieg J., Thompson R., Zhang M., Cutz E. 2006. Oxygen sensing in neuroepithelial and adrenal chromaffin cells. Novartis Found. Symp. 272:106–114 10.1002/9780470035009.ch9 [DOI] [PubMed] [Google Scholar]
- Ortega-Sáenz P., Pardal R., García-Fernandez M., López-Barneo J. 2003. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J. Physiol. 548:789–800 10.1113/jphysiol.2003.039693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P., Pascual A., Gómez-Díaz R., López-Barneo J. 2006. Acute oxygen sensing in heme oxygenase-2 null mice. J. Gen. Physiol. 128:405–411 10.1085/jgp.200609591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P., Pascual A., Piruat J.I., López-Barneo J. 2007. Mechanisms of acute oxygen sensing by the carotid body: lessons from genetically modified animals. Respir. Physiol. Neurobiol. 157:140–147 10.1016/j.resp.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Pardal R., López-Barneo J. 2002. Low glucose-sensing cells in the carotid body. Nat. Neurosci. 5:197–198 10.1038/nn812 [DOI] [PubMed] [Google Scholar]
- Pardal R., Ludewig U., García-Hirschfeld J., López-Barneo J. 2000. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc. Natl. Acad. Sci. USA. 97:2361–2366 10.1073/pnas.030522297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R., Ortega-Sáenz P., Durán R., López-Barneo J. 2007. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 131:364–377 10.1016/j.cell.2007.07.043 [DOI] [PubMed] [Google Scholar]
- Peers C. 1990. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci. Lett. 119:253–256 10.1016/0304-3940(90)90846-2 [DOI] [PubMed] [Google Scholar]
- Pérez-García M.T., Colinas O., Miguel-Velado E., Moreno-Domínguez A., López-López J.R. 2004. Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J. Physiol. 557:457–471 10.1113/jphysiol.2004.062281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piruat J.I., Pintado C.O., Ortega-Sáenz G.P., Roche M., López-Barneo J. 2004. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol. Cell. Biol. 24:10933–10940 10.1128/MCB.24.24.10933-10940.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant L.D., Kemp P.J., Peers C., Henderson Z., Pearson H.A. 2002. Hypoxic depolarization of cerebellar granule neurons by specific inhibition of TASK-1. Stroke. 33:2324–2328 10.1161/01.STR.0000027440.68031.B0 [DOI] [PubMed] [Google Scholar]
- Prabhakar N.R. 2006. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp. Physiol. 91:17–23 10.1113/expphysiol.2005.031922 [DOI] [PubMed] [Google Scholar]
- Rajan S., Wischmeyer E., Xin Liu G., Preisig-Müller R., Daut J., Karschin A., Derst C. 2000. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histidine as pH sensor. J. Biol. Chem. 275:16650–16657 10.1074/jbc.M000030200 [DOI] [PubMed] [Google Scholar]
- Sheehan J.J., Benedetti B.L., Barth A.L. 2009. Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia. 50:711–720 10.1111/j.1528-1167.2008.01888.x [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C.A. 1991. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 418:93–101 10.1007/BF00370457 [DOI] [PubMed] [Google Scholar]
- Trapp S., Aller M.I., Wisden W., Gourine A.V. 2008. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J. Neurosci. 28:8844–8850 10.1523/JNEUROSCI.1810-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. 1989. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J. Gen. Physiol. 93:979–999 10.1085/jgp.93.5.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J., Fernández-Chacón R., Benot A.R., Alvarez de Toledo G.A., López-Barneo J. 1994. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc. Natl. Acad. Sci. USA. 91:10208–10211 10.1073/pnas.91.21.10208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. 2002. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16:1792–1798 10.1096/fj.02-0211hyp [DOI] [PubMed] [Google Scholar]
- Weir E.K., López-Barneo J., Buckler K.J., Archer S.L. 2005. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 353:2042–2055 10.1056/NEJMra050002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.A., Buckler K.J. 2004. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L221–L230 10.1152/ajplung.00010.2003 [DOI] [PubMed] [Google Scholar]
- Wyatt C.N., Peers C. 1995. Ca(2+)-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J. Physiol. 483:559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt C.N., Peers C. 2009. Hetero or homo, hypoxia has them all. J. Physiol. 587:2717–2718 10.1113/jphysiol.2009.174078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Lande B., Kitajima T., Hori Y., Shirahata M. 2004. Patch clamp study of mouse glomus cells using a whole carotid body. Neurosci. Lett. 357:155–157 10.1016/j.neulet.2003.10.062 [DOI] [PubMed] [Google Scholar]
- Zhang M., Buttigieg J., Nurse C.A. 2007. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J. Physiol. 578:735–750 10.1113/jphysiol.2006.121871 [DOI] [PMC free article] [PubMed] [Google Scholar]