Upon opening, many ion channels spontaneously close by inactivation processes. As a form of negative feedback regulation, inactivation of ion channels helps to define a variety of physiological functions. The opening of voltage-gated Ca2+ (CaV) channels generates and maintains action potentials and permits Ca2+ ions to enter the cell and signal downstream biological events. Subsequently, CaV channels inactivate in response to both voltage (voltage-dependent inactivation [VDI]) and intracellular Ca2+ binding (Ca2+-dependent inactivation [CDI]). VDI also occurs in voltage-gated K+ (KV) and Na+ (NaV) channels, which is due to either the block of the open-channel pore by a structural component of the channel protein (Armstrong and Hille, 1998) or the collapse of the pore structure (Yellen, 2002). Channel opening exposes a receptor site for the intrinsic blocker (Zhou et al., 2001) and sets in motion a series of conformational changes that lead to pore closure (Cordero-Morales et al., 2007). CDI, on the other hand, is unique to CaV channels. Ca2+ entering the cell via open CaV channels binds to a calmodulin (CaM) molecule that is associated with the channel and causes inactivation (Lee et al., 1999; Peterson et al., 1999; Qin et al., 1999; Zühlke et al., 1999). Although the fascinating molecular details of Ca2+ sensing by CaV channel–associated CaM have been unveiled in the past decade, the downstream events of how the Ca2+–CaM complex reduces channel opening are not known. An overarching question is whether CDI and VDI share the same molecular endpoints, i.e., by pore block/collapse. In two papers published in the March 2010 issue of The Journal of General Physiology, Tadross et al. (Tadross et al. and Tadross and Yue) have studied both VDI and CDI in CaV1.3 channels and have provided some critical stepping-stones along the path of answering these questions.
VDI: a hinged-lid model of pore block
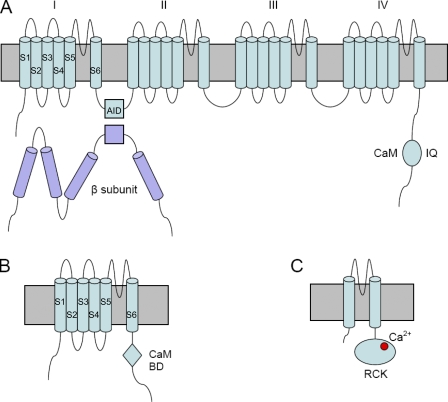
The pore-forming α1 subunit of CaV channels contains four repeated structural domains (I–IV), each containing six transmembrane segments (S1–S6; Fig. 1 A). S5 and S6 from all four domains form a central pore across the membrane, with S6 lining the inner face. S1–S4 of each domain form a voltage-sensing domain at the rim of the pore. This membrane topology and general structure are also shared by NaV and KV channels, except that KV channels are formed by four identical subunits, with each subunit analogous to one CaV domain. At rest, the S6 helices form a bundle crossing that occludes the pore at the cytoplasmic side. Upon activation of the voltage sensor by depolarization, an outward movement of the S4 segments across the membrane triggers the S6 helices to move apart, thereby opening the pore (Swartz, 2004). Thus, S6 also serves as an activation gate.
Figure 1.
Cartoon of the α1 and β subunits of CaV (A), SK (B), and MthK (C) channels.
One form of VDI in voltage-gated channels can be mimicked by the block by quaternary ammonium or channel peptides applied to the cytoplasmic side, and can be eliminated by intracellular protease digestion (Armstrong, 1971; Armstrong et al., 1973; Hoshi et al., 1990; Zagotta et al., 1990). A “ball and chain” model was proposed for VDI, i.e., a structural component of the channel protein attached by a peptide chain acts as a ball to plug the open-channel pore (Bezanilla and Armstrong, 1977). The structure of ball and chain in KV channels was subsequently identified as the N terminus of each α subunit (Hoshi et al., 1990; Zagotta et al., 1990). In NaV channels, three hydrophobic residues in the loop between domains III and IV (III-IV loop) were found to block the channel, resulting in VDI. This mechanism is called a “hinged-lid model” (West et al., 1992). A hinged lid in CaV channels was identified as the I-II loop (Fig. 1 A) based on the result that mutations in this region alter VDI (Stotz et al., 2004). The β subunits of CaV channels also affect VDI by interacting with a segment called the AID within the I-II loop (Fig. 1 A).
The intrinsic peptide blockers, whether “ball” or “lid,” dock on a receptor site formed by residues in the S6 helices to block the channel (Zhou et al., 2001). In Fig. 7 E of Tadross et al. (2010), the authors showed three mutations in I and II S6 that reduced the rate of VDI. These residues, which correspond to those in the receptor site of CaV1.2, are located above the putative bundle crossing and become exposed to the cytosol upon channel opening (Fig. 7 G in Tadross et al., 2010). Thus, these residues are likely to form the receptor site in CaV1.3, supporting the hinged-lid model for VDI.
Tadross et al. (2010) also revealed a twist in the hinged-lid model in CaV1.3, i.e., a “shield” that obstructs the hinged lid from closing, thereby reducing VDI. The shield concept is based on two observations. First, although the receptor site is located above the putative bundle crossing, a proline scan of S6 in all four domains shows that mutations producing the greatest alterations in VDI all cluster below the bundle crossing (Fig. 6 A in Tadross et al., 2010), forming a “hotspot” in between the hinged lid and its receptor site. Second, these hotspot mutations all increase VDI (Fig. 3 in Tadross et al., 2010), as if the mutations were removing an obstruction of VDI. To further demonstrate the shield phenomenon, the authors studied CaV1.3 coexpressed with either the β2a or β1b subunit, both of which interact with the hinged lid but show divergent effects on VDI when coexpressed with other CaV1 or CaV2 channels. Such a divergence of β subunit effects disappears with wild-type CaV1.3 but is unmasked by hotspot mutations. This indicates that the proposed shield structure reduces VDI, making it oblivious to the modulations by β subunits in the wild-type CaV1.3 channels (Fig. 6, D and E, in Tadross et al., 2010).
CDI: reduction of activation gating
CaM binds to the IQ domain (Fallon et al., 2005; Van Petegem et al., 2005; Mori et al., 2008), which is located in the cytoplasmic C-terminal tail of the α1 subunit (Fig. 1 A). Upon Ca2+ binding, the Ca2+–CaM complex interacts with channel effector sites (Tadross et al., 2008), leading to CDI. Despite the distinction between the initiation of CDI and VDI, whether CDI eventually reduces channel activity via the same inactivation gate as VDI is unknown. Early on, it was proposed that the mechanisms for CDI and VDI are different based on observations that VDI is accompanied by a reduction in gating currents, whereas photorelease of intracellular Ca2+ ions using photolabile Ca2+ chelators causes CDI with no change in gating currents (Hadley and Lederer, 1991). This result was recently confirmed by using physiological Ca2+ entering through CaV channels to drive CDI (Barrett and Tsien, 2008). Gating charge immobilization is commonly observed in VDI of voltage-gated channels. Therefore, these results are consistent with the hinged-lid model for VDI, while indicating that CDI may use a different mechanism. Moreover, the same study showed mutations that alter the kinetics of VDI but do not affect CDI, suggesting the dissociation of the two mechanisms (Barrett and Tsien, 2008). However, even if CDI and VDI share the same endpoint, the pathways leading to it may differ; on the other hand, the pathways for CDI and VDI may converge at certain points, although they eventually diverge to different endpoints. This data cannot decisively exclude either of these possibilities. Substantiating this concern, β subunits and mutations in the I-II loop affect both VDI and CDI, which have been used to argue for a shared mechanism underlying both forms of inactivation (Cens et al., 1999; Findeisen and Minor, 2009). Thus, to show independent mechanisms for CDI and VDI, a unique endpoint for CDI needs to be identified.
In Tadross et al. (2010), the authors considered two general models for the mechanism of CDI: one by pore block/collapse, as in VDI, and the other by an allosteric reduction of activation gating, a model that originates from a previous single-channel study in the Yue laboratory (Imredy and Yue, 1994). Structural perturbations may alter the free energy between the closed and open state of the activation gate, ΔΔGa. Theoretical derivations of the two models predict distinctive relationships between CDI and ΔΔGa (Fig. 1 in Tadross et al., 2010). For the first model, CDI depends only on Ca2+ concentration. Perturbations that favor channel opening will allow more Ca2+ ions to enter the cell and bind to CaM, which increases CDI until Ca2+ binding saturates. Therefore, CDI depends on ΔΔGa following a saturating curve. For the second model, CDI depends on both Ca2+ concentration and the changes in activation gating. Perturbations that favor channel opening allow more Ca2+ to enter the cell and bind to CaM, but at the same time, this bias toward the open state directly opposes the allosteric mechanism of CDI. As a result, CDI dependence on ΔΔGa follows a bell-shaped curve.
Two sets of structural perturbations are used to test these models. The first set is a tour de force proline scan of the S6 segments in all four domains (Fig. 3 in Tadross et al., 2010). These mutations in the activation gate produced a broad spectrum of changes in ΔΔGa that span the important parts of the CDI–ΔΔGa relationship. The data fit the prediction of the allosteric model and demonstrate an unambiguous decline of CDI as ΔΔGa increasingly favors the opening of the activation gate, strongly arguing for the idea that CDI reduces channel activity through allosteric hindrance of activation gate opening (Fig. 4 in Tadross et al., 2010). In the second set of perturbations, Bay K 8644 is applied to the wild-type CaV1.3 and several S6 mutants. Bay K 8644, which is known to interact with S5/S6 regions to enhance channel opening, also alters CDI in the direction predicted by the allosteric model (Fig. 5 in Tadross et al., 2010).
Supporting the idea that CDI and VDI have different endpoints, divergent behaviors of CDI and VDI are also demonstrated. A majority of proline scan mutations affect CDI but not VDI, whereas some affect VDI but not CDI. Only a handful of mutations alter both CDI and VDI (Fig. 5 in Tadross et al., 2010). A caveat for this comparison is that a “shield” largely reduces VDI in CaV1.3. In cases where VDI does not show a change by mutations, it is not clear whether the mutations genuinely do not affect VDI or the effect is shielded. In the second paper, Tadross and Yue (2010) analyzed the state dependence of VDI and CDI and found that VDI proceeds only from the open state, whereas CDI proceeds from the open as well as nearby closed states. With regard to VDI, the results suggest that the hinged lid can dock onto the receptor site only after channel opening exposes it. For CDI, inactivation from nearby closed states is consistent with the idea that CDI affects activation gating. Surprisingly, these results seem to suggest that apoCaM may cause CDI even before Ca2+ ions can enter the cell. Alternatively, it is also possible that Ca2+ influx through the neighboring CaV channel could bind CaM to drive CDI from the closed states (Tadross et al., 2008).
What is the molecular mechanism of CDI?
Since Hodgkin and Huxley elucidated the ionic basis of action potentials, experimental results as well as textbooks have taught us that activation and inactivation gating are distinctive molecular processes. The current papers seem to have blurred this distinction. Consequently, a possibility is now open that CDI may share mechanistic similarities with Ca2+-dependent allosteric gating of other ion channels, such as the small-conductance Ca2+-activated K+ (SK) channels and the calcium-activated prokaryotic K+ channel, MthK (Fig. 1, B and C).
SK also uses CaM as a Ca2+ sensor to modulate its activity (Xia et al., 1998). CaM binds to the CaM-binding domain (CaMBD) in its cytoplasmic C terminus. Upon Ca2+ binding, the CaMBD–CaM complex from two SK subunits forms a dimer, thereby pulling open the S6 activation gate (Schumacher et al., 2001). The Ca2+-binding sites of MthK reside in its cytoplasmic C terminus, called the RCK domain. In each MthK channel, four RCK domains tethered to the pore and another four isolated RCK domains from an alternative initiation codon form a complex called the gating ring (Jiang et al., 2002). Ca2+ binding triggers an expansion of the diameter of the gating ring that pulls the inner pore helices to open the activation gate (Jiang et al., 2002; Ye et al., 2006).
Is it possible that the Ca2+–CaM complex in CaV channels uses a similar mechanism to alter activation gating, i.e., by causing a conformational change to tug the activation gate? Unlike tetrameric K+ channels, in which each subunit contains a CaMBD or Ca2+-binding site, CaV channels have only one IQ domain for CaM binding. A tug by the Ca2+–CaM complex would only directly affect IV S6. Nevertheless, the four domains are cooperative during gating, and the final gate opening may involve a concerted movement of all four S6 helices (Zagotta et al., 1994). Therefore, a tug on IV S6 has the potential to modulate the movement of the entire activation gate. On the other hand, in CaV channels, the Ca2+–CaM complex can also enhance channel activity, a phenomenon known as Ca2+-dependent facilitation (CDF). CDI and CDF can occur in the same channel, driven by Ca2+ binding to the N and C lobe of CaM, respectively (DeMaria et al., 2001). It will be fascinating to elucidate the similarities and differences in the structures and mechanisms mediating CDI and CDF, which will shed light on the principles of Ca2+-dependent allosteric gating of ion channels.
Finally, although CDI and VDI may have different endpoints, ample opportunities exist for crosstalk between the two. For instance, S6 harbors the receptor site for VDI and also serves as the activation gate that is affected by CDI. Additionally, IQ-CaM may interact with the I-II loop (Kim et al., 2004). Therefore, perturbations of VDI may affect CDI by either a direct interaction or an allosteric connection via S6 movements, and vice versa.
Acknowledgments
Urvi Lee, Dick Wu, and Mark Zaydman made helpful suggestions on the manuscript. Dick Wu provided assistance in making Fig. 1.
This work was supported by a grant from the National Institutes of Health (R01-HL70393). J. Cui is Associate Professor of Biomedical Engineering on the Spencer T. Olin Endowment.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- CaM
- calmodulin
- CaMBD
- CaM-binding domain
- CaV
- voltage-gated Ca2+
- CDF
- Ca2+-dependent facilitation
- CDI
- Ca2+-dependent inactivation
- SK
- small-conductance Ca2+-activated K+
- VDI
- voltage-dependent inactivation
References
- Armstrong C.M. 1971. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58:413–437 10.1085/jgp.58.4.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M., Hille B. 1998. Voltage-gated ion channels and electrical excitability. Neuron. 20:371–380 10.1016/S0896-6273(00)80981-2 [DOI] [PubMed] [Google Scholar]
- Armstrong C.M., Bezanilla F., Rojas E. 1973. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J. Gen. Physiol. 62:375–391 10.1085/jgp.62.4.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett C.F., Tsien R.W. 2008. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc. Natl. Acad. Sci. USA. 105:2157–2162 10.1073/pnas.0710501105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C.M. 1977. Inactivation of the sodium channel. I. Sodium current experiments. J. Gen. Physiol. 70:549–566 10.1085/jgp.70.5.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cens T., Restituito S., Galas S., Charnet P. 1999. Voltage and calcium use the same molecular determinants to inactivate calcium channels. J. Biol. Chem. 274:5483–5490 10.1074/jbc.274.9.5483 [DOI] [PubMed] [Google Scholar]
- Cordero-Morales J.F., Jogini V., Lewis A., Vásquez V., Cortes D.M., Roux B., Perozo E. 2007. Molecular driving forces determining potassium channel slow inactivation. Nat. Struct. Mol. Biol. 14:1062–1069 10.1038/nsmb1309 [DOI] [PubMed] [Google Scholar]
- DeMaria C.D., Soong T.W., Alseikhan B.A., Alvania R.S., Yue D.T. 2001. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 411:484–489 10.1038/35078091 [DOI] [PubMed] [Google Scholar]
- Fallon J.L., Halling D.B., Hamilton S.L., Quiocho F.A. 2005. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Ca(v)1.2 calcium channel. Structure. 13:1881–1886 10.1016/j.str.2005.09.021 [DOI] [PubMed] [Google Scholar]
- Findeisen F., Minor D.L., Jr 2009. Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. J. Gen. Physiol. 133:327–343 10.1085/jgp.200810143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R.W., Lederer W.J. 1991. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J. Physiol. 444:257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., Aldrich R.W. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538 10.1126/science.2122519 [DOI] [PubMed] [Google Scholar]
- Imredy J.P., Yue D.T. 1994. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 12:1301–1318 10.1016/0896-6273(94)90446-4 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., MacKinnon R. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522 10.1038/417515a [DOI] [PubMed] [Google Scholar]
- Kim J., Ghosh S., Nunziato D.A., Pitt G.S. 2004. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 41:745–754 10.1016/S0896-6273(04)00081-9 [DOI] [PubMed] [Google Scholar]
- Lee A., Wong S.T., Gallagher D., Li B., Storm D.R., Scheuer T., Catterall W.A. 1999. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 399:155–159 10.1038/20194 [DOI] [PubMed] [Google Scholar]
- Mori M.X., Vander Kooi C.W., Leahy D.J., Yue D.T. 2008. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+. Structure. 16:607–620 10.1016/j.str.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.Z., DeMaria C.D., Adelman J.P., Yue D.T. 1999. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 22:549–558 10.1016/S0896-6273(00)80709-6 [DOI] [PubMed] [Google Scholar]
- Qin N., Olcese R., Bransby M., Lin T., Birnbaumer L. 1999. Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc. Natl. Acad. Sci. USA. 96:2435–2438 10.1073/pnas.96.5.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M.A., Rivard A.F., Bächinger H.P., Adelman J.P. 2001. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 410:1120–1124 10.1038/35074145 [DOI] [PubMed] [Google Scholar]
- Stotz S.C., Jarvis S.E., Zamponi G.W. 2004. Functional roles of cytoplasmic loops and pore lining transmembrane helices in the voltage-dependent inactivation of HVA calcium channels. J. Physiol. 554:263–273 10.1113/jphysiol.2003.047068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz K.J. 2004. Towards a structural view of gating in potassium channels. Nat. Rev. Neurosci. 5:905–916 10.1038/nrn1559 [DOI] [PubMed] [Google Scholar]
- Tadross M.R., Yue D.T. 2010. Systematic mapping of the state dependence of voltage- and Ca2+-dependent inactivation using simple open-channel measurements. J. Gen. Physiol. 135:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadross M.R., Dick I.E., Yue D.T. 2008. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell. 133:1228–1240 10.1016/j.cell.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadross M.R., Johny M.B., Yue D.T. 2010. Molecular endpoints of Ca2+/calmodulin- and voltage-dependent inactivation of Cav1.3 channels. J. Gen. Physiol. 135:197–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem F., Chatelain F.C., Minor D.L., Jr 2005. Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat. Struct. Mol. Biol. 12:1108–1115 10.1038/nsmb1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.W., Patton D.E., Scheuer T., Wang Y., Goldin A.L., Catterall W.A. 1992. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc. Natl. Acad. Sci. USA. 89:10910–10914 10.1073/pnas.89.22.10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.M., Fakler B., Rivard A., Wayman G., Johnson-Pais T., Keen J.E., Ishii T., Hirschberg B., Bond C.T., Lutsenko S., et al. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 395:503–507 10.1038/26758 [DOI] [PubMed] [Google Scholar]
- Ye S., Li Y., Chen L., Jiang Y. 2006. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 126:1161–1173 10.1016/j.cell.2006.08.029 [DOI] [PubMed] [Google Scholar]
- Yellen G. 2002. The voltage-gated potassium channels and their relatives. Nature. 419:35–42 10.1038/nature00978 [DOI] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Aldrich R.W. 1990. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 250:568–571 10.1126/science.2122520 [DOI] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Aldrich R.W. 1994. Shaker potassium channel gating. III: evaluation of kinetic models for activation. J. Gen. Physiol. 103:321–362 10.1085/jgp.103.2.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Morais-Cabral J.H., Mann S., MacKinnon R. 2001. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661 10.1038/35079500 [DOI] [PubMed] [Google Scholar]
- Zühlke R.D., Pitt G.S., Deisseroth K., Tsien R.W., Reuter H. 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 399:159–162 10.1038/20200 [DOI] [PubMed] [Google Scholar]