Abstract
In vertebrate olfactory receptor neurons (ORNs), odorant-induced activation of the transduction cascade culminates in production of cyclic AMP, which opens cyclic nucleotide–gated channels in the ciliary membrane enabling Ca2+ influx. The ensuing elevation of the intraciliary Ca2+ concentration opens Ca2+-activated Cl− channels, which mediate an excitatory Cl− efflux from the cilia. In order for the response to terminate, the Cl− channel must close, which requires that the intraciliary Ca2+ concentration return to basal levels. Hitherto, the extrusion of Ca2+ from the cilia has been thought to depend principally on a Na+–Ca2+ exchanger.
In this study, we show using simultaneous suction pipette recording and Ca2+-sensitive dye fluorescence measurements that in fire salamander ORNs, withdrawal of external Na+ from the solution bathing the cilia, which incapacitates Na+–Ca2+exchange, has only a modest effect on the recovery of the electrical response and the accompanying decay of intraciliary Ca2+ concentration. In contrast, exposure of the cilia to vanadate or carboxyeosin, a manipulation designed to block Ca2+-ATPase, has a substantial effect on response recovery kinetics. Therefore, we conclude that Ca2+-ATPase contributes to Ca2+ extrusion in ORNs, and that Na+–Ca2+exchange makes only a modest contribution to Ca2+ homeostasis in this species.
INTRODUCTION
Olfactory transduction takes place in the cilia of olfactory receptor neurons (ORNs) (for review see Kleene, 2008). The process begins with the activation of an odorant receptor in the ciliary membrane, followed by synthesis of cAMP through activation of adenylyl cyclase (Lowe et al., 1989) via a G protein–coupled cascade. The ensuing increase in cAMP concentration causes CNG channels to open (Nakamura and Gold, 1987; Zufall et al., 1994), leading to an increase in intraciliary Ca2+ concentration (Leinders-Zufall et al., 1997) and depolarization of the cell membrane. When the Ca2+ concentration rises, it opens a Ca2+-activated Cl− conductance (Kleene and Gesteland, 1991), enabling Cl− efflux from the cilia, which greatly amplifies the small Ca2+ current flowing through the CNG channels and further depolarizes the ORN (Kurahashi and Yau, 1993; Lowe and Gold, 1993).
Termination of the response to odor requires not only deactivation of the transduction cascade and closure of the CNG channels, but also cessation of Cl− efflux through the Ca2+-activated Cl− conductance. In frog and mouse ORNs, closure of the Ca2+-activated Cl− conductance, which contributes 70–90% of the total odor-induced current, depends directly on the restoration of the intraciliary Ca2+ concentration to pre-stimulus levels by a mechanism dependent on the Na+ electrochemical gradient (Reisert and Matthews, 1998, 2001a; Antolin and Matthews, 2007). It has therefore previously been concluded that in these species, extrusion of Ca2+ from the cilia is dominated by Na+–Ca2+ exchange (Reisert and Matthews, 1998, 2001a). Furthermore, the recovery kinetics of the electrical response of frog ORNs appear to be dominated by Ca2+ extrusion rather than the poststimulus decline in cAMP concentration (Reisert and Matthews, 1998; Antolin and Matthews, 2007), conferring upon Na+–Ca2+ exchange in this species a crucial role in shaping response dynamics.
In this study, we have revisited this question using simultaneous measurement of membrane current and intraciliary Ca2+ to investigate the relationship between Ca2+ dynamics and the kinetics of response termination in fire salamander ORNs. To our surprise, the results demonstrated that in this species, extracellular Na+ was not necessary for Ca2+ removal and near-normal response recovery. Instead, Ca2+ extrusion appeared to involve two distinct mechanisms. The first was eliminated by removal of external Na+, representing Na+–Ca2+ exchange. The second, in contrast, was independent of the Na+ gradient and was abolished instead by vanadate and carboxyeosin, both known blockers of Ca2+-ATPase. Therefore, these results demonstrate a functional role for Ca2+-ATPase in the termination of the vertebrate ORN response. Castillo et al. (2007) have also recently provided evidence that a Ca2+-ATPase is involved in Ca2+ extrusion from toad ORNs, using the photorelease of caged cAMP inside the cell. Here, by directly recording the receptor current and Ca2+ concentration in the cilia after a transient elevation of cAMP concentration, we show that removing external Na+ has only a modest effect on response recovery in fire salamander. These results indicate that in this species, Ca2+ extrusion by Ca2+-ATPase appears to play the dominant role in response recovery, in contrast to the other species so far investigated, in which the extent of its contribution remains to be determined. A preliminary report of these findings has been presented to the International Union of Physiological Sciences (Antolin, S., J. Reisert, and H.R. Matthews. 2009. The 36th Congress of the International Union of Physiological Sciences. Abstr. P1AM-14-4.).
MATERIALS AND METHODS
Preparation of isolated ORNs and dye loading
Terrestrial fire salamanders (Salamandra salamandra) were killed according to Schedule I of the Animals Scientific Procedures Act (1986) by stunning, followed by decapitation and rostral and caudal pithing. After dissection of the olfactory epithelia, the olfactory tissue was cut into two or three small pieces and stored at 4°C in glucose Ringer’s solution. Before dissociation, the cells were incubated in a low Na+ 1-µM Ca2+ solution for ∼30 min to facilitate dissociation and to dissolve the mucus. The cells were then dissociated mechanically by lightly dicing with a piece of razor blade. In experiments requiring Ca2+ measurement, the dissociated cells were then incubated for 30 min in an Eppendorf tube with 20 µM of the cell-permeant Ca2+-sensitive dye fluo-4 AM (TEFLabs) dissolved in Ringer’s solution, prepared from a stock solution in DMSO containing 20% of the nonionic surfactant Pluronic (final DMSO concentration 0.4%; Invitrogen). Sodium ascorbate and sodium pyruvate were added to the loading solution at a concentration of 10 mM to prevent cell damage during ester hydrolysis. The cells were then allowed to settle on the bottom of the recording chamber for an additional 20 min, after which excess dye was removed by bath perfusion.
Suction pipette recording
Receptor current responses were recorded using the suction pipette technique (Reisert and Matthews, 1998). The cell body of the ORN was drawn into the pipette tip, leaving the cilia exposed to the superfusion solution. This configuration isolates the receptor current flowing across the ciliary membrane and obviates contributions from K+ conductances, which are not present in the cilia (Lowe and Gold, 1991). The suction pipette current was recorded with a patch clamp amplifier (Warner PC-501A; Warner Instruments) and digitized (sampled at 200 Hz and filtered over a DC 50-Hz bandwidth with a digitally controlled Bessel filter) by an IBM PC-compatible microcomputer with an intelligent interface card (Cambridge Research Systems). The suction pipette current was plotted according to the convention that current flowing across the ciliary membrane into the suction pipette is negative and represents the inward receptor current of the olfactory cilia.
Solutions and solution changes
Amphibian Ringer’s solution contained 111 mM NaCl, 2.5 mM KCl, 1.6 mM MgCl2, 0.01 mM EDTA (to chelate impurity-heavy metal ions), and 3 mM HEPES; the solution was titrated to pH 7.7–7.8 with 1 M NaOH. 10 mM glucose was added to the bath Ringer’s solution. In solutions with reduced Na+ concentration, guanidinium chloride was substituted for NaCl, and pH was adjusted to 7.7–7.8 with 1 M TMAOH. The phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich) was dissolved in Ringer’s solution daily at the concentrations indicated in the text. The plasma membrane Ca2+ pump was inhibited either with sodium orthovanadate (Varecka and Carafoli, 1982) or carboxyeosin. Vanadate was diluted in Ringer’s solution daily from a 0.1-M stock solution (Tiffert and Lew, 1997, 2001). Carboxyeosin was prepared by dissolving in Ringer’s solution to form a 1-mM stock solution.
Fast solution changes were performed by one of two methods. In experiments in which only the receptor current was recorded, solution was delivered by gravity from a bank of four tubes formed between grooves milled in the lower face of the Perspex recording chamber and the coverslip that constituted its floor. The suction pipette holding an isolated ORN was placed in front of one of these tubes delivering control Ringer’s solution; the solution bathing the exposed cilia could be rapidly exchanged by translating the entire chamber sideways using a computer-controlled stepper motor (Reisert and Matthews, 1998). Between the command and the onset of the solution change was a delay of 0.24 ± 0.01 s (n = 28 cells), corresponding to the time taken for the interface between the two flowing streams of solution to translate to the cilia. The time required to change the solution bathing the cilia was estimated from the junction current, which typically increased from 10 to 90% of maximum amplitude in ∼50 ms. The figures illustrate the nominal times of the solution changes corresponding to the commands issued to the stepper motor.
In experiments in which both the receptor current and intraciliary Ca2+ concentration were recorded, solutions were delivered by gravity through a bundle of seven plastic-coated silica tubes with an internal diameter of 700 µm mounted in a common manifold, which exited via one of the grooves in the base of the Perspex chamber. The seven perfusing lines were independently controlled by solenoid valves (type LFAA; The Lee Company), which could be activated individually under computer control. A constant flow rate of ∼2 ml × min−1 was used to constrain the motile ciliary bundle during fluorescence measurements (Reisert and Matthews, 2001b). The time required to change the solution bathing the cilia was estimated from the junction currents between dissimilar solutions, which typically increased from 10 to 90% of maximum amplitude in 0.31 s (average from eight cells).
Individual suction pipette current traces have been corrected for the liquid junction currents accompanying changes between dissimilar solutions using the following procedure. First, the junction current was recorded for each cell immediately after receptor current recording using the same solutions, but without IBMX. Then, the receptor current was isolated by subtracting the averaged junction current from the original recording.
Intraciliary Ca2+ measurements
The laser spot technique originally developed to record Ca2+ fluorescence in skeletal muscle (Escobar et al., 1994) and later adapted to record cytoplasmic Ca2+ concentration from isolated photoreceptors and olfactory receptors was used to record the intraciliary Ca2+ concentration (Sampath et al., 1998; Reisert and Matthews, 2001b). Dye fluorescence was excited using an argon ion laser (model 60; American Laser Corporation), tuned to 488 nm. The laser beam illuminated a pinhole and, after reflection in a dichroic mirror with a 505-nm transition wavelength (505DRLP; Omega Optical, Inc.), entered an inverted microscope (Eclipse; Nikon) through its epifluorescence port. The laser spot image, with a diameter of 10 µm, was focused on the ciliary bundle using a 40× oil-immersion lens (1.3 NA; CFI60; Nikon), which had a working distance of 200 µm above the chamber floor. The motile cilia were constrained during fluorescence recording by a laminar flow of superfusing solution to minimize movement artifacts (see Fig. 1 of Reisert and Matthews, 2001b). The fixed laser spot was incident at the maximum possible distance from the cell body, where the cilia were approximately parallel to the flow stream. Typically, the spot diameter encompassed almost all the cilia in the bundle.
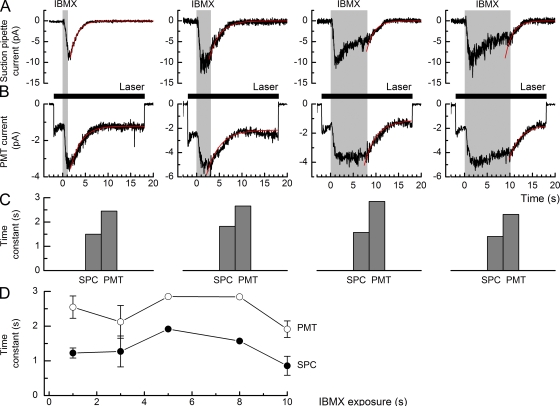
Figure 1.
Suction pipette and simultaneous intraciliary Ca2+ fluorescence recordings of IBMX-evoked responses in an isolated ORN. Representative recordings of (A) the suction pipette current (SPC) and (B) the PMT current evoked by 1-, 3-, 8-, and 10-s exposures of the same isolated ORN to 500 µM IBMX. Decaying single-exponential functions (solid red traces) were fitted to the recovery phase of each response using a least-squares algorithm. The PMT current, representing the Ca2+ dye signal, has been low-pass filtered at 10 Hz. Traces for the 1-, 3-, and 8-s IBMX exposures are each the average of three trials, and the recording for the 10-s IBMX exposure corresponds to a single trial. Gray bars denote the command for the presentation of the IBMX stimulus. Solid bars in B denote laser excitation of fluorescence. (C) Time constants for the decaying single-exponential functions fitted to the receptor current (suction pipette current) and PMT current traces for the cell in A and B. The mean ratio of the time constants fitted to the PMT and suction pipette current traces is 1.6 ± 0.2 for this cell. (D) Collected time constant data from experiments as in A and B for IBMX exposures of 1 s (10 cells), 3 s (3 cells), 5 s (1 cell), 8 s (1 cell), and 10 s (3 cells). Data points represent mean ± SEM. Statistical analysis was performed using the paired t test to compare the time constants fitted to the suction pipette and PMT current traces for a 1-s IBMX exposure and the Spearman rank correlation test to compare the time constants fitted to the current recovery after IBMX exposures of different durations.
The fluorescence intensity was measured with a restricted photocathode photomultiplier (PMT; model 9130/100A; ET Enterprises), after passing through an emission filter with a 510-nm peak transmission wavelength (510ALP; Omega Optical, Inc.). The output of the PMT was amplified by a low noise current to voltage converter (model PDA700; Terahertz Technologies Inc.), and the signal filtered, sampled, and stored in the same way as in the suction pipette recordings. The laser intensity was 7.6 × 1011 photons µm−2s−1. The laser shutter was normally opened 1 s before the IBMX stimulus was delivered, so as to measure baseline dye fluorescence before Ca2+ entry.
Data analysis
Decaying single-exponential functions were fitted to the recovery phase of the response using a least-squares algorithm (OriginLab Corporation). Statistical analysis was performed as indicated in the figure legends.
RESULTS
The strategy adopted in these experiments was to use the phosphodiesterase inhibitor IBMX to raise artificially the concentration of cAMP within the cilia in the absence of odor-induced activation of the transduction cascade. Upon exposure to the phosphodiesterase inhibitor IBMX, the basal levels of cAMP inside the cilia rise to a concentration sufficient to open the CNG channels, allowing Ca2+ influx and evoking an inward current. When IBMX is withdrawn, the cAMP concentration rapidly falls, the CNG and Cl− conductances then deactivate, and the current decays as extrusion of Ca2+ restores its intraciliary concentration to baseline levels (Fig. 1). Under these circumstances, the recovery of current is believed to be dominated by the kinetics of Ca2+ extrusion and the consequent closure of the Ca2+-activated Cl− conductance rather than by the kinetics of cAMP hydrolysis (Antolin and Matthews, 2007).
Fig. 1 illustrates simultaneous measurement of receptor current and the accompanying changes in intraciliary Ca2+ concentration from an isolated fire salamander ORN. The receptor current was recorded by a suction pipette (Fig. 1 A), while fluorescence from the Ca2+ indicator fluo-4 was excited using a laser spot positioned over the cilia alone (Fig. 1 B). The cilia were rapidly superfused with Ringer’s solution containing 500 µM IBMX for progressively increasing durations, evoking a rapid increase in receptor current and an accompanying rise in the intraciliary Ca2+ concentration. As the duration of the response increased, the amplitude of the receptor current relaxed progressively as the response adapted, falling in this cell to 0.43 and 0.36 of the initial peak amplitude for the 8- and 10-s exposures, respectively. In contrast, the elevated Ca2+ concentration in the cilia was more sustained, remaining at ∼0.75 of its peak amplitude after 10 s of IBMX stimulation.
Although both signals had a very similar time to peak during the rising phase of the response to IBMX, upon stimulus withdrawal, Ca2+ decayed more slowly when compared with the suction pipette current. The histograms in Fig. 1 C show the time constants obtained from decaying single exponentials fitted to the recovery time course of the suction pipette current and Ca2+ dye signal (PMT) in this cell (solid red curves in A and B, respectively). It can be seen that although the absolute value of the time constant for the decay of each of these variables was not systematically affected by the duration of the exposure to IBMX, the time constant for the decay of the Ca2+ dye signal was consistently longer than that for the receptor current in these experiments. Collected mean data are shown for several such experiments in Fig. 1 D. Increasing the duration of the IBMX exposure had no significant effect on the time constant for recovery of the receptor current or Ca2+ dye signal (Spearman rank correlation test; r = 0.087 and Z = 0.3595 for suction pipette current; r = −0.086 and Z = −0.3553 for PMT). In the case of the 1-s IBMX exposure, for which the data were the most extensive, the ratio of the mean PMT and suction pipette current time constants was 2.07 ± 0.36 (SEM; 10 cells), reflecting a significantly slower decay after stimulation of Ca2+ concentration than receptor current in the cilia (paired t test; P = 0.0008).
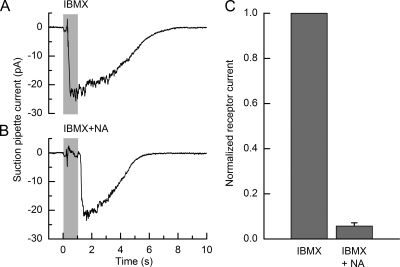
The fraction of the receptor current carried by Ca2+-activated Cl− channels in fire salamander ORNs was estimated by inhibiting this conductance during exposure to IBMX with a saturating concentration of the specific blocker niflumic acid (Kleene, 1993). Fig. 2 shows the response of an isolated ORN to a 1-s exposure to 500 µM IBMX with (B) or without (A) the addition of 2 mM niflumic acid. Exposure of the cilia to niflumic acid during the IBMX stimulus reduced the receptor current to a very small fraction of its control value in the absence of the blocker. However, upon the return to normal Ringer’s solution, the receptor current rapidly approached a magnitude similar to that in the control recording, reflecting the unblocking of Cl− channels opened by Ca2+ entry through CNG channels during the prior IBMX exposure.
Figure 2.
The Ca2+-activated Cl− conductance represents the major component of the olfactory receptor current. An isolated ORN was stimulated for 1 s with (A) 500 µM IBMX and with (B) 500 µM IBMX and 2 mM niflumic acid. Gray bars denote the command for the presentation of the IBMX stimulus. (C) The peak receptor current amplitude evoked by IBMX was reduced by ∼94% relative to the control response when niflumic acid was present during stimulus presentation (n = 15). Consequently, the cationic current through CNG channels represents only a very small fraction of the total receptor current (∼6%). Error bars represent SEM.
Fig. 2 C presents averaged results (n = 15 cells) for the normalized peak receptor current elicited by IBMX in the presence and absence of niflumic acid. Approximately 94% of the IBMX-evoked current was suppressed by niflumic acid, demonstrating that in fire salamander ORNs, as in other species, the overwhelming majority of the receptor current is carried by the Ca2+-activated Cl− conductance (Kurahashi and Yau, 1993; Lowe and Gold, 1993). Thus, the recovery of the response to IBMX will have been dominated by the closure of this conductance, which is governed by the kinetics of Ca2+ extrusion. The greater than unity ratio between the time constant for the decline in Ca2+ and that for the recovery of the receptor current seen in Fig. 1 C therefore indicates a positive cooperativity for the opening of these Cl− channels by Ca2+ (Kleene and Gesteland, 1991; Lowe and Gold, 1993; Reisert et al., 2003).
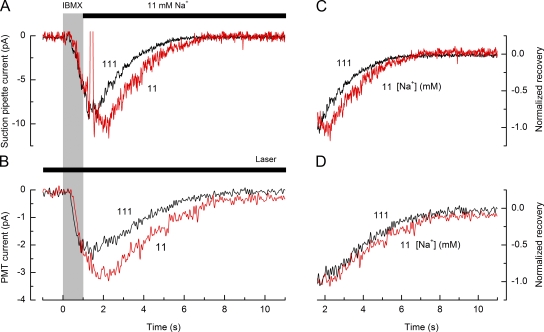
We investigated the processes responsible for extruding Ca2+ from fire salamander ORNs by first stimulating the cilia with IBMX and then allowing the response to recover in solutions with different Na+ concentrations. In frog ORNs, the Ca2+ ions that enter during stimulation are believed to be extruded by a Na+–Ca2+ exchanger that uses the Na+ gradient to transport Ca2+ across the ciliary membrane. Hence, in that species, removal or reduction of external Na+ prevents Ca2+ extrusion by incapacitating Na+–Ca2+ exchange (Reisert and Matthews, 1998; Antolin and Matthews, 2007). However, in fire salamander ORNs, a strikingly different result was obtained. A representative example of such an experiment is shown in Fig. 3. An isolated ORN was exposed for 1 s to 200 µM IBMX and then returned either to normal Ringer’s solution (black traces; 111 mM Na+) or to a modified Ringer’s solution in which 90% of the Na+ was substituted with guanidinium (red traces; 11 mM Na+), whose conductance through CNG channels is similar to Na+ (Picco and Menini, 1993) but does not support Na+–Ca2+ exchange (Yau and Nakatani, 1984; DiPolo and Beaugé, 1988; Fain et al., 1989). The graphs in Fig. 3 (A and B) show simultaneous recordings of the receptor current and the accompanying change in Ca2+ dye fluorescence under these two conditions. To facilitate comparison, each trace has been normalized according to its peak value, and the recovery phase replotted on an expanded time base in Fig. 3 (C and D). Despite a slight increase in response amplitude in the guanidinium-substituted solution, the substantial decrease in external Na+ concentration surprisingly had only a very modest effect on the recovery kinetics, both of the receptor current (Fig. 3 C) and of the accompanying fall in Ca2+ concentration (Fig. 3 D). For this cell, the time constant for recovery of the receptor current was slowed by only a factor of 1.1 (mean slowing ± SEM, 1.51 ± 0.06; 12 cells), which contrasts dramatically with the 17.4-fold slowing of response recovery evoked in frog ORNs by a 10-fold fall in external Na+ concentration (Antolin and Matthews, 2007). Similarly, the actual decline in intraciliary Ca2+ was only modestly affected, being prolonged by a factor of 1.5 in this case. It would therefore appear that incapacitation of Na+–Ca2+ exchange does not prevent Ca2+ extrusion in fire salamander ORNs. These results strongly implicate an additional mechanism for extrusion of Ca2+ from the ORN cilia of this species.
Figure 3.
Effect of external Na+ concentration on receptor current recovery. (A) Suction pipette recordings of receptor current from an isolated ORN in response to a 1-s stimulation with 200 µM IBMX. Afterward, the cilia were rapidly returned to normal Ringer’s solution with 111 mM Na+ (black trace) or to a partially guanidinium-substituted Ringer’s solution containing 11 mM Na+ (red trace). Gray bar denotes the command for the presentation of the IBMX stimulus. Solid bar denotes exposure to partially guanidinium-substituted Ringer’s solution with an 11-mM Na+ concentration. (B) PMT current evoked by the change in ciliary Ca2+ dye fluorescence recorded simultaneously with the suction pipette current traces shown in A. The Na+ concentration in the Ringer’s solution is indicated beside each trace. Suction pipette current traces have been junction corrected, and each trace represents the average of two to three trials. PMT current traces have been low-pass filtered at 10 Hz and baseline corrected to the fluorescence level before stimulus onset. Solid bar denotes laser excitation of fluorescence. (C and D) Recovery phases of the suction pipette and PMT current traces from A and B after normalization to their peak magnitude for the comparison of their time course.
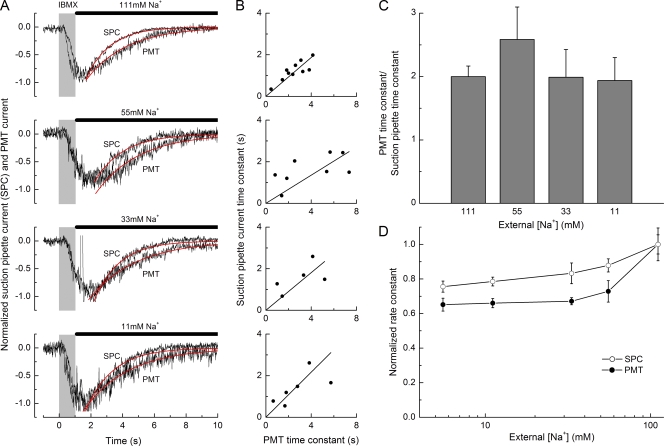
The dependence of Ca2+ extrusion upon external Na+ is investigated further in Fig. 4 A, which compares recovery of the receptor current (or suction pipette current) and intraciliary Ca2+ concentration (represented by the PMT current) from a single ORN for a range of different Na+ concentrations after 1 s of IBMX stimulation. In each case, the traces have been normalized according to their peak magnitudes to facilitate kinetic comparison and fitted with a single decaying exponential. It can be seen that at each Na+ concentration, the time constant for recovery of the receptor current was markedly faster than that of the intraciliary calcium concentration. This kinetic difference was investigated quantitatively for data from several such experiments in Fig. 4 C, which plots mean values of the ratio of the fitted time constant for the recovery of the dye fluorescence signal to that for the recovery of the receptor current calculated individually for each cell. It can be seen that at each Na+ concentration, the intraciliary Ca2+ concentration decayed after stimulation with a time constant around double that for the recovery of the receptor current. Nevertheless, these mean data conceal considerable variability in the absolute values of the time constants in different cells. Consequently, the data have been reexpressed in Fig. 4 B, which plots for each cell the time constant for the recovery of the receptor current against the time constant for the decay of the stimulus-induced increase in intraciliary Ca2+ concentration in the same cell. Despite considerable variation in the absolute magnitude of the time constants between different ORNs, which are known to exhibit considerable response diversity even within the intact epithelium (Rospars et al., 2003), the data could be well fitted by a straight line of constant slope of mean value 0.45 without systematic dependence upon external Na+ concentration (Spearman rank correlation test; r = −0.4; P > 0.1; see also Fig. 4 C). Since at the time of response recovery the receptor current is dominated by the Ca2+-activated Cl− conductance, these data provide an in-situ measure of the positive cooperativity for the activation of these channels by intraciliary Ca2+.
Figure 4.
The modest effect of Na+ on receptor current recovery indicates that Na+–Ca2+ exchange is not the dominant mechanism for ciliary Ca2+ clearance. (A) Suction pipette and simultaneous Ca2+ fluorescence recordings from an isolated ORN stimulated for 1 s with 200 µM IBMX. After stimulation, the cell was allowed to recover with the cilia exposed to either normal Ringer’s solution (111 mM Na+) or in low Na+ guanidinium–substituted Ringer’s solution containing 55, 33, or 11 mM Na+. All traces have been normalized to their peak magnitude and the baseline corrected to the zero current level before stimulation. Single exponentials (red traces) have been fitted to the decay phases of the responses using a least-squares algorithm. Gray bars denote the command for the presentation of the IBMX stimulus. Solid bars denote presentation of solutions of modified Na+ concentration. (B) Time constant for the decay of the receptor current plotted against the time constant for decay of the PMT current Ca2+ fluorescence signal in the same cell for each external Na+ concentration. A linear function constrained to pass through the origin fitted to the data yielded slopes of 0.47 ± 0.03, 0.34 ± 0.06, 0.45 ± 0.09, and 0.54 ± 0.08 for 111, 55, 33, and 11 mM [Na+], respectively (5–10 cells per panel). Statistical analysis was performed using the Spearman rank correlation test to compare these slopes, which were not significantly affected by the Na+ concentration (r = −0.4; P > 0.1). (C) Average ratio between the time constants for the decay of the Ca2+ fluorescence signal and the corresponding receptor current at each Na+ concentration; each bar represents the mean of 5–10 cells. (D) Rate constants, calculated as the reciprocal of the mean time constants for recovery of the responses evoked by 1-s IBMX stimulation obtained as in A at different Na+ concentrations (including 5 mM, not shown in A). Rate constants were normalized to the rate constant in normal Ringer’s solution and averaged for each concentration: 4–18 cells for suction pipette current (SPC; open circles) and 3–10 cells for PMT current (filled circles). Error bars represent SEM.
In contrast, the absolute value of each time constant was only modestly affected by the graded decrease in Na+ concentration compared with the much more substantial effects of such manipulations in frog ORN (Antolin and Matthews, 2007). These data are presented for several such experiments in Fig. 4 D, which plots the rate constant for recovery (reciprocal of the time constant) as a function of the external Na+ concentration, normalized for each cell to the rate constant for recovery in normal Ringer’s solution (111 mM Na+). Even when the external Na+ concentration was lowered to 5 mM, just 4.5% of its concentration in normal Ringer’s solution, the rate at which the receptor current decayed was only reduced to 0.76 ± 0.03 (mean ± SEM) of its value under control conditions, which contrasts with a rate of near zero in frog ORNs (Antolin and Matthews, 2007), while the rate of Ca2+ removal was slowed to 0.65 ± 0.04 of its control value. This relatively modest slowing of Ca2+ extrusion from the ORN cilia of fire salamander upon a substantial reduction in external Na+ suggests that Na+–Ca2+ exchange makes only a minor contribution in this species, and that some other process instead dominates Ca2+ extrusion. This conclusion is reinforced by the similarly modest effect of 5 mM Ni2+ (see Fig. 5 C), a known reversible inhibitor of Na+–Ca2+ exchange (Cervetto and McNaughton, 1986; Brommundt and Kavaler, 1987; Kimura et al., 1987), which is highly effective in slowing Ca2+ extrusion in frog ORNs (Antolin and Matthews, 2007). One possible candidate for such a Na+-independent Ca2+ extrusion process would be a plasma membrane Ca2+-ATPase. We investigated this possibility by attempting to inhibit pharmacologically a putative plasma membrane Ca2+-ATPase during the falling phase of the response to IBMX.
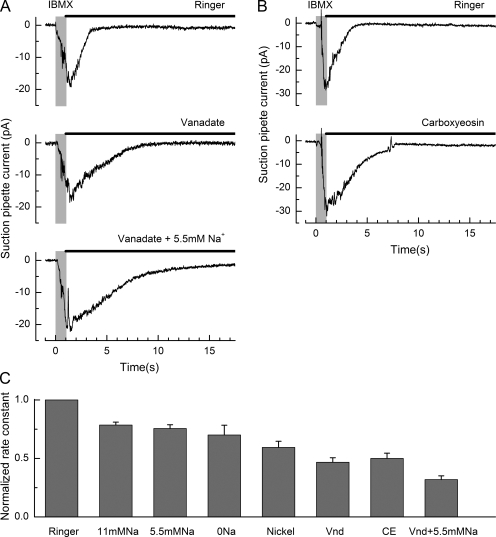
Figure 5.
Ca2+-ATPase inhibitors induce a drastic delay in receptor current recovery. (A) Suction pipette recordings from an isolated ORN, which was stimulated for 1 s with 500 µM IBMX and then allowed to recover either in normal Ringer’s solution, in Ringer’s solution containing 1 mM vanadate, or in a guanidinium-substituted solution with reduced external Na+ (5.5 mM) containing 1 mM vanadate. (B) Using the same protocol as in A, a different cell was allowed to recover either in normal Ringer’s solution or in Ringer’s solution containing 90 µM carboxyeosin. Each trace is the average of two to three trials and has been junction corrected for the experiments with vanadate. Gray bars denote the command for the presentation of the IBMX stimulus. Solid bars denote modified solution for response recovery. (C) Mean rate constants for receptor current decay in Ringer’s solution (n = 18), 11 mM Na+ (n = 14), 5.5 mM Na+ (n = 3), 0 mM Na+ (n = 5), nickel (n = 8), 1 mM vanadate (n = 11), vanadate with low Na+ (n = 8), and carboxyeosin (n = 46). All rate constants were normalized to the rate constant for current recovery upon return to normal Ringer’s solution in the same cell. Rate constants were obtained as the reciprocal of the time constant of the decaying single-exponential function fitted to each trace using a least-squares algorithm. Error bars represent SEM. Statistical analysis was performed using a one-way ANOVA followed by posthoc analysis using the Bonferroni test with significance set at P < 0.05 (confidence level = 95%) to assess differences between individual groups as specified in the text.
Vanadate has been shown to be a powerful inhibitor of P- and F-type ATPases and Ca2+-ATPases (Barrabin et al., 1980; Carafoli and Stauffer, 1994; Tiffert and Lew, 2001). Moreover, its effect on ATP-dependent Ca2+ efflux is reversible, and it has no reported effects on the Na+–Ca2+ exchanger. Vanadate exerts its inhibitory effect very rapidly, distributes uniformly to its inhibitory targets (Tiffert and Lew, 2001), and does not affect cytoplasmic Ca2+ buffering (Tiffert and Lew, 1997). Fig. 5 A illustrates the effect of vanadate on the recovery of the receptor current. An isolated fire salamander ORN was first stimulated for 1 s with 500 µM IBMX, and then stepped into a modified Ringer’s solution containing 1 mM vanadate. Vanadate induced a considerable prolongation of the time constant fitted to receptor current recovery (in this case by a factor of 2.87) relative to the control recovery in normal Ringer’s solution. When, in addition, the external Na+ concentration was reduced to 5.5 mM, recovery was slowed further (for this cell by a total factor of 5.06) compared with normal Ringer’s solution. The effect of vanadate was fully reversible.
Carboxyeosin is a potent membrane-permeant blocker of the plasma membrane Ca2+-ATPase (Gatto and Milanick, 1993; Shmigol et al., 1998) without affecting Na+–Ca2+ exchange (Gatto et al., 1995), and it has been shown to decrease the rate of Ca2+ decay in cardiac cells (Bassani et al., 1995), smooth muscle cells (Shmigol et al., 1998), and Purkinje cells (Fierro et al., 1998) in the rat. Results for a similar protocol using 90 µM carboxyeosin during the recovery phase of the response to IBMX are shown in Fig. 5 B. It can be seen that this inhibitor also resulted in a considerable slowing of recovery, although its magnitude was rather variable in different cells.
Averaged results from several such experiments for the rate constant for receptor current recovery are collected in Fig. 5 C. Reduction of the external Na+ concentration to 11mM and exposure of the cilia to nickel, vanadate, or carboxyeosin all significantly slowed the rate constant relative to that recorded in normal Ringer’s solution (Bonferroni test; P < 0.05). However, vanadate and carboxyeosin, which target Ca2+-ATPase, slowed the rate constant for response recovery to around half of its control value, whereas manipulations designed to inhibit Na+–Ca2+ exchange had a considerably lesser effect. Statistical comparison of the effects of blocking Ca2+-ATPase with those of inhibiting Na+–Ca2+ exchange showed that vanadate and carboxyeosin had a significantly greater effect than reduction of Na+ to 11 mM (Bonferroni test; t = 2.71 and 3.23, respectively). When these two approaches were combined by exposing the cilia to low Na+ solution in the presence of vanadate, the rate constant for response recovery was reduced further to 0.32 ± 0.03 (n = 11) of its control value. The nature of the residual recovery observed after blockade of both Ca2+ extrusion mechanisms is unknown, but it may represent in part diffusion of Ca2+ to the ORN cell body, which was exposed to normal Ringer’s solution in the suction pipette. Because response recovery at these late times is dominated by deactivation of the Ca2+-activated Cl− conductance, these values represent the rate of Ca2+ extrusion relative to that under control conditions. These results therefore suggest that plasma membrane Ca2+-ATPase rather than Na+–Ca2+ exchange dominates Ca2+ extrusion in fire salamander ORNs.
DISCUSSION
Calcium ions are known to play a central role in olfactory transduction (Matthews and Reisert, 2003; Kleene, 2008), serving not only to open the Cl− conductance that carries the majority of the receptor current, but also to exert feedback actions on the transduction mechanism, most especially on the CNG channels through which Ca2+ enters the cilia. Hitherto, Ca2+ extrusion has been believed to be principally mediated by Na+–Ca2+ exchange, which has been shown to be present in the olfactory dendrite and cilia (Jung et al., 1994; Noé et al., 1997; Castillo et al., 2007; Pyrski et al., 2007). When Na+–Ca2+ exchange is prevented by rapidly withdrawing Na+ from the external solution, the response of frog ORNs to odor (Reisert and Matthews, 1998) or elevated intraciliary cyclic nucleotide concentration (Antolin and Matthews, 2007) is greatly prolonged. This delay in recovery results from prolonged opening of the Ca2+-activated Cl− conductance, which deactivates with single exponential kinetics representing the progressive decline in intraciliary Ca2+ concentration. It has therefore been suggested that Na+–Ca2+ exchange serves as the principal means of ciliary Ca2+ extrusion in ORNs.
In contrast to these earlier results from the frog, the present study showed that in fire salamander ORNs, the recovery of both receptor current and Ca2+ concentration in the cilia after a transient elevation of cAMP concentration was only modestly slowed by the removal of external Na+ (Figs. 3 and 4). Because recovery of the receptor current is dominated by closure of the Ca2+-activated Cl− conductance (Fig. 2), these results indicate that Ca2+ extrusion in this species must instead be dominated by a Na+-independent mechanism. The ability of vanadate and carboxyeosin to slow further the time constant for receptor current recovery (Fig. 5) demonstrates that the principal mechanism for Ca2+ extrusion is likely to be the plasma membrane Ca2+-ATPase, for which these agents are known blockers (Barrabin et al., 1980; Gatto and Milanick, 1993; Carafoli and Stauffer, 1994; Shmigol et al., 1998; Tiffert and Lew, 2001). Because our experiments were not performed under clamped conditions, the voltage will have varied continuously during response recovery. This graded depolarization may have served to reduce further the relative contribution of electrogenic Na+–Ca2+ exchange, not only through a reduction in the driving electrochemical gradient, but also perhaps through voltage dependence of external Na+ binding (Lagnado et al., 1988).
Comparison of the time course of decline in receptor current with the decay in the ciliary Ca2+ dye signal also allowed us to determine in situ the cooperativity for the activation of the olfactory Cl− channel by Ca2+ because this channel dominates response recovery (see Fig. 2). Our estimate of between 2.0 (Fig. 4 C) and 2.2 (Fig. 4 B) contrasts with the higher value of 2.8 obtained from excised patches of rat ciliary membrane (Reisert et al., 2003), but matches quite well the value of 2 for the frog (Kleene and Gesteland, 1991). Furthermore, this comparison between the Ca2+ and receptor current signals also directly validates the use of the decay of current through the Ca2+-activated Cl− conductance as an indirect measure of intraciliary Ca2+ concentration (Antolin and Matthews, 2007; Castillo et al., 2007) and allows the time course of the decaying Ca2+ signal after transient elevation of cAMP to be derived unequivocally from the exponentially decaying Cl− current.
The plasma membrane Ca2+-ATPase has been shown to be expressed in the dendrite and cilia of ORNs (Weeraratne et al., 2006; Castillo et al., 2007; Klimmeck et al., 2008) and is capable of extruding Ca2+ from ciliary membrane vesicles (Castillo et al., 2007). However, the functional role of the plasma membrane Ca2+-ATPase in ORNs has proven to be somewhat controversial. Bath application of carboxyeosin has recently been shown to slow substantially the decline of the receptor current after transient release of caged cyclic nucleotide in toad ORNs, whereas the omission of ATP from the whole cell recording solution resulted in a more modest effect (Castillo et al., 2007). These observations have been taken to demonstrate that plasma membrane Ca2+-ATPase is likely to participate in Ca2+ extrusion after odor stimulation.
However, these results cannot be regarded as unequivocal because carboxyeosin also appears to increase substantially the current activated by subsaturating concentrations of cAMP (Kleene, 2009), thereby elevating Ca2+ influx and potentially prolonging the response. Furthermore, ATP depletion might be expected to affect not only Ca2+-ATPase, but also Na+,K+-ATPase, thereby potentially compromising Ca2+ extrusion by Na+–Ca2+ exchange via a reduction in the Na+ gradient because the diffusional remoteness of the cilia from the soma has been suggested to necessitate active Na+,K+ pumping within the cilia themselves (Lindemann, 2001). These concerns are reinforced by the recent demonstration that ATP is without effect on Cl− current activation by Ca2+ influx either by longitudinal diffusion from the bath or membrane influx in excised frog ORN cilia (Kleene, 2009). Thus, it has been suggested that the plasma membrane Ca2+-ATPase might play only a very limited role in clearing the micromolar levels of intraciliary Ca2+ produced during the odor response (Kleene, 2009), perhaps reflecting its lower capacity and higher affinity than Na+–Ca2+ exchange (Brini and Carafoli, 2000; Di Leva et al., 2008).
Our approach avoids these pitfalls by applying carboxyeosin and vanadate only during response recovery. Furthermore, use of the suction pipette technique ensures that only the cilia, and not the cell body, are exposed to these reagents, while the laser spot technique records dye fluorescence from the cilia alone, and therefore reports changes in [Ca2+] at the site of transduction. Because the CNG channels close rapidly after the withdrawal of IBMX (Antolin and Matthews, 2007), an effect of the exposure to carboxyeosin during response recovery on Ca2+ influx through this conductance can be excluded in our experiments. Although vanadate blocks not only Ca2+-ATPase but also Na+,K+-ATPase (Cantley et al., 1977), the brief duration of the exposure to this reagent of the cilia alone seems unlikely to have influenced the Na+ gradient during response recovery. Finally, in our experiments, both vanadate and carboxyeosin resulted in a reduction in the rate of Ca2+ extrusion from the cilia, which was not only larger than, but could also be enhanced further by, removal of external Na+ (Fig. 5 C). We therefore believe that our results indicate that plasma membrane Ca2+-ATPase plays a relatively larger role than Na+–Ca2+ exchange in Ca2+ extrusion from the cilia of fire salamander ORNs after stimulation.
The relative importance of these two mechanisms may, however, vary considerably in other species. In the ORN of the frog (both Rana temporaria [Reisert and Matthews, 1998; Antolin and Matthews, 2007] and Rana pipiens [Kleene, 2009]) and terrestrial tiger salamander (Ambystoma tigrinum, Reisert and Matthews, 2001b), the quantitative importance of the Ca2+-ATPase appears to be limited, whereas in toad ORN it may be functionally more significant (Castillo et al., 2007). A possible explanation for these differences is provided by the ecological niche occupied by each species. The amphibian olfactory system undergoes substantial changes during metamorphosis from the water-dwelling larval form to the adult, which is normally principally terrestrial. In most amphibians, the adult nasal cavity consists of two chambers, the principal cavity and the vomeronasal organ, in contrast to the water-dwelling Xenopus laevis, in which a third “middle chamber” is provided with microvillar rather than ciliated receptors and instead detects waterborne odorants (Hansen et al., 1998). The absence of such a “water nose” in other amphibian species suggests that in some cases, the principal cavity might play a dual role in the detection of both airborne and waterborne odorants. However, exposure to waterborne odorants would be accompanied by reduction of the ionic concentrations within the olfactory mucus overlaying the main olfactory epithelium, which might alter ORN response recovery kinetics if Na+–Ca2+ exchange were the sole mechanism for Ca2+ extrusion.
In the adult frog (Rana temporaria), which retains a partially aquatic habitat, the problems accompanying dilution are reduced but not abolished through a low Na+ affinity for the olfactory Na+–Ca2+ exchanger (Antolin and Matthews, 2007). In tiger salamander, despite its largely terrestrial habitat, the olfactory epithelium of the principal cavity contains both ciliated and microvillar receptors even in the adult (Breipohl et al., 1982; Eisthen and Schroeder, 1992), suggesting that it might continue to be used to detect waterbourne stimuli. However, in this species, metamorphosis is accompanied by a substantial enhancement of the electro-olfactogram responses to volatile odorants and a depression of the responses to amino acids (Arzt et al., 1986), indicating a functional shift to the detection of airborne stimuli upon the change from an aquatic to a terrestrial habitat. Consequently, the additional protection against the effects of dilution that would be provided by the plasma membrane Ca2+-ATPase may be unimportant in this species.
In contrast, different fire salamander populations, despite being derived from a common genetic lineage, exhibit diversity of habitat, subdividing into distinct subpopulations that deposit their larvae either in ponds or in small streams (Weitere et al., 2004). Because the former habitat is more liable to drying and limited food supply than the latter, pond larvae tend to metamorphose earlier than stream larvae at the cost of lower body mass. It therefore seems possible that the greater role played by plasma membrane Ca2+-ATPase in fire salamander ORNs may reflect this variability in the timing of the switch from an aquatic to a terrestrial environment, and serve to defend further the kinetics of ORN response recovery against mucus dilution in those subpopulations that remain aquatic for a longer period.
In mammalian ORNs, the relative importance of these two mechanisms for Ca2+ extrusion is just beginning to be evaluated. In mouse ORNs, although withdrawal of external Na+ dramatically slows recovery of the response to odor (Reisert and Matthews, 2001a), the response nevertheless relaxes over the next few seconds. Although this recovery may reflect diffusion of Ca2+ from the cilia to the soma, an alternative possibility would be the involvement instead of a low capacity, high affinity Ca2+ extrusion mechanism, such as a Ca2+-ATPase within the cilia themselves. In rodent ORNs, the plasma membrane Ca2+-ATPase has been found to be present not only in disrupted ciliary membranes (Castillo et al., 2007; Klimmeck et al., 2008; Mayer et al., 2009), which may potentially be contaminated with non-ciliary material (Mayer et al., 2008), but also immunocytochemically in the intact cilia (Weeraratne et al., 2006; Castillo et al., 2007; Saidu et al., 2009). It has recently been demonstrated using Ca2+ fluorescence imaging that carboxyeosin slows Ca2+ clearance from the dendritic knob of mouse ORN by around 30%, a result corroborated by the PMCA2 knockout (Saidu et al., 2009). It would be of great interest to determine the dynamics of Ca2+ clearance from the cilia of these knockout animals because the cilia appear to exhibit Ca2+ dynamics that are, to at least some extent, independent from those in the rest of the ORN (Leinders-Zufall et al., 1997). The true functional extent of Ca2+-ATPase involvement in Ca2+ extrusion from the cilia of mammalian ORNs remains to be determined.
Acknowledgments
We are grateful to V.L. Lew and T. Tiffert for providing vanadate stock solutions and to G.L. Fain for helpful comments on early versions of the manuscript.
This work was supported by the Wellcome Trust and by a studentship to S. Antolin from the Fundação para a Ciência e a Tecnologia, Portugal. J. Reisert is supported by the Monell Chemical Senses Center and a Morley Kare Fellowship. J. Reisert would like to thank Dr. K.-W. Yau for financial support during the early stages of the experiments.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- IBMX
- 3-isobutyl-1-methylxanthine
- ORN
- olfactory receptor neuron
- PMT
- photomultiplier
References
- Antolin S., Matthews H.R. 2007. The effect of external sodium concentration on sodium-calcium exchange in frog olfactory receptor cells. J. Physiol. 581:495–503 10.1113/jphysiol.2007.131094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzt A.H., Silver W.L., Mason J.R., Clark L. 1986. Olfactory responses of aquatic and terrestrial tiger salamanders to airborne and waterborne stimuli. J. Comp. Physiol. A. 158:479–487 10.1007/BF00603794 [DOI] [PubMed] [Google Scholar]
- Barrabin H., Garrahan P.J., Rega A.F. 1980. Vanadate inhibition of the Ca2+-ATPase from human red cell membranes. Biochim. Biophys. Acta. 600:796–804 10.1016/0005-2736(80)90482-4 [DOI] [PubMed] [Google Scholar]
- Bassani R.A., Bassani J.W., Bers D.M. 1995. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca ATPase. Pflugers Arch. 430:573–578 10.1007/BF00373894 [DOI] [PubMed] [Google Scholar]
- Breipohl W., Moulton D., Ummels M., Matulionis D.H. 1982. Spatial pattern of sensory cell terminals in the olfactory sac of the tiger salamander. I. A scanning electron microscope study. J. Anat. 134:757–769 [PMC free article] [PubMed] [Google Scholar]
- Brini M., Carafoli E. 2000. Calcium signalling: a historical account, recent developments and future perspectives. Cell. Mol. Life Sci. 57:354–370 10.1007/PL00000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommundt G., Kavaler F. 1987. La3+, Mn2+, and Ni2+ effects on Ca2+ pump and on Na+-Ca2+ exchange in bullfrog ventricle. Am. J. Physiol. 253:C45–C51 [DOI] [PubMed] [Google Scholar]
- Cantley L.C., Jr., Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. 1977. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J. Biol. Chem. 252:7421–7423 [PubMed] [Google Scholar]
- Carafoli E., Stauffer T. 1994. The plasma membrane calcium pump: functional domains, regulation of the activity, and tissue specificity of isoform expression. J. Neurobiol. 25:312–324 10.1002/neu.480250311 [DOI] [PubMed] [Google Scholar]
- Castillo K., Delgado R., Bacigalupo J. 2007. Plasma membrane Ca2+-ATPase in the cilia of olfactory receptor neurons: possible role in Ca2+ clearance. Eur. J. Neurosci. 26:2524–2531 10.1111/j.1460-9568.2007.05863.x [DOI] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P.A. 1986. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J. Physiol. 370:91–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva F., Domi T., Fedrizzi L., Lim D., Carafoli E. 2008. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch. Biochem. Biophys. 476:65–74 10.1016/j.abb.2008.02.026 [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. 1988. Ca2+ transport in nerve fibers. Biochim. Biophys. Acta. 947:549–569 [DOI] [PubMed] [Google Scholar]
- Eisthen H.L., Schroeder D.M. 1992. Ultrastructure of the nasal epithelial surface in land-phase tiger salamanders (Ambystoma tigrinum). Chem. Senses. 17:617 [Google Scholar]
- Escobar A.L., Monck J.R., Fernandez J.M., Vergara J.L. 1994. Localization of the site of Ca2+ release at the level of a single sarcomere in skeletal muscle fibres. Nature. 367:739–741 10.1038/367739a0 [DOI] [PubMed] [Google Scholar]
- Fain G.L., Lamb T.D., Matthews H.R., Murphy R.L. 1989. Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J. Physiol. 416:215–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L., DiPolo R., Llano I. 1998. Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices. J. Physiol. 510:499–512 10.1111/j.1469-7793.1998.499bk.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto C., Milanick M.A. 1993. Inhibition of the red blood cell calcium pump by eosin and other fluorescein analogues. Am. J. Physiol. 264:C1577–C1586 [DOI] [PubMed] [Google Scholar]
- Gatto C., Hale C.C., Xu W., Milanick M.A. 1995. Eosin, a potent inhibitor of the plasma membrane Ca pump, does not inhibit the cardiac Na-Ca exchanger. Biochemistry. 34:965–972 10.1021/bi00003a031 [DOI] [PubMed] [Google Scholar]
- Hansen A., Reiss J.O., Gentry C.L., Burd G.D. 1998. Ultrastructure of the olfactory organ in the clawed frog, Xenopus laevis, during larval development and metamorphosis. J. Comp. Neurol. 398:273–288 [DOI] [PubMed] [Google Scholar]
- Jung A., Lischka F.W., Engel J., Schild D. 1994. Sodium/calcium exchanger in olfactory receptor neurones of Xenopus laevis. Neuroreport. 5:1741–1744 10.1097/00001756-199409080-00013 [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. 1987. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J. Physiol. 384:199–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S.J. 1993. Origin of the chloride current in olfactory transduction. Neuron. 11:123–132 [DOI] [PubMed] [Google Scholar]
- Kleene S.J. 2008. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem. Senses. 33:839–859 10.1093/chemse/bjn048 [DOI] [PubMed] [Google Scholar]
- Kleene S.J. 2009. Limits of calcium clearance by plasma membrane calcium ATPase in olfactory cilia. PLoS One. 4:e5266 10.1371/journal.pone.0005266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S.J., Gesteland R.C. 1991. Calcium-activated chloride conductance in frog olfactory cilia. J. Neurosci. 11:3624–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimmeck D., Mayer U., Ungerer N., Warnken U., Schnölzer M., Frings S., Möhrlen F. 2008. Calcium-signaling networks in olfactory receptor neurons. Neuroscience. 151:901–912 10.1016/j.neuroscience.2007.11.023 [DOI] [PubMed] [Google Scholar]
- Kurahashi T., Yau K.W. 1993. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 363:71–74 10.1038/363071a0 [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P.A. 1988. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc. Natl. Acad. Sci. USA. 85:4548–4552 10.1073/pnas.85.12.4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T., Rand M.N., Shepherd G.M., Greer C.A., Zufall F. 1997. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J. Neurosci. 17:4136–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. 2001. Predicted profiles of ion concentrations in olfactory cilia in the steady state. Biophys. J. 80:1712–1721 10.1016/S0006-3495(01)76142-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Gold G.H. 1991. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J. Physiol. 442:147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Gold G.H. 1993. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 366:283–286 10.1038/366283a0 [DOI] [PubMed] [Google Scholar]
- Lowe G., Nakamura T., Gold G.H. 1989. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc. Natl. Acad. Sci. USA. 86:5641–5645 10.1073/pnas.86.14.5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R., Reisert J. 2003. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr. Opin. Neurobiol. 13:469–475 10.1016/S0959-4388(03)00097-7 [DOI] [PubMed] [Google Scholar]
- Mayer U., Ungerer N., Klimmeck D., Warnken U., Schnölzer M., Frings S., Möhrlen F. 2008. Proteomic analysis of a membrane preparation from rat olfactory sensory cilia. Chem. Senses. 33:145–162 10.1093/chemse/bjm073 [DOI] [PubMed] [Google Scholar]
- Mayer U., Küller A., Daiber P.C., Neudorf I., Warnken U., Schnölzer M., Frings S., Möhrlen F. 2009. The proteome of rat olfactory sensory cilia. Proteomics. 9:322–334 10.1002/pmic.200800149 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G.H. 1987. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 325:442–444 10.1038/325442a0 [DOI] [PubMed] [Google Scholar]
- Noé J., Tareilus E., Boekhoff I., Breer H. 1997. Sodium/calcium exchanger in rat olfactory neurons. Neurochem. Int. 30:523–531 10.1016/S0197-0186(96)00090-3 [DOI] [PubMed] [Google Scholar]
- Picco C., Menini A. 1993. The permeability of the cGMP-activated channel to organic cations in retinal rods of the tiger salamander. J. Physiol. 460:741–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrski M., Koo J.H., Polumuri S.K., Ruknudin A.M., Margolis J.W., Schulze D.H., Margolis F.L. 2007. Sodium/calcium exchanger expression in the mouse and rat olfactory systems. J. Comp. Neurol. 501:944–958 10.1002/cne.21290 [DOI] [PubMed] [Google Scholar]
- Reisert J., Matthews H.R. 1998. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J. Gen. Physiol. 112:529–535 10.1085/jgp.112.5.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J., Matthews H.R. 2001a. Response properties of isolated mouse olfactory receptor cells. J. Physiol. 530:113–122 10.1111/j.1469-7793.2001.0113m.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J., Matthews H.R. 2001b. Simultaneous recording of receptor current and intraciliary Ca2+ concentration in salamander olfactory receptor cells. J. Physiol. 535:637–645 10.1111/j.1469-7793.2001.00637.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J., Bauer P.J., Yau K.W., Frings S. 2003. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J. Gen. Physiol. 122:349–363 10.1085/jgp.200308888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospars J.P., Lánský P., Duchamp A., Duchamp-Viret P. 2003. Relation between stimulus and response in frog olfactory receptor neurons in vivo. Eur. J. Neurosci. 18:1135–1154 10.1046/j.1460-9568.2003.02766.x [DOI] [PubMed] [Google Scholar]
- Saidu S.P., Weeraratne S.D., Valentine M., Delay R., Van Houten J.L. 2009. Role of plasma membrane calcium ATPases in calcium clearance from olfactory sensory neurons. Chem. Senses. 34:349–358 10.1093/chemse/bjp008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath A.P., Matthews H.R., Cornwall M.C., Fain G.L. 1998. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. J. Gen. Physiol. 111:53–64 10.1085/jgp.111.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A., Eisner D.A., Wray S. 1998. Carboxyeosin decreases the rate of decay of the [Ca2+]i transient in uterine smooth muscle cells isolated from pregnant rats. Pflugers Arch. 437:158–160 10.1007/s004240050761 [DOI] [PubMed] [Google Scholar]
- Tiffert T., Lew V.L. 1997. Cytoplasmic calcium buffers in intact human red cells. J. Physiol. 500:139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffert T., Lew V.L. 2001. Kinetics of inhibition of the plasma membrane calcium pump by vanadate in intact human red cells. Cell Calcium. 30:337–342 10.1054/ceca.2001.0241 [DOI] [PubMed] [Google Scholar]
- Varecka L., Carafoli E. 1982. Vanadate-induced movements of Ca2+ and K+ in human red blood cells. J. Biol. Chem. 257:7414–7421 [PubMed] [Google Scholar]
- Weeraratne S.D., Valentine M., Cusick M., Delay R., Van Houten J.L. 2006. Plasma membrane calcium pumps in mouse olfactory sensory neurons. Chem. Senses. 31:725–730 10.1093/chemse/bjl014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitere M., Tautz D., Neumann D., Steinfartz S. 2004. Adaptive divergence vs. environmental plasticity: tracing local genetic adaptation of metamorphosis traits in salamanders. Mol. Ecol. 13:1665–1677 10.1111/j.1365-294X.2004.02155.x [DOI] [PubMed] [Google Scholar]
- Yau K.W., Nakatani K. 1984. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 311:661–663 10.1038/311661a0 [DOI] [PubMed] [Google Scholar]
- Zufall F., Firestein S., Shepherd G.M. 1994. Cyclic nucleotide-gated ion channels and sensory transduction in olfactory receptor neurons. Annu. Rev. Biophys. Biomol. Struct. 23:577–607 10.1146/annurev.bb.23.060194.003045 [DOI] [PubMed] [Google Scholar]