Abstract
The time scale of the photoresponse in photoreceptor cells is set by the slowest of the steps that quench the light-induced activity of the phototransduction cascade. In vertebrate photoreceptor cells, this rate-limiting reaction is thought to be either shutoff of catalytic activity in the photopigment or shutoff of the pigment's effector, the transducin-GTP–phosphodiesterase complex. In suction pipette recordings from isolated salamander L-cones, we found that preventing changes in internal [Ca2+] delayed the recovery of the light response and prolonged the dominant time constant for recovery. Evidence that the Ca2+-sensitive step involved the pigment itself was provided by the observation that removal of Cl− from the pigment's anion-binding site accelerated the dominant time constant for response recovery. Collectively, these observations indicate that in L-cones, unlike amphibian rods where the dominant time constant is insensitive to [Ca2+], pigment quenching rate limits recovery and provides an additional mechanism for modulating the cone response during light adaptation.
INTRODUCTION
A major focus of phototransduction research over the past decade has been to understand the mechanisms governing the recovery of the light response, as these influence the visual system's ability to respond to repeated or prolonged stimulation (for review see Burns and Baylor, 2001; Fain et al., 2001; Lamb and Pugh, 2006). Sensory transduction in rods and cones is initiated by the light activation of a G protein–coupled receptor, which, along with a covalently bound 11-cis retinal chromophore, forms the photopigment. Light-activated photopigment (R*) activates a heterotrimeric G protein (transducin), which disinhibits an effector enzyme, cyclic guanosine monophosphate (cGMP) phosphodiesterase (PDE). The increase in PDE activity hydrolyzes cGMP and allows CNG channels to close, hyperpolarizing the photoreceptor and reducing synaptic glutamate release. The ensuing reduction in Ca2+ influx through the CNG channels is accompanied by continuing Ca2+ efflux via Na-Ca,K exchange, leading to a decline in outer segment [Ca2+] during the light response (Yau and Nakatani, 1985), which acts to accelerate cGMP synthesis by guanylyl cyclase (Koch and Stryer, 1988), to speed R* quenching by phosphorylation (Kawamura, 1993) and increase the cGMP affinity of the CNG channel (Hsu and Molday, 1993).
The recovery of the photoresponse, which entails not only restoration of the dark current, but also recovery of sensitivity to its original dark-adapted level, requires the shutoff of all active intermediates in the phototransduction cascade and the restoration of cGMP by guanylyl cyclase. The translational invariance of the recovery of the responses to bright saturating flashes of increasing intensity has been taken as indicating the presence of a single dominant time constant governing the recovery of the supersaturating flash response (Hodgkin and Nunn, 1988; Pepperberg et al., 1992; Nikonov et al., 1998), which is taken to represent the slowest of these quenching processes (see Pugh, 2006). In rods, R* quenching requires the Ca2+-dependent phosphorylation of its C terminus by rhodopsin kinase (Bownds et al., 1972; Kühn and Dreyer, 1972) and subsequent capping by arrestin (Kühn et al., 1984). While R* remains active it will continue to activate PDE via transducin, whose shutoff is dependent on its GTPase activity (Arshavsky and Bownds, 1992). Whichever of these two intermediates is quenched more slowly will govern shutoff of the transduction cascade and dominate photoresponse recovery.
The balance of evidence suggests that the dominant mechanism controlling response recovery in amphibian rods is Ca2+ independent (Lyubarsky et al., 1996; Matthews, 1996). Instead, a Ca2+-sensitive step early in phototransduction, which decays more quickly than the dominant time constant (Matthews, 1997), can be prolonged to dominate response recovery by substituting 11-cis-9-demethylretinal for the normal chromophore (Matthews et al., 2001). Thus, this process appears to represent the rapid Ca2+-sensitive quenching of R* (Kawamura, 1993), which therefore does not normally dominate recovery of the amphibian rod photoresponse.
In mammalian rods, however, it remains unclear whether shutoff of catalytic activity of the photopigment or PDE limits response recovery. In mouse rods, the dominant time constant can be speeded by the overexpression of RGS-9 (Krispel et al., 2006), suggesting that deactivation of the G protein–effector complex limits recovery of the photoresponse and places a short upper bound on R* lifetime (Burns and Pugh, 2009). In contrast, overexpression of bovine rhodopsin kinase, with the intention of speeding R* phosphorylation and deactivation (Kühn, 1978), did not alter response kinetics (Krispel et al., 2006). However, the increased variability of the single-photon response in mouse rods with reduced levels of arrestin and rhodopsin kinase has recently been interpreted as indicating that R* lifetime might instead control response recovery (Doan et al., 2009). Thus, the rate-limiting step for the shutoff of the mammalian rod phototransduction cascade remains controversial and may depend upon the mouse model and recording conditions used.
Although the overall architecture of phototransduction is similar in cones and rods, the photoresponse in cones is approximately fivefold faster and ∼100-fold less sensitive than in rods. These considerable differences in kinetics and sensitivity may serve as key constraints on the mechanisms that dominate recovery of the photoresponse. For instance, the faster cone response necessitates a shorter lifetime for activated PDE (Holcman and Korenbrot, 2005), a higher cGMP turnover by the phototransduction cascade (Cornwall et al., 1995), and a faster decline in [Ca2+] (Sampath et al., 1999) than in rods (Hodgkin and Nunn, 1988; Rieke and Baylor, 1996; Sampath et al., 1998; Nikonov et al., 2000).
Here, we have investigated the termination of the photoresponse in salamander L-cones. We find that the dominant time constant for recovery in L-cones is shortened by the fall in internal [Ca2+] during the light response, in dramatic contrast to the situation in rods, whose dominant time constant is independent of [Ca2+] (Lyubarsky et al., 1996; Matthews, 1996). Furthermore, we surprisingly find that manipulating the anion-binding site of the L-cone pigment (Kleinschmidt and Harosi, 1992) also shortens the dominant time constant. Collectively, these results suggest that the Ca2+-dependent shutoff of the photopigment rate limits the recovery of the photoresponse in salamander L-cones.
MATERIALS AND METHODS
Preparation and external solutions
All experiments were performed in the Physiological Laboratory at the University of Cambridge, except those to characterize the Cl− shift in spectral sensitivity, which were performed at Stanford University. Details of the preparation, recording techniques, light stimuli, and fast solution changes have been described in detail previously (Matthews, 1995; Sampath and Baylor, 2002). In brief, aquatic tiger salamanders (Charles D. Sullivan Co. Inc.) were dark-adapted overnight and killed under dim red illumination by stunning by cranial concussion, followed by decapitation and pithing, according to Schedule I of the Animals Scientific Procedures Act. Their eyes were removed and hemisected, and pieces of eyecup were stored in darkness at 4°C until required. Photoreceptors were dissociated mechanically from the isolated retina under infrared illumination. The resulting cell suspension was injected into the recording chamber and superfused with amphibian Ringer's solution (111 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1.6 mM MgCl2, 3 or 10 mM HEPES, and 10 mM glucose, adjusted to pH 7.6–7.7 with NaOH).
Electrical recording, light stimuli, and fast solution exchange
An isolated cone photoreceptor was drawn inner segment first into a suction pipette so that the outer segment remained exposed to the bathing solution. The suction pipette current signal was filtered over the bandwidth DC-40 Hz (Bessel filter) and digitized continuously at 200 Hz for subsequent analysis using a PC equipped with an intelligent interface card (Cambridge Research Systems).
Light stimuli were delivered from a dual-beam optical bench. The light source for the first beam, which was used to provide intense flashes, was a high-intensity mercury discharge lamp (Lumatec SUV-DC; Ultrafine Technology). The light source for the second beam, which was used to provide steady background light (see Fig. 3), was a tungsten halogen lamp driven from a regulated DC supply. The two beams passed through narrow-band interference filters (Comar Instruments), calibrated neutral density filters (SCHOTT), and high-speed shutters (Vincent Associates). The absolute intensity of the light from the bench was measured with a calibrated silicon photodiode optometer (model S370; Graseby Optronics), whose detector was placed at the position normally occupied by the recording chamber.
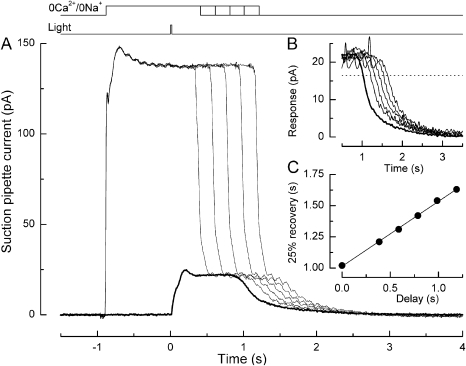
Figure 3.
Effect of prior steady illumination in Ringer's solution on the prolongation of the bright flash response in an L-cone by exposure to 0 Ca2+/0 Na+ solution. (A) Superimposed responses to bright flashes in Ringer's solution in darkness (heavy trace) or upon superfusion with 0 Ca2+/0 Na+ solution in darkness or after steady background illumination (light traces). The cone was exposed for 3 s to steady light, and then a bright flash was delivered and the background was extinguished. The solution superfusing the outer segment was rapidly changed from Ringer's solution to 0 Ca2+/0 Na+ solution 1 s before the flash, and then returned to Ringer's solution 600 ms thereafter. Each trace is the average of two responses in 0 Ca2+/0 Na+ solution and four responses in Ringer's solution; measurements were bracketed symmetrically in time. Top traces denote light and solution change monitors. Background intensities in 0 Ca2+/0 Na+ solution were 0, 6.00 × 102, 2.30 × 103, 9.86 × 103, and 3.96 × 104 photons µm−2 s−1 at 578 nm, evoking background responses of progressively increasing amplitude and flash responses of progressively decreasing duration. Bright flashes delivered 1.22 × 106 photons µm−2 at 578 nm. In 0 Ca2+/0 Na+ solution, sodium ions have been replaced with choline; the rising phase of the flash response in this solution is barely visible due to the virtual absence of permeant ions. (B) Recovery phases of the responses from A after subtraction of the junction current obtained upon the return to Ringer's solution after exposure to 0 Ca2+/0 Na+ solution during saturating light at the end of the experiment. Data have been digitally low-pass filtered at 20 Hz. Heavy trace denotes response in Ringer's solution. (C) Dependence of response duration after exposure to 0 Ca2+/0 Na+ solution on the circulating current during prior steady illumination (filled circles). Circulating current was measured in Ringer's solution just before the solution change. Response duration was measured as the time taken after the flash for the response to recover 25% of the original dark current in Ringer's solution (interrupted line in B); times of solution changes measured from the half-relaxation time of the junction current. Regression line fitted using a least-squares algorithm. Filled square represents time for 25% recovery of the flash response in Ringer's solution without prior background exposure.
Rapid solution changes from Ringer's solution to 0 Ca2+/0 Na+ solution were performed by translating the interface between two rapidly moving streams of solution across the exposed outer segment of the photoreceptor using a computer-controlled stepper motor coupled to the microscope stage (Matthews, 1995, 1996). The 0 Ca2+/0 Na+ solution, which was designed to minimize simultaneously influx and efflux of Ca2+, consisted of 111 mM choline chloride or guanidinium chloride (see individual figure legends), 2.5 mM KCl, 2 mM EGTA, and 3.0 mM HEPES, adjusted to pH 7.7–7.8 with tetramethylammonium hydroxide. Because CaCl2 and MgCl2 were entirely omitted from this solution, the EGTA buffer will have served to reduce the free divalent concentration to extremely low levels (Matthews, 1996). Previous experiments on salamander rods and cones indicate that exposure to such a 0 Ca2+/0 Na+ solution will hold [Ca2+] close to its initial value before the solution change for some 5–15 s thereafter (Matthews et al., 1988, 1990; Nakatani and Yau, 1988; Fain et al., 1989; Matthews and Fain, 2001). All experiments were performed at 20°C. Zero chloride solutions were prepared by substituting chloride with sulfate salts and adding 20 mM sucrose to equate approximately their osmolality to that of normal Ringer's solution. The osmolality of individual solutions was assessed using a vapor pressure osmometer (ELITech Group). Liquid junction currents between the dissimilar solutions in the suction pipette and bath were obtained by exposing the outer segment to 0 Ca2+/0 Na+ solution during steady light of sufficient intensity to completely suppress the dark current. The actual timings of solution changes were determined from the delay to 50% rise or fall of the junction current artifact.
RESULTS
Ca2+ modulates response recovery in L-cones
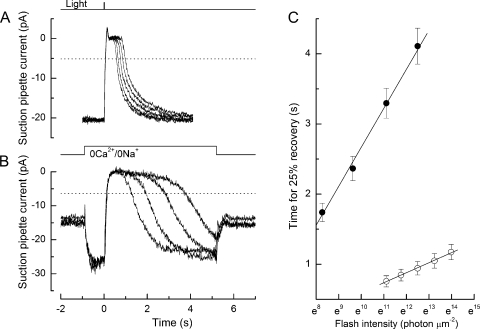
We investigated the influence of the light-induced decline of internal [Ca2+] in L-cones on the time course of response termination. The strategy for these experiments was to measure the time for recovery to a criterion level of the response to a bright flash of fixed strength, while the internal [Ca2+] was artificially maintained near the dark level for progressively increasing times after the flash (Matthews, 1997). This was accomplished by rapidly exposing the cone outer segment to a 0 Ca2+/0 Na+ solution designed to minimize simultaneously Ca2+ influx and efflux across the plasma membrane (Matthews et al., 1988; Nakatani and Yau, 1988). As shown in Fig. 1, the L-cone outer segment was first stepped into 0 Ca2+/0 Na+ solution, and a bright flash was delivered 1 s later. The change to this choline-substituted solution resulted in a substantial liquid junction current due to the dissimilar solutions in bath and suction pipette (Fig. 1 A); note that little photocurrent can be observed in this solution in response to the flash. At a variable time thereafter, the outer segment was returned to normal Ringer's solution, thereby allowing the internal [Ca2+] to fall to a greatly reduced common level before the onset of response recovery. Previous measurements of Ca2+ dye fluorescence indicate that when all the cGMP-gated channels are rapidly closed, this common [Ca2+] is achieved in <200 ms (Sampath et al., 1999). For flashes delivered in the absence of the solution change (Fig. 1, heavy trace), when internal [Ca2+] was allowed to fall immediately after the flash, the recovery of the response to a criterion level of 25% of the original dark current required ∼1 s (Fig. 1 B). However, holding the cone outer segment in 0 Ca2+/0 Na+ solution for a period after the flash delayed recovery to the criterion level, and this delay increased in direct proportion to the time spent in 0 Ca2+/0 Na+ solution (Fig. 1 C). Because internal [Ca2+] will have fallen rapidly to a common level upon the return to Ringer's solution, these changes in response duration cannot arise from rapid actions of Ca2+ upon guanylyl cyclase or the CNG channel. Instead, the progressive delay in the recovery of the bright flash response as the time spent in 0 Ca2+/0 Na+ solution increased indicates that recovery of the transduction cascade itself remained sensitive to Ca2+ for the entire duration of the flash response, in dramatic contrast to the situation in amphibian rods (Matthews, 1996, 1997). Collected data obtained from nine L-cones using this protocol are shown in Fig. 2. Response duration increased linearly with the time spent in 0 Ca2+/0 Na+ solution after the flash, with a slope of 0.49 ± 0.07 (SEM). The linearity of this relationship is consistent with the notion that the process that dominates L-cone recovery exhibits slow kinetics while the outer segment Ca2+ concentration is maintained at its initial dark-adapted level, and then accelerates once Ca2+ is allowed to fall to the much lower level that pertains during response saturation (Matthews et al., 2001). On the basis of this simple model, the halving of the slope of this relationship would correspond to an acceleration by at least twofold of a Ca2+-sensitive process that dominates L-cone response recovery (see Fig. 9 and Eq. 1 in Matthews et al., 2001).
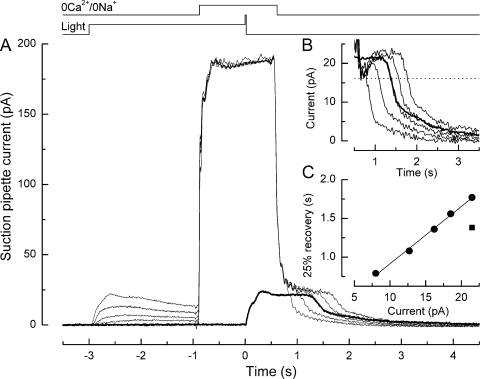
Figure 1.
Effect on the bright flash response in a salamander L-cone of superfusion with 0 Ca2+/0 Na+ solution. (A) Superimposed responses to bright flashes in Ringer's solution (heavy trace) and on exposure to 0 Ca2+/0 Na+ solution from 1 s before the flash until progressively increasing times thereafter (light traces). Top traces represent solution change and flash monitors. Suction pipette currents include a junction current resulting from the liquid junction potential between the dissimilar solutions in pipette and bath. Traces are the average of four responses in Ringer's solution and two responses in 0 Ca2+/0 Na+ solution; measurements were bracketed symmetrically in time. The bright flash delivered 1.22 × 106 photons µm−2 at 578 nm. In 0 Ca2+/0 Na+ solution, sodium ions have been replaced with choline; the virtual absence of external permeant ions abolished the inward dark current, but unlike the situation in rods, little outward potassium current was recorded, perhaps as a result of inner segment hyperpolarization (Matthews, 1995, 1996; Lyubarsky et al., 1996). (B) Recovery phases of the responses from A after subtraction of the junction current obtained upon the return to Ringer's solution after a 2-s exposure to 0 Ca2+/0 Na+ solution during saturating light at the end of the experiment. For each trace, the junction current has been offset in time to coincide with the return to Ringer's solution. Data have been digitally low-pass filtered at 20 Hz. Heavy trace denotes response in Ringer's solution. (C) Dependence of response duration on the time spent in 0 Ca2+/0 Na+ solution after the flash. Response duration was measured as the time taken after the flash for the response to recover 25% of the original dark current in Ringer's solution (interrupted line in B); times of solution changes measured from the half-relaxation time of the junction current. Regression line of slope 0.52 fitted using a least-squares algorithm.
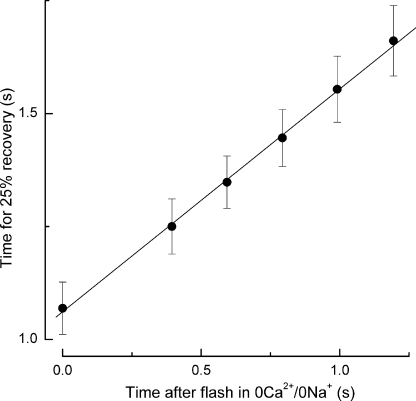
Figure 2.
Collected data illustrating the dependence of response duration on the time spent in 0 Ca2+/0 Na+ solution after a bright flash delivered in darkness, as in Fig. 1. Response duration was measured as the time taken after the flash for the recovery of 25% of the dark current in Ringer's solution. Mean data from nine L-cones; error bars represent SEM. Regression line of slope 0.49 ± 0.07 fitted using a weighted least-squares algorithm.
Background light–induced reduction in internal [Ca2+] speeds response termination
The Ca2+ dependence of response termination in L-cones provides a mechanism by which background light can modulate the sensitivity and time scale of the photoresponse. As the intensity of the background light increases and the photocurrent decreases, the internal [Ca2+] also declines in a graded manner (Younger et al., 1996; Sampath et al., 1999; Matthews and Fain, 2003; Leung et al., 2007). This graded decrease in [Ca2+] would be expected progressively to accelerate the Ca2+-dependent step dominating L-cone response recovery. The experiment in Fig. 3 investigates the effect of the background light–induced reduction in internal [Ca2+] on the recovery of the L-cone response. An L-cone was exposed to background light of several intensities (as well as darkness) for 2 s, and then stepped into 0 Ca2+/0 Na+ solution to hold the internal [Ca2+] close to the corresponding light-adapted level. After 1 s in 0 Ca2+/0 Na+ solution, a bright flash was delivered and the background was extinguished. Around 600 ms thereafter, the outer segment was returned to Ringer's solution to allow the further dynamic decline in Ca2+ to a common minimal level before the onset of response recovery. For the trial in darkness, recovery of the L-cone response to the criterion level required nearly 2 s, a value that progressively declined as the background light became brighter. In comparison, a flash delivered in Ringer's solution, which allows [Ca2+] to fall dynamically during the entire light response, evoked a response of intermediate duration (Fig. 3 B, heavy trace). When the outer segment was stepped to 0 Ca2+/0 Na+ solution before the flash, the time for 25% response recovery varied linearly with the prior steady-state circulating current in Ringer's solution during background illumination (Fig. 3 C), which due to balanced Ca2+ influx and efflux determines the subsequent level at which internal [Ca2+] is maintained after the solution change (Sampath et al., 1999).
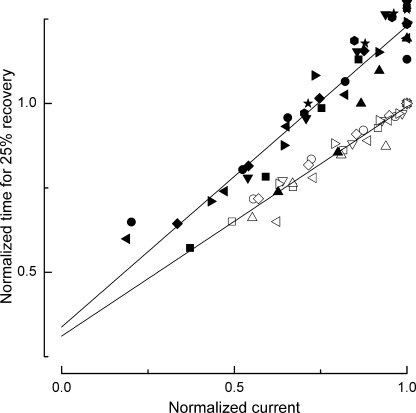
Collected data from nine L-cones obtained using this protocol are shown in Fig. 4 (filled symbols); in each case, the data have been normalized according to the duration and magnitude of the response to the same test flash in Ringer's solution. The linear relationship between response duration and the circulating current during prior background illumination also pertained when Ca2+ was allowed to change freely in Ringer's solution throughout the flash response (seven L-cones, open symbols); however, the slope of this relationship was more shallow because for any given degree of current suppression evoked by the background, the response recovered more rapidly in Ringer's solution than when the further dynamic fall in [Ca2+] was retarded by exposure to 0 Ca2+/0 Na+ solution. Linear fits to these data converged as the dark current approached 0, when [Ca2+] in the outer segment reaches its lowest value. Thus, the additional retardation of response recovery induced by delaying the dynamic fall in [Ca2+] with 0 Ca2+/0 Na+ solution immediately after the flash became ever smaller as the circulating current, and hence the outer segment [Ca2+] during prior background illumination, declined. Because, at the time at which the flash response recovers from saturation, [Ca2+] will have fallen to a common reduced level in each case. The response prolongation evoked by the prior exposure to 0 Ca2+/0 Na+ solution again must reflect actions of Ca2+ on the quenching of the transduction cascade, instead of fast actions on guanylyl cyclase or the CNG channel. Consequently, this result indicates that the Ca2+-sensitive process that dominates recovery of the transduction cascade in L-cones is accelerated in a graded manner as outer segment [Ca2+] falls, regardless of whether this decrease is achieved by prior background illumination or dynamically during the flash response.
Figure 4.
Collected data illustrating the dependence of response duration on the circulating current during prior steady illumination according to the protocol of Fig. 3. Filled symbols, flash delivered in 0 Ca2+/0 Na+ solution, as in Fig. 2 (nine L-cones); open symbols, flash delivered in Ringer's solution (seven L-cones). Circulating current was measured in Ringer's solution immediately before the solution change or flash and has been normalized for each cell to the dark current in Ringer's solution. Response duration was measured as the time taken after the flash for the recovery of 25% of the dark current in Ringer's solution and has been normalized for each cell to the time for 25% recovery of the flash response in Ringer's solution without prior background exposure. Regression lines of slope 0.89 ± 0.05 (0 Ca2+/0 Na+ solution) and 0.68 ± 0.03 (Ringer's solution) were fitted using a least-squares algorithm.
Preventing the light-induced fall in [Ca2+] prolongs the dominant time constant in L-cones
The experiments in Figs. 1 and 3 demonstrate that the duration of the L-cone flash response is strongly influenced by the internal [Ca2+]. We measured the dominant time constant more directly by monitoring the recovery of the circulating current after a series of bright flashes of increasing intensity. Over a range of supersaturating intensities, the slope relating the time for the response to recovery to a criterion level to the natural logarithm of flash intensity is believed to represent the dominant time constant for shutoff of the phototransduction cascade (τdom; Pepperberg et al., 1992; Nikonov et al., 1998).
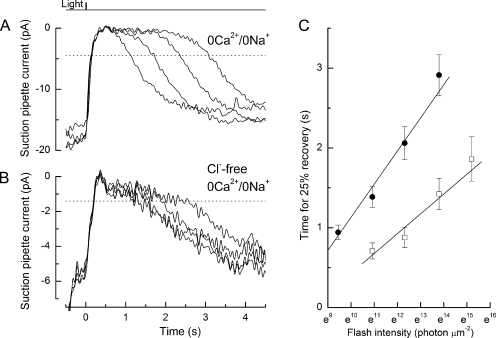
We compared values for τdom obtained from L-cones using bright flashes delivered either in normal Ringer's solution when Ca2+ is free to fall during the response (Fig. 5 A), or delivered after stepping the outer segment into 0 Ca2+/0 Na+ solution, which opposes this light-induced fall in Ca2+ concentration (Fig. 5 B). In these experiments, Na+ was replaced with the permeant cation guanidinium, which supports an inward dark current allowing the light response to be recorded during the 0 Ca2+/0 Na+ exposure (Nakatani and Yau, 1988; Matthews, 1995); response recovery was considerably retarded in this solution. The slope of the relationship between the time to reach a criterion level of recovery (interrupted lines) and the natural log of the flash intensity corresponds to τdom for L-cone response shutoff, provided that the flashes are of an intensity sufficiently high to evoke saturated responses, but not so great as to exceed the range of log linearity (Nikonov et al., 1998). Collected data obtained under these two conditions are plotted in Fig. 5 C. In Ringer's solution (open circles; seven L-cones), when [Ca2+] was free to fall during the response, the time for 25% recovery of the dark current varied only shallowly with the natural log of the flash intensity, yielding a value for τdom of 140 ms. In contrast, in 0 Ca2+/0 Na+ solution (filled circles; 12 L-cones) this relationship was considerably steeper, yielding a value for τdom of 560 ms, corresponding to a fourfold slowing of the dominant time constant.
Figure 5.
Determination of the dominant time constant from the dependence of L-cone response duration on flash intensity. (A) Flash delivered to a cone in Ringer's solution. (B) Flash delivered to another cone in 0 Ca2+/0 Na+ solution in which sodium was substituted with the permeant ion guanidinium, which does not support sodium–calcium exchange (Matthews et al., 1988; Nakatani and Yau, 1988). Dotted lines denote recovery of 25% of the dark current immediately preceding the flash in each solution. Flashes in Ringer's solution increased from 6.7 × 104 to 1.2 × 106 photons µm−2 by factors of ∼2; flashes in 0 Ca2+/0 Na+ solution increased from 3.9 × 103 to 1.2 × 106 by factors of ∼4. (C) Mean data for the dependence of response duration on flash intensity in Ringer's solution (open circles, as in A; seven cells) and 0 Ca2+/0 Na+ solution (filled circles, as in B; 12 cells); error bars denote SEM. Regression lines of slopes 0.14 ± 0.04 s (Ringer's solution) and 0.56 ± 0.06 s (0 Ca2+/0 Na+ solution), fitted by a weighted least-squares algorithm.
These results indicate that under conditions where [Ca2+] is free to fall, τdom becomes shorter for L-cones, which makes the interpretation of τdom in Ringer's solution ambiguous. Instead, the value for τdom is only meaningful if it is qualified based on the instantaneous Ca2+ concentration. Because recovery of the transduction cascade is progressively accelerated by the reduction in [Ca2+] and circulating current during increasing intensities of steady background illumination (see Fig. 3), we also expect a graded reduction in τdom. Indeed, when the circulating current is fully suppressed in Ringer's solution, because [Ca2+] falls to its lowest levels during response saturation, τdom must be near to its shortest value, allowing the full extent of its modulation by [Ca2+] to be appreciated. Thus, unlike rod photoreceptors, where τdom is independent of the internal [Ca2+] (Lyubarsky et al., 1996; Matthews, 1996), the Ca2+ concentration determines the time course of response recovery in L-cones.
Removal of external Cl− blue-shifts the spectral sensitivity and speeds the light response
Unequivocal identification of the site at which Ca2+ modulates the dominant time constant in L-cones requires a means of selectively modifying the shutoff of the transduction cascade (cf. Krispel et al., 2006). If the selective modification of one of the quenching steps causes a speeding of the dominant time constant, one can conclude that step contributes to the limitation of response recovery (Pugh, 2006). We exploited a unique property of the L-cone pigment to influence selectively the phototransduction cascade. In many species, the photopigment of long wavelength-sensitive cones carries an anion-binding site near the binding site for 11-cis retinal (Wang et al., 1993) that acts to further red-shift the wavelength of maximum spectral sensitivity (Kleinschmidt and Harosi, 1992). Because the cone outer segment consists of a continuously invaginated plasma membrane rather than the internally contained discs of rods (Nilsson, 1964), it is possible to manipulate this anion-binding site directly by changing the solution bathing the outer segment (cf. Sampath and Baylor, 2002).
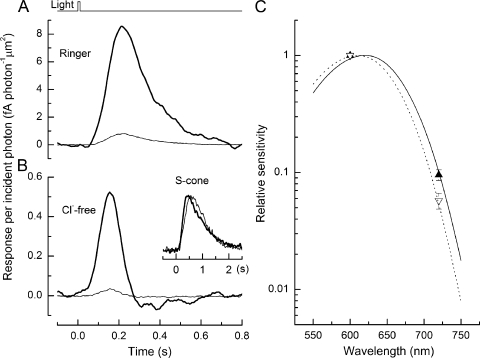
The effect of anions on the L-cone photopigment is illustrated in the experiment in Fig. 6, in which the responses to flashes of wavelength 600 nm (heavy trace) and 720 nm (light trace) in Ringer's solution (Fig. 6 A) are compared with the responses obtained to flashes at these two wavelengths when Cl− had been rapidly replaced with SO42− in the Ringer's solution bathing the outer segment (Fig. 6 B). Surprisingly and fortuitously, the removal of Cl− from the external Ringer's solution both accelerated the L-cone response and reduced the response sensitivity. Such effects were present within 1 s of the fast solution change (see Fig. 7). This rapid effect is too fast to result from changes in internal Cl− concentration because the outer segment lacks permeation mechanisms for anions, suggesting that removal of Cl− influences a site on the external surface of the L-cone outer segment membrane associated with response termination. To test the specificity of the removal of Cl− from the external Ringer's solution, similar experiments were performed on three salamander S-cones, which do not have an anion-binding site (Kleinschmidt and Harosi, 1992). As shown in Fig. 6 B (inset), these light responses exhibit essentially no change in dim flash response kinetics upon removal of Cl− from Ringer's solution, indicating that response speeding in L-cones is not due to changes in external Cl− per se or to alterations in internal Cl− concentration, but instead results from a specific action of anions on the pigment molecule.
Figure 6.
Dependence of dim flash response properties upon the anion present in the solution bathing the outer segment. Derived response per incident photon from an L-cone for 10-ms dim flashes of wavelength 600 nm (heavy trace) and 720 nm (light trace) in Ringer's solution (A), and in a Ringer's solution in which sulfate had been substituted for chloride (B). The difference in the average response amplitude per incident photon stems from the reduced sensitivity of the cone at the longer wavelength. In Ringer's solution, the average ratio of the sensitivity at 720 nm to that at 600 nm was 9.5 ± 1% (mean ± SEM; nine cells), whereas in Cl−-free Ringer's solution, the relative sensitivity decreased to 5.7 ± 0.9% (mean ± SEM; nine cells) at the longer wavelength. Note also that removal of external Cl− substantially reduced the sensitivity and speeded the kinetics of the response. (Inset) Responses of a salamander S-cone to a dim flash of 80 photons µm−2 at 460 nm in Ringer's solution (heavy trace) and Cl−-free solution (light trace). Plotted responses were averaged from five trials and normalized to their peak amplitude to compare time course. (C) Mean sensitivity at 600 and 720 nm, normalized to the sensitivity at the shorter wavelength. Filled upright triangles, Ringer's solution; open inverse triangles, Cl−-free solution. Spectral sensitivity curves are plotted according to the visual pigment nomogram of Govardovskii et al. (2000), with peak sensitivity at 610 nm (solid curve) and 620 nm (interrupted curve) adjusted by eye to pass through the 720-nm data point in each solution.
Figure 7.
Effect of anion substitution on the dominant time constant. Flashes delivered to two L-cones in guanidinium-substituted 0 Ca2+/0 Na+ solution containing either (A) chloride or (B) sulfate as anion. Dotted lines denote recovery of 25% of the dark current immediately preceding the flash in each solution. Flash intensities increased by factors of ∼4 from 1.3 × 104 to 9.8 × 105 photons µm−2 in chloride-containing 0 Ca2+/0 Na+ solution and 5.5 × 104 to 4.0 × 106 photons µm−2 in sulfate-containing 0 Ca2+/0 Na+ solution. Data have been digitally low-pass filtered at 10 Hz. (C) Mean data for the dependence of response duration on flash intensity in guanidinium-substituted 0 Ca2+/0 Na+ solution containing chloride (filled circles; 13 cells) or sulfate (open squares; seven cells) as anion. Regression lines of slopes 0.42 ± 0.05 s (chloride) and 0.25 ± 0.05 s (sulfate) fitted by a weighted least-squares algorithm. The cells in Cl−-containing solution exhibited a somewhat lower sensitivity and shorter dominant time constant than those of Fig. 5 C, probably reflecting their origin from a different batch of animals obtained at a later time, but within each figure the comparisons are contemporaneous.
Replacement of external Cl− with SO42− also blue-shifted the spectral sensitivity of the L-cone pigment (see also Kleinschmidt and Harosi, 1992). We estimated the spectral shift by comparing the sensitivity of the L-cone to flash stimuli at 600 nm, near the wavelength of peak sensitivity, and at the longer wavelength of 720 nm. Because the spectral sensitivity falls sharply at long wavelengths relative to the peak, a shift of the pigment spectrum toward shorter wavelengths should be accompanied by a decrease in the sensitivity at 720 nm relative to that at 600 nm. This effect can be observed in Fig. 6 (A and B), where the reduction in response amplitude per incident photon at 720 nm relative to that at 600 nm is greater in Cl−-free solution than in Ringer's solution. Fig. 6 C compares the relative sensitivity to 600- and 720-nm stimuli in Cl−-free solution (open inverse triangles) with that in normal Ringer's solution (filled upright triangles) for nine L-cones. The relative sensitivity at 720 nm decreased in every cone tested when Cl− was replaced with SO42− in the external solution (mean decrease to 60 ± 11%; nine L-cones; ±SEM), a difference significant at the 5% level (paired t test; P = 0.013), corresponding to a blue shift in the pigment spectrum. This spectral shift can be visualized directly by overlaying the pigment nomogram (Lamb, 1995; Govardovskii et al., 2000) upon the normalized mean spectral sensitivity as a function of wavelength in the presence (Fig. 6 C, solid curve) and absence (interrupted curve) of external Cl−. The 0.6-fold depression of the relative sensitivity at 720 nm corresponds to an ∼10-nm blue shift in spectral sensitivity. Thus, consistent with previous studies (Kleinschmidt and Harosi, 1992), the removal of Cl− from the external Ringer's solution shifts the spectral properties of the L-cone pigment and also causes a speeding of the dim flash response (Fig. 6 B).
Removal of external Cl− speeds τdom when the fall in internal Ca2+ is prevented
The dramatic speeding of the dim flash response and reduction in elementary response amplitude in L-cones when external Cl− is substituted with SO42− suggest the faster quenching of the phototransduction cascade, a mechanism that has not been previously documented. We investigated the effect of this anion substitution on τdom by exposing L-cones to supersaturating flashes of progressively increasing intensity in guanidinium-substituted 0 Ca2+/0 Na+ solution containing either Cl− (Fig. 7 A) or SO42− (Fig. 7 B) as the anion 1 s before the flash. Both of these solutions would be expected to hold internal [Ca2+] near the dark level, and therefore to differ solely in their expected influence on the photopigment. Collected data are plotted in Fig. 7 C for the time for 25% recovery of the responses to bright flashes delivered in 0 Ca2+/0 Na+ solution (filled circles), and for flashes delivered in a 0 Ca2+/0 Na+ solution in which Cl− had been replaced with SO42− (open squares), which as a divalent anion cannot associate with the L-cone anion-binding site (Shichida et al., 1990). Removal of external Cl− resulted in a near halving of the slope of the relationship between the time for 25% recovery and the natural log of the flash intensity, indicating a near twofold acceleration of τdom upon removal of Cl−, although the internal [Ca2+] remained around the dark level. Thus, the removal of external Cl− speeds the dominant time constant for shutoff in L-cones, the key criterion for establishing the identity of this step (Pugh, 2006). This result strongly supports the notion that the Ca2+-dependent process that dominates L-cone flash response recovery represents photopigment quenching.
DISCUSSION
Ca2+-dependent control of catalytic lifetime of the cone pigment
The experiments presented above reveal two distinct and important points about the dominant time constant for shutoff in salamander L-cones. First, the dominant time constant is modulated by the dynamic fall in Ca2+ that occurs naturally during the light response (Figs. 1–5). Second, the novel demonstration that the dominant time constant is speeded by a selective manipulation of the L-cone pigment: the removal of Cl− from the opsin anion-binding site (Figs. 6 and 7). When taken together, these two observations indicate that Ca2+-dependent quenching of photopigment serves to limit the recovery of the L-cone photoresponse, in dramatic contrast to the situation in amphibian rods, in which Ca2+-dependent pigment quenching takes place more rapidly (Matthews, 1997; Matthews et al., 2001).
Although our data do not uniquely specify the mechanism underlying this Ca2+-dependent pigment quenching, a role for pigment phosphorylation in rate-limiting recovery of the L-cone light response seems probable, given the wealth of biochemical evidence from rod photoreceptors. The phosphorylation of rhodopsin's C terminus is well known to be modulated by Ca2+ in amphibian and mammalian rods (Kawamura, 1993; Chen et al., 1995; Klenchin et al., 1995). Recent biochemical and molecular biological evidence from zebrafish cones also suggests that pigment phosphorylation may serve this limiting role. Analysis of the light-dependent phosphorylation of cone opsin C termini indicates a strong Ca2+ dependence of both the extent and sites of phosphorylation (Kennedy et al., 2004). Furthermore, the siRNA knockdown of GRK7 (a cone-specific kinase) leads to clear alterations in cone function (Rinner et al., 2005). Electroretinogram recordings from these fish indicate impaired cone photoresponse recovery, which is accompanied by a behavioral reduction in contrast sensitivity. A further constraint upon the Ca2+-dependent rate-limiting mechanism is provided by the observation in the present study of an approximately linear relationship between the magnitude of the dark current and the time of onset of photoresponse recovery to a fixed-intensity flash (Fig. 3 C). The linearity of this relationship suggests that the site of action for Ca2+ has a dissociation constant (Kd) above the dark [Ca2+] in the L-cone outer segment, so that changes in [Ca2+] lead to equal changes in the activity of this component of phototransduction cascade throughout the light response. Because the outer segment [Ca2+] in salamander L-cones is ∼400 nM in darkness (Sampath et al., 1999), the affinity of this site may be in the low micromolar range, a value consistent with a role for the protein recoverin, which has a Kd of 1–3 µM in cones (Chen et al., 1995; Klenchin et al., 1995). The distinction between the control of photoresponse recovery in rods and L-cones, which is reported here, may not necessarily apply to all cone classes. In many ways, individual cone classes are as different from each other as they are from rods. For instance, salamander S-cones are considerably more sensitive and have a slower photoresponse than their L-cones (Perry and McNaughton, 1991). In contrast, L-, M-, and S-cones in the primate all appear to exhibit similar sensitivities (Schnapf et al., 1990). It will be important in the future to determine whether the dominance of photopigment quenching in photoresponse recovery also applies to other cone classes.
Shutoff of phototransduction in rods versus cones: implications for background adaptation
Signaling cascades perform a multitude of critical biological functions; in each case, their detailed features need to be adjusted to meet specific functional requirements. In many cases, the precise temporal and spatial properties of a signaling cascade are determined by the activity or concentration of its individual components. Although rod and cone photoreceptors share a common G protein–coupled signaling cascade architecture, in most cases rods and cones express distinct proteins for its components. These differences are collectively responsible for shaping the light response in each cell type, allowing the cone photoresponse to be faster and less sensitive than that in rods. However, when the human or salamander L-cone pigment is expressed in mouse rods, or the human rod pigment is expressed in Xenopus L-cones, the kinetics of the responses generated by these pigment molecules are identical to those from the native pigment (Kefalov et al., 2003), whereas mice in which rhodopsin has been replaced with the mouse green cone pigment exhibit rod responses with normal kinetics but a reduced amplification constant (Sakurai et al., 2007). Thus, the subsequent components of the phototransduction cascade appear to be critical in shaping the sensitivity and time scale of the light response.
Differences in the concentration of the individual components of phototransduction are likely to further contribute to differences between rod and cone photoresponses. For instance, expression of the GTPase accelerating protein RGS-9 is ∼10-fold higher in cones than rods (Cowan et al., 1998; Zhang et al., 2003), a difference that would be expected to accelerate the time course of the light response if the rate of PDE shutoff were to limit response recovery. Indeed, the mean PDE lifetime has been shown to be ∼10 times shorter in fish cones than in nonmammalian rods (Holcman and Korenbrot, 2005). The present study suggests, however, that in L-cones this increased expression of RGS-9 accelerates G protein/effector shutoff to such an extent that it no longer limits response recovery, with the consequence that pigment quenching instead becomes dominant. Furthermore, the turnover of cGMP is at least sixfold faster in salamander L-cones than in rods (Perry and McNaughton, 1991), implying that this fast cascade shutoff will be reflected directly in the kinetics of the cone light response. The recent demonstration that guanylyl cyclase activity is >10 times higher in carp cones than in rods (Takemoto et al., 2009) implies that cGMP turnover is unlikely to be response limiting either in darkness or steady light.
Many of the adaptive changes in the salamander rod light response that accompany background adaptation can be achieved simply through the elevation in cGMP turnover that is evoked by steady light (Nikonov et al., 2000), which has been estimated to increase by 10- to 20-fold in salamander rods over the full range of background intensities (Hodgkin and Nunn, 1988; Cornwall and Fain, 1994; Nikonov et al., 2000). Although direct measurements for the increase in PDE rate during illumination are not available for salamander L-cones, measurements of cyclase rates suggest a much more modest increase of around 3.5-fold (Cornwall et al., 1995). Thus, the role of cGMP turnover in the control of the sensitivity and time course of cone light responses during background adaptation seems likely to be considerably more modest than in rods.
Given the tremendous capacity of cones for background adaptation (Baylor and Hodgkin, 1974; Matthews et al., 1990; Schneeweis and Schnapf, 1999), additional mechanisms must therefore largely be responsible for the background-dependent changes in the light response waveform (see also Soo et al., 2008). Although, at still higher light intensities, photopigment bleaching is likely to depress response sensitivity further (Burkhardt, 1994), at dimmer background light intensities, changes in [Ca2+] seem likely to play a major role in controlling response kinetics and sensitivity. In many ways, Ca2+ is ideal as a feedback messenger for controlling the properties of the cone photoresponse. From darkness to saturating light, outer segment [Ca2+] changes ∼80-fold in salamander L-cones (Sampath et al., 1999), a larger dynamic range than is observed in rods (∼10-fold in mice and ∼20-fold in salamander; Sampath et al., 1998; Woodruff et al., 2002), thereby allowing Ca2+-binding sites in the cone phototransduction cascade to be modulated to a greater extent during adaptation. Such effects of Ca2+ are apparent in earlier experiments in which changes in [Ca2+] are opposed during the cone light response (Matthews et al., 1988, 1990; Nakatani and Yau, 1988). The likely extent of these modulatory effects of [Ca2+] on the kinetics of the dim flash response can be appreciated from the fourfold reduction in dominant time constant that accompanies the fall in [Ca2+] from darkness to saturating light (Fig. 5 C). It will be important in the future to directly investigate the effects of these changes on the dynamics of the dim flash response in cones during adaptation to background light.
Acknowledgments
We thank Denis Baylor, Jeannie Chen, and Fred Rieke for helpful discussions and comments on the manuscript. A.P. Sampath would like to specially thank Denis Baylor and Fred Rieke for support and encouragement.
This work was supported by the Wellcome Trust (to H.R. Matthews), National Institutes of Health (grants NRSA EY14784 and EY17606), and a Karl Kirchgessner Foundation Vision Research Grant (to A.P. Sampath).
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- cGMP
- cyclic guanosine monophosphate
- PDE
- cGMP phosphodiesterase
References
- Arshavsky V.Y., Bownds M.D. 1992. Regulation of deactivation of photoreceptor G protein by its target enzyme and cGMP. Nature. 357:416–417 10.1038/357416a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D.A., Hodgkin A.L. 1974. Changes in time scale and sensitivity in turtle photoreceptors. J. Physiol. 242:729–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownds D., Dawes J., Miller J., Stahlman M. 1972. Phosphorylation of frog photoreceptor membranes induced by light. Nat. New Biol. 237:125–127 [DOI] [PubMed] [Google Scholar]
- Burkhardt D.A. 1994. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J. Neurosci. 14:1091–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns M.E., Baylor D.A. 2001. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu. Rev. Neurosci. 24:779–805 10.1146/annurev.neuro.24.1.779 [DOI] [PubMed] [Google Scholar]
- Burns M.E., Pugh E.N., Jr. 2009. RGS9 concentration matters in rod phototransduction. Biophys. J. 97:1538–1547 10.1016/j.bpj.2009.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.K., Inglese J., Lefkowitz R.J., Hurley J.B. 1995. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J. Biol. Chem. 270:18060–18066 10.1074/jbc.270.30.18060 [DOI] [PubMed] [Google Scholar]
- Cornwall M.C., Fain G.L. 1994. Bleached pigment activates transduction in isolated rods of the salamander retina. J. Physiol. 480:261–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Matthews H.R., Crouch R.K., Fain G.L. 1995. Bleached pigment activates transduction in salamander cones. J. Gen. Physiol. 106:543–557 10.1085/jgp.106.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.W., Fariss R.N., Sokal I., Palczewski K., Wensel T.G. 1998. High expression levels in cones of RGS9, the predominant GTPase accelerating protein of rods. Proc. Natl. Acad. Sci. USA. 95:5351–5356 10.1073/pnas.95.9.5351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan T., Azevedo A.W., Hurley J.B., Rieke F. 2009. Arrestin competition influences the kinetics and variability of the single-photon responses of mammalian rod photoreceptors. J. Neurosci. 29:11867–11879 10.1523/JNEUROSCI.0819-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G.L., Lamb T.D., Matthews H.R., Murphy R.L. 1989. Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J. Physiol. 416:215–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G.L., Matthews H.R., Cornwall M.C., Koutalos Y. 2001. Adaptation in vertebrate photoreceptors. Physiol. Rev. 81:117–151 [DOI] [PubMed] [Google Scholar]
- Govardovskii V.I., Fyhrquist N., Reuter T., Kuzmin D.G., Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17:509–528 10.1017/S0952523800174036 [DOI] [PubMed] [Google Scholar]
- Hodgkin A.L., Nunn B.J. 1988. Control of light-sensitive current in salamander rods. J. Physiol. 403:439–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D., Korenbrot J.I. 2005. The limit of photoreceptor sensitivity: molecular mechanisms of dark noise in retinal cones. J. Gen. Physiol. 125:641–660 10.1085/jgp.200509277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.T., Molday R.S. 1993. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 361:76–79 10.1038/361076a0 [DOI] [PubMed] [Google Scholar]
- Kawamura S. 1993. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 362:855–857 10.1038/362855a0 [DOI] [PubMed] [Google Scholar]
- Kefalov V., Fu Y., Marsh-Armstrong N., Yau K.-W. 2003. Role of visual pigment properties in rod and cone phototransduction. Nature. 425:526–531 10.1038/nature01992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.J., Dunn F.A., Hurley J.B. 2004. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 41:915–928 10.1016/S0896-6273(04)00086-8 [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Harosi F.I. 1992. Anion sensitivity and spectral tuning of cone visual pigments in situ. Proc. Natl. Acad. Sci. USA. 89:9181–9185 10.1073/pnas.89.19.9181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenchin V.A., Calvert P.D., Bownds M.D. 1995. Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J. Biol. Chem. 270:24127–24129 10.1074/jbc.270.41.24127 [DOI] [PubMed] [Google Scholar]
- Koch K.W., Stryer L. 1988. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 334:64–66 10.1038/334064a0 [DOI] [PubMed] [Google Scholar]
- Krispel C.M., Chen D., Melling N., Chen Y.J., Martemyanov K.A., Quillinan N., Arshavsky V.Y., Wensel T.G., Chen C.K., Burns M.E. 2006. RGS expression rate-limits recovery of rod photoresponses. Neuron. 51:409–416 10.1016/j.neuron.2006.07.010 [DOI] [PubMed] [Google Scholar]
- Kühn H. 1978. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 17:4389–4395 10.1021/bi00614a006 [DOI] [PubMed] [Google Scholar]
- Kühn H., Dreyer W.J. 1972. Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 20:1–6 10.1016/0014-5793(72)80002-4 [DOI] [PubMed] [Google Scholar]
- Kühn H., Hall S.W., Wilden U. 1984. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 176:473–478 10.1016/0014-5793(84)81221-1 [DOI] [PubMed] [Google Scholar]
- Lamb T.D. 1995. Photoreceptor spectral sensitivities: common shape in the long-wavelength region. Vision Res. 35:3083–3091 10.1016/0042-6989(95)00114-F [DOI] [PubMed] [Google Scholar]
- Lamb T.D., Pugh E.N., Jr. 2006. Phototransduction, dark adaptation, and rhodopsin regeneration. The proctor lecture. Invest. Ophthalmol. Vis. Sci. 47:5138–5152 10.1167/iovs.06-0849 [DOI] [PubMed] [Google Scholar]
- Leung Y.T., Fain G.L., Matthews H.R. 2007. Simultaneous measurement of current and calcium in the ultraviolet-sensitive cones of zebrafish. J. Physiol. 579:15–27 10.1113/jphysiol.2006.120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky A., Nikonov S., Pugh E.N., Jr. 1996. The kinetics of inactivation of the rod phototransduction cascade with constant Ca2+i. J. Gen. Physiol. 107:19–34 10.1085/jgp.107.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R. 1995. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J. Physiol. 484:267–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R. 1996. Static and dynamic actions of cytoplasmic Ca2+ in the adaptation of responses to saturating flashes in salamander rods. J. Physiol. 490:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R. 1997. Actions of Ca2+ on an early stage in phototransduction revealed by the dynamic fall in Ca2+ concentration during the bright flash response. J. Gen. Physiol. 109:141–146 10.1085/jgp.109.2.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R., Fain G.L. 2001. A light-dependent increase in free Ca2+ concentration in the salamander rod outer segment. J. Physiol. 532:305–321 10.1111/j.1469-7793.2001.0305f.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R., Fain G.L. 2003. The effect of light on outer segment calcium in salamander rods. J. Physiol. 552:763–776 10.1113/jphysiol.2003.050724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R., Murphy R.L., Fain G.L., Lamb T.D. 1988. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 334:67–69 10.1038/334067a0 [DOI] [PubMed] [Google Scholar]
- Matthews H.R., Fain G.L., Murphy R.L., Lamb T.D. 1990. Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J. Physiol. 420:447–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R., Cornwall M.C., Crouch R.K. 2001. Prolongation of actions of Ca2+ early in phototransduction by 9-demethylretinal. J. Gen. Physiol. 118:377–390 10.1085/jgp.118.4.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K.-W. 1988. Calcium and light adaptation in retinal rods and cones. Nature. 334:69–71 10.1038/334069a0 [DOI] [PubMed] [Google Scholar]
- Nikonov S., Engheta N., Pugh E.N., Jr. 1998. Kinetics of recovery of the dark-adapted salamander rod photoresponse. J. Gen. Physiol. 111:7–37 10.1085/jgp.111.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov S., Lamb T.D., Pugh E.N., Jr. 2000. The role of steady phosphodiesterase activity in the kinetics and sensitivity of the light-adapted salamander rod photoresponse. J. Gen. Physiol. 116:795–824 10.1085/jgp.116.6.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S.E. 1964. An electron microscopic classification of the retinal receptors of the leopard frog (Rana pipiens). J. Ultrastruct. Res. 10:390–416 10.1016/S0022-5320(64)80018-6 [DOI] [PubMed] [Google Scholar]
- Pepperberg D.R., Cornwall M.C., Kahlert M., Hofmann K.P., Jin J., Jones G.J., Ripps H. 1992. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis. Neurosci. 8:9–18 10.1017/S0952523800006441 [DOI] [PubMed] [Google Scholar]
- Perry R.J., McNaughton P.A. 1991. Response properties of cones from the retina of the tiger salamander. J. Physiol. 433:561–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E.N., Jr. 2006. RGS expression level precisely regulates the duration of rod photoresponses. Neuron. 51:391–393 10.1016/j.neuron.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Rieke F., Baylor D.A. 1996. Molecular origin of continuous dark noise in rod photoreceptors. Biophys. J. 71:2553–2572 10.1016/S0006-3495(96)79448-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinner O., Makhankov Y.V., Biehlmaier O., Neuhauss S.C. 2005. Knockdown of cone-specific kinase GRK7 in larval zebrafish leads to impaired cone response recovery and delayed dark adaptation. Neuron. 47:231–242 10.1016/j.neuron.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Sakurai K., Onishi A., Imai H., Chisaka O., Ueda Y., Usukura J., Nakatani K., Shichida Y. 2007. Physiological properties of rod photoreceptor cells in green-sensitive cone pigment knock-in mice. J. Gen. Physiol. 130:21–40 10.1085/jgp.200609729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath A.P., Baylor D.A. 2002. Molecular mechanism of spontaneous pigment activation in retinal cones. Biophys. J. 83:184–193 10.1016/S0006-3495(02)75160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath A.P., Matthews H.R., Cornwall M.C., Fain G.L. 1998. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. J. Gen. Physiol. 111:53–64 10.1085/jgp.111.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath A.P., Matthews H.R., Cornwall M.C., Bandarchi J., Fain G.L. 1999. Light-dependent changes in outer segment free-Ca2+ concentration in salamander cone photoreceptors. J. Gen. Physiol. 113:267–277 10.1085/jgp.113.2.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf J.L., Nunn B.J., Meister M., Baylor D.A. 1990. Visual transduction in cones of the monkey Macaca fascicularis. J. Physiol. 427:681–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis D.M., Schnapf J.L. 1999. The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. J. Neurosci. 19:1203–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y., Kato T., Sasayama S., Fukada Y., Yoshizawa T. 1990. Effects of chloride on chicken iodopsin and the chromophore transfer reactions from iodopsin to scotopsin and B-photopsin. Biochemistry. 29:5843–5848 10.1021/bi00476a028 [DOI] [PubMed] [Google Scholar]
- Soo F.S., Detwiler P.B., Rieke F. 2008. Light adaptation in salamander L-cone photoreceptors. J. Neurosci. 28:1331–1342 10.1523/JNEUROSCI.4121-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto N., Tachibanaki S., Kawamura S. 2009. High cGMP synthetic activity in carp cones. Proc. Natl. Acad. Sci. USA. 106:11788–11793 10.1073/pnas.0812781106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Asenjo A.B., Oprian D.D. 1993. Identification of the Cl(-)-binding site in the human red and green color vision pigments. Biochemistry. 32:2125–2130 10.1021/bi00060a001 [DOI] [PubMed] [Google Scholar]
- Woodruff M.L., Sampath A.P., Matthews H.R., Krasnoperova N.V., Lem J., Fain G.L. 2002. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 542:843–854 10.1113/jphysiol.2001.013987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K.-W., Nakatani K. 1985. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 313:579–582 10.1038/313579a0 [DOI] [PubMed] [Google Scholar]
- Younger J.P., McCarthy S.T., Owen W.G. 1996. Light-dependent control of calcium in intact rods of the bullfrog Rana catesbeiana. J. Neurophysiol. 75:354–366 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wensel T.G., Kraft T.W. 2003. GTPase regulators and photoresponses in cones of the eastern chipmunk. J. Neurosci. 23:1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]