Abstract
The cell wall protein Hwp1 was originally demonstrated to be expressed exclusively in hyphae of Candida albicans and cross-linked to human epithelium by mammalian transglutaminase. Hwp1 is expressed on the walls of hyphae formed by a/α, a/a, and α/α cells. Hence, it is expressed on hyphae independently of mating type. However, Hwp1 is selectively expressed on the wall of conjugation tubes formed by a/a cells, but not α/α cells, in the mating process. This was demonstrated in all possible crosses between four unrelated natural a/a strains and four unrelated α/α strains. In zygotes, Hwp1 is restricted to that portion of the wall of the conjugation bridge contributed by the a/a parent cell. Hwp1 staining further revealed that the first daughter bud that emerges from the conjugation bridge does so from the a/a-contributed portion. Hwp1 expression and localization during the mating process is, therefore, mating type specific, opaque phase specific, and α-pheromone induced. These results indicate that the mating type-specific contributions to the conjugation bridge during the mating process in C. albicans are qualitatively and functionally distinct and that the a/a portion of the bridge, which selectively contains Hwp1, bears the first daughter cell in the mating process.
INTRODUCTION
The mannoprotein Hwp1 is expressed on the surface of hyphae in the pathogen Candida albicans (Staab et al., 1996). This protein has been demonstrated to serve as a substrate for mammalian transglutaminase, which cross-links C. albicans to epithelial cells (Staab et al., 1999). HWP1 null mutants have been shown to be defective in forming stable attachments to buccal epithelium and to be less virulent in mouse systemic models (Staab et al., 1999; Tsuchimori et al., 2000; Sundstrom et al., 2002). It has been proposed, therefore, that Hwp1 plays a role in hypha-specific adhesion during C. albicans pathogenesis (Sundstrom, 1999; Sundstrom et al., 2002).
Recently, it was demonstrated that the majority of C. albicans strains are heterozygous for mating type (a/α) (Hull and Johnson, 1999; Lockhart et al., 2002). To mate, these cells must undergo homozygosis at the mating type locus. Mating has been demonstrated to occur between homozygous a and homozygous α cells both in vitro (Magee and Magee, 2000; Miller and Johnson, 2002; Lockhart et al., 2003a) and in vivo (Hull et al., 2000). Mating involves pheromone-induced conjugation tube formation, chemotropism, tube fusion, nuclear migration, and daughter cell formation from the conjugation bridge (Lockhart et al., 2003a). These stages are remarkably similar to those in the mating process of Saccharomyces cerevisiae. However, mating in C. albicans differs from that in S. cerevisiae in several respects, most notably in the addition of a developmental step between homozygosis of the MTL locus and mating competency (Miller and Johnson, 2002; Lockhart et al., 2003a; Soll et al., 2003a,b). Unlike S. cerevisiae, a/α cells that have undergone homozygosis at the MTL locus to either a/a or α/α are still not mating competent. To mate, they must first switch from the white to the opaque phase (Miller and Johnson, 2002; Lockhart et al., 2003a), a phenotypic transition (Slutsky et al., 1987) that involves dramatic changes both in cellular architecture and gene expression (Soll, 1992, 2002, 2003). Because physiological temperature (37°C) causes phenotypic conversion of opaque phase cells en masse to the white phase (Slutsky et al., 1987; Rikkerink et al., 1988; Srikantha and Soll, 1993; Soll, 2003), it has been suggested that mating, which requires expression of the opaque phase phenotype, may occur outside the human body, perhaps on skin, on catheters or in nonanimal reservoirs (Soll et al., 2003a,b), where the temperature is below 37°C and cocolonization can readily occur between multiple strains of C. albicans. Recently, this hypothesis was lent support by observations of mating on skin. Kvaal et al. (1999) had demonstrated that although white phase cells do not colonize the skin of newborn mice, opaque phase cells are highly efficient at colonizing skin. When opaque phase cells of natural a/a and α/α strains were mixed and incubated on skin, up to 50% of the cells fused, a proportion significantly higher than that observed in vitro (Lachke et al., 2003). These results demonstrate that skin facilitates mating between a/a and α/α cells of C. albicans.
Because adhesion to a substrate such as skin facilitates mating, we entertained the possibility that cell surface molecules involved in adherence may play a role in mating and may even be regulated by the mating process. We, therefore, investigated whether HWP1 was expressed in conjugation tubes induced by pheromones of the opposite mating types. Selective fluorescent staining techniques, including immunostaining with anti-Hwp1 antibody, reveal that Hwp1 is expressed exclusively on the conjugation tubes of a/a cells, not α/α cells, in mating mixtures. In addition, the first daughter bud formed in the mating process emerges from the a/a contribution to the conjugation bridge, which selectively contains Hwp1, demonstrating for the first time that the contributions of a/a and α/α cells to the contiguous conjugation bridge are functionally distinct.
MATERIALS AND METHODS
Maintenance of Stock Cultures
C. albicans a/α strain 3153A (Slutsky et al., 1985), α/α strain WO-1 (Slutsky et al., 1987), α/α strain P78048 (Lockhart et al., 2002), a/a strain P37005 (Lockhart et al., 2002), and a/a strain L26 (Lockhart et al., 2002) were maintained on agar containing modified Lee's medium (Bedell and Soll, 1979) for experimental purposes. Stock cultures of all strains were stored frozen at –80°C before experimental use. For distinguishing between white and opaque phase colonies, cells were plated on modified Lee's medium (e-mail davidsoll@uiowa.edu for recipe) containing 5 μg/l phloxine B, which differentially stains opaque phase colonies red (Anderson and Soll, 1987).
Hypha Induction
The methods for temperature-induced hypha formation have been described in detail previously (Buffo et al., 1984). In brief, cells were grown in shaking cultures to early stationary phase in liquid modified Lee's medium at 25°C. Cells were then diluted into fresh medium at 37°C at an original concentration of 107/ml and shaken for 18 h.
Pheromone Peptide Induction of MTLa Cells
The α-pheromone sequence GFRLTNFGYFEPGK was deduced from the Candida DB World Wide Web Server (http://genolist.pasteur.fr/CandidaDB) (Lockhart et al., 2003b). α-Pheromone was synthesized for this study by Alpha Diagnostic International (San Antonio, TX). The peptide was purified by high-performance liquid chromatography and mass spectroscopy to a purity of 96%. Cell cultures were grown in suspension on a rotary water bath shaker at 25°C in liquid modified Lee's medium to either mid-log phase (∼2 × 107 cells/ml) or to early stationary phase (∼2 × 108 cells/ml). Cells were then pelleted and resuspended in an equal volume of fresh liquid modified Lee's medium containing 10–6 M α-pheromone. Cells were then incubated in suspension on a rotary water bath shaker until ∼50% of the cell culture had formed shmoos (∼2 h).
Mating Experiments
Mating experiments were performed according to methods described previously (Lockhart et al., 2003a). In brief, cells were removed from 5-d-old clonal colonies and grown overnight in liquid modified Lee's medium at 25°C. Approximately 107 opaque phase a/a cells and 107 opaque phase α/α cells were mixed in a final volume of 1 ml of fresh liquid growth medium in a plastic 15-ml Falcon tube and incubated at 25°C for 6 h in a rotary water bath shaker at 250 rpm. Cells were examined for shmooing and fusion through an upright microscope fitted with a 100× objective by using bright field microscopy.
Immunofluorescence Techniques
To stain for Hwp1, cells were washed twice in HEPES balanced salt solution and fixed with 4% paraformaldehyde in Dulbecco's phosphate-buffered saline (PBS) (Invitrogen, Carlsbad, CA) for 30 min at room temperature. Cells were then rinsed in PBS and blocked in PBS containing 10% goat serum for 30 min. Rabbit polyclonal anti-Hwp1 antiserum (Staab et al., 1996) was then used to stain cells at a 1:300 dilution in PBS containing 1% bovine serum albumin and then treated with goat-anti-rabbit secondary antibody, either Alexa 488 (Molecular Probes, Eugene, OR) or Cy-5 (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were then stained with Calcofluor White M2R (Molecular Probes). Rinsed cells, stained either in suspension or on coverslips, were mounted on microscope slides in PBS containing glycerol and 1% azide. To label the walls of living cells to differentiate between a/a and α/α parental cells in fusions, a/a cells were stained for 30 min at room temperature with rhodamine-concanavalin A (rhodamine-ConA) (Vector Laboratories, Burlingame, CA) and α/α cells with fluorescein isothiocyanate-concanavalin A (FITC-ConA) (Vector Laboratories), both diluted 1:100 from 5 mg/ml stock solutions, before mixing in mating experiments. Cells were then fixed and stained with Calcofluor White M2R. Confocal and multiphoton images were gathered using the Bio-Rad Radiance 2100 MP multiphoton laser scanning confocal microscope (Bio-Rad, Hermel Hampstead, United Kingdom) equipped with a Mai-Tai IR laser (Spectra-Physics Lasers, Mountain View, CA). FITC-ConA, rhodamine-ConA and Cy-5 (Hwp1) were simultaneously visualized using four-line argon, helium-neon, and red diode lasers. Calcofluor was imaged using the Mai-Tai IR titanium-sapphire laser tuned to 818 nm. Simultaneous differential interference contrast (DIC) images were also obtained. Digital images and z-series image stacks were processed using Adobe Photoshop software.
3D-DIAS Reconstruction
Faceted reconstruction of the surface of zygotes using 3D-DIAS software has been described in detail previously (Soll, 1995; Soll and Voss, 1998; Wessels et al., 1998; Soll et al., 2000; Heid et al., 2002). Calcofluor and Hwp1 images of each optical section were merged, converted to a TIF file and outlined. Outlines were then stacked and converted to a faceted image. 3D-DIAS software then provided viewing of reconstructions at any angle in 3D.
Northern Analysis
Northern analysis was performed according to procedures described previously (Srikantha and Soll, 1993). In these analyses, Northern blots were probed with a PCR fragment of HWP1 obtained with the primers HWP1F and HWP1R (Zhao et al., 2002).
RESULTS
Pheromone Responses and Mating
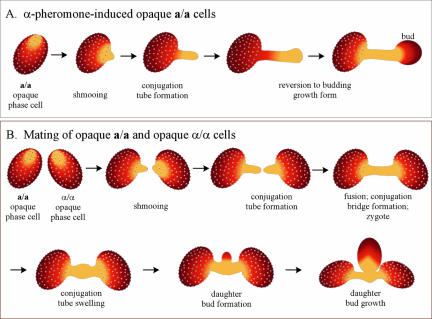
When opaque phase a/a cells were treated with synthetic α-pheromone peptide, they formed wide evaginations (the process of “shmooing”) that grew into conjugation tubes (Figure 1A) indistinguishable from those that form during mating of natural a/a and α/α strains (Lockhart et al., 2003a). Because the tubes formed by α-pheromone-treated a/a cells find no tubes of opposite mating type with which to fuse and/or because pheromone is rapidly degraded by secreted aspartyl proteinases, they reverted to the budding growth form at their apices (Figure 1A), in a manner similar to the reversion process described in mating mixtures of natural a/a and α/α strains (Lockhart et al., 2003a).
Figure 1.
Models of α-pheromone-induced conjugation tube formation and reversion to the budding growth form (A), and a/a × α/α mating in C. albicans (B).
When opaque phase a/a and opaque phase α/α cells were mixed, both cell types shmooed and the shmoos grew into conjugation tubes (Figure 1B) (Lockhart et al., 2003a). The tubes of opposite mating type then associated at their apices and fused, forming a conjugation bridge. A daughter cell then formed from the bridge (Figure 1B).
α-Pheromone Induction and Hwp1 Expression
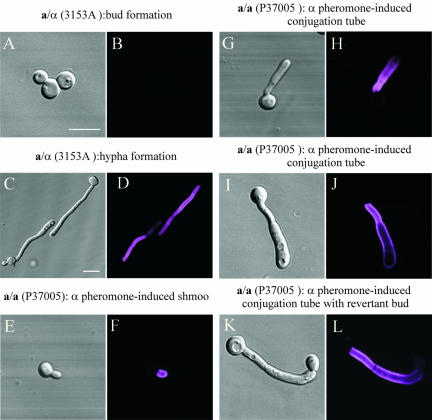
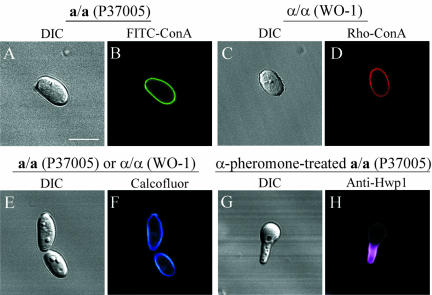
White cells and daughter buds of the a/α strain 3153A, the a/a strain P37005 or the α/α strain WO-1 did not stain with anti-Hwp1 antibody (Figure 2, A and B). However, when induced to form hyphae by suspending white cells in serum, the walls of the resulting germ tubes and hyphae of all three strains stained selectively with Hwp1 antiserum (Figure 2, C and D). In all three genotypes, mother cells remained unstained, demonstrating that differentially expressed Hwp1 localized exclusively in the hyphal wall, as demonstrated previously for a/α cells (Staab et al., 1996). In an analysis of 500 white budding cells from each of an a/α, a/a, and α/α strain stained with anti-Hwp1 antibody, 0% of mother and 0% of daughter cells stained. In a similar analysis of serum-induced hypha-forming cells, 0% of the mother cells and 100% of the hyphae of each strain stained. In an analysis of 500 untreated opaque phase cells of each of the MTL-homozygous strains P37005 (a/a) and WO-1 (α/α), 0% of the cells stained. These results demonstrate that Hwp1 is differentially expressed in hyphae, regardless of the MTL genotype, and that budding opaque phase cells do not express Hwp1, even though they have been shown to express other hypha wall antigens (Anderson and Soll, 1987).
Figure 2.
Anti-Hwp1 antibody staining reveals that Hwp1 is expressed in α-pheromone-induced a/a conjugation tubes as well as serum-induced hyphae. Cells are stained with anti-Hwp1 antibody. (A and B) White budding cell of the a/α strain 3153A. (C and D) Hypha formation of the a/α strain 3153A. (E and F) α-Pheromone-induced shmoo formation of the a/a strain P37005. (G and H and I and J) α-Pheromone-induced conjugation tube formation. (K and L) Reversion of a conjugation tube to the budding growth form at the tube apex. Each pair of images includes a DIC image and a confocal optical section through the center of the cell. Hwp1 stains purple. Bar, 5 μm.
When opaque phase cells of the a/a strain P37005 were treated with α-pheromone, the wall of the newly extended portion of each shmoo stained differentially with anti-Hwp1 antibody (Figure 2, E and F). The wall of the opaque phase parent cell did not stain (Figure 2F). As the extension of each shmoo expanded into a conjugation tube, the wall of the tube continued to stain intensely with anti-Hwp1 antibody (Figure 2, G and H, and I and J). However, when α-pheromone-induced tubes reverted at their apical ends to the budding growth form (Figure 1A), the wall of the newly formed apical bud did not stain (Figure 2, K and L). Only the long conjugation tube between parent cell and apical bud stained (Figure 2, K and L). These staining patterns held for 200 shmooing cells, 500 cells with conjugation tubes and 100 cells that had undergone reversion at the end of their conjugation tubes. These results demonstrate that in addition to selective expression in serum-induced hyphae, Hwp1 is selectively expressed in the wall of α-pheromone-induced conjugation tubes of a/a cells. The expression of Hwp1 in the conjugation tubes of a-pheromone-induced conjugation tubes of α/α cells could not be tested because the a-pheromone gene has not been identified in C. albicans.
Hwp1 Expression Early in a/a x α/α Mating Cultures
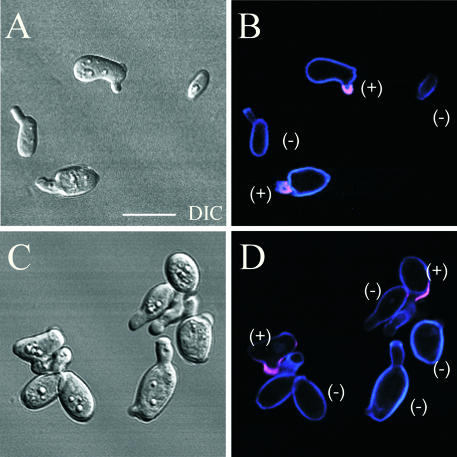
To test whether Hwp1 is expressed in the conjugation tubes formed by opaque a/a cells and opaque α/α cells during the mating process, cells of a/a x α/α cultures were stained with anti-Hwp1 antiserum 2 h after mixing. Only half of the mating projections stained on average (Figure 3, A–D). This was true for 500 shmoos analyzed after 2 h of incubation, and for 500 elongate conjugation tubes analyzed after 9 h of incubation. Because all shmoos and tubes formed by α-pheromone-induced a/a cells stained, these results suggested that only the projections formed by a/a cells in mating mixtures expressed HWP1.
Figure 3.
Anti-Hwp1 antibody stains only half of the shmoos and conjugation tubes of a/a × α/α mating cultures. Cells were stained with Calcofluor, which stains all cell wall blue, and anti-Hwp1 antibody, which stains Hwp1 purple. Each pair of images includes a DIC image and a confocal image taken through the center of cells. Bar, 5 μm.
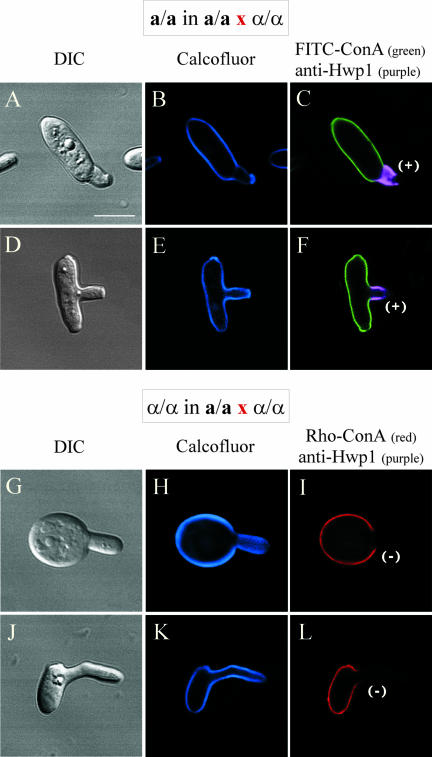
Hwp1 Localizes to Only Half of Each Conjugation Bridge
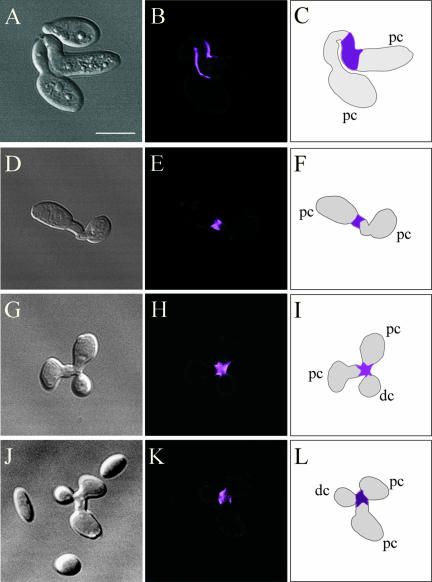
If Hwp1 is expressed exclusively in the wall of a/a conjugation tubes, then fusion of an a/a tube and an α/α tube in the process of mating would result in a half-stained conjugation bridge. This proved to be the case. When mixed opaque a/a × α/α cultures were incubated for 6–8 h, only a portion of each conjugation bridge associated with one parent cell, stained with anti-Hwp1 antibody (Figure 4, A–C and D–F). This pattern held for 100 conjugation bridges analyzed. In zygotes in which daughter buds had formed from the conjugation bridge, the same staining pattern was observed (Figure 4, G–I and J–L). Only a portion of the bridge, associated with one parent cell, stained. In addition, the first daughter bud seemed to form only from the stained portion of each bridge (Figure 4, G–I and J–L). The models drawn in Figure 4, I and L, were interpretations from z-axis scans by using confocal microscopy, in which the origin of the daughter bud could be identified. This pattern held for 50 analyzed conjugation bridges with daughter buds. These results demonstrate that only a portion of each tube expresses Hwp1 and that the first daughter bud forms from that portion of the bridge.
Figure 4.
Anti-Hwp1 antibody stains only half of each conjugation bridge, which represents the portion of the bridge from which the first daughter bud emerges. Each of the four sets of images includes a DIC image, a confocal optical section, and a diagram of the staining pattern. Bar, 5 μm.
HWP1 Is Expressed Exclusively on a/a Conjugation Tubes
To demonstrate definitively that Hwp1 is expressed exclusively on a/a and not on α/α conjugation tubes, parent a/a cells and α/α cells were vitally stained with FITC-ConA and rhodamine-ConA, respectively, before mixing. ConA permanently stains wall, and is not transferred to daughter cell, tube wall, or other cells (Lockhart et al., 2003a). After the mixed culture was incubated for 6 h, cells were fixed and additionally stained first with anti-Hwp1 antibody, and then with calcofluor, which stains the entire wall. Each cell or zygote was then imaged for the four stains by using a multiphoton laser scanning confocal microscope. In Figure 5, the staining patterns are demonstrated for FITC-ConA staining (green) of an a/a cell wall (Figure 5, A and B), rhodamine-ConA staining (red) of an α/α cell wall (Figure 5, C and D), calcofluor staining (blue) of cell wall of both a/a and α/α cells (Figure 5, E and F), and anti-Hwp1 antibody staining (purple) of Hwp1 (Figure 5, G and H).
Figure 5.
Multiple staining procedure. (A and B) DIC and FITC-ConA staining (green) of an a/a cell. (C and D) DIC and rhodamine-ConA staining (red) of an α/α cell. (E and F) DIC and calcofluor-staining of a/a or α/α cells. (G and H) DIC and anti-Hwp1 antibody-staining of an early conjugation tube formed by an α-pheromone-induced a/a cell. In the procedure, a/a and α/α parent cells are vitally stained with FITC-ConA and rhodamine-ConA, respectively, and then allowed to mate. The embryos are then fixed and stained with anti-Hwp1 antibody plus secondary antibody and calcofluor. Bar, 5 μm.
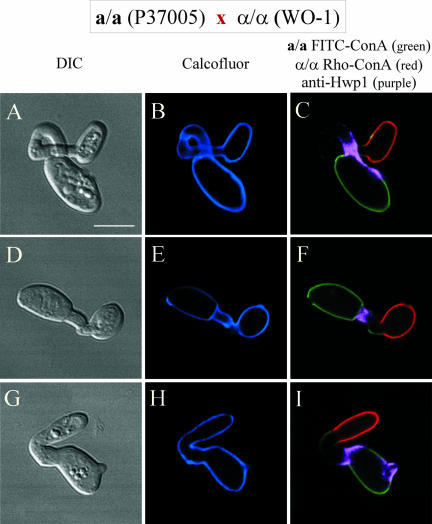
If Hwp1 is expressed exclusively in the conjugation tube of a/a cells and not α/α cells, then only tubes formed by green cells in an a/a (green) × α/α (red) mating culture will possess purple tubes. This was indeed the case. Of 100 green cells (a/a), 100% of their tubes stained for Hwp1 (purple). Examples of these are presented in Figure 6, A–C and D–F. Of 100 red cells (α/α), 0% of their tubes stained for Hwp1. Examples of these are presented in Figure 6, G–I and J–L. Similarly, if Hwp1 is expressed exclusively in the conjugation tube of a/a cells, then only that portion of a conjugation bridge contributed by the green cell (a/a) in a fusant should be purple. This was the case. Of 50 fusants, the purple portion of the bridge was associated with the green cell in 100% of cases. Examples of these are presented in Figure 7, A–C, D–F, and G–I.
Figure 6.
The multiple staining procedure demonstrates that in an a/a × α/α mating culture, only conjugation tubes formed by a/a cells contain Hwp1. FITC-ConA–stained (green) a/a cells and rhodamine-ConA–stained (red) α/α cells were mixed for 2 or 6 h and then fixed and stained with anti-Hwp1 antibody and calcofluor. (A–C; D–F) a/a cells with tubes. (G–I; J–L) α/α cells with tubes. +, staining of HWP1; –, no staining of Hwp1. Bar, 5 μm.
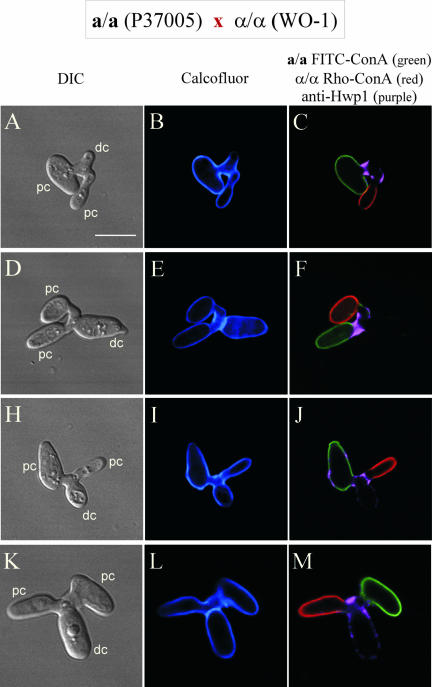
Figure 7.
The multiple staining procedure demonstrates that only the a/a contribution to the conjugation bridge contains Hwp1. FITC-ConA–stained (green) a/a cells and rhodamine-ConA–stained (red) α/α cells were mixed for 18 h and then fixed and stained with anti-Hwp1 antibody and calcofluor. (A–C; D–F; G–I) Individual fusants before bud formation.. Bar, 5 μm.
In Figures 6 and 7, crosses were performed between the α/α strain WO-1 and the a/a strain P137005. To verify that Hwp1 staining was in fact mating type (a/a) specific, we also performed the multiple staining experiment on the following crosses of natural a/a and α/α strains: 19F(α/α) × P137005(a/a), WO1(α/α) × L26(a/a), P137037(α/α) × L26(a/a), and WO1(α/α) × 12C(a/a). One hundred cells were analyzed in each cross. In every case, anti-Hwp1 antibody only stained the shmoo projections and the conjugation bridge contributions of a/a cells, never the projections or conjugation tube contributions of α/α cells. These results verify that HWP1 expression on the conjugation tube wall is mating type (a/a) specific.
Buds Form Exclusively from the a/a Portion of the Conjugation Bridge
If the first bud forms exclusively from the a/a portion of the conjugation bridge, then it should form exclusively from the purple portion of the bridge, which should in turn emanate directly from the green (a/a) parent cell. This was the case for 100% of 50 analyzed zygotes formed by a cross between stains WO-1 (α/α) and P137005 (a/a). Examples of these are presented in Figure 8, A–C, D–F, and G–I, and K–M. This was also true for the following crosses of natural a/a and α/α strains: 19F (α/α) × P137005 (a/a), WO1 (α/α) × L26 (a/a), P137037 (α/α) × L26 (a/a), and WO1 (α/α) × 12C (a/a).
Figure 8.
The multiple staining procedure demonstrates that daughter cells form only on the a/a contribution to the bridge. FITC-ConA–stained (green) a/a cells and rhodamine-ConA–stained (red) α/α cells were mixed for 18 h and then fixed and stained with anti-Hwp1 antibody and calcofluor. (A–C; D–F; G–I; J–L) Individual zygotes. Bar, 5 μm.
3D-DIAS Reconstruction of the Embryo Reveals Hwp1 and Daughter Bud Localization
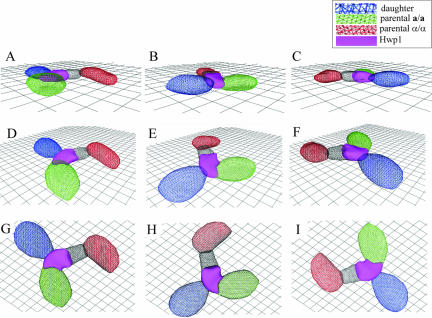
The staining patterns of single optical sections in Figures 6, 7, 8 support the conclusions that both Hwp1 and formation of the first daughter bud are localized to the a/a portion of the conjugation bridge. However, they fail to provide a complete 3D view of localization. We, therefore, used 3D-DIAS software (Soll and Voss, 1998; Soll et al., 2000; Heid et al., 2002) to reconstruct from a set of confocal sections obtained in the z-axis, a 3D faceted image of a zygote with daughter bud in which the a/a parent cell is color-coded green, the α/α parent cell is color-coded red, and Hwp1 is color-coded purple (Figure 9). This faceted representation is viewed from three angles: 0°, 45°, and 90°, and is rotated at each angle. It is clear from these different views that the daughter bud emanates from the Hwp1-containing portion of the conjugation bridge (pink) contributed by the a/a parent cell (green).
Figure 9.
3D-DIAS faceted reconstruction of a zygote demonstrates that the first bud is formed from the a/a contribution of the bridge. The a/a parent cell is color-coded green, the α/α parent cell is color-coded red, the Hwp1-containing portion of the bridge is color-coded purple, the portion of the bridge lacking Hwp1 is color-coded black, and the daughter cell is color-coded blue. (A–C) Zygote viewed at a 15° angle, rotated in the x,y-axis. (D–F) Zygote viewed at 45°, rotated in the x,y-axis. (G–I) Zygote viewed at 90°, rotated in the x,y-axis.
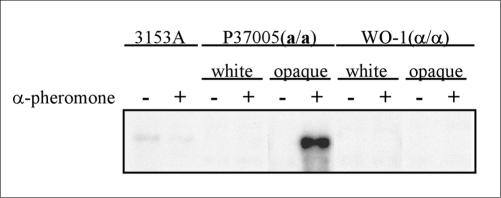
HWP1 Transcription Is Activated by α-Pheromone in Opaque a/a Cells
To test whether the selective staining of Hwp1 on the surfaces of pheromone-induced opaque a/a cells reflected differential gene transcription, Northern blots of untreated and α-pheromone treated a/α, a/a and α/α cells were probed with the HWP1 open reading frame. Extremely faint levels of HWP1 transcript were detected in both untreated and α-pheromone treated a/α cells, whereas negligible levels were detected in both untreated and α-pheromone-treated white and opaque α/α cells, in both untreated and α-pheromone–treated white a/a cells, and in untreated opaque a/a cells (Figure 10). However, a high level of HWP1 transcript was detected in α-pheromone-treated opaque phase a/a cells (Figure 10). The very low levels of HWP1 transcript in a/α cells were probably due to the low level of germ tubes formed in predominantly budding cultures. These results support the conclusion that HWP1 transcription occurs exclusively in α-pheromone–treated opaque phase a/a cells.
Figure 10.
A Northern blot analysis of HWP1 expression demonstrates that HWP1 transcription is induced only in opaque a/a cells by α-pheromone.
DISCUSSION
The mannoprotein HWP1 was originally demonstrated to be specifically localized in the walls of germ tubes and hyphae of C. albicans (Staab et al., 1996; Staab and Sundstrom, 1998). It was subsequently demonstrated that mammalian transglutaminase linked Hwp1 to epithelial cells, suggesting a unique role for the adhesin in pathogenesis (Staab et al., 1999). Because the conjugation tubes formed during mating share some morphological features with hyphae (Lockhart et al., 2003a), because adhesins have been shown to play a major role in fusion during the mating process (Lipke and Kurjan, 1992; Zhao et al., 2001) and because immobilization on skin has been shown to facilitate conjugation (Lachke et al., 2003), we tested whether Hwp1 was differentially expressed on conjugation tubes during the mating process. We first demonstrated that conjugation tubes formed by opaque a/a cells in response to synthetic α-pheromone peptide differentially stained with anti-Hwp1 antibody, just as germ tubes and hypha did. We further demonstrated that as conjugation tubes formed by a/a cells grew longer, they continued to stain, but when they reverted to the budding growth form at their apex, the new budding region did not stain. Reversion involved apical swelling followed by the formation of a bud with a constricted tube-bud junction. The absence of Hwp1 staining demarcated the junction, which is the point at which the mode of growth had changed.
To assess Hwp1 expression in the mating process, opaque a/a and opaque α/α cells were mixed and incubated at 25°C. Surprisingly, only half of the initial shmoos and conjugation tubes stained, suggesting to us either that our staining conditions were suboptimal or that tubes of only one of the two mating types stained. If only one of the two mating types stained, we expected it to be the a/a mating type, because all a/a tubes induced by synthetic α-pheromone peptide stained for Hwp1. The possibility that staining and hence Hwp1 expression was limited to only one of the two mating types was reinforced by the staining patterns of fusants. Only half of each conjugation bridge stained with anti-Hwp1 antiserum. To test definitively whether Hwp1 expression was restricted to a/a cells in the mating process, we mated a/a cells stained with FITC-ConA and α/α cells stained with rhodamine-ConA, and using anti-Hwp1 antibody tested whether Hwp1 localized to the wall of the a/a conjugation tubes and the a/a contribution to the conjugation bridge. Hwp1 localized exclusively to a/a tubes and the a/a portion of the conjugation bridge, demonstrating that α-pheromone induction of a/a cells activated HWP1, but a-pheromone induction of α/α cells, which occurs in the mating culture, did not. To confirm that Hwp1 expression was indeed type (a/a) specific, we demonstrated the same a/a-association in crosses between a number of additional unrelated natural a/a and α/α strains. We further demonstrated in these staining experiments that the first daughter bud that formed from the conjugation bridge did so exclusively from that portion contributed by the a/a parent cell, a distinction that to our knowledge has not been tested in S. cerevisiae. Northern analyses indicated that selective HWP1 induction by α-pheromone was at the level of transcription and limited to opaque a/a cells.
In S. cerevisiae, several classes of gene regulation associated with the mating process have been elucidated, including haploid-specific genes, mating type-specific genes, and pheromone induced or up-regulated genes (Kurjan and Herskowitz, 1982; Jensen et al., 1983; Miyajima et al., 1987; Nakafuka et al., 1987; Kuchler et al., 1989; McGrath and Varshavsky, 1989; Covitz et al., 1991). Because mating competency in C. albicans also involves the phenotypic switch from white to opaque, additional classes of mating-associated gene regulation will involve activation by the white-opaque transition (Lockhart et al., 2003b). In C. albicans, CAG1 was demonstrated to be specific for cells homozygous at the mating type locus, like its S. cerevisiae homolog GPA1 (Miyajima et al., 1987; Nakafuka et al., 1987); STE4 was demonstrated to be specific for cells homozygous at the mating type locus, like its S. cerevisiae homolog STE4 (Whiteway et al., 1988; Cole and Reed, 1991), but in contrast to S. cerevisiae, it was α-pheromone induced; and FIG 1 and KAR4 were α-pheromone induced, like their S. cerevisiae homologs FIG 1 (Erdman et al., 1998) and KAR4 (Kurihara et al., 1996), but in both cases, induction was opaque phase specific in C. albicans (Lockhart et al., 2003b). HWP1, like FIG 1 and KAR4, is also α-pheromone induced and opaque phase specific. To understand how HWP1 is regulated in this manner, a functional analysis of the HWP1 promoter is now in progress.
We initially analyzed HWP1 expression for two reasons. First, because conjugation tubes and hyphae are both elongate and, therefore, must restrict growth similarly to the apical growth zone (Staebell and Soll, 1985), we were interested in testing whether Hwp1 was also expressed on conjugation tubes. Second, we hypothesized that because immobilization could facilitate chemotropism and fusion, Hwp1 may play a role in the fusion process through cross-linking tubes to epithelium during chemotropism and fusion. We fully expected, therefore, to find Hwp1 on both a/a and α/α tubes. Our discovery that Hwp1 is expressed exclusively on a/a tubes and that daughter buds form exclusively on that portion of the conjugation bridge contributed by the a/a parent cell adds a new dimension to Hwp1 function, namely, a role in mating and bud emergence. Because the cytoskeleton plays a key role in bud location (Casamayor and Snyder, 2002), we are pursuing the relationship between daughter cell localization, Hwp1 localization, and cytoskeletal organization in the conjugation tube by generating an HWP1 null mutant in a natural a/a strain. Our results, therefore, demonstrate that the a/a and α/α portions of the conjugation bridge are functionally distinct. As far as we know, similar experiments have not been performed in S. cerevisiae to resolve a similar functional distinction between the a and α contributions to the conjugation bridge.
Acknowledgments
We acknowledge use of the microscopes and DIAS workstations in the W.M. Keck Dynamic Image Analysis Facility at University of Iowa. This research was supported by National Institutes of Health grants AI2392 to D.R.S. and DE11375 to P.S., and a grant from the Burroughs Wellcome Fund to P.S. K.J.D. was supported by the Developmental Studies Hybridoma Bank at University of Iowa.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–04–0264. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0264.
References
- Anderson, J.M., and Soll, D.R. (1987). Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 169, 5579–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell, G., and Soll, D.R. (1979). The effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc resistant and zinc sensitive pathways for mycelium formation. Infect. Immun. 26, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo, J., Herman, M., and Soll, D.R. (1984). A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia 85, 21–30. [DOI] [PubMed] [Google Scholar]
- Casamayor, A., and Snyder, M. (2002). Bud site selection and cell polarity in budding yeast. Curr. Opin. Microbiol. 5, 179–186. [DOI] [PubMed] [Google Scholar]
- Cole, G.M., and Reed, S.I. (1991). Pheromone-induced phosphorylation of a G protein beta subunit in S. cerevisiae is associated with an adaptive response to mating pheromone. Cell 64, 703–716. [DOI] [PubMed] [Google Scholar]
- Covitz, P.A., Herskowitz, I., and Mitchell, A.P. (1991). The yeast RME1 gene encodes a putative zinc finger protein that is directly repressed by a1-α2. Genes Dev. 5, 1982–1989. [DOI] [PubMed] [Google Scholar]
- Erdman, S., Lin, L., Malczynski, M., and Snyder, M. (1998). Pheromone-regulated genes required for yeast mating differentiation. J. Cell Biol. 140, 461–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid, P., Voss, E., and Soll, D.R. (2002). 3D-DIASemb: a computer-assisted system for reconstructing and behaviorally analyzing in 4D every cell and nucleus, and monitoring cytoplasmic flow in a developing embryo. Dev. Biol. 245, 329–347. [DOI] [PubMed] [Google Scholar]
- Hull, C.M., and Johnson, A.D. (1999). Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285, 1271–1275. [DOI] [PubMed] [Google Scholar]
- Hull, C.M., Raisner, R.M., and Johnson, A.D. (2000). Evidence for mating of the “asexual” yeast Candida albicans in mammals. Science 289, 307–310. [DOI] [PubMed] [Google Scholar]
- Jensen, R., Sprague, G.F., Jr., and Heskowitz, I. (1983). Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc. Natl. Acad. Sci. USA 80, 3035–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchler, K., Sterne, R.E., and Thorner, J. (1989). Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 8, 3973–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara, L.J., Stewart, B.G., Gammie, A.E., and Rose, M.D. (1996). Kar4p, a karyogamy-specific component of the yeast pheromone response pathway. Mol. Cell Biol. 16, 3990–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan, J., and Herskowitz, I. (1982). Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell 30, 933–943. [DOI] [PubMed] [Google Scholar]
- Kvaal, C., Lachke, S.A., Srikantha, T., Daniels, K., McCoy, J., and Soll, D.R. (1999). Misexpression of the opaque phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect. Immun. 67, 6652–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke, S.A., Lockhart, S.R., Daniels, K.J., and Soll, D.R. (2003). Skin facilitates Canadida albicans mating. Infect. Immun. 71, 4970–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke, P.N., and Kurjan, J. (1992). Sexual agglutination in budding yeast: structure, function and regulation of adhesion glycoproteins. Microbiol. Rev. 56, 180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart, S.R., Daniels, K.J., Zhao, R., Wessels, D., and Soll, D.R. (2003a). The cell biology of mating in Candida albicans. Eukaryot. Cell 2, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart, S.R., Pujol, C., Daniels, K., Miller, M.G., Johnson, A.D. Pfaller, M.A, and Soll, D.R. (2002) In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart, S.R., Zhao, R., Daniels, K.J., and Soll, D.R. (2003b). α-Pheromone-induced “Shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2, 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee, B.B., and Magee, P.T. (2000). Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289, 310–313. [DOI] [PubMed] [Google Scholar]
- McGrath, J.P., and Varshavsky, A. (1989). The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature 340, 400–404. [DOI] [PubMed] [Google Scholar]
- Miller, M.G., and Johnson, A.D. (2002). White-opaque switching in Candida albicans is controlled by the mating type (MTL) locus and allows efficient mating. Cell 110, 293–302. [DOI] [PubMed] [Google Scholar]
- Miyajima, I., Nakafuku, M., Nakayama, N., Brenner, C., Miyajima, A., Kaibuchi, K., Arai, K., Kaziro, Y., and Matsumoto, K. (1987). GPA1, a haploid-specific essential gene, encodes a yeast homolog of mammalian G protein which may be involved in mating factor signal transduction. Cell 50, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Nakafuku, M., Itoh, H., Nakamura, S., and Kaziro, Y. (1987). Occurrence in Saccharomyces cerevisiae of a gene homologous to the cDNA coding for the alpha subunit of mammalian G proteins. Proc. Natl. Acad. Sci. USA 84, 2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikkerink, E.H.A., Magee, B.B., and Magee, P.T. (1988). Opaque-white phenotypic transition: a programmed morphological transition in Candida albicans. J. Bacteriol. 170, 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky, B., Buffo, J., and Soll, D.R. (1985). High frequency switching of colony morphology in Candida albicans. Science 230, 666–669. [DOI] [PubMed] [Google Scholar]
- Slutsky, B., Staebell, M., Anderson, J., Risen, L., Pfaller, M., and Soll, D.R. (1987). “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll, D.R. (1992). High frequency switching in Candida albicans. Clin. Microbiol. Rev. 5, 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll, D.R. (1995). The use of computers in understanding how animal cells crawl. Int. Rev. Cytol. 163, 43–104. [PubMed] [Google Scholar]
- Soll, D.R. (2002). Phenotypic switching. In: Candida and Candidiasis, ed. R. Calderone, Washington, DC: Am. Soc. Microbiol., 123–144.
- Soll, D.R. (2003). Candida albicans. In: Antigenic Variation, ed. A. Craig and A. Scherf, London: Academic Press, London, 165–201.
- Soll, D.R., Lockhart, S.R., and Zhao, R. (2003a). Relationship between switching and mating in Candida albicans. Eukaryot. Cell 2, 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll, D.R., Lockhart, S., and Zhao, R. (2003b). Mating and virulence of Candida albicans. Mycologist 17, 64–69. [Google Scholar]
- Soll, D.R., and Voss, E. (1998). Two and three dimensional computer systems for analyzing how cells crawl. In: Motion Analysis of Living Cells, ed. D.R. Soll and D. Wessels, New York: John Wiley & Sons, 25–52.
- Soll, D.R., Voss, E., Johnson, O., and Wessels, D.J. (2000). Three-dimensional reconstruction and motion analysis of living crawling cells. Scanning 22, 249–257. [DOI] [PubMed] [Google Scholar]
- Srikantha, T., and Soll, D.R. (1993). A white-specific gene in the white-opaque switching system of Candida albicans. Gene 131, 53–60. [DOI] [PubMed] [Google Scholar]
- Srikantha, T., Tsai, L., Daniels, K., and Soll, D.R. (2000). EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J. Bacteriol. 182, 1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantha, T., Tsai, L.K., Klar, A., and Soll, D.R. (2002). The histone deacetylase genes HDA1 and RPD3 play distinct roles in the regulation of high frequency phenotypic switching in Candida albicans. J. Bacteriol. 183, 4614–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab, J.F., Bradway, S.D., Fidel, P.L., and Sundstrom, P. (1999). Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283, 1535–1538. [DOI] [PubMed] [Google Scholar]
- Staab, J.F., Ferrer, C.A., and Sundstrom, P. (1996). Developmental expression of a tandemly repeated, praline-and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J. Biol. Chem. 27, 6298–6305. [DOI] [PubMed] [Google Scholar]
- Staab, J.F., and Sundstrom, P. (1998). Genetic organization and sequence analysis of the hypha-specific cell wall protein gene HWP1 of Candida albicans. Yeast 14, 681–686. [DOI] [PubMed] [Google Scholar]
- Staebell, M., and Soll, D.R. (1985). Temporal and spatial differences in cell wall expansion during bud and mycelium formation in Candida albicans. J. Gen. Microbiol. 131, 1467–1480. [DOI] [PubMed] [Google Scholar]
- Sundstrom, P. (1999). Adhesins in Candida albicans. Curr. Opin. Microbiol. 2, 353–357. [DOI] [PubMed] [Google Scholar]
- Sundstrom, P., Balish, E., and Allen, C.M. (2002). Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J. Infect. Dis. 185, 521–530. [DOI] [PubMed] [Google Scholar]
- Tsuchimori, N., Sharkey, L.L., Fonzi, W.A., French, S.W., Edwards, J.E., Jr., and Filler, S.G. (2000). Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect. Immun. 68, 1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels, D., Voss, E., Von Bergen, N., Burns, R., Stites, J., and Soll, D.R. (1998). A computer-assisted system for reconstructing and interpreting the dynamic three-dimensional relationships of the outer surface, nucleus and pseudopods of crawling cells. Cell Motil. Cytoskeleton 41, 225–246. [DOI] [PubMed] [Google Scholar]
- Whiteway, M., Hougan, L., Dignard, D., Bell, L., Saari, G., Grant, F., O'Hara, P., MacKay, V.L., and Thomas, D.Y. (1988). Function of the STE4 and STE18 genes in mating pheromone signal transduction in Saccharomyces cerevisiae. Cold Spring Harb. Symp. Quant. Biol. 53, 585–590. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Shen, S.-M., Kahn, P.C., and Lipke, P.N. (2001). Interaction of α-agglutinin and a-agglutinin, Saccharomyces cerevisiae sexual cell adhesion molecules. J. Bacteriol. 183, 2874–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R., Lockhart, S.R., Daniels, K., and Soll, D.R. (2002). Roles of TUP1 in switching, phase maintenance and phase-specific gene expression in Candida albicans. Eukaryot. Cell 1, 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]