Abstract
Background
The purpose of our study was to compare differences in the prognosis of breast cancer (BC) patients at high (H) risk or intermediate slightly (IS) increased risk based on family history and those without a family history of BC, and to evaluate whether ten-year overall survival can be considered a good indicator of BRCA1 gene mutation.
Methods
We classified 5923 breast cancer patients registered between 1988 and 2006 at the Department of Oncology and Haematology in Modena, Italy, into one of three different risk categories according to Modena criteria. One thousand eleven patients at H and IS increased risk were tested for BRCA1/2 mutations. The overall survival (OS) and disease free survival (DFS) were the study end-points.
Results
Eighty BRCA1 carriers were identified. A statistically significantly better prognosis was observed for patients belonging to the H risk category with respect to women in the IS and sporadic groups (82% vs.75% vs.73%, respectively; p < 0.0001). Comparing only BRCA1 carriers with BRCA-negative and sporadic BC (77% vs.77% vs.73%, respectively; p < 0.001) an advantage in OS was seen.
Conclusions
Patients belonging to a population with a high probability of being BRCA1 carriers had a better prognosis than those with sporadic BC. Considering these results, women who previously had BC and had survived ten years could be selected for BRCA1 analysis among family members at high risk of hereditary BC during genetic counselling. Since only 30% of patients with a high probability of having hereditary BC have BRCA1 mutations, selecting women with a long term survival among this population could increase the rate of positive analyses, avoiding the use of expensive tests.
Background
The major breast cancer (BC) predisposing genes, BRCA1 and BRCA2 were identified in 1994 and 1995, respectively [1,2]. Unfortunately, the optimal clinical approach to women who develop hereditary breast cancer remains incompletely defined. Studies of the outcomes of women with BRCA1/BRCA2-related cancer have yielded conflicting results. Several reports suggested that women with germline mutations in BRCA1 are more likely to die from their disease than are women with sporadic breast cancer [3-6], whereas BRCA2 mutation carriers and non-mutation carriers seem to share a similar prognosis [7,8]. The poor prognosis in BRCA1 carriers may be consistent with the histological characteristics usually described for BRCA1-associated breast cancer, which show higher histologic grade and cancers that are more often hormone receptor-negative than sporadic breast cancer cases [9-12]. However, Bonadona et al. found no evidence for poorer short-term survival in BRCA1 mutation carriers compared with non-carriers in a prospective population-based cohort [13]. Apart from a simple interest in the epidemiological aspects of breast malignancy, knowledge of the associated mortality is important to the families of patients with BC and to clinicians and scientists involved in trying to improve the outcomes of breast cancer. The results of a recent study in an Ashkenazi Jewish population suggested that among the subgroup of patients with BC carrying a BRCA1 mutation, those who received chemotherapy had a better survival rate compared with those who did not [8].
The primary aim of our study was to calculate disease free survival (DFS) and overall survival (OS) of BC patients at high risk (H) or intermediate slightly (IS) increased risk based on family history and those without a family history of breast cancer using the population registered with the Breast Cancer Registry in Modena. Previous studies were aimed at evaluating the outcome in BRCA-positive and BRCA-negativepatients, but none showed a significant survival difference between different risk categories. In case of statistically significant differences in OS between the three groups, a secondary aim was to determine whether patients with a better prognosis were BRCA1 mutation carriers, showing that the outcome could be considered an indicator of BRCA1 inheritance. Additionally, we evaluated whether chemotherapy could play a role in the prognosis of BRCA1 carriers, providing more benefit in this patient population than in patients with sporadic breast cancer.
Methods
Patients
Patients included in our analysis were diagnosed between 1988 and 2006 at the Department of Oncology and Haematology in Modena. All newly-diagnosed, biopsy-proven primary breast cancer patients were evaluated. The family history was collected and classified according to the Modena criteria (without family history, IS increased risk, or H risk)[14] and a blood sample, preserved with EDTA, was obtained with informed consent and frozen at -80°C for biological studies. On the basis of the Modena criteria for familial risk, patients who did not have any family history were considered to have sporadic breast cancer, patients with one or two breast cancers at ≥ 40 years, without a first-degree relationship, were considered at IS increased risk, and patients with breast cancer at ≤ 35 years, three or more breast cancers with a first-degree relationship, and at least one case at ≤ 40 years or bilateral breast cancer had a H risk of being hereditary.
All research regarding the identification, counselling, genetic testing, and clinical data regarding individuals at risk of developing breast cancer were ethically approved by the Ethics Commitee of Modena (reference number 45/00).
Mutational analysis
In 1995, DNA started to be extracted from frozen whole blood using the Invisorb Blood Universal kit (Invitek, Berlin, Germany), amplified by PCR using primers specific for the coding sequence and exon-intron boundaries of BRCA1, and analyzed by Direct Automated Sequencing using an ABI Prism 3100 (Applied Biosystems, Foster City, CA). Subsequently, patients with breast cancer provided consent for genetic testing that was completed for each case.
Samples negative for BRCA1 mutations were tested for BRCA1 rearrangements using the multiplex ligation-dependent probe amplification assay (MRC Holland, Amsterdam, The Netherlands) following the manufacturer's protocol.
Statistical analysis
The χ2 test was used to determine differences in clinicopathological features between groups. Survival curves were estimated using the Kaplan-Meier method including the log-rank test group comparison. Patients who were BRCA1 carriers were matched with patients with sporadic breast cancer from the Modena cancer registry. This registry, initiated in 1988, covers an area with approximately 650,000 inhabitants in Northern Italy. A database with a total of 3858 cases of sporadic breast cancer was used to find four matched controls for each case and a randomized matches were assigned between BRCA1 and patients with sporadic BC of the same age at diagnosis (range, between 26 and 76 ± 4 years), tumour grade (I, II, and III), and stage (I, II, and III). Multivariate analyses of DFS and OS were conducted using a proportional hazards Cox regression model. All statistical analyses were done with SPSS, version 12.0 (SPSS Inc, Chicago, IL).
Results
Patient Characteristics
From January 1988 and December 2006, 5923 patients were diagnosed with breast cancer. According to their family history, 4912 were considered sporadic breast cancers, 691 were at IS increased risk, and 320 were considered H risk. The patients' characteristics are detailed in Table 1. The H risk group presented with a medullary carcinoma histotype more frequently than the other two groups (p < .0001). The H risk group was more likely to have stage II disease than the sporadic and IS increased risk groups (p < .0001), and to have a significantly lower estrogen receptor (ER) and progesterone receptor (PgR) expression levels (p < .0001) as measured by immunohistochemical analysis (clone 6F11, Ventana, for ER; and clone 1E2, Ventana, for PgR) and stained by Ventana Benchmark autostainer. The ER and PgR receptor status was tested by evaluating the percentage of nuclear immunoreactivity with respect to all the nuclei in the neoplastic cells, independently of the staining intensity. No differences were seen in the Ki67 level between the groups (p = .17). The proportion of patients who received adjuvant chemotherapy was greater in the IS increased risk group than in the other two groups (p < .0001). The most frequent chemotherapy strategy was anthracycline-based followed by taxanes and the CMF regimen. The most frequently used first line chemotherapy regimen was platinum based, followed by a CMF scheme. The proportion of patients who received adjuvant hormone therapy was lower in the H risk group than in the other two groups, according to the negative hormonal receptor status (p < .0001).
Table 1.
Clinicopathological characteristics of patients according to familial risk group
| Family Risk Group | |||||||
|---|---|---|---|---|---|---|---|
| No family history | Intermediate-Slightly increased risk | High Risk | p | ||||
| (n = 4912) | (n = 691) | (n = 320) | |||||
| Characterics | N° | % | N° | % | N° | % | |
| Histotype | |||||||
| Ductal | 3936 | 80.1 | 565 | 81.8 | 251 | 78.4 | NS |
| Lobular | 606 | 12.3 | 83 | 12.0 | 44 | 13.7 | NS |
| Tubular | 93 | 1.9 | 13 | 1.9 | 2 | 0.6 | NS |
| Colloid | 64 | 1.3 | 10 | 1.4 | 0 | 0 | NS |
| Medullary | 26 | 0.5 | 6 | 0.9 | 13 | 4.1 | < .0001 |
| Other | 187 | 3.9 | 14 | 2.0 | 10 | 3.2 | NS |
| Stage | |||||||
| I | 2322 | 47.3 | 312 | 45.1 | 141 | 44.1 | NS |
| II | 1546 | 31.5 | 255 | 36.9 | 127 | 39.7 | < .0001 |
| III | 802 | 16.4 | 112 | 16.2 | 48 | 15.0 | NS |
| IV | 216 | 4.4 | 11 | 1.8 | 1 | 0.3 | <.0001 |
| X | 23 | 0.5 | 1 | 0.1 | 3 | 0.9 | NS |
| ER | |||||||
| Positive | 3286 | 66.8 | 448 | 64.8 | 147 | 45.9 | < .0001 |
| Negative | 757 | 15.4 | 139 | 20.2 | 149 | 46.6 | < .0001 |
| Unknown | 869 | 17.8 | 103 | 15.0 | 24 | 7.5 | < .0001 |
| PgR | |||||||
| Positive | 2796 | 56.9 | 408 | 59.1 | 135 | 42.2 | < .0001 |
| Negative | 1109 | 22.5 | 173 | 25.0 | 162 | 50.6 | < .0001 |
| Unknown | 1007 | 20.6 | 110 | 15.9 | 23 | 7.2 | < .0001 |
| Ki67 | |||||||
| Low (0-19) | 2523 | 51.4 | 383 | 55.4 | 185 | 57.8 | NS |
| High (20-100) 1109 | 22.5 | 167 | 24.2 | 103 | 32.2 | NS | |
| Unknown | 1007 | 20.6 | 141 | 20.4 | 32 | 10.0 | NS |
| Chemotherapy | |||||||
| Yes | 1851 | 37.7 | 350 | 50.7 | 106 | 33.1 | < .0001 |
| No | 3061 | 62.3 | 341 | 49.3 | 214 | 66.9 | < .0001 |
| Hormone therapy | |||||||
| Yes | 2804 | 57.1 | 380 | 55.0 | 106 | 33.1 | < .0001 |
| No | 2108 | 42.9 | 311 | 45.0 | 214 | 66.9 | < .0001 |
Abbreviations: ER, estrogen receptor; PgR, progesterone receptor
Mutational Analysis
The BRCA1 mutational analysis was only performed in patients belonging to the H and IS risk groups, with a 22.8% (73/320) and 1.0% (7/691) mutation rate, respectively. In total, 80 patients were BRCA1 carriers.
Survival analysis
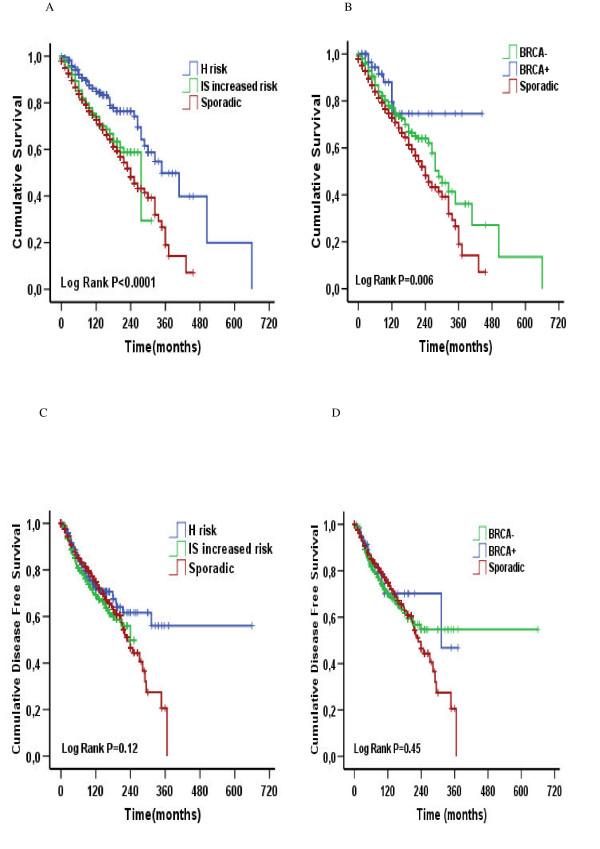
Overall, 5923 patients were followed until December 2006, resulting in a median follow-up time of 72 months. In all, 1302 deaths (1074 sporadic, 174 IS increased risk, and 54 H risk) and 1137 relapses (884 sporadic, 186 IS increased risk, and 67 H risk) were reported. The estimated 10-year overall survival rate was 82% for the H risk patients, 75% for the IS increased risk group, and 73% for patients with sporadic breast cancer (log-rank p < .0001; Fig. 1A). The estimated 10-year DFS was 72% in the H risk group, 70% in the IS increased risk group, and 75% in patients with sporadic breast cancer (log-rank p = .12; Fig. 1C). After the genetic analysis, 80 patients were classified as BRCA1 carriers and 931 were considered BRCA negative. In the BRCA1 carrier group, 8 deaths (5 caused by a second tumour arising after breast cancer) and 13 relapses (6 local recurrences and 7 distant recurrences) were reported, whereas in the BRCA negative group 220 deaths and 240 relapses occurred. The 10-year overall survival rate was 77% for BRCA1 carriers, 77% for BRCA-negative patients, and 73% for sporadic breast cancer (log-rank p < .001; Fig. 1B). The 10-year DFS was 75% in sporadic breast cancer, 70% in the BRCA-negative patients, and 70% in the BRCA1 group (log-rank p = .45; Fig. 1D).
Figure 1.
Overall survival and disease free survival according to risk group (A, C) and to BRCA1 status (B, D).
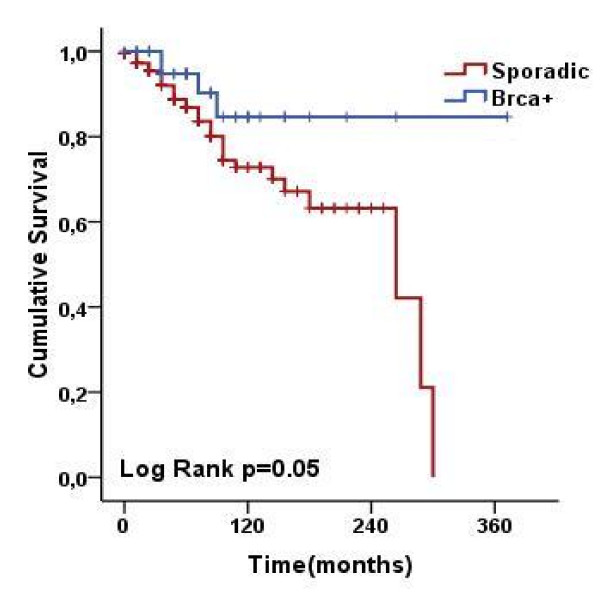
The study group of 80 BRCA1 cases was compared with 320 matched sporadic cases from the Modena cancer registry which were the same age, and had the same tumour grade and stage. Results are shown in Fig. 2. A statistically significant difference in OS was seen for BRCA1 patients compared with sporadic BC (85% vs. 73%, respectively, p = .05).
Figure 2.
Probability of survival in 80 BRCA1 breast cancer cases and 320 sporadic breast cancer controls matched for age and tumour grade and stage, using Kaplan-Meier analysis. There was a statistically significant difference between the two groups (log-rank test, p = .05).
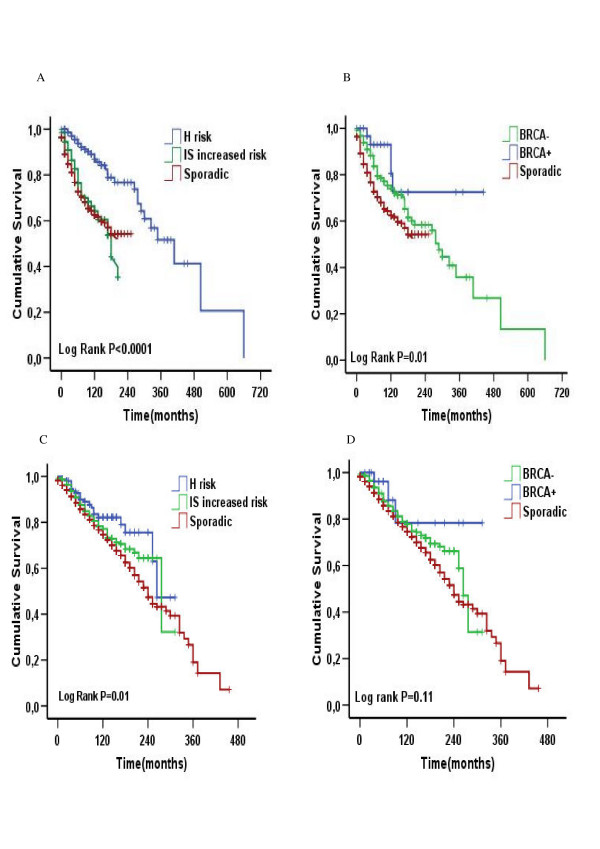
According to the hormone receptor status, the hormone receptor-negative patients in the H risk group and the BRCA1 patients had a better OS (Fig. 3A, B) while a hormone receptor-positive advantage was only seen in the H risk group, but not in the BRCA1 group (Fig. 3C, D).
Figure 3.
Overall survival according to risk group in hormone receptor negative (A) and positive (C) patient subgroups, and according to BRCA1 status in hormone receptor negative (B) and positive (D) patient subgroups.
Multivariate survival analysis according to prognostic factors and chemotherapy
Of the 5923 patients, 5012 were included in multivariate analyses, which included BRCA1 status, disease stage, ER status, PgR status, grading, age, and chemotherapy. The BRCA1 positive and negative status was a significant independent factor for improved OS (HR = 0.29; p = .002 and HR = 0.76; p = < .001, respectively) compared with the sporadic breast cancer cases. Positive ER and PgR status was associated with a better OS (HR = 0.82; p = .03 and HR = 0.79; p = .004, respectively). Also, chemotherapy was an important factor in reducing the mortality rate (HR = 0.68; p < .0001). Stage > I (HR = 3.49; p < .0001) and grading III (HR = 1.39; p < .0001) were significant factors for poorer OS. Younger age (≤ 35 years) represented a risk factor for a shorter OS, but the trend was not statistically significant. In a model that included 2213 patients receiving chemotherapy, BRCA1-positive status (HR = 0.38; p = .05) but not BRCA1-negative status (HR = 0.97; p = .79) reduced the mortality rate. Positive PgR status (HR = 0.76; p = .021) was a favourable factor as was positive ER status (HR = 0.76; p = .083), but did not reach statistical significance. Stage > I (HR = 3.55; p < .0001) and grading III (HR = 1.38; p < .0001) were factors statistically associated with an increased risk of death. There was a similar but non significant trend in age ≤ 35 years (HR = 1.15; p = .44). Conversely, in a model without chemotherapy, the BRCA1-positive status lost statistical significance in reducing mortality (HR = 0.23; p = 0.056), whereas the BRCA1-negative status maintained its favourable impact (HR = 0.56; p < .0001). Also, positive ER (HR = 0.82, p = .17) and PgR status (HR = 0.82; p = .08) and age less than 35 years (HR = 0.95;p = .85) did not affect OS. Finally, stage > 1 (HR = 3.43; p < .0001) and grading III (HR = 1.39; p < .0001) were statistically associated with an increased risk of death. Detailed results for multivariate analysis are shown in Table 2.
Table 2.
Cox's proportional hazard regression models for overall survival
| Variable | N° (%) | HR | p | [95% CI] |
|---|---|---|---|---|
| All cases | 5012 (85) | |||
| Sporadic | 4172 (83) | 1.00 | - | - |
| BRCA - | 785 (16) | 0.76 | < .001 | 0.64 to 0.89 |
| BRCA1+ | 55 (1) | 0.29 | .002 | 0.13 to 0.62 |
| stage > 1 | 2372 (47) | 3.49 | < .0001 | 3.01 to 4.04 |
| Estrogen receptor positive | 4090 (82) | 0.82 | .03 | 0.69 to 0.98 |
| Progesterone receptor positive | 3138 (64) | 0.79 | .004 | 0.67 to 0.93 |
| Chemotherapy, yes | 2213 (44) | 0.68 | < .0001 | 0.59 to 0.77 |
| Age ≤ 35 | 199 (4) | 1.12 | .42 | 0.84 to 1.49 |
| Grading III | 1778 (35) | 1.39 | < .0001 | 1.22 to 1.58 |
| Model with chemotherapy | 2213 (44) | |||
| Sporadic | 1780 (36) | 1.00 | - | - |
| BRCA- | 404 (8) | 0.97 | 0.79 | 0.78 to 1.20 |
| BRCA1+ | 29 (2) | 0.38 | .05 | 0.14 to 0.99 |
| Stage > I | 1689 (76) | 3.55 | < .0001 | 2.65 to 4.76 |
| Estrogen receptor positive | 1571 (71) | 0.81 | .083 | 0.65 to 1.02 |
| Progesterone receptor positive | 1492 (67) | 0.76 | .021 | 0.61 to 0.96 |
| Age ≤ 35 years | 128 (6) | 1.15 | .44 | 0.81 to 1.62 |
| Grading III | 1116 (50) | 1.38 | < .0001 | 1.15 to 1.64 |
| Model without chemotherapy | 2799 (56) | |||
| Sporadic | 2392 (85) | 1.00 | - | - |
| BRCA- | 381 (14) | 0.56 | < .0001 | 0.43 to 0.73 |
| BRCA1+ | 26 (1) | 0.23 | .056 | 0.14 to 1.02 |
| stage > I | 951 (34) | 3.43 | < .0001 | 2.89 to 4.09 |
| Estrogen receptor positive | 2519 (90) | 0.82 | .17 | 0.63 to 1.08 |
| Progesterone receptor positive | 2298 (82) | 0.82 | .08 | 0.65 to 1.03 |
| Age ≤ 35 | 71 (3) | 0.95 | .85 | 0.56 to 1.60 |
| Grading III | 662 (24) | 1.39 | < .0001 | 1.15 to 1.68 |
Abbreviations: HR, hazard ratio; CI, confidence interval
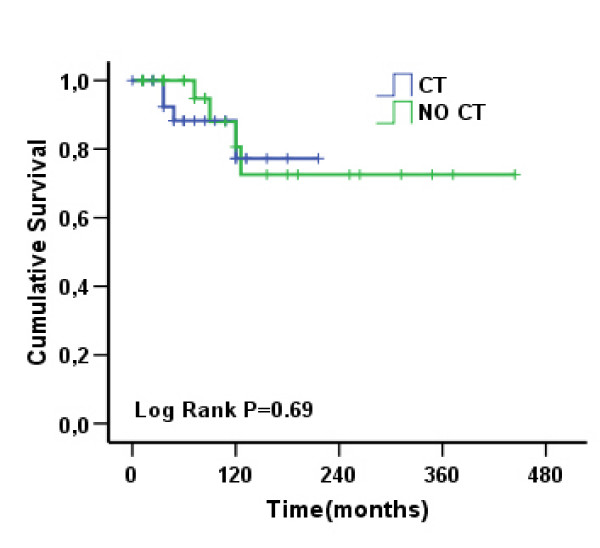
In comparing BRCA1 patients who did or did not receive chemotherapy, no difference was seen in terms of OS (see Fig. 4), even if all the death events in the treated group of patients were derived from second tumours (4 cases).
Figure 4.
A comparison of the group of BRCA1 patients who received or did not receive chemotherapy did not show any difference in overall survival (log-rank test, p = .69).
Discussion
The results of this large analysis show that patients considered at H risk of being BRCA1 carriers had a better OS than patients considered at IS increased risk or to have sporadic breast cancer. This difference was also maintained in BRCA1 carriers with respect to BRCA1-negative and sporadic breast cancer patients. Notably, 91.2% (73/80) of BRCA1 carriers were identified in the H risk group, and may explain the survival advantage in this group of patients, even if other reasons may be involved. Patients who know they are at high risk of developing breast cancer may be more likely to participate in surveillance programs and start at a younger age, receive an earlier diagnosis, and subsequently, experience a better outcome.
One of the most important findings of our study was that the survival difference was attributable, in a multivariate analysis, to the patient's BRCA1 status and was observed in patients treated with chemotherapy. This finding is important when, as happens in a family cancer centre like in ours, we are faced with a patient or a healthy woman who has a family history of breast cancer. By collecting all the information about individuals affected by breast cancer, very long-term survivors can be identified by predicting a predisposition for being a BRCA1-mutation carrier in the descendants. Furthermore, BRCA1 carriers may be more likely to be found among women who have a high probability of hereditary breast cancer and who have a long term survival. If BRCA1 carriers could be identified through these characteristics, the use of expensive tests could be avoided and the rate of positive analyses increased.
Our study involves a very large number of Caucasian patients with breast cancer (N = 5923) who were first evaluated for their family history of breast cancer and subsequently for BRCA1 status. The most common weakness of previous studies includes the small number of patients or selection bias, such as a retrospective analysis for BRCA1 status. Owing to the large number of subjects, we could calculate OS differences between risk groups, and even in subgroup analyses.
Unexpectedly, the survival difference according to H risk and BRCA1 status was not related to the DFS. This finding shows that the H risk and BRCA1 patients have the same DFS as the other two groups, but seem to respond better to chemotherapeutic agents. This finding is in contrast with the results of Rennert et al. [8] who showed an insignificant difference in OS between BRCA carriers and non carriers and between BRCA1 carriers and BRCA-negative patients treated with chemotherapy. Our argument is that, in BRCA1 patients, 41% of relapses were local recurrences, whereas in patients with sporadic BC the local recurrence rate was 25%, and distant metastases accounted for 75%. Consequently, it is not surprising that a difference in OS was found and could be explained by the use of alkylating agents, such as platinum-derived drugs, in metastatic disease that are well known to be more effective in BRCA-related tumours [15]. Furthermore, since BRCA1 patients maintain a statistically significant OS advantage, even after matching each case to four controls for age and tumour grade and disease stage, this result is very important in terms of its practical value, because all confounders have been removed.
Robson et al [12] suggested that a BRCA1 mutation was an independent predictor of breast cancer mortality in a multivariate analysis of a group of women who did not receive chemotherapy, but not in women who received adjuvant chemotherapy.
In a multivariate analysis, we found that chemotherapy is a prognostic factor for better survival in all patients combined; furthermore, we found that a BRCA1-positive status was an independent predictor for a better survival in all patients and also in the subgroup of patients who received chemotherapy, but not in patients who did not receive chemotherapy. No differences are shown for BRCA1 patients, independently of chemotherapy or not. Also, this result might be explained by the fact that deaths in the treated group of patients were all related to a second tumour, while in the non treated group one of three deaths was caused by breast cancer. In conclusion, chemotherapy has a greater protective effect in BRCA1 mutation carriers compared with patients who are BRCA-negative and those with sporadic breast cancer. Furthermore, since BRCA1-related tumours are more likely to be triple negative, the greatest advantage was shown for ER-negative breast cancer, were chemotherapy is the most active. Hence, increasing the number of BRCA1 carriers identified by correctly selecting patients with a high probability of having hereditary breast cancer through a 10-year survival analysis could improve the benefit derived from specific chemotherapy agents (i.e., alkylating or PARP-inhibitors).
In fact, the chemotherapy benefit is well known because normal BRCA1 and BRCA2 proteins participate with RAD51 in the repair of double-stranded DNA breaks induced by DNA-damaging agents [16-19]. The better prognosis could be due to the deficiency of the BRCA proteins, which confer substantial cellular sensitivity to the inhibition of poly (ADP-Ribose) polymerase enzyme (PARP). This polymerase is a key enzyme in the repair of single-stranded DNA damage via the base excision repair pathway. The loss of PARP activity in BRCA mutant cells might lead to the persistence of DNA lesions normally repaired by homologous recombination, resulting in increased chromosome instability and programmed cell death specifically in tumour cells [20,21]. Therefore, because there is no functional protein within the tumour cells, they lose their capacity to repair DNA damage. This might be specifically pronounced for drugs, such as cisplatin, acting through the induction of DNA damage leading to cell death and to a better therapeutic response. In a small clinical study, Chappuis et al [22] found a benefit in Ashkenazi Jews with locally advanced breast cancer who were BRCA-positive and treated with anthracycline-based chemotherapy regimens. In this study, all patients received anthracycline-based neoadjuvant chemotherapy, and 10 of 11 patients with BRCA mutations had a clinical complete response compared with only 8 of 27 BRCA-negative patients with sporadic breast tumours. From this study, it was inferred that tumours with BRCA1 mutations are highly sensitive to anthracycline-based chemotherapy regimens.
In our study, patients were considered part of a hospital-based population and were not selected for age, tumour stage, or treatment, which could influence survival rates. We were successful in obtaining blood samples from all patients and were able to genotype all the samples we received. The patients were followed for a median of 72 months. Treatment regimens were chosen on the basis of disease staging. The Modena Cancer Registry captures outcome data from almost all patients with cancer who are treated in the province. Data on pathological diagnosis are verified by a pathologist, and incomplete information is retrieved from medical records when possible. All patients were Caucasian, and it is possible that the chemotherapy sensitivity can be associated with specific modifier genes not found in other populations.
Our study has a number of limitations. Tumour stage and hormone receptor status were not routinely recorded, particularly during the first period. Since we tested only a subpopulation of patients with a positive family history of breast cancer, it is possible that some hereditary cases were misclassified; but we found that only 1% of mutations occurred in the IS increased risk group suggesting that the number of BRCA1 carriers in the sporadic group would be very low. Since we identified only 80 mutation carriers, the subgroup analysis relied on a small number of subjects.
In conclusion, our study is the first to evaluate a relationship between familial risk of breast cancer and a genetic assessment in terms of DFS and OS. We propose that the increased survival associated with a family history of breast cancer, suggesting hereditary breast cancer according to the Modena criteria, should be considered with respect to BRCA1 analysis as a predictor of mutations. We feel that randomized studies need to be designed to further address the relationship between BRCA1 mutations and sensitivity to chemotherapy, and further clinical investigations are required to determine whether the BRCA1 status can be used to predict treatment outcomes.
Conclusions
In conclusion, our data show that patients belonging to a population with a high probability of being BRCA1 carriers have a better prognosis than other risk groups, and this could be considered an indicator of BRCA1 inheritance. This paper provides, for the first time, evidence-based proof that women at high risk of being BRCA1 carriers have a favorable prognosis after 10 years follow-up.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LC participated in the design of the study and drafted the manuscript. CM acquired the data and performed the statistical analysis. CC participated in the statistical analysis. VM performed the genetic testing. IM acquired the data from the centre. GC participated in the collection of patient data from Mantua Hospital. GP participated in the collection of patient data from Rimini Hospital.
DT participated in the collection of patient data from Bologna Hospital. MF designed the study and revised the final manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Laura Cortesi, Email: hbc@unimo.it.
Cristina Masini, Email: cristinamas@libero.it.
Claudia Cirilli, Email: cirilli.claudia@policlinico.mo.it.
Veronica Medici, Email: veronica.medici@gmail.com.
Isabella Marchi, Email: robertoisa@alice.it.
Giovanna Cavazzini, Email: giovanna.cavazzini@ospedalimantova.it.
Giuseppe Pasini, Email: Giuseppe.Pasini@auslrn.net.
Daniela Turchetti, Email: danielat@med.unibo.it.
Massimo Federico, Email: mfederico@unimore.it.
Acknowledgements
Associazione Angela Serra per la Ricerca sul Cancro; Euro Mediterranean Network for Genetic Services-Coordination Action (Grant to the Budget) - Contract n° INCO-CT-2006-031968
References
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Wong N, Brunet JS, Begin LR, Zhang JC, Martinez JJ, Rozen F, Tonin PN, Narod SA, Karp SE. Germ-line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin Cancer Res. 1997;3(12 Pt 1):2465–2469. [PubMed] [Google Scholar]
- Stoppa-Lyonnet D, Ansquer Y, Dreyfus H, Gautier C, Gauthier-Villars M, Bourstyn E, Clough KB, Magdelenat H, Pouillart P, Vincent-Salomon A. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol. 2000;18(24):4053–4059. doi: 10.1200/JCO.2000.18.24.4053. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Chappuis PO, Wong N, Brunet JS, Vesprini D, Rozen F, Yuan ZQ, Pollak MN, Kuperstein G, Narod SA. Primary node negative breast cancer in BRCA1 mutation carriers has a poor outcome. Ann Oncol. 2000;11(3):307–313. doi: 10.1023/A:1008340723974. [DOI] [PubMed] [Google Scholar]
- Goffin JR, Chappuis PO, Begin LR, Wong N, Brunet JS, Hamel N, Paradis AJ, Boyd J, Foulkes WD. Impact of germline BRCA1 mutations and overexpression of p53 on prognosis and response to treatment following breast carcinoma: 10-year follow up data. Cancer. 2003;97(3):527–536. doi: 10.1002/cncr.11080. [DOI] [PubMed] [Google Scholar]
- Hampl JA, Hampl M, Reiss G, Koch R, Saeger HD, Schackert HK. Loss of BRCA2 correlates with reduced long-term survival of sporadic breast cancer patients. Anticancer Res. 2004;24(1):281–290. [PubMed] [Google Scholar]
- Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, Narod SA. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357(2):115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- Cortesi L, Turchetti D, Bertoni C, Bellei R, Mangone L, Vinceti M, Federico M, Silingardi V, Ferrari S. Comparison between genotype and phenotype identifies a high-risk population carrying BRCA1 mutations. Genes Chromosomes Cancer. 2000;27(2):130–135. doi: 10.1002/(SICI)1098-2264(200002)27:2<130::AID-GCC3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26(14):2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25(43):5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR, Hudis C, Roberge D, Norton L, Begin LR. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6(1):R8–R17. doi: 10.1186/bcr658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadona V, Dussart-Moser S, Voirin N, Sinilnikova OM, Mignotte H, Mathevet P, Bremond A, Treilleux I, Martin A, Romestaing P. Prognosis of early-onset breast cancer based on BRCA1/2 mutation status in a French population-based cohort and review. Breast Cancer Res Treat. 2007;101(2):233–245. doi: 10.1007/s10549-006-9288-7. [DOI] [PubMed] [Google Scholar]
- Cortesi L, Turchetti D, Marchi I, Fracca A, Canossi B, Battista R, Ruscelli S, Pecchi AR, Torricelli P, Federico M. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer. 2006;6:210. doi: 10.1186/1471-2407-6-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, Goel A, Barbieri V, Costanzo F, Boland CR, Venuta S. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88(8):1285–91. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275(31):23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- Fedier A, Steiner RA, Schwarz VA, Lenherr L, Haller U, Fink D. The effect of loss of Brca1 on the sensitivity to anticancer agents in p53-deficient cells. Int J Oncol. 2003;22(5):1169–1173. [PubMed] [Google Scholar]
- Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8(12):571–576. doi: 10.1016/S1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96(22):1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279(53):55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Chappuis PO, Goffin J, Wong N, Perret C, Ghadirian P, Tonin PN, Foulkes WD. A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. J Med Genet. 2002;39(8):608–610. doi: 10.1136/jmg.39.8.608. [DOI] [PMC free article] [PubMed] [Google Scholar]