Abstract
Estrogens control cell growth and viability in target cells via an interplay of genomic and extragenomic pathways not yet elucidated. Here, we show evidence that cell proliferation and survival are differentially regulated by estrogen in rat pituitary tumor PR1 cells. Pico- to femtomolar concentrations of 17β-estradiol (E2) are sufficient to foster PR1 cell proliferation, whereas nanomolar concentrations of the same are needed to prevent cell death that occurs at a high rate in these cells in the absence of hormone. Activation of endogenous (PRL) or transfected estrogen-responsive genes occurs at the same, higher concentrations of E2 required to promote cell survival, whereas stimulation of cyclin D3 expression and DNA synthesis occur at lower E2 concentrations. Similarly, the pure antiestrogen ICI 182,780 inhibits estrogen response element-dependent trans-activation and cell death more effectively than cyclin-cdk activity, G1-S transition, or DNA synthesis rate. In antiestrogen-treated and/or estrogen-deprived cells, death is due predominantly to apoptosis. Estrogen-induced cell survival, but not E2-dependent cell cycle progression, can be prevented by an inhibitor of c-Src kinase or by blockade of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling pathway. These data indicate the coexistence of two distinguishable estrogen signaling pathways in PR1 cells, characterized by different functions and sensitivity to hormones and antihormones.
INTRODUCTION
Estrogen regulation of target cell proliferation results from promotion of both cell growth and survival (Altucci and Gronemeyer, 2001). Estrogens, in fact, control the function of several genes and proteins directly involved in cell cycle regulation, including cyclins, proto-oncogenes, and negative cell cycle regulators (Altucci et al., 1996; Dinda et al., 1997), although they are well known modulators of cell life and death in different cell types, including uterine epithelia (Pollard et al., 1987), breast cancer cells (Kyprianou et al., 1991), and neurons (Singer et al., 1996; Sawada et al., 2000). The cellular mechanism(s) mediating estrogen prevention of programmed cell death has not been clarified yet. 17β-Estradiol (E2) causes up-regulation of Bcl-2, the antiapoptotic factor that is able to suppress cytochrome c release ensuing caspase-3 activation in neurons (Dubal et al., 1999) and breast cancer cell lines (El Etreby et al., 1998), whereas the antiestrogen tamoxifen down-regulates Bcl-2 and mediates apoptosis via caspase-3 and c-Jun NH2-terminal kinase (JNK)-1 activation in breast carcinoma cells (Mandlekar et al., 2000), where estrogen receptor (ER)-α directly regulates Bcl-2 gene transcription (Perillo et al., 2000). On the other hand, other evidence suggests an inhibitory effect of estrogen on nongenomic apoptotic signals. Kousteni et al. (2001) have shown that the antiapoptotic effects of estrogens on bone cells are mediated by the extracellular signal-regulated kinase (ERK) cascade via an ER-α region that is not involved in genotropic actions of these steroids. Moreover, it has been reported that E2 prevents UV- or Taxol-induced apoptosis in breast cancer cells by inhibiting JNK activation and stimulating ERK activity (Razandi et al., 2000). These authors observed the same also with membrane-impermeable E2-bovine serum albumin conjugates, suggesting the possible involvement of a putative membrane receptor in mediating these hormonal effects. The causal role of these nongenotropic estrogen-signaling pathways and their correlation with the classical, gene transcription-mediated actions of these hormones are far from being understood. For example, although some reports indicate a central role for ERKs and protein kinase C-α signaling in estrogen regulation of cell cycle progression (Castoria et al., 1999; Manole et al., 2001; Marino et al., 2001), other authors have shown cell cycle effects of these hormones independently from these cascades. Furthermore, a growing body of evidence suggests the existence of cross talk between estrogen receptors and other signaling pathway effectors (Tolon et al., 2000; Coleman and Smith, 2001).
PR1 is an estrogen-responsive cell line derived from diethylstilbestrol-treated F344 female rats (Pastorcic et al., 1995) that shows a unique response to E2, because in these cells the concentration of E2 required for cell proliferation is 1000-fold lower than that required to increase expression of the hormone-responsive prolactin (PRL) gene (Chun et al., 1998), suggesting biphasic or distinct mechanisms for estrogen control of cell proliferation and gene activity. Furthermore, the ERK-dependent signaling is rapidly activated in this cell line in response to estrogen, and hormone-induced PRL production is prevented by mitogen-activated protein kinase kinase (MEK) inhibition (Watters et al., 2000). These observations indicate that this cell line represents a useful model to test the possibility that multiple, independent hormone-responsive signaling cascades may coexist in the same cellular background and control distinct cellular functions.
We have thus investigated here the effects of estrogen deprivation and stimulation on cell cycle progression, target gene trans-activation, and cell survival in PR1 cells, aiming at understanding the mechanism(s) that underlie promotion of tumor cell growth and survival by estrogens. Results show that estrogens control cell growth, DNA synthesis, and cell fate via distinguishable mechanisms in this cell line.
MATERIALS AND METHODS
Culture Conditions
PR1 is a pituitary cell line derived from E2-treated F344 female rats (Pastorcic et al., 1995). Cells were cultured in DMEM-F12 medium (Cambrex Bioproducts Europe, Verviers, Belgium) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT) and antibiotics (Sigma Chemical, Poole, Dorset, United Kingdom). Culture media were replaced every 2 d. Estrogen deprivation was obtained by culturing the cells in DMEM-F12 phenol red-free (Invitrogen, Carlsbad, CA) medium containing 10% charcoal (DCC)-treated FBS (DCC-FBS). For treatment with 17β-estradiol, MEK inhibitors (50 μM PD98059, New England Biolabs, Beverly, MA; 30 μM U0126, Cell Signaling Technology, Beverly, MA) and/or c-Src inhibitor (2 μM PP1, Alexis, Läufelfingen, Switzerland) cells were kept in hormone-free medium, as indicated. Culture media were changed every day.
Cell Growth Analysis
Colorimetric Assay. Cells (16,000/well) were loaded in 96-well dishes. After appropriate stimulation, cells were washed in phosphate-buffered saline (PBS) and fixed with 12.5% glutaraldehyde for 20 min at room temperature, before further washing with H2O and incubation with 0.05% methylene blue for 30 min, washing, and incubation with 0.33 M HCl for 18 h. Absorption was measured at 595 nm (Dufourny et al., 1997).
[3H]Thymidine Incorporation. Cells were loaded in six-well dishes with or without E2, ICI 182,780, or kinase inhibitors. The rate of DNA synthesis or the cell number was estimated by measuring [3H]thymidine ([methyl-3H]TdR 5 Ci/mmol; Amersham Italia, Milan, Italy) incorporation in DNA during 1 or 24 h, respectively. DNA was precipitated by 5% trichloroacetic acid and resolved in 0.1 M NaOH and 2% Na2CO3. Samples were then neutralized with 1 N HCl, and radioactivity was measured by liquid scintillation counter (PerkinElmer Wallac, Gaithersburg, MD). For [3H]thymidine pulse, data were normalized for total DNA content.
Cell Death Analysis
For cytofluorimetric analysis, cells were cultured in 100-mm dishes and collected in 0.1% sodium citrate and 50 μg/ml propidium iodide (PI). After an 18-h incubation, the percentage of cells with sub-G1 DNA was evaluated for flow cytometry with a FACS analyzer (FACScalibur; BD Biosciences, San Jose, CA).
For Annexin V-BOBO-1 labeling, cells were plated (30,000 cells/well) in 24-well dishes and, after stimulation, the medium was removed and cells were incubated in the Annexin V solution (10 mM HEPES, 140 mM NaCl and CaCl2, and 1 μg/ml Annexin V-Alexa-568; Roche Diagnostics, Mannheim, Germany), 1 μg/ml BOBO-1 (Molecular Probes). Simultaneous application of DNA stain and BOBO-1 (Molecular Probes), for dye exclusion tests, was used and cells were analyzed by fluorescence microscopy (Leica, Wetzlar, Germany).
Transient Transfections
For transient transfection, triplicate 60-mm culture dishes (1 × 106 cells/dish) were transfected by liposome-mediated (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate; Roche Diagnostics) gene transfer with ERE-tk luc (1.5 μg/transfection), a luciferase reporter gene, including estrogen responsive elements. Six hours after addition of the liposome-DNA mixture, media were changed and cells further incubated for 24 h. Cells were then harvested by washing, scraped in lysis buffer (Promega Italia, Milan, Italy), and lysed with three cycles of rapid freeze-thawing. After clearing the cell lysates by 5-min centrifugation at 4°C, protein concentration was determined in the crude extracts with a colorimetric assay (Bio-Rad Italia, Milan, Italy). Luciferase activity was assayed with luciferase assay reagent (Promega), following manufacturer's instructions. Results were normalized for protein concentration or controlled using a dual luciferase assay kit (Promega).
Immunoblot Analysis
Cell extracts were prepared in NP-40 buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 10 μM sodium fluoride, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, leupeptin, pepstatin A, and 20 nM okadaic acid).
Cells were washed twice in ice-cold PBS, scraped into ice-cold NP-40 buffer, and lysed for 10 min. Cell lysates were cleared by 15-min centrifugation at 4°C; protein content in the supernatant was assayed by the Bradford protein assay (Bio-Rad). The cell extracts were diluted 1:1 in 2× Laemmli sample buffer, followed by boiling for 5 min. Equal amounts of protein were loaded and separated for the SDS-PAGE gel. Protein smears were transferred to cellulose nitrate filters (Schleicher & Schüll, Dassel, Germany) and blocked for 30 min at 37°C in 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20. Membranes were incubated with the primary antibody for 1 h at room temperature in Tris-buffered saline with 0.1% Tween 20. Primary antibodies were the following: anti-Cyclin D3 (Transduction Laboratories, Lexington, KY), 1:1000; anti-rat prolactin antibodies (provided by the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases), 1 μg/ml; and anti-ERK (K23; Santa Cruz Biotechnology, Santa Cruz, CA), 100 μg/ml. After washing, a horseradish peroxidase-linked secondary antibody (Amersham Biosciences, Piscataway, NJ) was added and target protein bands were detected using enhanced chemiluminescence (Amersham Biosciences).
Caspase-3 Colorimetric Assay
Caspase-3 activity was assayed with a colorimetric assay kit (Biovision) from Alexis according to standard protocols suggested by the producer. Briefly, the assay is based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA. After the treatment, cells were lysed with the cell lysis buffer included in the kit, incubated for 10 min on ice, and then centrifuged at 13,000 rpm for 1 min at 4°C. The supernatant was added to the reaction buffer with dithiothreitol and DEVD-p-NA, and incubated 18 h at 37°C. Relative caspase-3 activity was measured as optical density at 405 nm. Two hundred micrograms of total proteins of each samples was used.
Cdc2 Kinase Assay
Cdc-2 kinase activity was assayed with Signatect cdc2 protein kinase assay (Promega Italia) according to the protocol suggested by the producer. A biotinylated peptide substrate derived from histone H1 (PKTPKKAKKL) was used as substrate. One microgram of proteins was assayed and radiolabeled. Phosphorylated substrate was recovered from the reaction mix with SAM2 biotin capture membrane. Results are expressed as picomoles per minute per microgram of proteins.
RNA Analysis by Multiplexed RNase Protection
Total RNA was extracted with Eurozol (Euroclone, Paris, France) as indicated by the producer. Rat apoptosis template sets (rAPO-1, 45601P; BD PharMingen, San Diego, CA) were labeled with [32P]uridine triphosphate (PerkinElmer Life Sciences, Boston, MA). The ribonuclease (RNase) protection assay was performed according to the supplier's instructions (BD PharMingen). Briefly, total RNA (4 μg) and 6-8 × 105 labeled probes were used for hybridization. After RNase treatments, the protected probe fragments were resolved on a 5% urea-polyacrylamide-bis-acrylamide gel. Dried gels were placed on film (Hyperfilm-MP; Amersham Biosciences) in a cassette with an intensifying screen. Exposure time varied depending upon application.
RESULTS
Characterization of the Mitogenic Responses of PR1 Cells to Estrogen
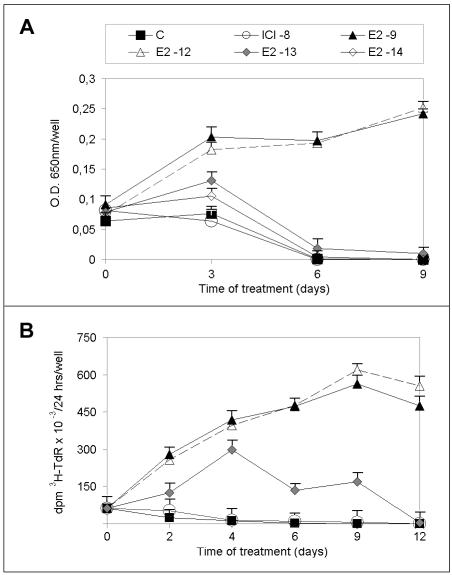
PR1 cells derive from F344 rat pituitary tumors induced by prolonged in vivo treatment with estrogens. As reported previously (Chun et al., 1998) PR1 cells are highly responsive to estrogen, being capable to respond to very low, subphysiological concentrations of E2. Analysis of estrogen receptor expression in this cell line reveals that both ER-α and ER-β subtypes can be detected (Chun et al., 1998; our unpublished data). The proliferative response of PR1 cells to different concentrations of E2 is shown in Figure 1, as assessed by a colorimetric cell quantitation assay (Figure 1A), or by [3H]thymidine incorporation into DNA (Figure 1B). The results indicate that concentrations of the hormone >10-12 M induce a substantial and progressive increase of cell number, both determining an increase during up to 9 d of treatment, as detected by either of the two quantitative methods applied. On the contrary, induction of cell cycle proliferation by lower concentrations of hormone (10-13-10-14 M) is transient, increasing slightly during the first 3-4 d of treatment and decreasing thereafter. Interestingly, in the absence of estrogen, a progressive and drastic reduction in the initial number of cells can be observed (Figure 1, A and B), so that by days 4-6, few cells can be detected, suggesting that massive cell death has occurred.
Figure 1.
Effects of estrogen and the pure antiestrogen ICI 182,780 on PR1 cell proliferation. (A) PR1 cells were maintained in estrogen-free medium (C) for 4 d, before replating and stimulation with the indicated concentrations of E2 or ICI 182,780 (ICI). At each indicated time, cells were collected and their number was evaluated by a colorimetric assay. (B) For [3H]TdR incorporation assays, cells were incubated as described above for the last 24 h before collection with radioactive thymidine and then processed as described under MATERIALS AND METHODS. The data shown (± SD) are representative of at least three, separate experiments carried out with six or eight replicate determinations.
Effects of Low Concentrations of E2 on Target Gene Transcription and Cell Cycle Control in PR1 Cells
To determine the activity of PR1 cells' ERs to their cognate hormone, we measured the effects of different concentrations of E2 on transcription of a transiently transfected estrogen receptor (ER)-responsive reporter gene (ERE-tk-luc) and compared the results obtained with what observed in the results reported in Figure 1. Results show that a concentration of hormone of 10-12 M, able to induce a maximal stimulatory effect of PR1 cell proliferation (Figure 1, A and B), is able to promote only half-maximal ER-mediated trans-activation (Figure 2A). This result was confirmed by analysis of PRL gene activation by E2, an endogenous marker of ER activity of PR1 cell genome (Chun et al., 1998), that shows a comparable response to the different concentrations of hormone tested (Figure 2B). Interestingly, 10-12 M E2 promotes a limited induction of PRL expression, compared with higher concentrations such as 10-9 M, whereas both concentrations are equally effective in promoting cell proliferation (compare data reported in Figures 1 and 2, A and B). This was further verified by assessing the sensitivity of key cell cycle progression markers to E2. As shown in Figure 2B, bottom, the expression of cyclin D3, an estrogen-regulated gene product that is rate-limiting for G1 progression in this cell line (Watters et al., 2000), increases in response to 10-12 M E2 to a degree comparable with that observed in response to higher concentrations of the hormone. Furthermore, this same concentration of E2 is sufficient to trigger maximal increase of the DNA synthesis rate (Figure 2C), a marker of S-phase entry under these test conditions. These results indicate that two distinct estrogen effector pathways show a different sensitivity to the hormone in PR1 cells.
Figure 2.
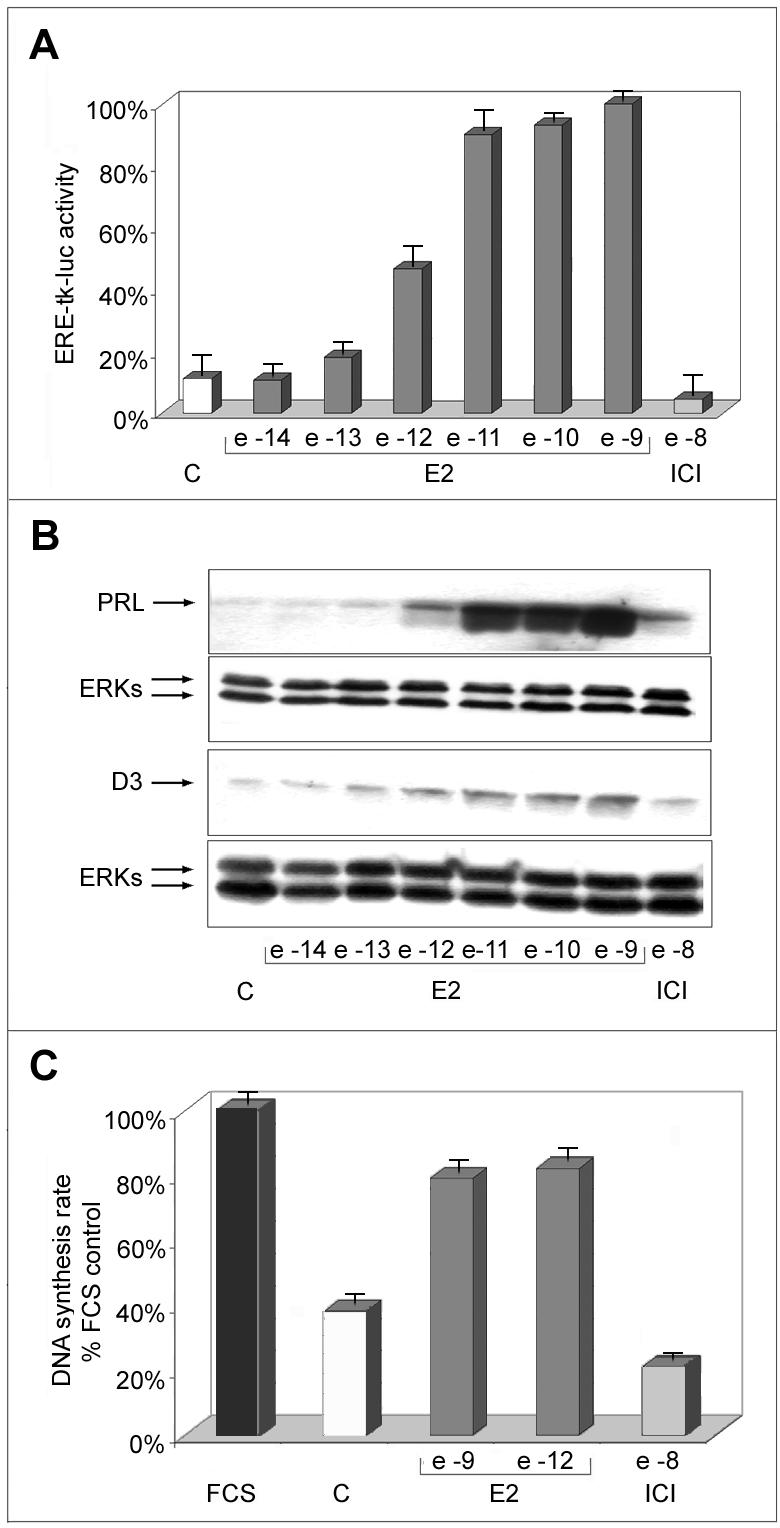
Effects of estrogen on ERE-mediated reporter gene transcription, DNA synthesis rate, and endogenous gene expression in PR1 cells. (A) Cells were maintained in estrogen-free medium (C) for 4 d, before transfection with the ERE-tk-luc reporter gene, followed by stimulation for 24 h with the indicated molar concentrations of either E2 or ICI 182,780 (ICI) and assay of luciferase activity in whole cell extracts, carried out as described in the text. (B) Intracellular prolactin (PRL) and cyclin D3 (D3) protein content was evaluated in whole cell lysates by Western blotting analysis, after 48-h stimulation carried out as described above. ERK1/2 assessment in the same blot or sample have been used for normalization, and the results are reported (ERKs). (C) Pulse labeling with [3H]TdR for 1 h was used to assess the effects of E2 and ICI treatment for 48 h on DNA synthesis rate. FCS, cells were maintained in medium containing 10% fetal bovine serum throughout the experiment. The data shown (± SD) are representative of multiple determinations carried out in replicate.
Biphasic Response of PR1 Cells to Estrogen Signaling Blockade with the Pure Anti-Estrogen ICI 182,780
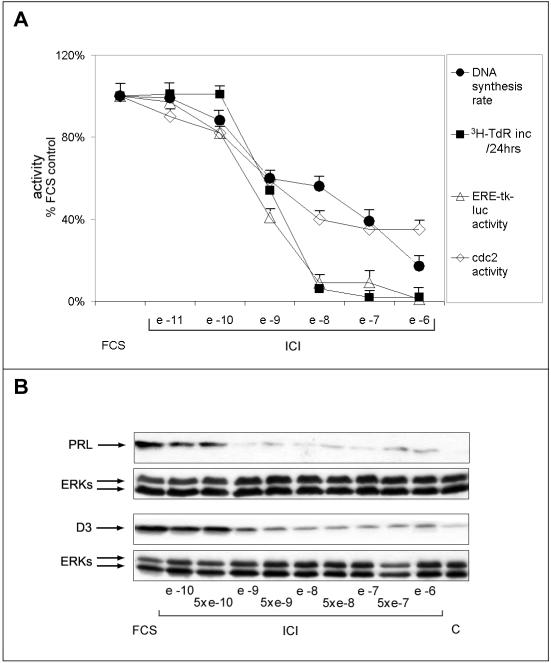
Given the high sensitivity of PR1 cells to estrogens, it cannot be excluded that traces of these hormones still present in the culture media containing DCC-FBS may influence interpretation of the experimental results. Indeed, when analyzing the effects of hormone withdrawal on cell cycle phasing in PR1 cells by cytofluorimetry, we observed consistent cell cycle arrest only when an estrogen antagonist was added to the culture medium 24-36 h before analysis (our unpublished data). Furthermore, charcoal treatment of the serum might affect the growth characteristics of PR1 cells by removal or reduction of one or more essential serum components. For these reasons, we decided to control the results described above by evaluating the effects on PR1 cells of estrogen signaling ablation by addition of the pure antiestrogen ICI 182,780 to standard culture medium. Under these experimental conditions, we compared DNA synthesis rate (measured by [3H]TdR incorporation), cdc2 kinase activity, ERE-tk-luc trans-activation, and cell proliferation in the absence and presence of increasing concentrations of ICI 182,780. As shown in Figure 3A, a variable degree of responsiveness to estrogen ablation was observed for the different effectors studied. In particular, estrogen response element (ERE)-mediated transcriptional activation and cell proliferation were more sensible to inhibition by the antiestrogen then DNA synthesis rate or cdc2 activity (Figure 3A; IC50 of 10-9 and 10-7 M, respectively). Furthermore, whereas ERE-tk-luc activity and cell number decreased abruptly as the concentration of ICI 182,780 in the medium was raised above 10-10 M, reaching a value close to 8-12% of the untreated control in the presence of 10-8 M antiestrogen, DNA synthesis rate and cdc-2 activity decreased more gradually in response to the antihormone, reaching a minimum value of 20-35% in the presence of >10-7 M ICI 182,780 (Figure 3A). These measures were independently confirmed by analysis of PRL and cyclin D3 expression changes under the same experimental conditions. In line with the data shown in Figure 3A, in fact, PRL expression was completely inhibited by 10-9 M ICI, a concentration that only partly inhibited cyclin D3 expression (Figure 3B).
Figure 3.
Distinct responses of PR1 cells to progressive estrogen receptor blockade with the antiestrogen ICI 182,780. (A) Effects of 48-h blockade of estrogen signaling with the indicated concentrations of ICI 182,780 on DNA synthesis rate (full circles), measured by pulse-labeling with radioactive thymidine, cell growth (full squares), measured by labeling with radioactive thymidine for 24 h, cdc-2 cyclin-dependent kinase activity (open lozenges), ERE-tk-luc activity (open triangle). Each point is the mean ± SD of at least two determinations carried out in triplicate. (B) Whole cell lysates were prepared for evaluating by Western blotting intracellular prolactin (PRL) and cyclin D3 (D3) protein content after 48-h treatment of the cells as described above. ERK1/2 assessment in the same blot or sample have been used for normalization and the results are reported (ERKs). FCS, cells were maintained in medium containing 10% fetal bovine serum throughout the experiment. The data shown (± SD) are representative of multiple determinations carried out in replicate.
These results, when combined, confirm the existence of at least two classes of estrogen-responsive effector pathways in PR1 cells, the first characterized by low sensitivity to E2 and higher sensitivity to hormone blockade with ICI 182,780, controlling cell number and ERE-mediated gene transcription, and the second one more sensitive to E2 and less responsive to the antihormone, controlling cell cycle progression.
Estrogen Deprivation Induces Apoptosis in PR1 Cells That Can be Prevented by Stimulation with E2
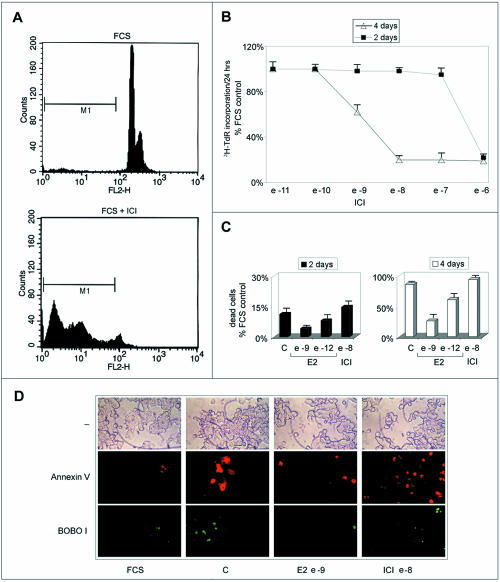
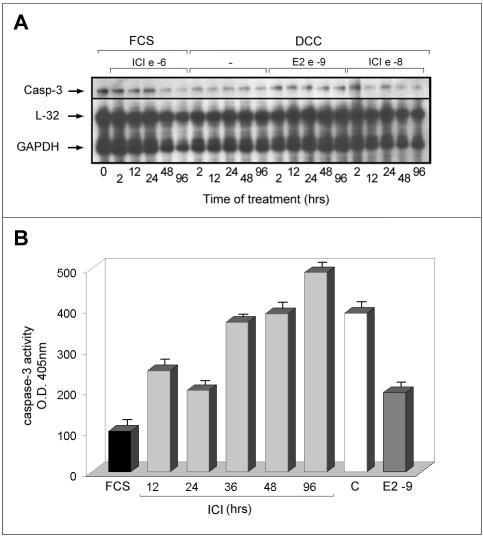
During prolonged treatment of PR1 cultures with ICI 182,780, or estrogen-free media, the number of cells decreases well below the values observed in control cultures (Figures 1 and 3A). We observed that under these conditions, cells undergo drastic changes in their morphology and assume features reminiscent of apoptosis. Cytofluorimetric analysis of estrogen-deprived or antiestrogen-treated PR1 cell cultures shows a progressive increase of the “pre-G1” population, representing dead cells, as shown, after 4-d treatment with ICI 182,780, at the bottom of Figure 4A (M1 gate). This is confirmed by the data reported graphically in Figure 4B, where the effects of increasing concentrations of ICI 182,780 on the cell number are reported, to show that after 4 d of treatment a significant reduction of this parameter (up to 85% decrease) is observed at concentrations of antiestrogen >10-10 M. This decrease seems to be due primarily to cell loss that becomes evident starting from day 3 of treatment by a great number of floating, dead cells (Figure 4B; our unpublished data). Notably, this dramatic response to estrogen signaling blockade is superimposable on that observed for ER-mediated trans-activation (Figure 3), in particular, for what concerns the steep decrease in ERE-tk-luc activity detectable at concentrations >10-10 M. The same effect observed after treatment with the antihormone could be detected upon estrogen withdrawal, in which case it was reversed by stimulation with ≥10-9 M E2 (Figure 4C). Using fluorescence microscopy, cell death by apoptosis was confirmed to occur in PR1 cells after estrogen withdrawal or ICI 182,780 treatment, as shown in Figure 4D by double fluorescent staining of the cultures with Annexin V (red), revealing cells undergoing apoptosis, and BOBO-1 (green), staining only dead cells. ICI 182,780 was found to induce an increase of Annexin V-positive cells in 24 h, whereas treatment of hormone-deprived cells with 10-9 M E2 for the same time was sufficient to prevent the appearance of dying or dead cells. The nature of the cell death induced by estrogen withdrawal was further investigated at a molecular level by multiprobe RNase mapping, by which the expression level of genes encoding known apoptosis regulators, including Fas, Fas-ligand, Bcl-2, Bax, Bcl-x, and caspase-1, -2, and -3, was assessed in PR1 cells before and after treatment. Although significant differences in expression of the investigated mRNAs were not detected (our unpublished data), caspase-3 gene was found highly expressed in PR1 cells (Figure 5A). Caspase-3 has been reported to play a central effector role in multiple apoptotic pathways and may thus represent one of the endpoints of the proapoptotic pathway(s) inhibited by estrogen in PR1 cells and released upon estrogen withdrawal or ICI 182,780 treatment. After this observation, the enzymatic activity of caspase-3 was measured with a colorimetric assay to assess activation of this proapoptotic effector in PR1 cells after estrogen signaling blockade. As shown in Figure 5B, treatment of the cells with ICI 182,780 resulted in the progressive, time-dependent increase in caspase-3 activity, reaching a four- to fivefold increase after 1.5-4 d of treatment. This effect of ICI 182,780 was dose dependent, inducing a significant increment in caspase-3 activity at concentrations ≥10-9 M (our unpublished data). 0
Figure 4.
Analysis of PR1 cells viability after estrogen deprival and restimulation or antiestrogen treatment. (A) Representative cytofluorimetric profile, cells growing in medium containing 10% FCS medium were analyzed before (top) or after treatment with ICI 182,780 (10-6 M; bottom). M1 indicate the pre-G1 fraction of dead cells. (B) Kinetics of induction of cell death by ICI 182,780 treatment of PR1 cells. (C) Kinetics of induction of PR1 cell death upon culture in estrogen-free medium (C), without or with ICI 182,780 (ICI), and reversal by stimulation with E2. (D) Cells were maintained in estrogen-free medium (C) for 2 d, before treatment with E2 10-9 M or ICI 10-8 M for 24 h and then were analyzed for Annexin V/BOBO-1 double staining by fluorescence microscopy. FCS, cells were maintained in medium containing 10% fetal bovine serum throughout the experiment. The data shown (± SD) are representative of multiple determinations carried out in replicate.
Figure 5.
Caspase-3 expression level and activity in PR1 cells after estrogen blockade with ICI 182,780. (A) Expression levels of caspase 3 mRNA in cells treated as indicated with either E2 or ICI 182,780 (ICI) were assessed by a multiple RNase protection assay. The signals corresponding to the mRNA of the housekeeping genes L32 and GAPDH are also shown for normalization. Cells were maintained in whole medium containing 10% fetal calf serum (FCS) or phenol red-free medium containing charcoal-stripped serum (DCC) throughout the experiment. (B) Cells were treated with 10-6 M ICI 182,780 for the indicated times before evaluation of caspase-3 enzyme activity in cell extracts by a colorimetric assay, as described under MATERIALS AND METHODS. Where indicated, cells were maintained in DCC medium without (C) or with (E2) 10-9 M 17β-estradiol for 48 h before analysis. The data shown (± SD) are representative of multiple determinations carried out in replicate.
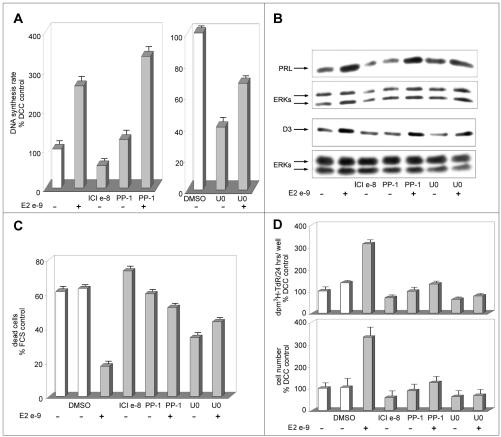
ERK and Src Kinase Inhibitors Do Not Block Estrogen-induced Increase in DNA Synthesis Rate in PR1 Cells
ERK and Src family kinases have been reported to play a pivotal role in estrogen prevention of apoptotic cell death (Razandi et al., 2000). To gain further insights into estrogen-dependent pathways that control cell cycle progression and cell survival in PR1 cells, the effects of ERK and Src kinase inhibition on hormone-mediated stimulation of DNA synthesis rate, inhibition of apoptotic cell death, and activation of target gene expression were investigated. In PR1 cells, estrogens have been described to stimulate ERK signaling, and it has been reported that inhibition of the ERK-activating MEK or Src enzymes prevents certain effects of estrogens, such as PRL gene activation (Watters et al., 2000). As shown in Figure 6A, the Src kinase inhibitor PP-1 (2 μM) does not affect basal or estrogen-induced DNA synthesis (measured as [3H]TdR incorporation rate), suggesting that neither of these activities is strictly dependent on Src activity. On the other hand, the MEK-1 and -2 inhibitor U0126 shows a marked inhibitory effect on estrogen-independent DNA synthesis, but it does not prevent completely the estrogen-dependent increase of this parameter (Figure 6A). Comparable results were obtained when using a different MEK inhibitor (PD98059; our unpublished data). These last results suggest that although the activity of this MEK-ERK signaling cascade is a necessary prerequisite for efficient DNA synthesis in PR1 cells, its blockade reduces but does not obliterate the ability of estrogen to enhance this cell cycle-dependent process. Cyclin D3 accumulation in response to E2 was not prevented by MEK inhibition with U0126 (Figure 6B), despite the fact that this compound determined a significant (50-60%) reduction in basal expression of the cyclin in hormone-starved control cells, further suggesting that estrogen-mediated cell cycle progression in PR1 cells is not fully dependent on the activity of the ERK signaling cascade. Similarly, Src kinase inhibition by PP-1 did not affect the ability of E2 to promote expression of this G1 cyclin (Figure 6B). The ability of the hormone to stimulate PRL gene expression, instead, was fully prevented by cell treatment with U0126, as reported for PD98059 (Watters et al., 2000), but not by Src kinase blockade with PP-1 (Figure 6B). Interestingly, MEK and Src kinase inhibitors both effi-ciently prevented the positive effects of E2 on cell survival (Figure 6C), resulting in a marked overall reduction in estrogen-dependent PR1 cell proliferation, as determined by both the standard tritiated thymidine incorporation assay and direct cell count (Figure 6D, top and bottom). U0126 alone seems to induce a marked inhibition of apoptosis in PR1 cells, independently of estrogen (Figure 6C), similarly to what reported previously in other cellular backgrounds (Bacus et al., 2001).
Figure 6.
Effects of MEK and c-Src inhibition on estrogen-induced stimulation evaluated on different PR1 cells parameters: DNA synthesis rate, endogenous gene expression, cell proliferation, and prevention of cell death. PR1 cells cultured in estrogen-free medium (C) were treated with the indicated concentration of E2 in the presence of 2 μM c-Src kinase inhibitor PP-1 or of 30 μM MEK inhibitor U0126 (dissolved in dimethyl sulfoxide). (A) DNA synthesis rate was assessed after 48 h of treatment by pulse-labeling with radioactive thymidine. (B) Prolactin (PRL) and cyclin D3 (D3) expression in the cells were evaluated by Western blotting after normalization for ERK protein expression in the same blot or sample. (C) Extent of cell death in the cultures evaluated by cytofluorimetric analysis. (D) Growth of the cultures assessed by cell labeling with radioactive thymidine for 24 h or by direct cell count (top and bottom, respectively). The data shown (± SD) are representative of multiple replicate determinations.
When combined, these results support the possibility that estrogen controls survival, apoptosis, and cell cycle progression in PR1 cells via distinct signaling cascades, among which the ERKs pathway is likely to play an important role in mediating the proliferative effects of E2 not through mitogenesis but via prevention of cell loss by apoptosis.
DISCUSSION
Fisher 344 is a rat strain highly sensitive to the carcinogenic effects of estrogens. In these animals, 6-10 wk of treatment with estrogenic compounds induces at high frequency pituitary prolactinomas (Kaplan and De Nicola, 1976), which are often hormone responsive, and for this reason can be exploited to investigate the molecular and genetic bases of hormone-mediated carcinogenesis and tumor progression and of the hormone-responsive phenotypes of cancer cells. The PR1 cell line, derived from one such F344 lactotroph tumors, expresses both ER-α and ER-β receptor subtypes and shows a high sensitivity to estrogen, representing a good model to analyze the signal transduction cascades-mediating estrogen effects in target cells. Indeed, very low concentrations of E2, inducing as low as 0.1% receptor occupancy, are sufficient for half-maximal stimulation of PR1 cell proliferation, PRL gene activation requires 1000-fold more hormone (Chun et al., 1998). This result suggests that either a small pool of active ER is sufficient to promote proliferation of this cell line or, alternatively, that hormone-responsive pathways exist here that are triggered by very low concentrations of estrogen and are independent from the “classical” ER. In the first case, the high sensitivity of the cell replication machinery to estrogen might be explained with the existence of cellular factors, critical for cell cycle control, interacting with ER with higher affinity than the factors involved in PRL gene control.
The results reported here demonstrate that different signaling pathways are implicated in the control of two key estrogen-regulated functions in PR1 cells, namely cell cycle progression and cell survival. Indeed, we found that E2 promotes cell cycle progression and prevents cell death and, more importantly, that the hormone differently regulates these events. We observed in fact a dissociation between the mitogenic and the antiapoptotic effects of E2, because concentrations of E2 ≤ 10-12 M are effective in enhancing DNA synthesis rate and cell proliferation (Figures 1 and 2B), whereas a preventive effect toward apoptosis requires 100-fold more E2 (Figure 4C). Interestingly, only the latter response occurs under conditions that clearly reflect optimal activation of the trans-activating functions of ERs. Expression of the PRL gene, an estrogen-responsive gene directly targeted by ERs, is minimal upon cell exposure to 10-12 M E2 (Figure 2B), and the same applies to ERE-mediated transcription of an exogenous reporter gene (Figure 2A). Promotion of PR1 cell survival shows an identical sensitivity to E2, because cell death occurring spontaneously in hormone-deprived cultures can be efficiently prevented only by concentrations of E2 well above 10-12 M. On the contrary, expression of cyclin D3, a component of the cell cycle regulatory machinery that marks G1-phase progression, or DNA synthesis rate, an indicator of S-phase activity, is already significantly activated at concentration of hormone ≤10-12 M (Figures 2, B and C). A possible functional explanation to these observations could reside on the fact that the diethylstilbestrol-driven carcinogenic process that led to clonal expansion in the tumor might have required that primary functions, such as the capacity of cells to replicate, be supported also by very low concentrations of circulating estrogens. On the other hand, promotion of cell survival is more critical during tumor initiation, when clonal selection might have been dependent upon higher concentrations of estrogenic compounds. Indeed, tumorigenesis and tumor growth are known to result from the combined activities of signaling pathways that control the balance between cell proliferation and arrest and cell death and survival (Altucci and Gronemeyer, 2001).
The comparable sensitivity to E2 of ERE-mediated promoter regulation and the cell survival phenotype might reflect also a functional link between the two cellular processes they represent. Indeed, the products of primary, ER-responsive “master” genes might be responsible for modulation of “secondary” gene programs, or even a complex circuitry of different and overlapping genetic programs, that permit PR1 cells survival. Similar control circuitries have been postulated for estrogen control of epithelial-to-mesenchymal transition and, possibly, the metastatic phenotype in breast cancer (Fujita et al., 2003) or of cell cycle progression in hormone-responsive cells (Weisz and Bresciani, 1993).
Cell cycle progression, DNA synthesis, trans-activation of target genes, and prevention of apoptosis are thus inscribed in distinct regulatory circuitries with different priorities in these cells. This dissociation among estrogen-dependent functions of PR1 cells becomes even more evident when the pure antiestrogen ICI 182,780 is used to block hormonal signaling. The concentrations of this compound that abolish ERE-mediated transcription or promote significant cell loss are, in fact, only partly inhibitory upon DNA synthesis rate and cyclin-dependent cdc2 activity (Figure 3A). More importantly, whereas the activity of the former two markers tends to drop steeply when antihormone concentration is raised above 10-10 M, that of the latter decreases gradually and less markedly. Similarly, abrupt PRL gene inhibition occurs when ICI 182,780 concentration is raised above 5 × 10-10 M, whereas suppression of cyclin D3 levels is once again more gradual and resistant to the antihormone, occurring only in the presence of higher concentrations of compound (Figure 3B). These evidences fully support the idea that estrogens and antiestrogens both exploit different pathways to intervene on each different vital function of PR1 cells. Apoptosis, be it induction by ICI 182,780 or prevention by E2, is strictly dependent upon the transcriptional activity of the ERs, whereas different signaling cascades molecules mediate cell cycle control.
ICI 182,780 induces apoptosis by activating the proteolytic activity of caspase-3 (Figure 5B). Caspase-3 is a downstream apoptotic effector, reported to be activated in response to either of at least two different pathways, one involving Bcl-2 and cytochrome c release, whereas the other dependent upon activation of death receptors. Both pathways can be regulated by estrogen. In fact E2 up-regulates Bcl-2 gene expression and two EREs have been reported to be present in the promoter region of the Bcl-2 gene (Perillo et al., 2000). Moreover, estrogen-mediated regulation of the expression of TRAIL-R2/Killer/DR5 gene (Wang and Jeng, 2000) and induction of TNFR1 and TRADD gene expression by ICI 182,780 and tamoxifen (Smolnikar et al., 2000) have been reported. The induction of apoptosis mediated by ICI 182,780 is dependent on ER trans-activation, most likely being controlled by a signaling molecule functionally located downstream of ER activation. In this respect, it is worth mentioning that this pure antiestrogen, known to block ER-α activity by increasing protein turnover and thereby reducing its intracellular content (Dauvois et al., 1992) as well as by disrupting nucleocytoplasmic shuttling of the receptor (Dauvois et al., 1993) can also affect directly ER trans-acting functions by inducing corepressor binding to the LXXLL motif of the receptor molecule (Huang et al., 2002; Webb et al., 2003).
MEK inhibitors can interfere with estrogen signaling in PR1 and other pituitary tumor cells (Watters et al., 2000). Interestingly, mitogen-activated protein kinase blockade by MEK inhibition has been reported by these authors to block the ability of estrogens to regulate PRL gene expression. Analysis of the data obtained here with protein kinase inhibitors (Figure 6) strongly supports the possibility that different estrogen-dependent pathways control cell cycle progression and apoptosis/gene trans-activation. The MEK inhibitor U0126 and the Src tyrosine kinase inhibitor PP-1, in fact, can prevent induction of PR1 cell survival by E2 (Figure 6C), whereas being ineffective toward hormone-dependent DNA synthesis rate (Figure 6A) and cyclin D3 expression (Figure 6B), suggesting that G1-phase progression in response to estrogen can occur in these cells also in the absence of Src- and MEK-dependent signaling. PP1 has a minimal, if any, influence on E2-dependent up-regulation of PRL levels, whereas U0126 abrogates this response completely. This implies that Src may act in concert with estrogen in regulating PRL, and possibly other genes, also via effector molecules disjunct from the MEK-ERK cascade. Interestingly, both PP-1 and U0126 prevent estrogen-mediated increase in cell number, suggesting that promotion of cell survival is dominant over induction of cell division in determining tumor cell population control by estrogen. This implies that cell death can occur even in the presence of residual hormonal signaling, once the level of estrogen stimulation decreases below a critical threshold.
In conclusion, the results reported here demonstrate that estrogens can control multiple cellular functions in target cells by means of an integrated network of signaling pathways, involving distinct effector molecules. These different hormone-responsive pathways can be activated in parallel, in concert, thanks to functional cross talk, or independently from each other, whereas they can cooperate toward the same function or exert a selective role. The knowledge that several signaling pathways are differently regulated by estrogens suggests ways to improve our knowledge of the master players involved in the regulation of complex cellular functions, such as cell transformation, proliferation, differentiation, and death. At the same time, the results reported here indicate that PR1 cells represent a unique tool for the molecular and genetic dissection of estrogen action, to foster our understanding of the mechanisms by which estrogens influences different biological functions in the cell.
Acknowledgments
We thank W. Basile, T. Battista, J. Gorski, and A.F. Harlow (National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases) for cell lines, reagents and technical assistance. This research was supported by Associazione Italiana per la Ricerca sul Cancro (research grants 2001-2003 to A.W.), Ministero dell'Istruzione, Università e Ricerca (grants PRIN 2001067229_002 to F.B., PRIN 2002067514_002, and FIRB RBNE0157EH to A.W.), European Commission (contracts BMH4-CT98-3433 to A.W., QLG1-CT-2000-01935 and QLK3-CT-2002-02029 to L.A.), Regione Campania (Fondi per la Ricerca di Base, L. 41/99 to A.W. and L. 41/00 to L.A.), and Seconda Università degli Studi di Napoli (Fondi per la Ricerca di Ateneo 2001-02).
References
- Altucci, L., Addeo, R., Cicatiello, L., Dauvois, S., Parker, M.G., Truss, M., Beato, M., Sica, V., Bresciani, F., and Weisz, A. (1996). 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene 12, 2315-2324. [PubMed] [Google Scholar]
- Altucci, L., and Gronemeyer, H. (2001). Nuclear receptors in cell life and death. Trends Endocrinol. Metab. 12, 460-468. [DOI] [PubMed] [Google Scholar]
- Bacus, S.S., Gudkov, A.V., Lowe, M., Lyass, L., Yung, Y., Komarov, A.P., Keyomarsi, K., Yarden, Y., and Seger, R. (2001). Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 20, 147-155. [DOI] [PubMed] [Google Scholar]
- Castoria, G., Barone, M.V., Di Domenico, M., Bilancio, A., Ametrano, D., Migliaccio, A., and Auricchio, F. (1999). Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 18, 2500-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, T.Y., Gregg, D., Sarkar, D.K., and Gorski, J. (1998). Differential regulation by estrogens of growth and prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. Proc. Natl. Acad. Sci. USA 95, 2325-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, K.M., and Smith, C.L. (2001). Intracellular signaling pathways, nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front. Biosci. 6, 1379-1391. [DOI] [PubMed] [Google Scholar]
- Dauvois, S., Danielian, P.S., White, R., and Parker, M.G. (1992). Antiestrogen ICI 164, 384 reduces cellular estrogen receptor content by increasing its turnover. Proc. Natl. Acad. Sci. USA 9, 4037-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois, S., White, R., and Parker, M.G. (1993). The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J. Cell Sci. 104, 1377-1388. [DOI] [PubMed] [Google Scholar]
- Dinda, S., Kodali-Gali, S., Sevilla, L., Burkley, M., Hurd, C., and Moudgil, V.K. (1997). Inhibition of proliferation of T47D human breast cancer cells: alterations in progesterone receptor and p53 tumor suppressor protein. Mol. Cell. Biochem. 175, 81-89. [DOI] [PubMed] [Google Scholar]
- Dubal, D.B., Shughrue, P.J., Wilson, M.E., Merchenthaler, I., and Wise, P.M. (1999). Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 19, 6385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourny, B., Alblas, J., van Teeffelen, H.A., van Schaik, F.M., van der Burg, B., Steenbergh, P.H., and Sussenbach, J.S. (1997). Mitogenic signaling of insulin-like growth factor I in MCF-7 human breast cancer cells requires phosphatidylinositol 3-kinase and is independent of mitogen-activated protein kinase. J. Biol. Chem. 272, 31163-31171. [DOI] [PubMed] [Google Scholar]
- El Etreby, M.F., Liang, Y., Wrenn, R.W., and Schoenlein, P.V. (1998). Additive effect of mifepristone and tamoxifen on apoptotic pathways in MCF-7 human breast cancer cells. Breast Cancer Res. Treat. 51, 149-168. [DOI] [PubMed] [Google Scholar]
- Fujita, N., Jaye, D.L., Kajita, M., Geigerman, C., Moreno, C.S., and Wade, P.A. (2003). MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113, 207-219. [DOI] [PubMed] [Google Scholar]
- Huang, H.J., Norris, J.D., and McDonnell, D.P. (2002). Identification of a negative regulatory surface within estrogen receptor alpha provides evidence in support of a role for corepressors in regulating cellular responses to agonists and antagonists. Mol. Endocrinol. 16, 1778-1792. [DOI] [PubMed] [Google Scholar]
- Kaplan, S.E., and De Nicola, A.F. (1976). Protein and RNA synthesis in pituitary tumors from F344 rats given implants of estrogen. J. Natl. Cancer Inst. 56, 37-42. [DOI] [PubMed] [Google Scholar]
- Kousteni, S., et al. (2001). Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719-730. [PubMed] [Google Scholar]
- Kyprianou, N., English, H.F., Davidson, N.E., and Isaacs, J.T. (1991). Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 51, 162-166. [PubMed] [Google Scholar]
- Mandlekar, S., Yu, R., Tan, T.H., and Kong, A.N. (2000). Activation of caspase-3 and c-Jun NH2-terminal kinase-1 signaling pathways in tamoxifen-induced apoptosis of human breast cancer cells. Cancer Res. 60, 5995-6000. [PubMed] [Google Scholar]
- Manole, D., Schildknecht, B., Gosnell, B., Adams, E., and Derwahl, M. (2001). Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J. Clin. Endocrinol. Metab. 86, 1072-1077. [DOI] [PubMed] [Google Scholar]
- Marino, M., Di Stefano, E., Caporali, S., Ceracchi, G., Pallottini, V., and Trentalance, A. (2001). beta-estradiol stimulation of DNA synthesis requires different PKC isoforms in HepG2 and MCF7 cells. J. Cell. Physiol. 188, 170-177. [DOI] [PubMed] [Google Scholar]
- Pastorcic, M., De, A., Boyadjieva, N., Vale, W., and Sarkar, D.K. (1995). Reduction in the expression and action of transforming growth factor beta 1 on lactotropes during estrogen-induced tumorigenesis in the anterior pituitary. Cancer Res. 55, 4892-4898. [PubMed] [Google Scholar]
- Perillo, B., Sasso, A., Abbondanza, C., and Palumbo, G. (2000). 17beta-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol. Cell. Biol. 20, 2890-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, J.W., Pacey, J., Cheng, S.V., and Jordan, E.G. (1987). Estrogens and cell death in murine uterine luminal epithelium. Cell Tissue Res. 249, 533-540. [DOI] [PubMed] [Google Scholar]
- Razandi, M., Pedram, A., and Levin, E.R. (2000). Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol. Endocrinol. 14, 1434-1447. [DOI] [PubMed] [Google Scholar]
- Sawada, H., Ibi, M., Kihara, T., Urushitani, M., Honda, K., Nakanishi, M., Akaike, A., and Shimohama, S. (2000). Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. FASEB J. 14, 1202-1214. [DOI] [PubMed] [Google Scholar]
- Singer, C.A., Rogers, K.L., Strickland, T.M., and Dorsa, D.M. (1996). Estrogen protects primary cortical neurons from glutamate toxicity. Neurosci. Lett. 212, 13-16. [DOI] [PubMed] [Google Scholar]
- Smolnikar, K., Loffek, S., Schulz, T., Michna, H., and Diel, P. (2000). Treatment with the pure antiestrogen faslodex (ICI 182780) induces tumor necrosis factor receptor 1 (TNFR1) expression in MCF-7 breast cancer cells. Breast Cancer Res. Treat. 63, 249-259. [DOI] [PubMed] [Google Scholar]
- Tolon, R. M., Castillo, A. I., A. M., J.-L., and A, A. (2000). Association with Ets-1 causes ligand- and AF2-indipendent activation of nuclear receptors. Mol. Cell. Biol. 20, 8793-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.T., and Jeng, J. (2000). Coordinated regulation of two TRAIL-R2/KILLER/DR5 mRNA isoforms by DNA damaging agents, serum and 17beta-estradiol in human breast cancer cells. Breast Cancer Res. Treat. 61, 87-96. [DOI] [PubMed] [Google Scholar]
- Watters, J.J., Chun, T.Y., Kim, Y.N., Bertics, P.J., and Gorski, J. (2000). Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol. Endocrinol. 14, 1872-1881. [DOI] [PubMed] [Google Scholar]
- Webb, P., Nguyen, P., and Kushner, P.J. (2003). Differential SERM effects on corepressor binding dictate ERalpha activity in vivo. J. Biol. Chem. 278, 6912-6920. [DOI] [PubMed] [Google Scholar]
- Weisz, A., and Bresciani, F. (1993). Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit. Rev. Oncog. 4, 361-388. [PubMed] [Google Scholar]