Abstract
In a chronic disease such as glaucoma, a therapy that provides a long lasting local effect, with minimal systemic side effects, while circumventing the issue of patient compliance, is very attractive. The field of gene therapy is growing rapidly and ocular applications are expanding. Our understanding of the molecular pathogenesis of glaucoma is leading to greater specificity in ocular tissue targeting. Improvements in gene delivery techniques, refinement of vector construction methods, and development of better animal models combine to bring this potential therapy closer to reality.
Keywords: animal models, gene delivery, gene therapy, glaucoma, viral vectors
Introduction
Glaucoma, the second most common cause of blindness in the world, is becoming increasingly prevalent as life expectancy increases. Currently, there is no treatment that reverses the glaucomatous optic neuropathy that characterizes this disease. Elevated intraocular pressure (IOP) appears to be one of the main risk factors. Medically or surgically treating ocular hypertension to lower IOP by even a few mmHg can slow progression of the disease. [1, 57] As such, lowering IOP remains the mainstay therapy in the management of glaucoma. Topical drops are the most common first treatment option for elevated IOP.
Surgical therapies carry certain risks and may not free patients from the need for additive topical therapies. Once a patient is diagnosed with glaucoma, he/she remains a glaucoma patient and may need to be treated for the rest of his/her life, often with more than one therapy. Patient compliance may be problematic, especially in the long term. Over the past decades, the molecular pathogenesis of glaucoma has been progressively delineated, and gene therapy techniques have been developed and refined to allow targeting different ocular tissues to potentially treat glaucoma. This review is a summary of current knowledge and the possible applications of gene therapy to glaucoma.
Potential Glaucoma Gene Therapy Strategies
Genes and targets for glaucoma gene therapy are summarized in Table 1 and Fig. 1.
Table 1.
Summary of strategies used in glaucoma gene therapy.
| Genes studied | Target genes, proteins, mechanisms | Target tissues | Main cellular and molecular changes induced in vitro or in vivo | Outcome expected | Refs |
|---|---|---|---|---|---|
| DN Rho | Inhibiting Rho | TM | Reduction of actin and focal adhesions in cultured TM cells; loss of intercellular adhesions in cultured SC cells. | Increased conventional outflow | 117 |
| C3 | Inactivating Rho by rebosylation | TM | Cell rounding; disruption of actin and loss of cellular adhesions in cultured HTM cells. | Increased conventional outflow | 69 |
| DNRK | Inhibiting Rho kinase | TM | Cell rounding, cell-cell detachment; reduction of actin, focal adhesion and myosin light chain phosphorylation in cultured TM cells. | Increased conventional outflow | 95 |
| Caldesmon | Inhibiting actin-tropomyosin activating myosin MgATPase | TM | Formation of unique curvy actin networks and disruption of focal adhesions in cultured HTM cells | Increased conventional outflow | 33, 37 |
| MMPs | Disrupting MMP/TIMP balance | TM/CM | Degrading the ECM | Increased uveoscleral, conventional outflow | 83 |
| PG synthase genes | Increasing MMP expression | CM | Upregulation of MMP expression acting to degrade ECM | Increased uveoscleral, conventional outflow | 74 |
| Specific siRNAs | Silencing gene expression | TM/retina | Suppression of target genes e.g. myocilin | e.g., increased outflow, decreased AHF, neuroprotection | 20, 124 |
| Bcl-2 | Protecting the integrity of the mitochondrial membrane | Retina | RGC cells remained morphologically intact and survived | Neuroprotection | 73, 77 |
| BIRC4 | Inhibiting caspase | Retina | Increased optic nerve axon survival | Neuroprotection | 78,107 |
| BDNF | Neuroprotection | Retina | Increased neuronal survival | Neuroprotection | 23, 64, 76 |
| TrkB | BDNF receptor | Retina | Increased neuronal survival | Neuroprotection | 19 |
| Erk | Mediating neuroprotective activity of extracellular factors, including neurotrophins | Retina | Increased RGC survival | Neuroprotection | 89 |
| MEK1 | The upstream activator of Erk1/2 | Retina | Increased RGC survival | Neuroprotection | 127 |
| CNTF | Neuroprotection | Retina | Increased RGC survival | Neuroprotection | 12, 101 |
| TNF-alpha | Inhibiting TNF-alpha | Retina | Increased RGC survival | Neuroprotection | 82, 110 |
| p21 | Regulating cell cycle and preventing proliferation of fibroblasts | Conjun-ctiva | Decreased fibroproliferation | Prevention of wound healing in filtering surgery | 43,88 |
Abbreviations: DN: dominant negative; DNRK: dominant negative Rho kinase; C3: exoenzyme C3 transferase; MMPs: matrix metalloproteinases; PG: prostaglandin; ERK1/2: extracellular signal-regulated kinase 1 and 2; BIRC4(XIAP): baculoviral IAP (inhibitor of apoptosis) repeat-containing protein-4; Apaf-1: apoptotic protease-activating factor 1. BDNF: brain-derived neurotrophic factor; MAPK1: mitogen-activated protein kinase 1; CNTF: ciliary neuronotrophic factor; p21: p21 WAF-1/Cip-1 (a cyclin kinase inhibitor); TNF-alpha: tumor necrosis factor alpha.
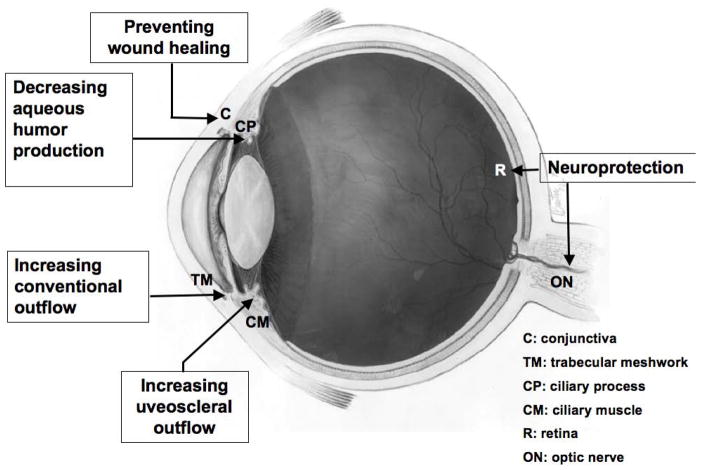
Figure 1.
Diagram showing different ocular target tissues and strategies for glaucoma gene therapy. C: conjunctiva; TM: trabecular meshwork; CP: ciliary process; CM: ciliary muscle; R: retina; ON: optic nerve. Eye diagram adapted from National Eye Institute, National Institutes of Health Ref#: NEA04
LOWERING IOP
Increasing Conventional Outflow
Ocular hypotensive agents are the standard pharmacotherapy for glaucoma. Except for pilocarpine and epinephrine, no agents have been developed to enhance outflow facility through the conventional outflow pathway (the trabecular meshwork (TM) and Schlemm’s canal (SC)), which usually accounts for 50% to 75% of aqueous humor outflow. [44]
Cholinergic drugs, such as pilocarpine, indirectly enhance conventional outflow and have been used in glaucoma therapy for over a century. [111] Their effect on IOP is consequent to agonist-induced, muscarinic receptor-mediated contraction of the ciliary muscle. Ciliary muscle contraction via [11, 14, 107]an intimate anatomic relationship between the anterior tendons of the ciliary muscle bundles and the scleral spur, peripheral cornea, TM, and inner wall of SC, [77] results in an unfolding of the meshwork and widening of the canal, facilitating aqueous outflow from the anterior chamber through the meshwork into the canal lumen, and thence into the venous collector channels and the general venous circulation. [10, 87]
Epinephine, which, like pilocarpine, is no longer commonly used for glaucoma therapy, also enhances outflow facility via β2-adrenergic receptors on the trabecular endothelial cells.[32] One hypothesis for this is that epinephrine induces disruption of actin filaments within the TM cells, altering cell shape, and increasing hydraulic conductivity across the meshwork. Relaxation of the TM could also play a role in the outflow facility response to epinephrine. [37]
The cells in the juxtacanalicular region of the TM and the SC inner wall, where most of the resistance to aqueous humor outflow resides, [58] have unique morphological and functional properties. It is thought that an increase in outflow facility can be achieved by modulating these properties. New therapies are being developed to target the structures and enzymes involved in maintaining actin associated cell shape, cell-cell and cell-ECM interactions that could ultimately reduce the resistance to outflow by affecting cellular and tissue contractility/relaxation in these areas.
Disrupting Actin
Reduction of cellular contractility lowers IOP and increases outflow facility in organ cultured ocular anterior segments, [112] [125] and in primates in vivo. [100] [121] Disruption of the actin cytoskeleton by proteins and enzymes also increases outflow facility in live animals. [50]
Rho GTPase (Rho) regulates actin dynamics by interacting with specific effectors, such as Rho kinase, and plays a key role in mediating a variety of biological properties and functions in cells. Inhibition of Rho kinase increases outflow facility in organ cultured porcine and human eyes and in monkey eyes in vivo. [50,105, 120] Interfering with other proteins associated with the rho cytoskeleton-regulating cascade including myosin II ATPase, [134] protein kinase C, [62, 119] and G-protein coupled receptors [85, 104] also increases outflow facility via the conventional outflow pathway. It is reasonable to hypothesize increasing outflow facility by using gene therapy strategies to express protein inhibitors of this cascade, or by expressing dominant negative mutants of the actin effectors. However, caution is advised because of the possibility of triggering cell migration and cell division when the Rho kinase pathway is manipulated.
Dominant negative forms of regulators of actin cytoskeleton assembly have also been investigated. Over-expressing dominant negative forms of rho or its downstream effector, Rho-kinase (DNRK), increased outflow facility in organ cultured human eye anterior segments. The percentage increase in facility was much greater with the latter (DNRK). [103, 125] This difference between the outflow facility increasing effects of inhibiting Rho compared to Rho-kinase indicates that direct targeting of Rho-kinase represents a more effective strategy for modulating aqueous outflow. These observations strongly indicate the role of the Rho/Rho-kinase pathway in the regulation of aqueous humor outflow through the TM and the potential of increasing outflow facility by selectively down-regulating Rho/Rho-kinase.
Rho is the preferred intracellular target of bacterial protein toxins. Exoenzyme C3 from Clostridium botulinum produces ADP-ribosylation, which specifically blocks Rho at R43 and prevents the recruitment of Rho on the cell membrane, resulting in inactivation of its downstream effectors. [3] Liu el al showed that adenovirus (Ad)-mediated C3 expression significantly disrupted the cytoskeleton, focal adhesions and cell-cell adhesions of cultured HTM cells, and furthermore, increased outflow facility by 90% in organ cultured monkey anterior segments.[74]
Caldesmon is an actin-binding protein, which binds F-actin reversibly in the presence or absence of Ca2+. It has two isoforms, smooth muscle and non-muscle, both of which are potent inhibitors of actin-tropomyosin activated myosin MgATPase. The non-muscle isoform is a regulatory factor in the microfilament network and is involved in the disassembly and destabilization of microfilaments. [51] Over-expression of non-muscle caldesmon induces changes in the distribution of the actin cytoskeleton. Unique curvy actin networks are formed and focal adhesions in cultured HTM cells are disrupted. [42] Over-expression also increases outflow facility in organ-cultured human and monkey anterior segments. [38]
Regulating Extracellular Matrix Interactions
In addition to actin targeting strategies, matrix protein regulation in the TM is another area that can be targeted with gene therapy.utations in myocilin, a protein that is present both intra- and extracellularly, have been linked to glaucoma. [33] Caballero et al [20] transduced both primary HTM cells and perfused human anterior segment cultures with an adenoviral vector expressing a truncated mutant of myocilin that accumulated inside the cell, and reduce secretion of endogenous myocilin, leading to an increase in outflow facility at 48 h post-transduction. [20] Other studies showed that perfusion of organ cultured anterior segments with recombinant myocilin caused an increase in IOP over a 12-hour period, increasing outflow resistance 94%.[34]
Myocilin also modified signaling events mediated by the heparin II domain of fibronectin (Hep II), possibly through direct interactions with it. [98] Hep II increased outflow facility in cultured human anterior segments[108] and its addition to plated HTM cells increased cell spreading and the numbers of focal adhesions and stress fibers. [99] These studies suggest that fibronectin-mediated interactions in the TM may have a role in modulating aqueous hydrodynamics.
Enzymes that degrade the ECM in the TM may also regulate outflow resistance. Studies have shown the feasibility of transferring genes encoding matrix metalloproteinase (MMP) to cultured HTM cells and the TM of human donor and rat eyes. [52] [60] In the latter two cases, outflow facility was increased and IOP was decreased, respectively.
Increasing Uveoscleral Outflow
Uveoscleral outflow, which accounts for up to half of the aqueous drainage in young, healthy human and nonhuman primate eyes, declines with age. [39, 55, 124] Uveosceleral outflow is also significantly decreased in ocular hypertensive human eyes compared to normotensive controls. [123] The ciliary muscle is an appropriate target structure for increasing uveoscleral outflow via gene therapy. Altering the ECM in the ciliary muscle is an important mechanism by which prostaglandins lower IOP. [90, 91] A recent finding suggests that latanoprost may shift the balance of MMPs and tissue inhibitors of the MMPs (TIMPs) toward greater levels of MMPs, resulting in the observed changes in the ECM in the ciliary body. [90] Gene therapy may be used to enhance or suppress the endogenous targets that are ultimately responsible for the outflow enhancement triggered by these agents.
Using an adenoviral vector, Kee et al showed that the stromelysin gene was expressed in cultured HTM cells, and in the TM, iris, and uveoscleral outflow pathway of the rat eye. In addition, in vitro work demonstrated that the gene products possessed enzymatic activity. [60] These results indicate the possibility of treating glaucoma by expressing this functional gene within the uveoscleral outflow pathway.
Over-expression of PG synthase genes may be another approach to increase MMP synthesis. For example, synthesis of MMPs such as collagenase I (MMP-1), gelatinase B (MMP-9) and matrilysin (MMP-7) is dependent on the synthesis of PGE2, suggesting that alterations in MMP expression may be instigated by alterations in PG synthesis. [80] In addition, the role of the prostaglandin FP receptor in IOP lowering effects by prostaglandin analogues currently used clinically has been demonstrated using FP knockout mice. [25, 91] Transferring genes for PGE2 synthase or the FP receptor into ciliary muscle has potential as a strategy to lower IOP by enhancing uveoscleral outflow. Very recently, lentiviral vectors mediated expression of PG pathway genes, including Cyclooxygenase-2 (COX-2), PGF synthase and FP receptor genes, significantly lowered IOP in live animals. Fluorophotometry suggested increased outflow rather than decreased aqueous production as the mechanism (Barraza RA, McLaren J, Poeschla EM. Glaucoma: Sustained IOP Reduction with Integrated Prostaglandin Biosynthesis and Response Transgenes. ARVO Asbract 2881: 2007.)
Decreasing Aqueous Humor Production
Aqueous humor and its production are essential to the normal function of the eye. Lowering IOP by long-term reduction in aqueous humor production may not be an ideal approach to treat glaucoma. Nevertheless, medication targeting aqueous humor secretion remains one of the mainstays of glaucoma treatment. Used in combination with classes of drugs that reroute fluid away from the TM, suppressors of aqueous humor formation can cause underperfusion of the TM and a secondary decrease in outflow facility. [64]
The aqueous humor secretory process is not fully known. Current thought is that aqueous humor is formed mostly by active transport of ions and solutes across the ciliary epithelium. [122] The feasibility of transducing the relevant tissue/cells has been demonstrated. A replication-competent herpes simplex virus (HSV) type 1 ribonucleotide reductase mutant (hrR3) expressing a reporter gene was delivered to monkey eyes via intracameral injection, and reporter gene expression was detected in multiple ocular tissues/cells including non-pigmented ciliary epithelial cells (NPE). [73]
Inhibition of aqueous humor secretion with RNA interference based gene therapeutic strategies has been developed. Topical administration of specific siRNAs targeting carbonic anhydrase genes (Jiménez A, Sesto A, Pintor J, et al. RNAi: A New Strategy for Treating Ocular Hypertension Silencing Carbonic Anhydrases. ARVO Abnr 405, 2006) and alpha and beta adrenoceptors (Pintor JJ, Mediero A, Jimenez A, et al. SiRNA in the Treatment of Ocular Hypertension Targeting Alpha and Beta Adrenoceptors. ARVO Abnr 403, 2006) lowered IOP in rabbits. The IOP lowering effects of these strategies were comparable to those obtained by commercial products. The siRNA strategies are desirable because of the potential for producing a longer lasting effect than commercial pharmaceutical products (Pintor JJ, Mediero A, Jimenez A, et al. SiRNA in the Treatment of Ocular Hypertension Targeting Alpha and Beta Adrenoceptors. ARVO Abnr 403, 2006) (Jiménez A, Sesto A, Pintor J, et al. RNAi: A New Strategy for Treating Ocular Hypertension Silencing Carbonic Anhydrases. ARVO Abnr 405, 2006).
NEUROPROTECTION
Glaucoma is a neurodegenerative disease in which the retinal ganglion cells and optic nerve axons are vulnerable to degeneration and may also be amenable to neuroprotection. Selective loss of retinal ganglion cells (RGC) is the hallmark of virtually all diseases of the optic nerve, including glaucomatous optic neuropathy. At present, the pathophysiology underlying RGC death in glaucoma is not completely clear. However, accumulating evidence has mainly indicated the following mechanisms, with apoptosis of RGC as a final common denominator: 1) neurotrophic factor deprivation; 2) hypoperfusion or ischemia of the anterior optic nerve; 3) glial cell activation; 4) glutamate excitotoxicity, and 5) abnormal immune response. [69] Neuroprotective strategies, such as providing inhibitor and survival factors, are used to block the mechanisms associated with RGC loss in experimental glaucoma. [69]
Targeting Apoptosis
Interfering with the apoptosis cascade through expression of genes or their protein products can have a neuroprotective effect in animal eyes. Martinou et al [83] reported a transgenic mouse line expressing Bcl-2, an apoptosis inhibitor that protects the integrity of the mitochondrial membrane. Compared to normal mice, the transgenic line showed a 50% increase in retinal ganglion cell numbers, accompanied by an increase in the thickness of the inner plexiform layer. [83] Malik et al showed that RGC cells transduced with AAV vector expressing Bcl-2 remained morphologically intact and survived up to 8 weeks after axotomy. [79]
An essential element of the apoptosis cascade is the activation of cysteine proteases, termed caspases. [69] Delivering genes coding caspase inhibitors is a potential strategy to protect RGCs from glaucomatous damage. AAV- chicken β-actin (CBA) vector mediated expression of human baculoviral IAP repeat-containing protein-4 (BIRC4), a potent caspase inhibitor, promoted optic nerve axon survival in a rat model of experimental glaucoma. [84] This was supported by the observation that inhibition of retinal caspase-3 activity with BIRC4 reduced caspase-3-mediated cleavage of alpha-fodrin, a neuronal cytoskeletal protein and a known caspase-3 target. [115]
Successful AAV-mediated, X-linked inhibitor of apoptosis protein (XIAP) gene therapy has been reported in rat eyes with high IOP-induced ischemic damage to retinal neurons. Compared to control-treated eyes, the number of cells in the inner nuclear layer (INL) and the thickness of the inner retina was significantly preserved in XIAP-treated eyes. In addition, functional analysis showed that XIAP-only-treated eyes retained larger electroretinography b-wave amplitudes than green fluorescent protein (GFP)-only-treated eyes up to 4 weeks post-ischemia. [106]
Tumor necrosis factor-alpha (TNFa) is a proinflammatory and immunomediator cytokine. It is present in higher amounts in the retinas of glaucoma patients compared to age-matched normal donor eyes. [117] Determination of neuronal cell fate in response to TNFa depends on the combination/mediation of TNFa with other cytokines and proteins. In rodent crush and ocular hypotension models, inhibition of TNFa led to increased survival of RGCs. [89, 118] No significant apoptosis was detected in the retina of rats treated with saline or TNFa alone, however TNFa in the presence of a PKCzeta-inhibitor induced retinal cell apoptosis. [71] The potential of TNFa inhibition as a neuroprotective strategy in glaucoma and other neurodegenerative diseases depends on the development of a greater understanding of its role in the cellular events surrounding apoptosis.
It should be noted that apoptosis is an evolutionarily conserved pathway needed for embryonic development and tissue homoeostasis, and gene therapy strategies based on perturbations of the apoptosis, and anti-apoptosis processes may have unwanted effects. In vivo studies are needed to elucidate this point.
Increasing Neurotrophic Factors
Apoptotic death of RGCs after axonal injury can be prevented in the short term in animal models by repeated intravitreal injection of neurotrophins such as neurotrophin-4 (NT-4), [95] brain-derived neurotrophic factor (BDNF), ciliary neuronotrophic factor (CNTF), and sciatic nerve (ScN)-derived medium. [67,86,95] This approach has disadvantages, which include side effects induced by multiple intraocular injections and the short half-lives of the proteins. Recent studies with intraocular delivery of the genes for neuronotrophic factors were encouraging. [106]
Neurotrophins are proteins capable of stimulating and controlling neurogenesis and survival, with BDNF being one of the most active. BDNF is considered to be an important neurotrophic factor for RGCs. [69] In a rat optic nerve transection model, Ad-mediated intravitreal delivery of BDNF selectively transduced Müller cells and the expressed BDNF from these cells protected RGCs. [27] In a rat model of glaucoma, following a single intravitreal injection of AAV, a highly efficient transduction of RGCs was achieved. In this model AAV-mediated gene therapy with BDNF had a significant neuroprotective effect compared to saline or control virus injections. [82] BDNF receptor TrkB is down-regulated after axotomy of the optic nerve. AAV-mediated TrkB gene transfer into RGCs combined with exogenous BDNF administration markedly increased neuronal survival. [23]
Neuroprotection afforded by BDNF appears to be mediated by extracellular signal-regulated kinase (ERK) and phosphatidylinositol-3 kinase (PI3K). [97] The Erk1/2 pathway is involved in the protection of RGCs, as demonstrated by experiments using AAV to transduce axotomized rat RGCs in vivo. [97] With genes encoding constitutively active or wild-type mitogen-activated protein kinase 1 (MEK1), the upstream activator of Erk1/2. RGC survival increased markedly, indicating that the Erk1/2 pathway plays a key role in the protection of RGCs from ocular hypertensive damage. [136]
Other neurotrophic factors delivered by gene transfer approaches have been reported. Ad-mediated expression of CNTF led to better preservation of intraretinal RGCs but did not support regeneration of axotomized RGCs into peripheral nerve grafts. [15] [109] Cooperative effects of bcl-2 and CNTF on retinal ganglion cell survival and axonal regeneration were also studied. Bi-cistronic AAV mediated expression of a secretable form of CNTF in eyes of bcl-2 over-expressing transgenic mice significantly increased the survival and axonal regeneration of RGCs following axotomy in both wt and bcl-2 transgenic mice; for the latter, however, the effects were greater. [68]
Antioxidation
It is believed that an oxidative component is involved in glaucomatous RGC death initiated by elevated IOP and/or tissue hypoxia. Reactive oxygen species (ROS) form as by-products of cellular metabolism in the mitochondria. ROS are cytotoxic resulting in damage to proteins, lipids and DNA, which may play a role in the neurodegenerative process in diseases such as glaucoma. [116] Antioxidative gene therapy has potential as a glaucoma treatment. Recently, AAV-mediated intravitreal delivery of antioxidant genes for human extracellular superoxide dismutase (ECSOD) or catalase (CAT) reduced RGC loss by 29%, optic nerve demyelination by 36% and axonal loss by 44%, compared to contralateral eyes that received AAV-GFP, in an experimental autoimmune encephalomyelitis (EAE) DBA/1J mouse model. [102]
PREVENTING WOUND HEALING IN GLAUCOMA FILTRATION SURGERY
The most commonly used surgical procedure for glaucoma treatment is filtration surgery. The procedure was developed to introduce a guarded ostomy through the sclera into the anterior chamber of the eye to allow the escape of aqueous humor. A common problem after a trabeculectomy is scarring of the opening that prevents fluid drainage from the eye and interferes with the proper functioning of the bleb, reducing the long-term success of the surgery. To reduce scarring at the conjunctival level at the bleb and sclerostomy sites, it has become common to use antimetabolites, such as 5-fluorouracil and mitomycin C (MMC). [56] A drawback to antimetabolite therapy is the nonspecific nature of the inhibition of the wound-healing process, which can result in an increased risk of bleb leaks and hypotony, with a possible increased risk of infection. [56] The wound healing process involves multiple growth factors, angiogenic factors, enzymes, and inhibitory substances, the expressions of which are gene-regulated. This makes it feasible to use a gene therapy approach to regulate the wound healing process, via inhibiting or increasing selected gene expression.
p21(WAF-1/Cip-1) (p21) is a cell cycle regulating protein associated with G1 arrest. [7] Topical application of p21-expressing adenoviral vector (Ad p21), had an inhibitory effect on the wound healing process and fibroproliferation after filtering surgery in rabbits, with none of the complications associated with MMC detected. A cellulose sponge soaked with Ad p21 was placed under the surgically created conjunctival flap before the sclerotomy. In vitro experiments showed a dose-dependent inhibition of DNA synthesis and cell growth. [96] The results were supported by another study characterizing transgene expression in target tissue, inflammatory response, biodistribution to non-target tissues, and immune response after local delivery of the vector. [128] The same vector was also delivered subconjunctivally to monkeys with ocular hypertension undergoing trabeculectomy. [48] The p21 treated eyes exhibited open surgical ostomies by both functional and histological criteria. There was less tissue damage in the area around the surgical site and equivalent or better IOP control than seen in animals treated with MMC. These studies present a gene therapy alternative to antimetabolites by using the p21 gene to induce cell cycle arrest of surrounding cells rather than destroying the cells.
Potential Vectors Suitable for Glaucoma Gene Therapy
NON-VIRAL DELIVERY SYSTEMS
Non-viral vectors can be useful in gene transfer and have several advantages over the virus-based vectors, including low toxicity and immunogenicity, virtually infinite capacity, lack of infectious or mutagenic potentials, ease of manipulation and large scale production. However, non-viral vectors are generally less efficient in gene delivery and achieve lower therapeutic levels compared to virus-based vectors, perhaps because the vector DNA is not stably maintained in the nucleus. [5]
Naked DNA Injection
Naked DNA in the form of a plasmid can be simply and directly injected into tissues. Angella et al [6] compared the efficiency of two delivery techniques for plasmid vectors expressing chloramphenicol acetyltransferase (CAT) and found that delivery of the plasmid DNA into the bleb through a collagen shield increased CAT activity 30-fold over injection of plasmid in saline. Their data indicate gene therapy using naked plasmid DNA and a simple collagen shield delivery vehicle may be useful for regulating wound healing after glaucoma surgery. [6]
Exogenous genes were transferred to photoreceptor cells of the primate retina using a vascular route and an ocular specific promoter (opsin promoter). This method requires repeat injections because plasmid-based gene expression is transient as plasmid DNA is not integrated in the host chromosome. [135] 122 Non-viral, in vivo gene transfer was found to have increased efficiency when mannitol was included in the plasmid solution, in adult C57Bl6 mouse RGCs (Chung DC, Wei Z, Bennett J. In vivo Non-Viral Gene Transfer to Retinal Ganglion Cells. ARVO Abnr 836 2006).
Interfering RNA (RNAi)
The main advantage of RNAi is its ability to silence the expression of deleterious genes of known sequence. Clinical trials to test safety and efficacy are underway using short-interfering RNAs (siRNAs) targeting vascular endothelial growth factor (VEGF) or its receptor (VEGFR1) to treat AMD. [66] [31] Of relevance to glaucoma therapy, siRNA molecules have been delivered to organ cultured, perfused human TM tissue, and the delivered naked siRNA was able to inhibit the targeted genes myocilin (MYOC) and matrix GLA protein and downstream effectors. [24] MYOC has been linked to open-angle glaucoma. MYOC-specific short hairpin RNAS (shRNAs) were able to suppress the expression of myocilin proteins and the related cytotoxicity of mutant myocilins in vitro. [133] Both siRNA and shRNAs are desirable because of the potential for producing a longer lasting effect than pharmaceuticals. They each have specific advantages and disadvantages to be considered in designing therapeutic strategies. [66]
Physical Methods
Electroporation, one of the newer physical methods for ocular gene delivery, consists of local application of electric pulses after DNA injection. This strategy was used recently in a rat optic nerve transection model to deliver a glial cell derived neurotrophic factor (GDNF) gene into RGCs. [53] The plasmid DNA was injected intravitreally, after which electric pulses were delivered to the rat eye using a contact lens-type electrode attached to the cornea and a needle electrode (anodal) inserted to the middle of the forehead. After gene transfer, a significant increase in the number of surviving RGCs was observed and a decrease of caspase 3 and 9 was detected by RT-PCR. While a number of other physical methods for gene transfer have been developed such as pressure-perfusion and ultrasound, they have not been used in experimental glaucoma gene therapy.
Chemical Approaches
Cationic lipids (liposomes) have been widely used for gene transfer in cell culture and have also shown promise as vectors for in vivo gene therapy. Fusogenic liposomes transduced LacZ DNA and phosphorothioate oligonucleotide in adult rat and primate TM following injection into the anterior chamber. [46]
VIRAL DELIVERY SYSTEMS
Both precisely defined integrating and non-integrating viral vectors holds promise for ocular gene therapy.
Herpes Simplex Viruses (HSV)
HSV is a large enveloped DNA virus with a wide host range. HSV vectors have mainly been used for neuronal gene delivery but a reporter gene has been delivered into rat and monkey eyes using a replication-competent HSV type 1 ribonucleotide reductase mutant (hrR3) expressing the E. coli LacZ gene. TM, ciliary body epithelia, and RGCs were efficiently transduced, indicating the viability of utilizing the HSV vector for glaucoma gene therapy. [73] The advantages of this vector - it is helper independent and high titers [109 to 1010 transducing units (TU) per ml] can be achieved - may be outweighed by its drawbacks: limited promoter selection (CMV), inflammatory responses, possible cytotoxicity, and limited duration of transgene expression.
Adenovirus (Ad)
Ad is an intermediate-sized, non-enveloped DNA virus. Ad vectors were one of the earliest systems developed. The advantages to their use include relatively high titers (> 1011 TU per ml), the ability to infect a variety of dividing and postmitotic or terminally differentiated cells, and the ability to express foreign genes at an abundant level. [41] [131] Of particular relevance to glaucoma therapy, recombinant Ads are able to transduce all TM cell types with high efficiency in many species (mice, rats, dogs, monkeys, and anterior segments from postmortem human donors). [4] [19] [38] [60] Ad vectors have also been used to deliver transgenes to Müller cells and the RPE. [21] Delivery of Ad vectors expressing GFP permits noninvasive monitoring of delivery and length of gene expression. [16] Ad vectors have been used often in experimental glaucoma gene therapy, although repeated applications can result in complications such as severe inflammatory reactions and development of high neutralizing antibody titers, which limits their usefulness for long term therapy. [41] [52] [131]
Adeno-associated Viruses (AAV)
AAV (titer: about 1010 TU per ml) is a naturally replication-deficient virus that requires Ad for helper functions. It requires integration into the host genome and is capable of transducing terminally-differentiated and non-dividing cells. Efficient gene delivery to photoreceptors and pigmented epithelial cells following subretinal injection of AAV has been achieved in various animal models. [47] [94] [30] [45] AAV-mediated gene therapy slows photoreceptor loss in rodent models of primary photoreceptor diseases and in dogs with a naturally occurring disease similar to human Leber’s congenital amaurosis (LCA). AAV contains no viral genes and lacks apparent pathogenicity and high immunogenicity. AAV vectors do not produce significant inflammation but instead generate a neutralizing antibody response. [49] The most current AAV system is a helper virus free preparation, which will avoid the inflammation induced by the Ad helpers. [29] It should be noted that the severity of virus based vector induced inflammation varies significantly between rodents and primates. For example, we observed hrR3 induced inflammation in monkey eyes but not in mice eyes, even after multiple intraocular injections. [18] [73]
Transduction efficiency of an AAV vector is serotype dependent. The different capsid protein of the three AAV serotypes (AAV1, 2 and 5) determines the cell trophism in the retina. AAV2 has better tropism for RGCs than AAV1 and 5, whereas AAV5 transduces RPE photoreceptor cells more efficiently than AAV2. AAV1 primarily transduces RPEs. [8, 130]
A modified AAV incorporating a CBA promoter and the woodchuck hepatitis posttranscriptional regulatory element has been successfully used to introduce genes into RGCs. Within 2 weeks of a single intravitreal virus injection, approximately 85% of rat RGCs were transduced. [81]
Intravitreal injection of an AAV-2 vector encoding for a tetracycline (Tet)-regulated destabilized reporter gene, displayed sustained long-term regulation of the reporter gene in RGCs. [35] AAV-mediated gene therapy shows promise in a number of retinal applications and may also be useful in neuroprotective approaches for glaucoma.
Although AAV appears to be a promising vector for gene therapy of the posterior segment, it has been unable to transduce the TM in vivo. The underlying mechanism for that remains unclear. It was recently reported that the rate-limiting step of AAV transduction in TM was not viral entry failure but, at least in part, host down regulation of DNA replication. Self-replicating vectors appear to circumvent this problem.[17]
Lentiviruses
Lentiviral (LV) vectors also require integration into the host genome, which facilitates long-term expression. With nondividing or slowly dividing TM cells this could be an advantage. These vectors must be pseudotyped by replacing the envelope gp120 with vesicular stomatitis virus envelope glycoproteins to broaden the host range. [59] HIV and feline immunodeficiency virus (FIV) vector-mediated gene expression have been detected in several species [22] [76] and efficient delivery to the TM has been reported with both vectors. FIV vectors principally transduced the TM after injection into the anterior chamber of organ-cultured human eyes. No change in morphology or significant cellular loss in the TM was noticed. [75] Transgene expression persisted stably for at least 10 months in a cat model, after a single transcorneal, lentiviral vector injection. Expression was monitored serially and noninvasively. Expression terminated only at the highest (1×108 TU) dose and was associated with a period of inflammation. [76] In a rabbit model, termination of LV transduced GFP expression in RPE was also associated with inflammation but one month of systemic immunosuppression, starting the day of injection, prevented loss of GFP expression. [28] In monkeys, FIVmediated transgene expression in the TM lasted more than 1 year after a single intracameral injection (Poeschla EM, Loewen N, Rasmussen C, et al. Transduction of Nonhuman Primate Trabecular Meshwork With Lentiviral Vectors. ARVO Abnr 2696, 2006). In cats, FIV-mediated GFP expression, using either 5′ cap-translation or internal ribosome entry site (IRES)-translation, was achieved in the TM and observed non-invasively in vivo for 1.2-2.3 years. In most cases, post-mortem examination revealed transgene expression in the TM greater than that observed in vivo. [61]
For gene therapy of glaucoma, long-term transgene expression appears to be promising. However, the non-specificity of integration of viral DNA into the human genome may potentially lead to insertional mutagenesis and cell transformation. Designing a vector by which the transgene could be integrated into a site that does not induce cell transformation would be ideal. Montini et al reported a strategy of gene therapy using lentiviral vectors with low risk of oncogenic potential from insertional mutagenesis, [88] highlighting a major rationale for gene therapy.
Animal Models for Glaucoma Gene Therapy
Animal models that imitate aspects of the glaucomatous disease process are needed to better evaluate therapies. The differences in rodent and primate responses to therapeutic manipulations need to be studied further. Immune response differences are particularly relevant to the field of gene therapy.
MODELS WITH ELEVATED IOP
Rodent models with experimentally elevated IOP provide valuable opportunities to study glaucomatous optic neuropathy. Techniques include acute IOP elevation through injection of saline into the anterior chamber for an hour, [12] episcleral vessel manipulations such as ligation, hypertonic saline injection, and cauterization. [54] [63] [65] [26] [132] Injection of an Ad based TGFβ2 construct increased IOP in rats (Clark AF, Millar C, Pang IH, et al. Adenoviral Gene Transfer of Active Human Transforming Growth Factor-?2 Induces Elevated Intraocular Pressure in Rats. ARVO Abnr 4771, 2006). Non-human primate models of elevated IOP rely on laser scarification of the TM. Models that physically damage the TM hamper efforts to study reduction of elevated IOP by modulating TM resistance. While not common, spontaneous glaucoma does occur in dogs and the effects of IOP lowering compounds can be evaluated using this model. [40] [101] [126] Monitoring reduction of IOP in normotensive animals is more difficult than assessing IOP changes in ocular hypertensive ones, though a consistent small decrease in IOP can be sufficient for proof of concept for IOP lowering compounds. Exploitation of the mouse as a model for glaucoma has been advanced by the development of methods to measure mouse IOP. [93] The DBA/2J mouse is a model for secondary angle-closure glaucoma, due to iris atrophy and pigment dispersion, which ultimately lead to increased IOP. [113] A domestic cat model in which long-term in vivo transgene expression in TM was achieved after a single transcorneal LV vector injection, [76] indicates the possibility of creating an animal model with elevated IOP by over-expressing a relevant glaucoma gene in the TM. These studies provide a basis for developing realistic disease models and administering glaucoma gene therapy.
MODELS WITH RGC DAMAGE UNRELATED TO IOP
Axotomized RGCs in adult cats offer a good experimental model to understand mechanisms of RGC deterioration in ophthalmic diseases such as glaucoma. A number of rodent models have also been developed to examine RGC death. Techniques include intravitreal injection of staurosporine [43] or the glutamate analog N-methyl-D-aspartate (NMDA). [70] Bilateral common carotid artery occlusion produces moderate levels of ischemia in the retina of rats [129] and an optic nerve crush models have been developed for rats [114] and mice. [118] Non-human primate models of RGC death and optic nerve injury generally use optic nerve transection. [2] [36] [127]
MODELS WITH GENETIC MUTATIONS LINKED TO GLAUCOMA
Establishment of the mouse as a model for glaucoma has been advanced by the identification of mutant mouse strains with elevated IOP. These developments enable investigations that directly test the influence of specific gene product alterations on the progression of glaucoma. Moreover, new transgenic mouse models have been produced with genetic mutations that parallel human gene mutations that have been linked to the onset of glaucoma. These new mouse models and technologies have potential for uncovering the biological basis of glaucoma as well as for evaluating new treatments. [72]
MODELS THAT OVER-EXPRESS APOPTOSIS-INHIBITING GENE IN NEURONS
Martinou et al [83] have successfully generated a transgenic mouse line that allows expression of the apoptosis-inhibiting gene Bcl-2 in rat neurons. The strain showed a 50% increase in RGC numbers accompanied by an increase in the thickness of the inner plexiform layer. [83] This animal model is valuable not only for understanding the molecular basis for the survival of neurons during development and adulthood, but also for designing neuroprotection-based glaucoma gene therapy.
MODELS WITH FILTERING SURGERY
Gene therapy using naked plasmid DNA and a simple collagen shield delivery vehicle may useful for regulating wound healing after rabbit glaucoma surgery. [6] rAd.p21 was effective in preventing fibroproliferation and wound healing in rabbit and monkey models of glaucoma surgery. [48, 96] The standard animal model for studying glaucoma filtering surgery is the rabbit but newer investigative tools that examine changes induced in biologic systems at a genetic level have made development of a rat model desirable. Cannulated filtering surgery in the rat, which involves introducing a silicone cannula through a penetrating scleral tunnel, under a limbal-based conjunctival flap and suturing the conjunctiva closed, provides a longer lasting and more predictable model than needle tract sclerostomy for studying wound healing following filtering surgery and may facilitate the study of induced changes at the gene level. [110]
It should be noted that gene therapy for glaucoma lags behind that in retinal diseases. Lack of suitable models appears to be the major reason. For example, the rd12 mouse, a naturally occurring rodent model of Leber congenital amaurosis (LCA) with a recessive nonsense mutation of Rpe65 (retinal pigment epithelium-specific protein 65 kDa, a protein essential for the visual cycle), displayed a profoundly diminished rod electroretinogram (ERG), an absence of 11-cis-retinaldehyde and rhodopsin, an over-accumulation of retinyl esters in retinal pigmented epithelial (RPE) cells, and photoreceptor degeneration. Gene therapy with AAV-mediated expression of hRPE65 was tried in rd12 mice and improved rhodopsin levels with ERG signals restored to near normal. Retinyl ester levels were maintained at near normal, and fundus and retinal morphology remained normal. [92] Similar studies also showed success in large animal (dog) models. Subretinal delivery of an amount of 8.25 × 10(10) vector genome (vg) AAV vector in affected dogs showed improved visual behavior and pupillary responses, and reduced nystagmus within 2 weeks of injection. ERG responses confirmed the reversal of visual deficit. [13] These studies demonstrate that AAV vector holds great promise for treatment of retinal disorders such as LCA and provides useful information relevant to glaucoma gene therapy such as neuroprotection or rescue of glaucomatous optic nerve damage at an early stage. In two separate clinical trials, research teams have reported partially restoring vision using gene therapy to treat advanced cases of LCA. [9] [78]
Summary
The chronic nature of glaucoma makes it a good target for long-term therapeutic strategies, such as gene therapy, that can target and correct relevant pathophysiology. It has the potential to provide a long-lasting IOP lowering effect with minimal systemic side effects, while circumventing the issue of patient compliance with multiple topical drop therapies. Nevertheless, significant challenges remain before gene therapy can be used to treat glaucoma. The tolerance to viral vectors seen in posterior segment applications has not been duplicated in the anterior segment. Continued research to screen specific genes and proteins, improve delivery vectors, and establish more suitable animal models will help realize the potential clinical applications of this promising new therapeutic strategy.
Method of Literature Search
The PubMed database was used with search words including, but not limited to, glaucoma, gene therapy, intraocular pressure, nonviral and viral vectors, neuroprotection, glaucoma animal models, in articles from 1965 to 2008, with most being from 2000 to 2008. This review compiles evidence coming from human and non-human primate and animal studies, and articles only in English are cited.
Table 2.
Summary of Virus-based Vectors Used in Ocular Gene Therapy.
| Properties | HSV[73] | AD [41] [131] | AAV[47] [29] [49] | LV[22] [76] |
|---|---|---|---|---|
| DNA or RNA virus | dsDNA | dsDNA | ssDNA | RNA |
| Size of genome (kb) | 152 | 36 | 4.7~6 | 10 |
| Enveloped or non-enveloped | Enveloped | Non-enveloped | Non-enveloped | Enveloped |
| Titers obtained (transducing units per ml) | 109 to 1010 | > 1011 | 5×1010 | 1×108 |
| Capacity for foreign DNA (up to x kb) | 40~50 | 37 | < 4 | 10 |
| Integrating or non-integrating | Non-integrating | Non-integrating | Integrating | Integrating |
| Transient or permanent expression | Transient | Transient | Permanent expression | Permanent expression |
| Host range | Capable of transducing terminally-differentiated and non-dividing cells | Wide host range | Capable of transducing terminally-differentiated and non-dividing cells | Capable of transducing terminally-differentiated and non-dividing cells |
| Neurotropism | Yes | None | None | None |
| Side effects/safety | Relatively high cytotoxicity | Relatively high pathogenicity and immunogenicity | Low pathogenicity and immunogenicity; relatively safe | Low immunogenicity |
ds=double-stranded; ss=single-stranded
Acknowledgments
Support :EY02698 (PLK), EY016665 (Core Grant for Vision Research), RPB, OPREF, RRF, NNSF 30772379 (XL). Drs Kaufman and Liu are inventors on patents for gene therapy for glaucoma that have been assigned to the Wisconsin Alumni Research Foundation (WARF), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Advanced Glaucoma Intervention Study (AGIS):7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–40. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 2.Agapova OA, Kaufman PL, Lucarelli MJ, et al. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003;967(1–2):132–43. doi: 10.1016/s0006-8993(02)04234-8. [DOI] [PubMed] [Google Scholar]
- 3.Aktories K, Mohr C, Koch G. Clostridium botulinum C3 ADP-ribosyltransferase. Curr Top Microbiol Immunol. 1992;175:115–31. doi: 10.1007/978-3-642-76966-5_6. [DOI] [PubMed] [Google Scholar]
- 4.Andrawiss M, Maron A, Beltran W, et al. Adenovirus-mediated gene transfer in canine eyes: a preclinical study for gene therapy of human uveal melanoma. J Gene Med. 2001;3(3):228–39. doi: 10.1002/1521-2254(200105/06)3:3<228::AID-JGM186>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Andrieu-Soler C, Bejjani RA, de Bizemont T, et al. Ocular gene therapy: a review of nonviral strategies. Mol Vis. 2006;12:1334–47. [PubMed] [Google Scholar]
- 6.Angella GJ, Sherwood MB, Balasubramanian L, et al. Enhanced short-term plasmid transfection of filtration surgery tissues. Invest Ophthalmol Vis Sci. 2000;41(13):4158–62. [PubMed] [Google Scholar]
- 7.Atencio IA, Chen Z, Nguyen QH, et al. p21WAF-1/Cip-1 gene therapy as an adjunct to glaucoma filtration surgery. Curr Opin Mol Ther. 2004;6(6):624–8. [PubMed] [Google Scholar]
- 8.Auricchio A, Kobinger G, Anand V, et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10(26):3075–81. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- 9.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 10.Bárány EH. The immediate effect on outflow resistance of intravenous pilocarpine in the vervet monkey. Invest Ophthalmol. 1967;6:373–80. [Google Scholar]
- 11.Bárány EH, Rohen JW. Localized contraction and relaxation within the ciliary muscle of the vervet monkey (Cercopithecus ethiops) In: Rohen J, editor. The Structure of the Eye, Second Symposium. FK Schattauer Verlag; Stuttgart: 1965. pp. 287–311. [Google Scholar]
- 12.Ben Simon GJ, Bakalash S, Aloni E, Rosner M. A rat model for acute rise in intraocular pressure: immune modulation as a therapeutic strategy. Am J Ophthalmol. 2006;141(6):1105–11. doi: 10.1016/j.ajo.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 13.Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16(3):458–65. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bill A. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus) Exp Eye Res. 1967;6(2):120–5. doi: 10.1016/s0014-4835(67)80062-9. [DOI] [PubMed] [Google Scholar]
- 15.Bok D, Yasumura D, Matthes MT, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74(6):719–35. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- 16.Borrás T, Gabelt BT, Klintworth GK, et al. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001;3(5):437–49. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- 17.Borrás T, Xue W, Choi VW, et al. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J Gene Med. 2006;8(5):589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- 18.Brandt CR, Imesch P, Spencer B, et al. The herpes simplex virus type 1 ribonucleotide reductase is required for acute retinal disease. Arch Virol. 1997;142(5):883–96. doi: 10.1007/s007050050126. [DOI] [PubMed] [Google Scholar]
- 19.Budenz DL, Bennett J, Alonso L, Maguire A. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995;36(11):2211–5. [PubMed] [Google Scholar]
- 20.Caballero M, Rowlette LL, Borrás T. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim Biophys Acta. 2000;1502(3):447–60. doi: 10.1016/s0925-4439(00)00068-5. [DOI] [PubMed] [Google Scholar]
- 21.Cashman SM, Sadowski SL, Morris DJ, et al. Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors--implications for gene therapy. Mol Ther. 2002;6(6):813–23. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- 22.Challa P, Luna C, Liton PB, et al. Lentiviral mediated gene delivery to the anterior chamber of rodent eyes. Mol Vis. 2005;11:425–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng L, Sapieha P, Kittlerova P, et al. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22(10):3977–86. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comes N, Borras T. Functional delivery of synthetic naked siRNA to the human trabecular meshwork in perfused organ cultures. Mol Vis. 2007;13:1363–74. [PubMed] [Google Scholar]
- 25.Crowston JG, Lindsey JD, Morris CA, et al. Effect of bimatoprost on intraocular pressure in prostaglandin FP receptor knockout mice. Invest Ophthalmol Vis Sci. 2005;46(12):4571–7. doi: 10.1167/iovs.05-0723. [DOI] [PubMed] [Google Scholar]
- 26.Danias J, Shen F, Kavalarakis M, et al. Characterization of retinal damage in the episcleral vein cauterization rat glaucoma model. Exp Eye Res. 2006;82(2):219–28. doi: 10.1016/j.exer.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Polo A, Aigner LJ, Dunn RJ, et al. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95(7):3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi K, Kong J, Hargitai J, et al. Transient immunosuppression stops rejection of virus-transduced enhanced green fluorescent protein in rabbit retina. J Virol. 2004;78(20):11327–33. doi: 10.1128/JVI.78.20.11327-11333.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drittanti L, Jenny C, Poulard K, et al. Optimised helper virus-free production of high-quality adeno-associated virus vectors. J Gene Med. 2001;3(1):59–71. doi: 10.1002/1521-2254(2000)9999:9999<::AID-JGM152>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Dudus L, Anand V, Acland GM, et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res. 1999;39(15):2545–53. doi: 10.1016/s0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 31.Emerson MV, Lauer AK. Emerging therapies for the treatment of neovascular age-related macular degeneration and diabetic macular edema. BioDrugs. 2007;21(4):245–57. doi: 10.2165/00063030-200721040-00005. [DOI] [PubMed] [Google Scholar]
- 32.Erickson K, Liang L, Shum P, Nathanson JA. Adrenergic regulation of aqueous outflow. J Ocul Pharmacol. 1994;10(1):241–52. doi: 10.1089/jop.1994.10.241. [DOI] [PubMed] [Google Scholar]
- 33.Fan BJ, Wang DY, Lam DS, Pang CP. Gene mapping for primary open angle glaucoma. Clin Biochem. 2006;39(3):249–58. doi: 10.1016/j.clinbiochem.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41(13):4163–8. [PubMed] [Google Scholar]
- 35.Folliot S, Briot D, Conrath H, et al. Sustained tetracycline-regulated transgene expression in vivo in rat retinal ganglion cells using a single type 2 adeno-associated viral vector. J Gene Med. 2003;5(6):493–501. doi: 10.1002/jgm.367. [DOI] [PubMed] [Google Scholar]
- 36.Gaasterland D, Kupfer C. Experimental glaucoma in the rhesus monkey. Invest Ophthalmol. 1974;13(6):455–7. [PubMed] [Google Scholar]
- 37.Gabelt BT, Kiland JA, Tian B, PLK . Aqueous humor: Secretion and Dynamics. In: KJ, editor. Duane’s Foundations of Clinical Ophthalmology. Lippincott Williams &Wilkins; Philadelphia: 2006. [Google Scholar]
- 38.Gabelt BT, Hu Y, Vittitow JL, et al. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res. 2006;82(6):935–44. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24(5):612–37. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Gelatt KN, Mackay EO, Dashiell T, Biken A. Effect of different dose schedules of 0.15% unoprostone isopropyl on intraocular pressure and pupil size in the glaucomatous beagle. J Ocul Pharmacol Ther. 2004;20(5):411–20. doi: 10.1089/jop.2004.20.411. [DOI] [PubMed] [Google Scholar]
- 41.Ginsberg HS. The ups and downs of adenovirus vectors. Bull N Y Acad Med. 1996;73(1):53–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Grosheva I, Vittitow JL, Goichberg P, et al. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp Eye Res. 2006;82(6):945–58. doi: 10.1016/j.exer.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Guo L, Salt TE, Maass A, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47(2):626–33. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta D. Glaucoma, diagnosis and management. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 45.Guy J, Qi X, Muzyczka N, Hauswirth WW. Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Arch Ophthalmol. 1999;117(7):929–37. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- 46.Hangai M, Tanihara H, Honda Y, Kaneda Y. Introduction of DNA into the rat and primate trabecular meshwork by fusogenic liposomes. Invest Ophthalmol Vis Sci. 1998;39(3):509–16. [PubMed] [Google Scholar]
- 47.Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant adeno-associated virus. Methods Enzymol. 2000;316:743–61. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- 48.Heatley G, Kiland J, Faha B, et al. Gene therapy using p21WAF-1/Cip-1 to modulate wound healing after glaucoma trabeculectomy surgery in a primate model of ocular hypertension. Gene Ther. 2004;11(12):949–55. doi: 10.1038/sj.gt.3302253. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez YJ, Wang J, Kearns WG, et al. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J Virol. 1999;73(10):8549–58. doi: 10.1128/jvi.73.10.8549-8558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honjo M, Tanihara H, Inatani M, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Investigative Ophthalmology and Visual Science. 2001;42:137–44. [PubMed] [Google Scholar]
- 51.Huber PA. Caldesmon. Int J Biochem Cell Biol. 1997;29(8–9):1047–51. doi: 10.1016/s1357-2725(97)00004-6. [DOI] [PubMed] [Google Scholar]
- 52.Hudde T, Apitz J, Bordes-Alonso R, et al. Gene transfer to trabecular meshwork endothelium via direct injection into the Schlemm canal and in vivo toxicity study. Curr Eye Res. 2005;30(12):1051–9. doi: 10.1080/02713680500323350. [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa H, Takano M, Matsumoto N, et al. Effect of GDNF gene transfer into axotomized retinal ganglion cells using in vivo electroporation with a contact lens-type electrode. Gene Ther. 2005;12(4):289–98. doi: 10.1038/sj.gt.3302277. [DOI] [PubMed] [Google Scholar]
- 54.Ji J, Chang P, Pennesi ME, et al. Effects of elevated intraocular pressure on mouse retinal ganglion cells. Vision Res. 2005;45(2):169–79. doi: 10.1016/j.visres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Johnson DH, Johnson M. How does nonpenetrating glaucoma surgery work? Aqueous outflow resistance and glaucoma surgery. J Glaucoma. 2001;10(1):55–67. doi: 10.1097/00061198-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Jones LD, Ramanathan S, Sandramouli S. Trabeculectomy with mitomycin C. Ophthalmology. 2007;114(6):1231. doi: 10.1016/j.ophtha.2007.03.004. author reply −2. [DOI] [PubMed] [Google Scholar]
- 57.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman PL, Tian B, Gabelt BT, XL . Outflow enhancing drugs and gene therapy in glaucoma. In: Weinreb KGR, Kitazawa Y, editors. Glaucoma in the 21st Century. Harcourt-Mosby; London: 2000. pp. 117–28. [Google Scholar]
- 59.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 60.Kee C, Sohn S, Hwang JM. Stromelysin gene transfer into cultured human trabecular cells and rat trabecular meshwork in vivo. Invest Ophthalmol Vis Sci. 2001;42(12):2856–60. [PubMed] [Google Scholar]
- 61.Khare PD, Loewen N, Teo W, et al. Durable, Safe, Multi-gene Lentiviral Vector Expression in Feline Trabecular Meshwork. Mol Ther. 2008;16(1):97–106. doi: 10.1038/sj.mt.6300318. [DOI] [PubMed] [Google Scholar]
- 62.Khurana RN, Deng PF, Epstein DL, Vasantha Rao P. The role of protein kinase C in modulation of aqueous humor outflow facility. Exp Eye Res. 2003;76(1):39–47. doi: 10.1016/s0014-4835(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 63.Kielczewski JL, Pease ME, Quigley HA. The effect of experimental glaucoma and optic nerve transection on amacrine cells in the rat retina. Invest Ophthalmol Vis Sci. 2005;46(9):3188–96. doi: 10.1167/iovs.05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiland JA, Gabelt BT, Kaufman PL. Studies on the mechanism of action of timolol and on the effects of suppression and redirection of aqueous flow on outflow facility. Exp Eye Res. 2004;78(3):639–51. doi: 10.1016/j.exer.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Kim DH, Kim HS, Ahn MD, Chun MH. Ganglion cell death in rat retina by persistent intraocular pressure elevation. Korean J Ophthalmol. 2004;18(1):15–22. doi: 10.3341/kjo.2004.18.1.15. [DOI] [PubMed] [Google Scholar]
- 66.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8(3):173–84. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 67.Klocker N, Braunling F, Isenmann S, Bahr M. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport. 1997;8(16):3439–42. doi: 10.1097/00001756-199711100-00005. [DOI] [PubMed] [Google Scholar]
- 68.Leaver SG, Cui Q, Bernard O, Harvey AR. Cooperative effects of bcl-2 and AAV-mediated expression of CNTF on retinal ganglion cell survival and axonal regeneration in adult transgenic mice. Eur J Neurosci. 2006;24(12):3323–32. doi: 10.1111/j.1460-9568.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- 69.Levin LA. Neuroprotection and regeneration in glaucoma. Ophthalmol Clin North Am. 2005;18(4):585–96. vii. doi: 10.1016/j.ohc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40(5):1004–8. [PubMed] [Google Scholar]
- 71.Liang H, Baudouin C, Behar-Cohen F, et al. Protein kinase C-zeta mediates retinal degeneration in response to TNF. J Neuroimmunol. 2007;183(1–2):104–10. doi: 10.1016/j.jneuroim.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 72.Lindsey JD, Weinreb RN. Elevated intraocular pressure and transgenic applications in the mouse. J Glaucoma. 2005;14(4):318–20. doi: 10.1097/01.ijg.0000169411.09258.f6. [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Brandt CR, Gabelt BT, et al. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp Eye Res. 1999;69(4):385–95. doi: 10.1006/exer.1999.0711. [DOI] [PubMed] [Google Scholar]
- 74.Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–21. [PubMed] [Google Scholar]
- 75.Loewen N, Bahler C, Teo WL, et al. Preservation of aqueous outflow facility after second-generation FIV vector-mediated expression of marker genes in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2002;43(12):3686–90. [PubMed] [Google Scholar]
- 76.Loewen N, Fautsch MP, Teo WL, et al. Long-term, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Invest Ophthalmol Vis Sci. 2004;45(9):3091–8. doi: 10.1167/iovs.04-0366. [DOI] [PubMed] [Google Scholar]
- 77.Lütjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981;21(4):563–73. [PubMed] [Google Scholar]
- 78.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malik JM, Shevtsova Z, Bahr M, Kugler S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol Ther. 2005;11(3):373–81. doi: 10.1016/j.ymthe.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 80.Marshall B, Keskin DB, Mellor AL. Regulation of prostaglandin synthesis and cell adhesion by a tryptophan catabolizing enzyme. BMC Biochem. 2001;2:5. doi: 10.1186/1471-2091-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28(2):267–75. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 82.Martin KR, Quigley HA. Gene therapy for optic nerve disease. Eye. 2004;18(11):1049–55. doi: 10.1038/sj.eye.6701579. [DOI] [PubMed] [Google Scholar]
- 83.Martinou JC, Dubois-Dauphin M, Staple JK, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13(4):1017–30. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 84.McKinnon SJ, Lehman DM, Tahzib NG, et al. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol Ther. 2002;5(6):780–7. doi: 10.1006/mthe.2002.0608. [DOI] [PubMed] [Google Scholar]
- 85.Mettu PS, Deng PF, Misra UK, et al. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2004;45(7):2263–71. doi: 10.1167/iovs.03-0960. [DOI] [PubMed] [Google Scholar]
- 86.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602(2):304–17. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- 87.Mindel J. Cholinergic Pharmacology. In: Tasman WaJ, EA, editors. Duane’s Foundations of Clinical Ophthalmology. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 88.Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24(6):687–96. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 89.Nakazawa T, Nakazawa C, Matsubara A, et al. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26(49):12633–41. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oh DJ, Martin JL, Williams AJ, et al. Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest Ophthalmol Vis Sci. 2006;47(3):953–63. doi: 10.1167/iovs.05-0516. [DOI] [PubMed] [Google Scholar]
- 91.Ota T, Aihara M, Narumiya S, Araie M. The effects of prostaglandin analogues on IOP in prostanoid FP-receptor-deficient mice. Invest Ophthalmol Vis Sci. 2005;46(11):4159–63. doi: 10.1167/iovs.05-0494. [DOI] [PubMed] [Google Scholar]
- 92.Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13(3):565–72. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Pease ME, Hammond JC, Quigley HA. Manometric calibration and comparison of TonoLab and TonoPen tonometers in rats with experimental glaucoma and in normal mice. J Glaucoma. 2006;15(6):512–9. doi: 10.1097/01.ijg.0000212276.57853.19. [DOI] [PubMed] [Google Scholar]
- 94.Peel AL, Klein RL. Adeno-associated virus vectors: activity and applications in the CNS. J Neurosci Methods. 2000;98(2):95–104. doi: 10.1016/s0165-0270(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 95.Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37(4):489–500. [PubMed] [Google Scholar]
- 96.Perkins TW, Faha B, Ni M, et al. Adenovirus-mediated gene therapy using human p21WAF-1/Cip-1 to prevent wound healing in a rabbit model of glaucoma filtration surgery. Arch Ophthalmol. 2002;120(7):941–9. doi: 10.1001/archopht.120.7.941. [DOI] [PubMed] [Google Scholar]
- 97.Pernet V, Hauswirth WW, Di Polo A. Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo. J Neurochem. 2005;93(1):72–83. doi: 10.1111/j.1471-4159.2005.03002.x. [DOI] [PubMed] [Google Scholar]
- 98.Peters DM, Herbert K, Biddick B, Peterson JA. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp Cell Res. 2005;303(2):218–28. doi: 10.1016/j.yexcr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 99.Peterson JA, Sheibani N, David G, et al. Heparin II domain of fibronectin uses alpha4beta1 integrin to control focal adhesion and stress fiber formation, independent of syndecan-4. J Biol Chem. 2005;280(8):6915–22. doi: 10.1074/jbc.M406625200. [DOI] [PubMed] [Google Scholar]
- 100.Peterson JA, Tian B, Bershadsky AD, et al. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40(5):931–41. [PubMed] [Google Scholar]
- 101.Plummer CE, MacKay EO, Gelatt KN. Comparison of the effects of topical administration of a fixed combination of dorzolamide-timolol to monotherapy with timolol or dorzolamide on IOP, pupil size, and heart rate in glaucomatous dogs. Vet Ophthalmol. 2006;9(4):245–9. doi: 10.1111/j.1463-5224.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 102.Qi X, Sun L, Lewin AS, et al. Long-term suppression of neurodegeneration in chronic experimental optic neuritis: antioxidant gene therapy. Invest Ophthalmol Vis Sci. 2007;48(12):5360–70. doi: 10.1167/iovs.07-0254. [DOI] [PubMed] [Google Scholar]
- 103.Rao PV, Deng P, Maddala R, et al. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005;11:288–97. [PubMed] [Google Scholar]
- 104.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80(2):197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 105.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–37. [PubMed] [Google Scholar]
- 106.Renwick J, Narang MA, Coupland SG, et al. XIAP-mediated neuroprotection in retinal ischemia. Gene Ther. 2006;13(4):339–47. doi: 10.1038/sj.gt.3302683. [DOI] [PubMed] [Google Scholar]
- 107.Rohen JW, Lütjen E, Bárány E. The relation between the ciliary muscle and the trabecular meshwork and its importance for the effect of miotics on aqueous outflow resistance. A study in two contrasting monkey species, Macaca irus and Cercopithecus aethiops. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1967;172(1):23–47. doi: 10.1007/BF00577152. [DOI] [PubMed] [Google Scholar]
- 108.Santas AJ, Bahler C, Peterson JA, et al. Effect of heparin II domain of fibronectin on aqueous outflow in cultured anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2003;44(11):4796–804. doi: 10.1167/iovs.02-1083. [DOI] [PubMed] [Google Scholar]
- 109.Schlichtenbrede FC, MacNeil A, Bainbridge JW, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10(6):523–7. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- 110.Sherwood MB, Esson DW, Neelakantan A, Samuelson DA. A new model of glaucoma filtering surgery in the rat. J Glaucoma. 2004;13(5):407–12. doi: 10.1097/01.ijg.0000131482.86547.5a. [DOI] [PubMed] [Google Scholar]
- 111.Shields M. In: Cholinergic Agents. Shields’ textbook of glaucoma. Allingham R, editor. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 501–8. [Google Scholar]
- 112.Song J, Deng PF, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005;46(7):2424–32. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- 113.Steele MR, Inman DM, Calkins DJ, et al. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47(3):977–85. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 114.Swanson KI, Schlieve CR, Lieven CJ, Levin LA. Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest Ophthalmol Vis Sci. 2005;46(10):3737–41. doi: 10.1167/iovs.05-0155. [DOI] [PubMed] [Google Scholar]
- 115.Tahzib NG, Ransom NL, Reitsamer HA, McKinnon SJ. Alpha-fodrin is cleaved by caspase-3 in a chronic ocular hypertensive (COH) rat model of glaucoma. Brain Res Bull. 2004;62(6):491–5. doi: 10.1016/S0361-9230(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 116.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25(5):490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42(8):1787–94. [PubMed] [Google Scholar]
- 118.Tezel G, Yang X, Yang J, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996(2):202–12. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 119.Tian B, Brumback LC, Kaufman PL. ML-7, chelerythrine and phorbol ester increase outflow facility in the monkey Eye. Exp Eye Res. 2000;71(6):551–66. doi: 10.1006/exer.2000.0919. [DOI] [PubMed] [Google Scholar]
- 120.Tian B. Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp Eye Res. 2005;80(2):215–25. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Tian B, Kaufman PL, Volberg T, et al. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol. 1998;116:633–43. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- 122.To CH, Kong CW, Chan CY, et al. The mechanism of aqueous humour formation. Clin Exp Optom. 2002;85(6):335–49. [PubMed] [Google Scholar]
- 123.Toris CB, Koepsell SA, Yablonski ME, Camras CB. Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma. 2002;11(3):253–8. doi: 10.1097/00061198-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 124.Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999;127(4):407–12. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- 125.Vittitow JL, Garg R, Rowlette LL, et al. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol Vis. 2002;8:32–44. [PubMed] [Google Scholar]
- 126.Volopich S, Mosing M, Auer U, Nell B. Comparison of the effect of hypertonic hydroxyethyl starch and mannitol on the intraocular pressure in healthy normotensive dogs and the effect of hypertonic hydroxyethyl starch on the intraocular pressure in dogs with primary glaucoma. Vet Ophthalmol. 2006;9(4):239–44. doi: 10.1111/j.1463-5224.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 127.Wang RF, Schumer RA, Serle JB, Podos SM. A comparison of argon laser and diode laser photocoagulation of the trabecular meshwork to produce the glaucoma monkey model. J Glaucoma. 1998;7(1):45–9. [PubMed] [Google Scholar]
- 128.Wen SF, Chen Z, Nery J, Faha B. Characterization of adenovirus p21 gene transfer, biodistribution, and immune response after local ocular delivery in New Zealand white rabbits. Exp Eye Res. 2003;77(3):355–65. doi: 10.1016/s0014-4835(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 129.Yamamoto H, Schmidt-Kastner R, Hamasaki DI, Parel JM. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp Eye Res. 2006;82(5):767–79. doi: 10.1016/j.exer.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 130.Yang GS, Schmidt M, Yan Z, et al. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J Virol. 2002;76(15):7651–60. doi: 10.1128/JVI.76.15.7651-7660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Y, Nunes FA, Berencsi K, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91(10):4407–11. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu S, Tanabe T, Yoshimura N. A rat model of glaucoma induced by episcleral vein ligation. Exp Eye Res. 2006;83(4):758–70. doi: 10.1016/j.exer.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 133.Yuan C, Zins EJ, Clark AF, Huang AJ. Suppression of keratoepithelin and myocilin by small interfering RNAs (siRNA) in vitro. Mol Vis. 2007;13:2083–95. [PubMed] [Google Scholar]
- 134.Zhang M, Rao PV. Blebbistatin a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci. 2005;46(11):4130–8. doi: 10.1167/iovs.05-0164. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, Schlachetzki F, Li JY, et al. Organ-specific gene expression in the rhesus monkey eye following intravenous non-viral gene transfer. Mol Vis. 2003;9:465–72. [PubMed] [Google Scholar]
- 136.Zhou Y, Pernet V, Hauswirth WW, Di Polo A. Activation of the extracellular signal-regulated kinase 1/2 pathway by AAV gene transfer protects retinal ganglion cells in glaucoma. Mol Ther. 2005;12(3):402–12. doi: 10.1016/j.ymthe.2005.04.004. [DOI] [PubMed] [Google Scholar]