Abstract
Tbx18 is a T-Box transcription factor that has specific expression and indispensible function in the lower urinary tract (Airik et al., 2006). Here, we report the generation and characterization of a bacterial artificial chromosome (BAC) transgene expressing Cre under the control of Tbx18 regulatory elements. When crossed to the ROSA26R-lacZ reporter mice, the Tbx18-Cre transgene mediates loxP recombination in the mesenchymal derivatives in the lower urinary tract, especially in the smooth muscle cells (SMCs) and the stromal cells. There is no expression of this transgene in the urothelium or in the kidney. This Tbx18-Cre transgene recapitulates the endogenous Tbx18 expression in the urinary system and can be used for the study of the development, physiology, and diseases in the urinary tract. Its additional expression in the epicardium, limb, vibrissae, and other structures would be useful for studies in the relevant fields.
The urinary system can be divided into the kidney and the lower urinary tract (everything posterior to the kidney). In mid-gestation, the ureteric bud (UB) emerges from the Wolffian duct (WD) to invade the metanephric mesenchyme (MM) (Dressler, 2006; Schedl, 2007) for the initiation of nephrogenesis. As development proceeds, the UB becomes the ureteric epithelium and the collecting ducts system inside the kidney. The ureteric epithelium is surrounded by ureteric mesenchyme (UM) that later becomes the smooth muscles (SM) of the ureter. The bladder epithelium is derived from the endodermal hindgut. Recent studies have shown that both the bladder mesenchyme and UM originate from the tailbud-derived mesenchyme (Brenner-Anantharam et al., 2007). Tbx18 has expression in the tailbud-derived mesenchyme and later, in the UM. Inactivation of Tbx18 leads to an absence of Bmp4 expression in the UM and defective differentiation of the mesenchymal cells into functional smooth muscle cells (SMCs) (Airik et al., 2006). It is becoming clear that development of the urinary tract requires precise integration of progenitor cell populations of distinct embryonic origins. The complexity of such integration is the basis for a high percentage of congenital kidney and urinary tract diseases.
The Cre/loxP system has seen ever increasing applications in studies of the kidney and urinary tract (Gawlik and Quaggin, 2004; Wu, 2007) with its potential limited only by the availability of various Cre transgenes and floxed alleles. To provide a new genetic tool in the study of the development and physiology of the mesenchymal derivatives of the lower urinary tract, we set out to make a transgene that will direct Cre expression specifically in these tissues. Although a number of genes have expression in the mesenchymal derivatives in the lower urinary tract, most of them also express in MM derivatives in the kidney (Chang et al., 2004; Kobayashi et al., 2005). Only Tbx18 has been shown to have the desired specificity for the lower urinary tract and was thus chosen to provide the transcription control for Cre expression.
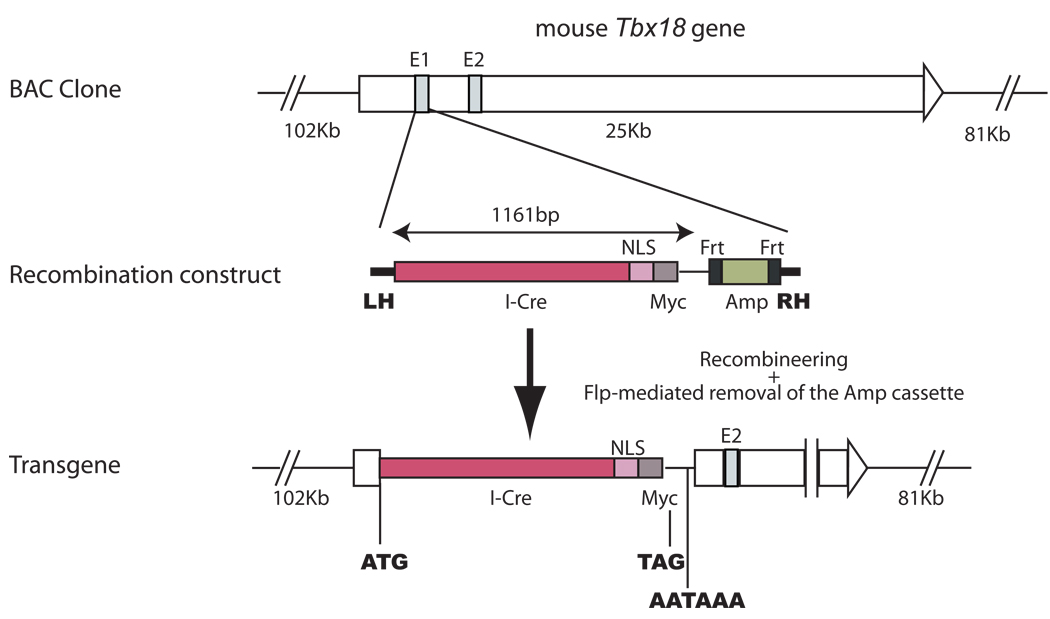
Since BAC (Bacterial artificial chromosome) transgenes have higher capacity to carry most, if not all, of the regulatory sequences necessary to recapitulate the expression pattern of the endogenous gene, we set out to construct the Cre transgene in a BAC clone covering the Tbx18 gene but not any other known genes. We used recombineering (recombination-mediated genetic engineering) (16) to replace the first exon of Tbx18 with the coding sequence for an I-Cre (codon Improved Cre) fused to a nuclear localization signal (NLS) (Fig. 1). Thus, Cre expression is effectively under the control of the regulatory elements of the Tbx18 gene. This cassette destroys the coding sequence of Tbx18 and terminates the transcription (by the transcription termination in the Cre cassette) upstream of exon 2 of the Tbx18 gene.
Figure 1. Generation of a Tbx18-Cre transgene using recombineering in a BAC clone.
The entire Tbx18 gene resides in the middle portion of the BAC clone we used to build the Cre transgene. No other complete genes are present in this BAC clone. The recombination construct has two homology arms (LH: left homology; RH: right homology) that are homologous to the sequences immediately 5’ and immediately 3’ to exon 1 of Tbx18, respectively. The recombination construct also has the coding sequence for a codon-improved Cre (I-Cre), a nuclear localization signal (NLS), and a Myc tag. In addition, it has an Frt-flanked ampicillin resistance gene cassette for selection. Exon 1 (E1) of Tbx18 in the BAC clone was replaced by the recombination construct between the two homology arms after recombineering. An additional round of transient Flp expression eliminates the Amp cassette to avoid bringing unecessary prokaryotic sequences into the mammalian genome.
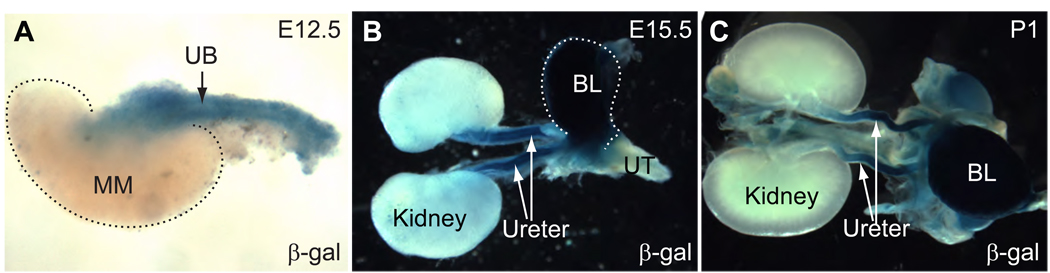
After pronuclei injection, three healthy and fertile founders were produced in a C57Bl/6xCBA hybrid background. They were crossed with ROSA26R-lacZ reporter mice (Soriano, 1999) for the analysis of Cre-mediated loxP recombination. All three lines have similar lacZ expression pattern though line 2 appeared to have a lower level of Cre activity in the tissues examined. The results described herein were primarily from line 1 and 3. At E12.5, Tbx18-Cre-mediated loxP recombination was detected in the ureteric stalk as revealed by the blue staining from the whole-mount β-galactosidase assay (Fig. 2A). The metanephros proper (the future kidney) is free of labeled cells. At E15.5, blue cells were again detected in the ureter. The urogenital sinus/bladder was also heavily labeled. The developing kidney, however, had no staining. The developing urethra also was not labeled (Fig. 2B). The same pattern continued in newborn samples (Fig. 2C): The kidney remains free of labeled cells while the major components of the lower urinary tract (the ureter and the bladder) have significant lacZ expression, indicating Tbx18-Cre expression in these structures and/or their progenitors.
Figure 2. The Tbx18-Cre transgene has expression specifically in the lower urinary tract.
At E12.5, whole-mount β-galactosidase assay revealed the occurrence of Cre-mediated loxP recombination in the developing ureteric stalk but not in the metanephros proper (A). The dotted line outlines the metanephric mesenchyme (MM). UB: Ureteric bud. At E15.5, blue cells were observed in the ureter and bladder (BL) but not in the kidney or urethra (UT) (B). The same pattern of Cre expression continues to birth (C).
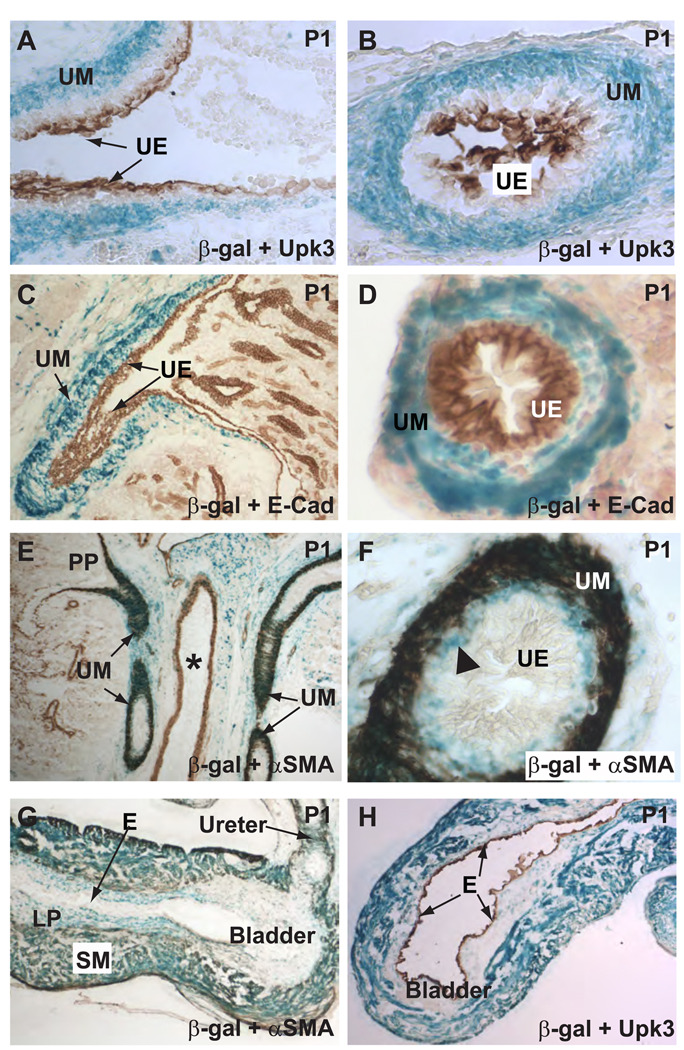
β-galactosidase assay on cryostat sections of the urogenital system from newborn mice showed that Tbx18-Cre-mediated loxP recombination occurred in cells surrounding the urothelium. No blue signal was found in cells positive for ureteric epithelial markers uroplakin III and E-Cadherin (Fig. 3A–D). The Cre-positive cells reached the renal pelvis but did not enter the kidney proper (Fig. 3C). The ureter had a layer of stromal cells sandwiched between the urothelium and the SMCs. Although the function of these stromal cells is unclear, these cells were reportedly abnormal or missing in a couple of animal models with urinary tract obstruction (Mahoney et al., 2006; Yu et al., 2002). Unlike Uroplakin III that only labels the innermost layers of the urothelium, E-Cadherin labels all layers of the urothelium but not in the stromal cells (Fig. 3C–D). Cre-positive cells were present directly adjacent to the E-Cadherin positive cells, indicating that the stromal layer had loxP recombination. This is further supported by the α-SM actin (α-SMA) staining (Fig. 3E–F). There is a layer of Cre-positive but α-SMA negative cells just inside the SM layers, corresponding to the stromal cells between the SM and the urothelium. Within the SM layers, almost all α-SMA positive cells had lacZ expression, indicating high and uniform expression of Cre in these cells and/or their progenitors. loxP recombination was specific to the urinary tract SMCs. Vascular SMCs of the adjacent blood vessels were not labeled (Fig. 3E).
Figure 3. Tbx18-Cre expression is in the mesenchymal derivatives but not in the epithelium.
The urothelium (UE) was labeled by an anti-Uroplakin III (Upk3) antibody (A–B) and an E-Cadherin (E-Cad) antibody (C–D). LacZ expression (blue), indicative of loxP recombination, was in the mesenchymal derivatives but not in the epithelium as evidenced by the lack of colocalization of the blue signal and the epithelial markers (brown). Blue cells were largely absent in the kidney proper. UM: ureteric mesenchyme; UE: urothelium. E and F, most of the blue cells were also α-SMA-positive (brown) urinary tract SMCs. Vascular SMCs in the adjacent blood vessels did not have significant loxP recombination. PP: Papilla of the kidney. * indicates the lumen of a blood vessel. In addition to urinary tract SMCs, the ureteric stromal cells were also positive for β-galactosidase (triangle in F), indicating Cre transgene expression in this cell population or their progenitors. G–H, within the bladder, SMCs and the α-SMA-negative cells within the lamina propria (LP) had extensive lacZ expression while the transitional epithelium (E) is completely void of any staining.
Cre-mediated loxP recombination in the bladder mesenchyme is very similar to that observed in the UM. Both the SMCs and the α-SMA negative cells within the lamina propria showed extensive blue labeling (Fig. 3G–H). The transitional epithelium lining the lumen of the bladder was not labeled (Fig. 3G–H). It is still unclear if the observed loxP recombination in the bladder results from de novo Tbx18-Cre expression in the bladder, from the migration of UM cells into the bladder, or the combination of the two. High level of Tbx18 expression in the urogenital sinus/bladder has not been reported. However, even low level of Tbx18 expression in the bladder mesenchymal progenitors at any stage of development prior to detection could produce the blue cells. It is worth noting that at least parts of the bladder (trigone in particular) have been shown to have intercalating SMCs of both bladder and ureter origins (Viana et al., 2007). Although careful examination of Tbx18 expression and further lineage tracing experiments will eventually distinguish these possibilities, the extensive labeling of the bladder SMCs not restricted to the ureterovesical junction area favors the possibility that Tbx18-Cre expression in the bladder mesenchyme contributed significantly to the observed recombination within the bladder.
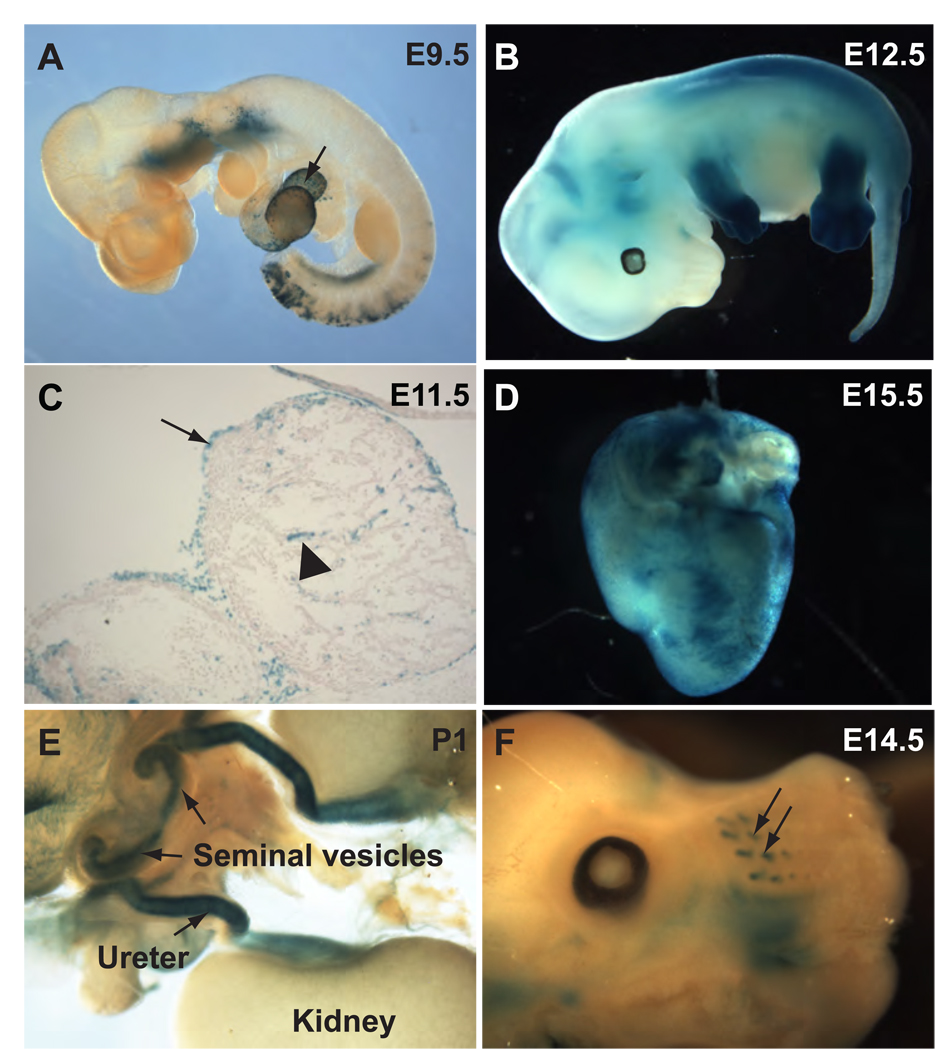
Outside the urinary system, Cre expression was evident in the pharyngeal area, the posterior somites, and in the epicardium starting from as early as E9.5 (Fig. 4A). The overall pattern of transgene expression at E10.5 resembled that of E9.5 with the exception that staining became visible in the emerging limb buds at E10.5 (data not shown). At E12.5, Tbx18-Cre expression in the distal portions of the limb buds became very strong (Fig. 4B). By E14.5, however, the expression spread to most of the limbs (data not shown).
Figure 4. Tbx18-Cre expression in other tissues.
A, Whole-mount β-galactosidase assay at E9.5 revealed loxP recombination in the pharyngeal area, the epicardium (arrow) and somites. B, Staining in all four limb buds became very prominent at E12.5. C, lacZ-positive cells were detected in the epicardium (arrow) and sparsely in the myocardium (triangle). D, an image of the E15.5 heart showed epicardial staining. Additional expression in the seminal vesicle (arrows in E) was observed. Cre-mediated recombination was also detected in progenitor cells for the vibrissae (arrows in F).
The expression of Tbx18 in the pericardium and the presence of small number of blue cells in the myocardium are consistent with previous reports and the recent observation that epicardial participation in the ventricular septum as well as the atrial and ventricular walls (Fig. 4C–D) (Airik et al., 2006; Cai et al., 2008; Haenig and Kispert, 2004; King et al., 2006). Blue cells were also seen in the seminal vesicles (Fig. 4E) and the progenitors for vibrissae at about E14.5 (Fig. 4F). Most of these sites have been reported to express Tbx18, suggesting that the Tbx18-Cre BAC transgene closely mimics the expression pattern of the endogenous gene (Airik et al., 2006; Cai et al., 2008; Haenig and Kispert, 2004; King et al., 2006). The expression of the Tbx18-Cre transgene in other structures (such as the limbs, the epicardium, and the vibrissae) may present complications to studies on the urinary tract for some genes but may be regarded as a valuable feature by researchers interested in these structures. In fact, Cai et al recently generated a mouse line with Cre coding sequence knocked-in to the Tbx18 locus for the study of the proepicardial cells (Cai et al., 2008). Cre expression in the urogenital system was not described in this study. Tbx18 is known to have important functions in the development of the UM and Tbx18 deficiency leads to early postnatal lethality due to urinary tract anomalies (Airik et al., 2006). Although no phenotype was reported for theTbx18+/− mice, it is not uncommon to see deteriorating effects of the heterozygous loss of a gene when mutations in other genes are present. The Tbx18-Cre transgene neither disrupts the endogenous Tbx18 expression nor carries additional copies of Tbx18, thus is better suited for studies of the urinary system than the Cre knock-in allele. Conceivably, this transgene can also be used for lineage tracing studies in normal development and in diseases states. In addition to studying development, the Cre transgene can be used for studies on the physiology of the ureter and the bladder. For example, mice with conditional inactivation of a gene of interest postnatally may be used to assess the role of the gene in the regulation of peristalsis or in the response to injury such as lithiasis.
Methods
Generation of the BAC transgene construct by Recombineering
A BAC clone carrying the murine Tbx18 gene was purchased from Invitrogene (Clone # RP23-353O7). This BAC clone (Tbx18BAC), carrying a chloramphenicol (Cm) resistant cassette, was transformed into DH10B host cells. We then introduced another plasmid pRedET, carrying a tetracycline (Tet) resistant cassette, into these DH10B cells that already have Tbx18BAC. The presence of the pRedET plasmid restores the ability for selected types of recombination in the DH10B cells. At the same time, we used PCR to introduce 2 homology arms to the plasmid phCre2.myc.nuc.FRTN1.amp.FRT that expresses a codon-improved Cre with a small Myc tag and nuclear localization sequence (NLS) (a gift from Dr. Günther Schütz) (Casanova et al., 2001). The Frt.Amp.Frt cassette provides ampicillin resistance (Amp) for the intermediate cloning steps and was later removed before the construct was finalized. The homology arms were designed for replacing a stretch of the Tbx18 gene with the Cre cassette by recombineering. The left homology arm consists of the 50 nucleotides directly 5’ of the ATG start codon of Tbx18. The right homology arm consists of a 50 nucleotides long sequence in intron 1 of Tbx18. In this design, after recombineering, the coding sequence (CDS) in the first exon of Tbx18 would be replaced by the Cre-expressing cassette. Therefore, the expression of Cre would be subjected to the control of the Tbx18 promoter. The multiple transcriptional stop signals would ensure that the partial Tbx18 gene left on the BAC clone is not transcribed. The purified PCR product (Tbx18.left.arm_phCre2.myc.nuc.FRTN1.amp.FRT_Tbx18.right.arm) was electroporated into the competent DH10B cells that already had both Tbx18Bac/DH10B and pRedET. The electroporated cells were selected on plates with Cm, Tet, Amp, and Arabinose (for inducing the required recombinases from the pRedET plasmid). The Tbx18.left.arm_phCre.myc.nuc.FRT.AMP.FRT_Tbx18.right.arm was a PCR product and could not be reproduced by the bacteria without recombination that incorporates it into the BAC. Thus, the Amp resistant clones were screened for the ones where recombination occurred between the homology arms of the PCR product and the corresponding Tbx18 sequence on the BAC. The Frt-Amp-Frt cassette was subsequently removed by the introduction of a p706FLPE plasmid (a gift from Dr. Stewart) (Zhang et al., 1998). Every cloning step was confirmed by restriction digestion, PCR, and/or sequencing. The final transgene construct (Tbx18-Cre/BAC) was subjected to sequencing to ensure that everything went as planned and no unwanted mutation was introduced.
Generation of the transgenic mice
All animal studies have been approved by IACUC (Institutional Animal Care and Use Committee) at Washington University School of Medicine. The transgene construct was purified by using the Qiagen large DNA Construct kit and was dialyzed by using the transgene injection buffer (10mM TRIS, pH7.4, 0.1mM EDTA). The construct was injected into the pronuclei of fertilized oocytes from C57Bl/6xCBA hybrids. The presence of the transgene in the founders and their offspring was detected by PCR using TCTF 5’ CCATCCAACAGCACCTGGGCCAGCTCAACA 3’ and TCTR 5’ CCACCATCGGTGCGGGAGATGTCCTTCACT 3’. The ROSA26R-lacZ reporter mice were described previously (Soriano, 1999) and were genotyped by using primers WS268 5’ GTTATCAGTAAGGGAGCTGCAGTGG 3’, WS270 5’ AAGACCGCGAAGAGTTTGTCCTC 3’, and WS271 5’ GGCGGATCACAAGCAATAATAACC 3’ to amplify a wild-type band of 500 bp and a band of 250 bp corresponding to the ROSA26R-lacZ allele. PCR conditions were: 95°C, 2’, 35 × (94°C 30”; 59.5°C, 30”; 72°C, 30”), 72°C, 5’.
Histology and Immunohistochemistry
10 µm cryostat sections of embryos or tissues were collected. 5-Bromo-4-chloro-3-indolyl-D-galactoside (Xgal) staining on cryostat sections was performed as described (Chang et al., 2004). Immunostaining on cryostat sections was performed as previously described (McDill et al., 2006). Antibodies used were: anti-α-SMA antibody (Sigma, 1:500), anti-Uroplakin III antibody (APR, 1:100), anti-E-Cadherin antibody (BD, 1:100). Appropriate HRP-conjugated secondary antibodies (Jackson ImmunoResearch, 1:1000) were used to detect the corresponding primary antibodies.
Acknowledgements
We thank the Washington University Mouse Genetics Core and the Renal Disease Model Core within the George M. O'Brien Washington University Center for Kidney Disease Research (NIHP30DK079333) for assistance in the generation of the Tbx18-Cre transgenic mice. We thank Drs. Radek Skoda, Ralph Tiedt, Günther Schütz, Francis Stewart, Gerald Crabtree, as well as Hong Chen and Ann Kuo for providing plasmids for the recombineering of the Tbx18-Cre transgene construct. F.C. was supported in part by an NIH grant (NIHR01DK067386) and a March of Dimes Award (FY06-343).
References
- Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–674. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–1975. doi: 10.1242/dev.004234. [DOI] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, Schutz G. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31:37–42. doi: 10.1002/gene.1078. [DOI] [PubMed] [Google Scholar]
- Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Gawlik A, Quaggin SE. Deciphering the renal code: advances in conditional gene targeting. Physiology (Bethesda) 2004;19:245–252. doi: 10.1152/physiol.00009.2004. [DOI] [PubMed] [Google Scholar]
- Haenig B, Kispert A. Analysis of TBX18 expression in chick embryos. Dev Genes Evol. 2004;214:407–411. doi: 10.1007/s00427-004-0415-3. [DOI] [PubMed] [Google Scholar]
- King M, Arnold JS, Shanske A, Morrow BE. T-genes and limb bud development. Am J Med Genet A. 2006;140:1407–1413. doi: 10.1002/ajmg.a.31250. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, Stappenbeck TS, Miner JH, Swat W. Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc Natl Acad Sci U S A. 2006;103:19872–19877. doi: 10.1073/pnas.0609326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci U S A. 2006;103:6952–6957. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A. Renal abnormalities and their developmental origin. Nature Reviews genetics. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, Shapiro E, Hensle T, Lambert S, Mendelsohn C. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134:3763–3769. doi: 10.1242/dev.011270. [DOI] [PubMed] [Google Scholar]
- Wu F. Conditional targeting in the kidney. Nephron Physiol. 2007;107:p10–p16. doi: 10.1159/000106483. [DOI] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]