Abstract
Cytoplasmic dynein and dynactin are megadalton-sized multisubunit molecules that function together as a cytoskeletal motor. In the present study, we explore the mechanism of dynein-dynactin binding in vitro and then extend our findings to an in vivo context. Solution binding assays were used to define binding domains in the dynein intermediate chain (IC) and dynactin p150Glued subunit. Transient overexpression of a series of fragments of the dynein IC was used to determine the importance of this subunit for dynein function in mammalian tissue culture cells. Our results suggest that a functional dynein-dynactin interaction is required for proper microtubule organization and for the transport and localization of centrosomal components and endomembrane compartments. The dynein IC fragments have different effects on endomembrane localization, suggesting that different endomembranes may bind dynein via distinct mechanisms.
INTRODUCTION
Cytoplasmic dynein is a minus end-directed, microtubule-based motor that provides the force for translocation, tension, and organization of cellular components. Each cytoplasmic dynein molecule contains two enzymatically active heads that convert the energy of ATP hydrolysis into mechanical work. These are connected to a basal cargo binding unit (for reviews of dynein structure, see King, 2000a,b). Cytoplasmic dynein requires another multisubunit protein complex, dynactin, for full activity (Gill et al., 1991; Schroer and Sheetz, 1991; Boylan et al., 2000). Dynactin contributes to cytoplasmic dynein function by acting as an “adapter” that expands the range of cargoes dynein can move (for review, see Karki and Holzbaur, 1999) and by increasing motor processivity (King and Schroer, 2000). Dynactin can also act independently of cytoplasmic dynein to anchor microtubules at the centrosome (Quintyne and Schroer, 2002). Dynactin is thought to accomplish these diverse tasks by using its distinct cargo-, cytoplasmic dynein- and microtubule-binding domains.
The interaction between dynein and dynactin seems to be tightly regulated, because dynactin and dynein are not always colocalized in cells (for reviews, see Holleran et al., 1998; Karki and Holzbaur, 1999). For example, dynactin is concentrated at centrosomes throughout the cell cycle, whereas dynein accumulates between the time of centriole duplication (mid-S phase) and the onset of mitosis (Quintyne and Schroer, 2002). Both proteins are found at kinetochores, spindle poles, and along spindle microtubules during mitosis, but after mitosis dynein is lost from the centrosome, whereas dynactin remains. Similarly, dynactin, but not dynein, associates with microtubule plus ends (Vaughan et al., 1999; Habermann et al., 2001). Finally, dynein and dynactin colocalize on numerous endomembranes destined to move along microtubules (Habermann et al., 2001) and decrease in concert at the onset of mitosis (Niclas et al., 1996). Apparently, dynein and dynactin can be either associated or separate in cells, which highlights the importance of understanding how dynein-dynactin binding is controlled in time and space.
Previous in vitro studies have identified a region of direct interaction between the two complexes. Native dynactin can bind immobilized dynein IC (IC) molecules and native cytoplasmic dynein can bind an immobilized fragment of dynactin p150Glued (AA 133-899; Karki and Holzbaur, 1995). Furthermore, an in vitro translated N-terminal 123-amino acid fragment of dynein IC can bind a recombinant fragment of p150Glued (AA 150-811) in blot overlays (Vaughan and Vallee, 1995). A recent study (Vaughan et al., 2001) has identified a serine residue (S84) in the dynein IC that is proposed to regulate the dynein-dynactin interaction. Together, these results show that the N terminus of the dynein IC can bind directly to the central portion of p150Glued and suggest that this interaction might, in part, be regulated by the phosphorylation of S84 of the IC.
Dynein and dynactin function can be inhibited by blocking their interaction. Antibodies that bind either dynein IC or p150Glued interfere with dynein-based motility in vitro and in vivo (Burkhardt et al., 1997; Gaglio et al., 1997; Steffen et al., 1997; Waterman-Storer et al., 1997; King and Schroer, 2000). Overexpression of full-length p150Glued disrupts dynein-based motility, as assessed by immunolocalization of centrosome and endomembrane components (Quintyne et al., 1999). In addition, overexpression of the dynactin subunit dynamitin, which destroys dynactin function by releasing its dynein-binding p150Glued subunit, causes dispersion of the Golgi complex and endosomes (Burkhardt et al., 1997) and inhibits organelle movement (Valetti et al., 1999). These effects are proposed to be the result of competitive inhibition of the dynein-dynactin interaction by excess free dynein-binding polypeptide (Quintyne et al., 1999).
To better understand the interaction between cytoplasmic dynein and dynactin, we analyzed the ability of dynein IC and p150Glued fragments to bind directly in solution. These assays allowed us to identify the smallest interaction domains within IC and p150Glued yet described. We then characterized the effects of overexpression of the dynactin-binding domain of dynein IC on microtubule organization, centrosome integrity, and endomembrane localization and found all to be altered. Full-length IC and another IC fragment had a different set of effects, suggesting that cellular endomembrane cargoes may bind to dynein via separate domains and mechanisms.
MATERIALS AND METHODS
Expression Constructs
Previously described expression constructs were used for CC1, CC2, and kinesin stalk (pVEX-CC1 and pVEX-CC2, Quintyne et al., 1999; DsRed1-CC1, Quintyne et al., 1999; Quintyne and Schroer, 2002; and pET-STK(2B), de Cuevas et al., 1992). The ubiquitous IC2C isoform of dynein IC was used in all work (Vaughan and Vallee, 1995; Brill and Pfister, 2000). Full-length mouse IC2C (IC2-FL) was cloned by polymerase chain reaction (PCR) with primers that added BglII and HindIII sites onto the ends of the IC2C expressed sequence tag (EST AA667372; Image clone no. 1162066). The original PCR fragment was cloned into a PCR 2.1 TA cloning vector (Invitrogen, Carlsbad, CA) to yield PCR2.1-IC2. Green fluorescent protein (GFP)-tagged IC2-FL was cloned by subcloning the BglII-HindIII insert from PCR2.1-IC2 into the N-terminal GFP fusion vector pEGFP-C2 (BD Biosciences Clontech, Palo Alto, CA). A bacterially expressed His-tagged construct encoding the N-terminal 237 amino acids of IC2C (pRSET:IC2-N237) was engineered by subcloning the PCR2.1-IC2 BglII-HindIII insert into pRSET-a (Invitrogen), digesting with BlpI to remove the coding region for AA 238 to the end of the IC, filling-in with Klenow, and ligating. GFP-tagged IC2-N237, IC2-N106, and the IC2 C-terminal 375 AA (IC2-C375) constructs for mammalian expression were engineered from PCR2.1-IC2 via PCR. IC2-N237 used primers IC2UP (5′ ACCACCT GACAGATCTCAACCATGTC) and IC2N237DN (5′ GATTTAATGAAAGCTTAGCACCTG); the PCR fragment was digested with BglII and HindIII and ligated into pEGFP-C2. IC2-N106 used primers IC2UP and IC2N106DN (5′ TCCAAGTTTAATGGATCCTCATCTAGATCC); the PCR fragment was digested with BglII and BamHI and ligated into pEGFP-C2 at the BglII site. IC2-C375 used primers IC2CTUP (5′ CAGGTGCTAAGATCTCATTAAATC) and IC2DN (5′ GATTTAATGAAAGCTTAGCACCTG); the PCR fragment was digested with BglII and HindIII and cloned into pEGFP-C2. PRSET:IC2-N237 S84A and S84D were constructed using PCR-based site-directed mutagenesis with primers containing a silent mutation for a unique restriction endonuclease by using standard techniques (Lum and Schildebach, 1999). EGFP-IC2-N237 S84A and S84D were constructed using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla CA). The orientation and open reading frames of all constructs were confirmed by sequencing.
Antibodies, Gels, and Blots
Primary antibodies to the following proteins were used: dynein IC: monoclonal antibody (mAb) 74.1 (Chemicon International, Temecula, CA) and polyclonal antibody D1 (Gill et al., 1994); Golgi complex: mAb to giantin (Linstedt and Hauri, 1993); endoplasmic reticulum-Golgi intermediate compartment (ERGIC)-53: mAb G1/93 (Schweizer et al., 1988); early endosomes: mAb EEA1 (BD Biosciences PharMingen, San Diego, CA); human transferrin receptor: mAb HTR-H68.4 (White et al., 1992); γ-tubulin: mAb GTU88 (Sigma-Aldrich, St. Louis, MO); α-tubulin: mAb DM1A (Sigma-Aldrich); p150Glued: mAb 150B (Gaglio et al., 1996; Blocker et al., 1997), mAb 150N (BD Biosciences Transduction Laboratories, Lexington, KY); Arp1: mAb 45A (Schafer et al., 1994); centrin: 20H5 (Salisbury et al., 1986); GFP: GFP mAb (Covance Research Products, Richmond, CA); and RP3: pAb CVMC 123 (Tai et al., 2001). The lysosomal antibody to LAMP-1 (mAb H4A3) developed by August and Hildreth was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by Department of Biological Sciences, The University of Iowa, Iowa City, IA. Texas Red-conjugated horse anti-mouse (Vector Laboratories, Burlingame, CA) was used as the secondary antibody for immunofluorescence. SDS-PAGE (Laemmli, 1970) and immunoblotting (Towbin et al., 1979) were performed as described. For immunoblotting, appropriate alkaline phosphatase-conjugated secondary antibodies were detected by chemiluminescence (Tropix, Bedford, MA).
Cell Culture, Transfection, Immunofluorescence, and Microscopy
COS-7 cells were grown, transfected, fixed, and processed for microtubule or centrosome immunofluorescence, as described previously (Quintyne et al., 1999). HeLa cells were grown in Dulbecco's modified eagle medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO), 1% l-glutamine (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 1% nonessential amino acids (Sigma-Aldrich). Cytosols from COS-7 and HeLa cells were prepared by detergent lysis and subjected to velocity sedimentation as described previously (Quintyne et al., 1999). For giantin, ERGIC-53, EEA-1, and human transferrin receptor immunofluorescence, cells were fixed with 4% formaldehyde (Polysciences, Warrington, PA) for 15 min., blocked with 50 mM NH4Cl (Mallinckrodt, Paris, KY) for 10 min, and then permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) before addition of primary antibody. For LAMP-1, cells were fixed in formaldehyde and blocked as above; 0.2% saponin (Sigma-Aldrich) was used for permeabilization. All cells were transfected by electroporation with 10-15 μg of DNA, as described previously (Quintyne et al., 1999). Transfection frequencies of ∼60% were commonly observed for all constructs except IC2-C375 (see text for details). For every transfection condition, at least 200 cells on multiple coverslips were scored per experiment and at least two independent experiments were evaluated per condition.
For scoring of endomembrane compartments, GFP intensity was assessed visually and cells were categorized as either nonexpressing, or low, moderate, high, or very high expressing. Only cells expressing moderate or high levels of GFP-tagged protein were evaluated. Within these populations, only cells showing complete fragmentation with some degree of dispersion (Golgi, ERGIC) or complete dispersion (early endosomes, recycling endosomes, late endosomes/lysosomes) were scored as abnormal. Dispersion was assessed by loss of juxtanuclear accumulation. Epifluorescence microscopy was performed using an Axiovert 35 microscope (Carl Zeiss, Thornwood, NY). Microtubule and centrosome images were recorded using a DeltaVision deconvolving microscope system (Applied Precision, Issaquah, WA). Endomembrane images were captured and recorded using a Photometrics CoolSNAP cf. camera (Roper Scientific, Trenton, NJ) and IPLab imaging software (Scanalytics, Fairfax, VA). All images were imported into Adobe Photoshop (Adobe Systems, San Jose, CA) for contrast manipulation and assembled using Canvas (Deneba Systems, Miami, FL) or Microsoft Power-Point (Microsoft, Redmond, WA).
Protein Expression and Purification
CC1 was expressed from pVEX-CC1 (Quintyne et al., 1999) in BL21(DE3) cells and purified as described previously (de Cuevas et al., 1992) with minor modifications. Briefly, after overnight protein expression, cells were resuspended in Bugbuster reagent (Novagen, Madison, WI) with 25 U/ml benzonase DNase (Novagen), rocked at room temperature for 20 min, and spun at 16,000 × g for 20 min at 4°C. The supernatant was fractionated by 25% ammonium sulfate precipitation, the pellet was resuspended in phosphate buffer (16 mM Na2HPO4, 4 mM NaH2PO4, 2 mM dithiothreitol, 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, pH 7.2), brought to 0.75 M KCl, and boiled in a water bath for 3 min. After clarification by centrifugation, the supernatant was loaded onto a MonoQ HR 5/5 anion exchange column (Amersham Biosciences, Piscataway, NJ) at 1 ml/min. The protein was eluted using a 0-1000 mM KCl gradient in 20 mM Tris-Cl, pH 7.0. The protein was concentrated using an Amicon centricon (3000 MW cut-off; Millipore, Bedford, MA). CC2 was expressed from pVEX-CC2 (Quintyne et al., 1999) in BL21(DE3)pLysS cells and purified as described above, except that a 50% ammonium sulfate precipitation was used.
The IC2-N237 polypeptide was expressed from the pRSET:IC2-N237 plasmid in BL21-Codon Plus (DE3)-RP cells (Stratagene). Cells were grown at 37°C to an OD600 of ∼0.4, at which point 0.1 mM isopropyl β-d-thiogalactoside was added to the medium and the cells were grown overnight at 37°C. Cells were collected by centrifugation at 3000 × g, and the pellets frozen at -80°C. Thawed pellets were resuspended into sonication buffer (20 mM Tris-Cl, pH 8.0, 100 mM KCl, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 10 μg/ml Nα-benzoyl-L-arginine methyl ester, 10 μg/ml Nα-p-tosyl-L-arginine methyl ester, 10 μg/ml N-tosyl-l-lysine chloromethyl ketone, 10 μg/ml N-tosyl-l-phenylalanine chloromethyl ketone), 0.7 mg/ml lysozyme was added, and the cells incubated 20 min at room temperature. The cells were sonicated and cell debris was removed by centrifugation at 16,000 × g at 4°C for 20 min. The cell supernatant was run over Talon metal affinity resin (BD Biosciences Clontech) by gravity flow. The protein was washed in 20 mM Tris-Cl, pH 8.0, 100 mM KCl, 10 mM imidizole and eluted with 20 mM Tris-Cl, pH 8.0, 100 mM KCl, 100 mM imidizole. Pooled fractions were diluted twofold and loaded onto a MonoQ HR 5/5 (Amersham Biosciences) column at 1.0 ml/min equilibrated with 20 mM Tris-Cl, pH 8.0, as the column buffer. The protein was eluted with a three component gradient: 0-200 mM KCl in 7 ml, 200-400 mM KCl in 30 ml, and 400-1000 mM KCl in 10 ml, all in 20 mM Tris-Cl, pH 8.0. The protein was concentrated using a centricon as described above.
Gel Filtration Chromatography
Protein samples were dialyzed against column buffer (25 mM Tris-Cl, pH 8.0, 100 mM KCl, 1 μM β-mercaptoethanol) overnight. The samples were incubated at room temperature for 30 min before injection onto a Superose 12 HR 10/30 column (Amersham Biosciences) run at 0.3 ml/min. Fractions were collected and analyzed by SDS-PAGE. Individual runs of CC1 and IC2-N237 were examined at 24 and 6 μM. Equimolar mixes of CC1 (dimer) and IC2-N237 (monomer) were examined at 24, 6, 1.5, and 0.4 μM final concentrations.
RESULTS
Direct Interaction between Dynein IC and Dynactin p150Glued
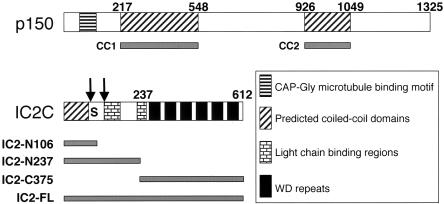
Previous work has shown that the N-terminal 123 AA of the dynein IC can bind AA 150-811 of p150Glued in vitro (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995). However, the blot overlay and affinity chromatography techniques used in these studies relied on denatured or immobilized proteins and did not address the nature or in vivo relevance of the observed binding. The IC and p150Glued primary sequences suggest some interesting structural features in these regions (Figure 1). About one-half of the p150Glued interaction domain (AA 217-548; referred to as CC1) is α-helical in nature (Quintyne et al., 1999) and is predicted to assemble into a stable coiled-coil structure. Based on the predicted dimensions of this coiled-coil and what is known of dynactin structure, CC1 is believed to constitute the projecting stalk and part of the dynactin shoulder (Schafer et al., 1994; Eckley et al., 1999; Quintyne et al., 1999). The 123 AA IC fragment that binds p150Glued exhibits several notable features, including an N-terminal predicted coiled-coil (AA 1-66), a serine-rich region (AA 81-99) and binding sites for at least two light chains (LCs; Lo et al., 2001; Mok et al., 2001; Tai et al., 2001; Makokha et al., 2002; Susalka et al., 2002).
Figure 1.
Schematic representations of expression constructs used in this study. (A) p150Glued contains a CAP-Gly microtubule-binding motif near the N terminus and two predicted coiled-coil domains, CC1 (AA 217-548) and CC2 (AA 926-1049). (B) The IC 2C isoform contains a predicted N-terminal coiled-coil domain (AA 1-66), light chain binding regions (RP3, Tctex-1, and LC8: AA 107-138; roadblock: AA 223-261) and WD repeats (AA 262-612). Arrows indicate two sites of alternative splicing (after AA 76 and 106), and the S indicates a region (AA 81-99) that contains eight serine residues. The regions encoded by different expression constructs are shown beneath.
To examine the IC-p150Glued interaction in greater detail, we expressed and purified polypeptide fragments corresponding to dynactin p150Glued CC1 and the N terminus of the ubiquitous 2C isoform of IC (Figure 1). The CC1 domain of p150Glued was used for this analysis because it inhibits dynein function when overexpressed in vivo (Quintyne et al., 1999). The IC2C fragment IC2-N237 was engineered to completely exclude the WD repeats thought to mediate interactions with DHC/DLIC (Vaughan and Vallee, 1995; Habura et al., 1999; Ma et al., 1999).
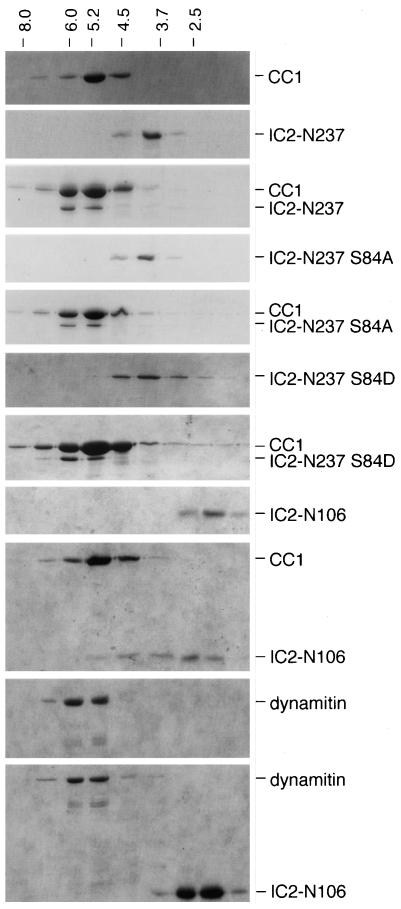
We assayed the ability of these polypeptides to interact in solution using Superose 12 gel filtration chromatography. IC2-N237 and CC1 were first examined separately. The IC2-N237 polypeptide eluted in a single peak with an apparent Stokes' radius of 4.1 nm, and the CC1 polypeptide eluted as a single peak with an apparent Stokes' radius of 5.3 nm (Figure 2). To determine whether the two polypeptides could associate into a larger complex, they were combined in a 1:1 M ratio and subjected to gel filtration. A species containing both CC1 and IC2-N237 that eluted slightly larger than CC1 were observed. Similar results were obtained using samples at different concentrations but still in an equimolar ratio (our unpublished data). The interaction with CC1 is specific, because IC2-N237 did not show a shift in elution volume when analyzed at higher concentrations by itself or cochromatographed with other coiled-coil proteins, such as the kinesin stalk (de Cuevas et al., 1992), the second coiled coil domain of p150Glued (CC2: AA 926-1048; Quintyne et al., 1999) or dynamitin (our unpublished data). A recent study has proposed that the phosphorylation of IC serine 84 may regulate the interaction between dynein and dynactin (Vaughan et al., 2001). To evaluate how this might affect IC-p150Glued binding in solution, we engineered the phospho-mimic, “low-affinity” S84D substitution into the IC2-N237 construct. The dephospho-mimic mutant, S84A, which should behave the same as wild type, was prepared and analyzed in parallel. The S84D mutant chromatographed indistinguishably from wild-type IC2-N237 and S84A alone or in combination with CC1 (Figure 2). Apparently, a mutation that mimics the phosphorylated state of S84 does not dramatically affect its ability to bind CC1 in solution. However, it is possible that S84 phosphorylation controls the interaction of IC with a different region of p150Glued and/or that it contributes to other dynein-related functions in cells.
Figure 2.
Direct interaction between dynein IC fragments and dynactin p150Glued CC1. Recombinant proteins (CC1, IC2-N237, IC2-S84A, IC2-S84D, IC2-N106, and dynamitin) were used alone or mixed pairwise and chromatographed on a Superose 12 gel filtration column. The two S84 mutants (S84A and S84D) were made in the IC2-N237 fragment. Individual column fractions were analyzed by SDS-PAGE and Coomassie Blue staining. The identity of polypeptides present in each column run is provided to the right. The A280 chromatographic positions of molecular standards of known Stokes' radii are indicated at the top.
To determine whether a dynein IC lacking the LC binding regions could bind p150Glued, we next examined the interaction of the N-terminal 106 amino acids of IC2C (IC2-N106) with CC1. Superose 12 chromatography of IC2-N106 by itself revealed a single peak with a Stokes' radius of 2.5 nm (Figure 2). When IC2-N106 was combined with CC1 at equimolar concentration and chromatographed, it eluted in a broad peak that overlapped with the position of the CC1 polypeptide (Stokes' radius ≈5.3 nm). Such peak broadening was not seen at higher concentrations of IC2-N106 alone or when IC2-N106 was mixed with control proteins, such as dynamitin (Figure 2). CC1 itself showed no shift in elution volume in the presence of IC2-N106. This suggests that IC2-N106 can bind CC1 without dramatically affecting its hydrodynamic properties. Moreover, IC2-N106 does not seem to bind CC1 as well as IC2-N237. To pursue these findings further, we examined the effects of overexpression of the N-terminal IC fragments, as well as full-length IC2 (IC2-FL), on a variety of dynein-dependent cellular phenomena.
Overexpression Analyses of Intermediate Chain Domains
Overexpression of dynactin subunits in cells has been widely used to determine how the dynein-dynactin complex contributes to endomembrane trafficking and localization, centrosome and microtubule organization, and a number of other phenomena (Waterman-Storer et al., 1995; Echeverri et al., 1996; Holleran et al., 1996; Burkhardt et al., 1997; Quintyne et al., 1999; Valetti et al., 1999; Eckley and Schroer, 2003). Overexpression of dynamitin, a protein that causes dynactin disassembly in vitro (Karki et al., 1998; Eckley et al., 1999; Wittman and Hyman, 1999) and in vivo (Echeverri et al., 1996), has profound effects on cellular architecture, causing fragmentation and dispersal of the Golgi complex, dispersion of endocytic compartments, loss of pericentriolar components, and microtubule defocusing (Burkhardt et al., 1997; Quintyne et al., 1999; Valetti et al., 1999). Overexpression of full-length dynein IC in mammalian cells has also been reported to affect Golgi complex and endosome organization, although not as profoundly as dynamitin overexpression (Vaughan et al., 2001). Effects of IC overexpression on centrosome and microtubule organization have not been previously examined, but it has been demonstrated that loss of dynein IC from Dictyostelium cells results in perturbation of the normal microtubule array (Ma et al., 1999). Neither of these previous studies of IC function have specifically evaluated the importance of the dynactin-binding, N-terminal domain of IC in any cellular process.
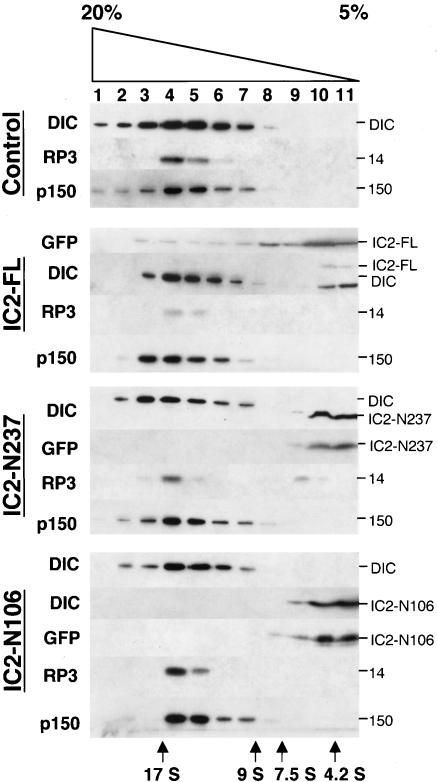
We engineered and overexpressed by transient transfection IC2-FL, -N237, -N106, and the C-terminal fragment IC2-C375 (Figure 1) in COS-7 and HeLa mammalian tissue culture cells. All proteins were expressed as GFP chimeras to facilitate detection. Velocity sedimentation of cytosol isolated from transfected cell populations revealed that the majority of GFP-IC2-FL, -N237, and -N106 was present in light sucrose fractions (Figure 3). A small amount of GFP-IC2-FL was also detected at about 20S, suggesting that the tagged, full-length protein could be incorporated into dynein. This seemed to displace a small amount of endogenous IC, which now sedimented in the light sucrose gradient fractions. The majority of endogenous IC, as well as the dynein light chain, rp3, cosedimented at 20S, indicating that dynein was largely intact. IC2-N237 overexpression caused release of a small amount of rp3, but did not alter the behavior of endogenous IC, once again showing the majority of dynein is intact. IC2-N106, which is not expected to bind LCs, did not affect the sedimentation behavior of either rp3 or endogenous IC. These data indicate that dynein structure is not significantly perturbed in cells overexpressing IC2-FL, IC2-N237, or IC2-N106. The dynactin subunits p150 (Figure 3) and Arp1 (our unpublished data) also sedimented normally, indicating that none of the IC2 constructs had an impact on dynactin structure.
Figure 3.
Velocity sedimentation of detergent lysates from HeLa cells overexpressing dynein IC fragments. Lysates were sedimented into 5-20% sucrose gradients. Fractions were analyzed by Western blotting with antibodies to GFP, dynein IC, the dynein LC, rp3, or the dynactin subunit p150Glued. The construct being expressed and the size of each immunoreactive species are indicated; sedimentation standards are at the bottom. Similar sedimentation patterns were obtained when IC polypeptides were overexpressed in COS-7 cells (our unpublished data).
To our surprise, only trace amounts of IC2-C375 could be detected in transfected cell lysates harvested ∼20 h after transfection (the standard time used in our dynactin subunit overexpression experiments (Quintyne et al., 1999; our unpublished data). Inspection by epifluorescence microscopy revealed only a small number of IC2-C375-expressing cells in the population, suggesting that the protein might be toxic. To test this hypothesis we analyzed IC2-C375-overexpressing cell populations at earlier times after transfection. Between 8 and 12 h posttransfection, significantly more IC2-C375 cells (30 vs. 5% of controls) seemed rounded-up, and many dead cells, as detected by trypan blue exclusion, were found floating in the culture medium. The IC2-C375 construct was not analyzed further owing to its apparently toxic effects on cells.
Microtubule and Centrosomal Phenotypes
Overexpression of some dynactin subunits results in centrosome instability and disorganization of the microtubule array (Waterman-Storer et al., 1995; Quintyne et al., 1999). We have proposed that CC1 and full-length p150Glued (either overexpressed or released by dynamitin) can bind dynein directly and competitively inhibit dynactin binding. This prevents dynein-based transport of dynactin and other pericentriolar proteins to the centrosome (Quintyne et al., 1999), leading to a deficit in microtubule-anchoring activities (Quintyne et al., 1999; Quintyne and Schroer, 2002). According to this model, overexpression of any other protein or protein domain that interferes with dynein-dynactin binding should have similar effects on the integrity of centrosomes and the microtubule array. Full-length IC2 (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995), IC2-N237, and IC2-N106 can all bind p150Glued in vitro, suggesting that they might competitively inhibit dynein-dynactin binding in vivo. This is predicted to lead to disorganized microtubule arrays and centrosome fragmentation.
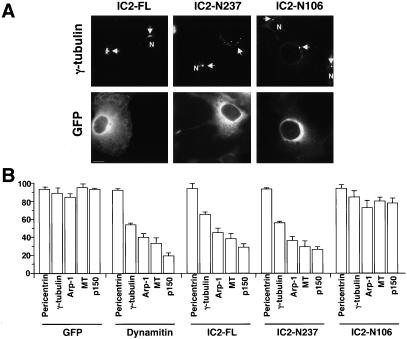
COS-7 cells were transiently transfected with IC2 constructs and then fixed and stained for microtubules and centrosome components. We chose COS-7 cells for this analysis because they have a well-organized radial microtubule array. To better evaluate the effects of IC2 overexpression, we compared the effects of the three constructs with dynamitin (Quintyne et al., 1999) and GFP alone. Under normal conditions, COS-7 cells contain a well-organized radial microtubule array that emanates from a centrosome whose pericentriolar material is focused into one or two juxtanuclear spots. Dynamitin overexpression causes microtubules to become defocused and results in the spreading or loss of centrosomal components (Table 1; see also Quintyne et al., 1999). Dynactin subunits were lost from centrosomes altogether. γ-Tubulin, which is normally found in one or two spots (depending on cell cycle stage), was now found in multiple foci that, in some cases, were dispersed throughout the cytoplasm. Pericentrin distribution was unaffected (Table 1). Cells overexpressing either IC2-FL or -N237 also displayed disorganized microtubules and altered staining for centrosome markers (Figure 4). For both constructs, the prevalence of the different phenotypes (Figure 4 and Table 1) was very similar to dynamitin overexpression, although IC2-FL overexpression affected slightly fewer cells than IC2-N237.
Table 1.
Effects of dynein IC overexpression on microtubule, centrosome, and endomembrane organization
| Structure scored | GFP | Dynamitin | IC2-FL | N237 | N237 S84A | N237 S84D | N106 |
|---|---|---|---|---|---|---|---|
| Microtubule array | 95.3 | 33.1 | 38.3 | 29.3 | ND | ND | 79.7 |
| γ-Tubulin | 88.8 | 54.0 | 65.3 | 56.0 | ND | ND | 84.3 |
| Centrosomal Arp1 | 84.3 | 39.8 | 45.3 | 36.0 | ND | ND | 72.3 |
| Centrosomal p150 | 93.0 | 19.3 | 28.7 | 26.3 | ND | ND | 77.3 |
| Pericentrin | 93.0 | 92.5 | 94.0 | 93.3 | ND | ND | 94.0 |
| Golgi complex | 97.7 | 8.9 | 84.2 | 23.4 | 22.7 | 55.5 | 90.2 |
| ERGIC | 97.8 | 6.9 | 92.6 | 43.4 | 49.0 | 63.2 | 91.0 |
| Early endosomes | 97.3 | 13.8 | 87.0 | 30.2 | 31.8 | 45.0 | 53.1 |
| Late endosomes | 99.1 | 32.8 | 89.6 | 49.5 | 44.5 | 56.7 | 74.7 |
| Recycling endosomes | 96.8 | 7.4 | 86.4 | 45.2 | 48.9 | 48.3 | 81.5 |
Summary of effects of dynein IC overexpression on subcellular organization. COS-7 cells were used for experiments examining microtubules and centrosomes; HeLa cells were used to evaluate endomembrane compartments. Cells were scored as described in the legends to Figures 4 and 5. The mean percentage of cells showing a normal phenotype is given for each overexpression condition; standard deviations are provided in Figures 4 and 5.
Figure 4.
Effects of dynein IC overexpression on the integrity of the microtubule array and pericentriolar material in COS-7 cells. (A) Representative images of the centrosome component, γ-tubulin (top), in cells transfected with IC2-FL, -N237 or -N106. GFP staining (to identify overexpressing cells) is shown below. N indicates the nuclei of untransfected control cells in the population. (B) Bar graphs (percentage of cells) illustrating how each expression construct affected the integrity of the microtubule array (MT) or pericentriolar material (pericentrin, γ-tubulin, Arp1, p150). The construct being expressed is indicated below each set of bars. Overexpressing cells were scored as normal if they had radially focused microtubules, one or two perinuclear foci of γ-tubulin, or a perinuclear focus of Arp1, p150Glued, or pericentrin; anything else was considered abnormal. The numbers given are the percentage of overexpressing cells with normal phenotypes ± SD.
IC2-N106 had only modest effects on microtubule organization and centrosomal dynactin compared with controls (Table 1). No change was seen in the localization of γ-tubulin to centrosomes. The reduced “potency” of IC2-N106 does not seem to be due to a lower expression level, because similar amounts of overexpressed protein as seen in cell lysates of IC2-N237- and -N106-overexpressing cells (Figure 3). Moreover, in cells showing altered behavior, the severity of the phenotypes was comparable with what was seen in cells overexpressing dynamitin, IC2-FL, or -N237. These results indicate that IC2-N106 is considerably less effective than IC2-N237 or IC2-FL at inhibiting dynein-based transport of components of the pericentriolar material to centrosomes.
Membranous Organelle Phenotypes
Dynactin, which is anchored to the Golgi complex and possibly other structures via βIII-spectrin (Holleran et al., 2001; Muresan et al., 2001), plays the important role of attaching dynein to membranous cargoes. Previous studies have shown that overexpression of dynactin subunits, such as dynamitin (Burkhardt et al., 1997; Valetti et al., 1999) or the CC1 fragment of p150Glued (Quintyne et al., 1999) result in the dispersion of endomembrane systems toward the cell periphery, presumably due to inhibition of dynactin-mediated dynein-endomembrane binding. However, dynein may also be able to bind endomembranes directly via interactions between LCs and transmembrane proteins, such as rhodopsin (Tai et al., 1999). To further test the hypothesis that the dynein-dynactin interaction is required for endomembrane function, we examined the effects of overexpression of the IC2 constructs on the localization of various endomembrane compartments in HeLa cells. We reasoned that IC overexpression might also reveal roles, beyond simply binding p150Glued, for the dynein IC in endomembrane organization. As with the analyses of microtubule and centrosomal phenotypes, percentages of cells with abnormal phenotypes were compared with both control cells and cells overexpressing dynamitin. For all constructs analyzed, similar results were obtained in HeLa (Figure 5 and Table 1) and COS-7 (our unpublished data).
Figure 5.
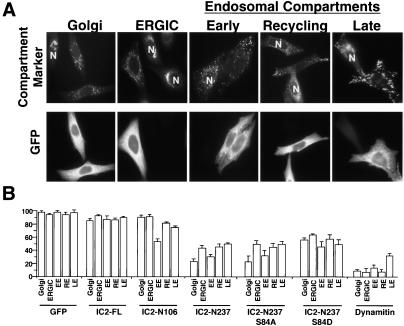
Effects of dynein IC overexpression on endomembrane localization. HeLa cells were used in this experiment because they exhibit more homogeneous endomembrane localization patterns than COS-7 cells. (A) Representative images of cell populations overexpressing IC2-N237 stained for giantin (Golgi complex; Golgi), ERGIC-53 (endoplasmic reticulum-Golgi intermediate compartment; ERGIC), EEA1 (early endosomes; EE), transferrin receptor (recycling compartment; RE), and LAMP-1 (late endosomes and lysosomes; LE) are shown. GFP staining (to identify overexpressing cells) is shown below. N indicates the nuclei of untransfected control cells in the population. (B) Bar graphs (percentage of cells) illustrating how each expression construct affected endomembrane compartment morphology. The construct being expressed is indicated below each set of bars. Only cells expressing moderate-to-high levels of GFP-tagged protein were counted and of these, only cells showing complete fragmentation or dispersion were scored as abnormal. The numbers given are the percentage of overexpressing cells with normal membrane distribution ± SD.
Dynamitin overexpression caused dispersion of biosynthetic membranes (Golgi complex or endoplasmic reticulum-Golgi intermediate compartment), recycling endosomes and early endosomes in nearly all cells (Figure 5 and Table 1), similar to what has been observed previously (Quintyne et al., 1999). Late endosomes seem to be somewhat less sensitive, with only about two-thirds of cells showing marked dispersion (see also Burkhardt et al., 1997). Overexpression of IC2-FL had only a minor effect on the localization of these endomembrane compartments. Approximately 20% of overexpressing cells showed fragmentation and dispersal (secretory compartments) or complete dispersal (endocytic compartments; Figure 5 and Table 1), as reported previously (Vaughan et al., 2001).
IC2-N237 overexpression resulted in redistribution of all five endomembrane compartments to the cell periphery, but in no case was the percentage of cells affected as high as with dynamitin overexpression (Figure 5 and Table 1). IC2-N106 overexpression had little, if any, impact on biosynthetic membranes and only a modest effect on late or recycling endosomes. However, early endosomes were dispersed toward the periphery in nearly half of the overexpressing cells.
The phosphorylation status of IC2 serine 84 did not significantly affect the ability of the IC2-N237 fragment to bind CC1 in solution (Figure 2). To determine whether the phosphorylation status of S84 affected dynein-dependent processes in vivo, we evaluated the effects of IC2-N237 S84 mutants on endomembrane localization. The S84D mutant (i.e., proposed low affinity), which is predicted to be less potent than wild-type IC2-N237 (Vaughan et al., 2001), had a slightly weaker effect on all compartments with the exception of late endosomes (Figure 5 and Table 1). The S84A mutant, which cannot be phosphorylated and should therefore have maximal affinity for dynactin, acted similarly to IC2-N237.
DISCUSSION
The present study extends our understanding of the dynein-dynactin interaction in vitro and in vivo. We have identified polypeptide fragments of the dynein IC and p150Glued that bind each other in solution. Our overexpression studies support previous work indicating that dynein-dynactin binding is required for maintenance of pericentriolar material and the morphology of a variety of endomembrane compartments. However, dynein-based transport to centrosomes and endomembrane localization seem differentially sensitive to IC fragment overexpression. Finally, our results suggest that the molecular details of the dynein-cargo interaction are different for different endomembranes.
Solution Binding of IC and p150Glued
The gel filtration chromatography binding assay shows, for the first time, that the free N terminus of the dynein IC can bind to a fragment of dynactin p150Glued in solution. For dynactin, we find that AA 217-548 of p150Glued (i.e., CC1), which form an α-helix that is predicted to form a coiled-coil structure, are sufficient for binding. For dynein, N-terminal dynein IC fragments encoding either 237 or 106 amino acids are both competent to bind CC1, although the larger fragment binds better. This suggests that AA 107-237, or some element thereof, contributes positively to CC1 binding. The identification of small IC and p150Glued fragments that can interact directly in vitro should facilitate future studies of the interaction between the megadalton-sized dynein and dynactin complexes.
CC1 and IC2-N237 seem to form a slightly larger complex upon binding each other, whereas CC1 and IC2-N106 do not. This might simply be due to the different sizes of the IC2-N237 and -N106 polypeptides. However, preliminary analytical velocity sedimentation analysis suggests that, at the concentration used in our solution binding assays, IC2-N237 is a monomer that can exist in two possible conformations (our unpublished data). Barbar and coworkers (Makokha et al., 2002) find that AA 1-289 of the Drosophila IC undergoes a conformational change upon binding to the LC8 dimer. A conformational change and/or dimerization of IC2-N237 that occurs upon binding the CC1 dimer might explain the apparent increase in size of the complex.
We did not detect a reduction in CC1 binding with a mutation (S84D) previously shown to diminish the interaction between full-length IC and p150Glued AA 1-811 in blot overlays (Vaughan et al., 2001). One possible explanation for the difference between our results is that the relevant domains of the denatured, immobilized proteins used previously have different conformations from these polypeptides in solution. Alternatively, the dynein IC and p150Glued may contact each other at more than one site. A complex, multisite “footprint” that anchors dynein to dynactin (and/or other cargo components) may be necessary to support the flexible, long-lived interaction that allows this motor to move cargoes through the cytoplasm. In keeping with this hypothesis, it was recently reported that just AA 600-811 of p150Glued is sufficient to bind full-length IC in a blot overlay (Deacon et al., 2003). It will be interesting to determine whether this fragment shows better binding to IC2-N106 compared with CC1.
Overexpression of Dynein IC Domains
It is widely accepted that dynein and dynactin contribute to the recruitment of pericentriolar material to centrosomes and the localization and dynamics of many endomembrane systems. However, compartment dispersion is not an automatic consequence of the disorganized microtubule arrays seen when pericentriolar material is disrupted. Many cell types that lack a radial microtubule array still contain tightly clustered Golgi complexes and juxtanuclear endosomes. Moreover, we have identified dynactin subunits and fragments (“dynactin inhibitors”) that can perturb microtubule organization without altering the architecture of membranous organelles (Quintyne et al., 1999).
We have extended and refined this work by transiently overexpressing dynein IC and its fragments in mammalian cells. On the basis of previous findings in Dictyostelium (Ma et al., 1999), we were surprised to discover that overexpression of a C-terminal fragment of IC2 (IC2-C375) had no obvious effects. Closer examination revealed IC2-C375 to be toxic in mammalian cells, leaving only cells that expressed the fragment at very low levels. Dynein polypeptides have been shown to be involved in early stages of apoptotic cell signaling (Dick et al., 1996; Chang et al., 2000; Lukashok et al., 2000) and dynein IC is an early target of apoptotic cleavage (Lane et al., 2001). All this is consistent with the notion that IC2-C375 overexpression triggers cell death in our system.
IC2-FL is predicted to bind free dynactin and compete for binding to endogenous holodynein. Overexpressed IC2-FL is competent to associate with its normal binding partners, because some incorporates into 20S dynein and displaces a small amount of endogenous IC. However, the overall effect on endogenous dynein integrity is minimal. Microtubule organization and centrosome integrity were obviously perturbed, suggesting that full-length IC competes well with dynein for binding soluble dynactin. However, IC2-FL overexpression had a weak effect upon endomembrane localization in HeLa cells, with only 20% of overexpressing cells (at most) showing profoundly dispersed compartments. Full-length IC2 overexpression in COS-7 cells resulted in complete disruption of Golgi complex and late endosomes in ≈20 and 40% of cells, respectively (Vaughan et al., 2001). It is possible that the difference in our results is due to the position of the GFP tag. Our IC2 was tagged at the N terminus, which might interfere slightly with binding of this domain to dynactin. The full-length IC used by Vaughan's group, in contrast, carries a C-terminal tag.
IC2-N237 binds p150Glued CC1 well in vitro, so we expected it would perturb dynein-dynactin binding in vivo. Endogenous dynein continues to sediment at 20S and the overexpressed species is not incorporated into dynein, indicating that IC2-N237 does not have a major disruptive effect on endogenous dynein. Its effects on microtubule and centrosome organization were very similar to what we observed when we overexpressed CC1 (Quintyne et al., 1999). IC2-N237 overexpression also affected the morphology of all the endomembrane compartments we examined.
We noted only a small difference in the effects of wild-type IC2-N237 and the phospho-mimic mutant S84D, and no difference with the mutant S84A. S84D mutation reduces the strength of the interaction between full-length IC and full-length p150Glued in blot overlays, and both mutants altered the extent of endomembrane disruption (Vaughan et al., 2001). Our data indicate that that IC2-N237 affects membrane localization more strongly than IC2-FL. A mutation that strengthens the effect of a weak inhibitor (IC2-FL) might not affect a stronger inhibitor (IC2-N237). All three IC2-N237 species affected late endosomes to an equal extent, suggesting that the factors that govern the localization of this compartment are not sensitive to the phosphorylation state of IC2 at position S84. All are GFP-tagged at the N terminus, so it is clear that a large tag at this site does not completely block its effects.
Overexpression of IC2-N106 yielded two surprising results. Microtubule and centrosome organization were largely normal, and early endosomes were the only endomembrane system affected. One potential explanation for the weaker effects of this species in vivo is the absence of AA 106-237, which contains the sites for LC binding (Lo et al., 2001; Mok et al., 2001; Tai et al., 2001; Makokha et al., 2002; Susalka et al., 2002). IC2-N237 might act by depleting endogenous dynein of LCs. However, only a small amount of the LC rp3 was released in cells overexpressing IC2-N237, suggesting this is not the explanation for its more potent inhibitory effects. We believe that the weaker effects of IC2-N106 reflect its lower affinity for p150Glued relative to IC2-N237.
Implications for Dynein Function in Different Endomembrane Systems
Our results are consistent with the emerging view that cytoplasmic dynein associates with membranes via multiple, distinct linkages. We believe the differential effects of the three IC species reflect their accessibilities to and affinities for their binding sites on dynactin. Previous work from our laboratory (Quintyne et al., 1999; Valetti et al., 1999) has revealed the linkage of dynein to membrane-bound dynactin to be very robust and to potentially involve a complicated molecular footprint comprising contacts with multiple subunits. We also proposed that binding of dynactin to membranes might constrain the p150Glued projecting arm (Quintyne et al., 1999). The present data suggest that IC2-N237 binds membrane-bound dynactin readily and interferes with its binding to intact dynein. Binding may be especially favored because IC2-N237 is monomeric (our unpublished data). Full-length IC, by contrast, is considerably larger and can dimerize (King et al., 2002), which may interfere with its access to membrane-bound dynactin.
IC2-N106 is expected to associate easily with dynactin, but our in vitro data indicate it binds p150Glued with lower affinity. This may explain its weak inhibitory effects in vivo. However, the ability of IC2-N106 to selectively disperse early endosomes suggests that the mode by which dynein binds this compartment is distinct from other endomembranes. IC2-N106 cannot bind dynein LCs, suggesting that LCs are not a major component of the dynein footprint on early endosomes. It is also possible that the p150Glued arm is more accessible on early endosomes than other membranous organelles, allowing for easier interactions with IC2-N106.
As we have seen for dynactin subunits, overexpression of different dynein IC species can have distinct effects on mammalian tissue culture cells. The dynein and dynactin components that perturb cellular architecture most extensively seem to be those that are predicted to contribute directly to dynein-dynactin binding. We propose that, when present in excess, these polypeptide species titrate out free dynein or dynactin and prevent them from interacting with their binding partners. We expect that future overexpression studies will allow us to map the dynein-dynactin interaction, as well as the interactions between dynein, dynactin, and their molecular cargoes, in more detail.
Acknowledgments
We thank Drs. H.-P. Hauri (Biozentrum, Basel, Switzerland), Adam Linstedt (Carnegie Mellon University, Pittsburgh, PA), Jeff Salisbury (Mayo Clinic, Rochester, MN), Ching-Hwa Sung (Cornell University, Ithaca, NY) and Ian Trowbridge (Salk Institute, San Diego, CA), for antibodies. Drs. Larry Goldstein (University of California at San Diego) and Maggie de Cuevas (University of Maryland, College Park, MD) generously provided the kinesin stalk construct. We also thank Dr. Kevin Vaughan (University of Notre Dame, Indianapolis, IN) for advice on IC expression in bacteria. This work was supported by National Institutes of Health grants GM-44589 and DK-44375 to T.A.S.
References
- Blocker, A., Severin, F.F., Burkhardt, J.K., Bingham, J.B., Yu, H., Olivo, J.-C., Schroer, T.A., Hyman, A.A., and Griffiths, G. (1997). Molecular requirements for bi-directional movement of phagosomes along microtubules. J. Cell Biol. 137, 113-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan, K., Serr, M., and Hays, T. (2000). A molecular genetic analysis of the interaction between the cytoplasmic dynein intermediate chain and the Glued (dynactin) complex. Mol. Biol. Cell. 11, 3791-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill, 2nd, L.B., and Pfister, K.K. (2000). Biochemical and molecular analysis of the mammalian cytoplasmic dynein intermediate chain. Methods 22, 307-316. [DOI] [PubMed] [Google Scholar]
- Burkhardt, J.K., Echeverri, C.J., Nilsson, T., and Vallee, R.B. (1997). Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y.W., Jakobi, R., McGinty, A., Foschi, M., Dunn, M.J., and Sorokin, A. (2000). Cyclooxygenase 2 promotes cell survival by stimulation of dynein light chain expression and inhibition of neuronal nitric oxide synthase activity. Mol. Cell. Biol. 20, 8571-8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon, S.W., Serpinskaya, A.S., Vaughan, P.S., Lopez Fanarraga, M., Vernos, I., Vaughan, K.T., and Gelfand, V.I. (2003). Dynactin is required for bidirectional organelle transport. J. Cell Biol. 160, 297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas, M., Tao, T., and Goldstein, L.S. (1992). Evidence that the stalk of Drosophila kinesin heavy chain is an alpha-helical coiled coil. J. Cell Biol. 116, 957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, T., Ray, K., Salz, H.K., and Chia, W. (1996). Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. Mol. Cell. Biol. 16, 1966-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri, C.J., Paschal, B.M., Vaughan, K.T., and Vallee, R.B. (1996). Molecular characterization of 50kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckley, D.M., Gill, S.R., Melkonian, K.A., Bingham, J.B., Goodson, H.V., Heuser, J.E., and Schroer, T.A. (1999). Analysis of dynactin subcomplexes reveals a novel actin-related protein associated with the Arp1 minifilament pointed end. J. Cell Biol. 147, 307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckley, D.M., and Schroer, T.A. (2003). Association of Arp11, an evolutionarily conserved actin-related protein, with actin and Arp1. Mol. Biol. Cell 14, 2645-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio, T., Dionne, M.A., and Compton, D.A. (1997). Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 138, 1055-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio, T., Saredi, A., Bingham, J.B., Hasbani, J., Gill, S.R., Schroer, T.A., and Compton, D.A. (1996). Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J. Cell Biol. 135, 399-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, S.R., Cleveland, D.W., and Schroer, T.A. (1994). Characterization of DLC-A and DLC-B, two families of cytoplasmic dynein light chain subunits. Mol. Biol. Cell. 5, 645-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, S.R., Schroer, T.A., Szilak, I., Steuer, E.R., Sheetz, M.P., and Cleveland, D.W. (1991). Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 115, 1639-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann, A., Schroer, T.A., Griffiths, G., and Burkhardt, J.L. (2001). Immunolocalization of cytoplasmic dynein and dynactin subunits in cultured macrophages: enrichment on early endocytic organelles. J. Cell Sci. 114, 229-240. [DOI] [PubMed] [Google Scholar]
- Habura, A., Tikhonenko, I., Chisholm, R.L., and Koonce, M.P. (1999). Interaction mapping of a dynein heavy chain. J. Biol. Chem. 274, 15447-15453. [DOI] [PubMed] [Google Scholar]
- Holleran, E.A., Karki, S., and Holzbaur, E.L. (1998). The role of the dynactin complex in intracellular motility. Int. Rev. Cytol. 182, 69-109. [DOI] [PubMed] [Google Scholar]
- Holleran, E.A., Ligon, L.A., Tokito, M., Stankewich, M.C., Morrow, J.S., and Holzbaur, E.L.F. (2001). Beta III spectrin binds to the Arp1 subunit of dynactin. J. Biol. Chem. 276, 36598-36605. [DOI] [PubMed] [Google Scholar]
- Holleran, E.A., Tokito, M.K., Karki, S., and Holzbaur, E.L.F. (1996). Centractin (Arp1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J. Cell Biol. 135, 1815-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki, S., and Holzbaur, E.L.F. (1995). Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 270, 28806-28811. [DOI] [PubMed] [Google Scholar]
- Karki, S., LaMonte, B., and Holzbaur, E.L.F. (1998). Characterization of the p22 subunit of dynactin reveals the localization of cytoplasmic dynein and dynactin to the midbody of dividing cells. J. Cell Biol. 142, 1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki, S., and Holzbaur, E.L.F. (1999). Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 11, 45-53. [DOI] [PubMed] [Google Scholar]
- King, S.J., Bonilla, M., Rodgers, M.E., and Schroer, T.A. (2002). Subunit organization in cytoplasmic dynein subcomplexes. Prot. Sci. 11, 1239-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.J., and Schroer, T.A. (2000). Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2, 20-24. [DOI] [PubMed] [Google Scholar]
- King, S.M. (2000a). AAA domains and organization of the dynein motor unit. J. Cell Sci. 113, 2521-2526. [DOI] [PubMed] [Google Scholar]
- King, S.M. (2000b). The dynein microtubule motor. Biochim. Biophys. Acta 1496, 60-75. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Lane, J.D., Vergnolle, M.A., Woodman, P.G., and Allan, V.J. (2001). Apoptotic cleavage of cytoplasmic dynein intermediate chain and p150(Glued) stops dynein-dependent membrane motility. J. Cell Biol. 153, 1415-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt, A.D., and Hauri, H.P. (1993). Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 4, 679-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, K.W.-H., Naisbitt, S., Fan, J.S., Sheng, M., and Zhang, M. (2001). The 8 kDa dynein light chain binds to its targets via a conserved “K/R-X-T-Q-T” motif. J. Biol. Chem. 276, 14059-14066. [DOI] [PubMed] [Google Scholar]
- Lukashok, S.A., Tarassishin, L., Li, Y., and Horwitz, M.S. (2000). An adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis complexes with dynein and a small GTPase. J. Virol. 74, 4705-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum, P.L., and Schildebach, J.F. (1999). Specific DNA recognition by F factor TraY beta-sheet residues. J. Biol. Chem. 274, 19644-19648. [DOI] [PubMed] [Google Scholar]
- Ma, S., Trivinos-Lagos, L., Graf, R., and Chisholm, R.L. (1999). Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 147, 1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makokha, M., Hare, M., Li, M., Hays, T., and Barbar, E. (2002). Interactions of cytoplasmic dynein light chains Tctex-1 and LC8 with the intermediate chain IC74. Biochemistry 41, 4302-4311. [DOI] [PubMed] [Google Scholar]
- Mok, Y.-K., Lo, K.W.-H., and Zhang, M. (2001). Structure of Tctex-1 and its interaction with cytoplasmic dynein intermediate chain. J. Biol. Chem. 276, 14067-14074. [DOI] [PubMed] [Google Scholar]
- Muresan, V., Stankewich, M.C., Steffen, W., Morrow, J.S., Holzbaur, E.L., and Schnapp, B.J. (2001). Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids. Mol. Cell 7, 173-183. [DOI] [PubMed] [Google Scholar]
- Niclas, J., Allan, V.J., and Vale, R.D. (1996). Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J. Cell Biol. 133, 585-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne, N.J., Gill, S.R., Eckley, D.M., Crego, C.L., Compton, D.A., and Schroer, T.A. (1999). Dynactin is required for microtubule anchoring at fibroblast centrosomes. J. Cell Biol. 147, 321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne, N.J., and Schroer, T.A. (2002). Distinct cell-cycle dependent roles for dynactin and dynein at centrosomes. J. Cell Biol. 159, 245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury, J.L., Baron, A.T., Coling, D.E., Martindale, V.E., and Sanders, M.A. (1986). Calcium-modulated contractile proteins associated with the eucaryotic centrosome. Cell Motil. Cytoskeleton 6, 193-197. [DOI] [PubMed] [Google Scholar]
- Schafer, D.A., Gill, S.R., Cooper, J.A., Heuser, J.E., and Schroer, T.A. (1994). Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles f-actin. J. Cell Biol. 126, 403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer, T.A., and Sheetz, M.P. (1991). Two activators of microtubule-based vesicle transport. J. Cell Biol. 115, 1309-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, A., Fransen, J.A., Bachi, T., Ginsel, L., and Hauri, H.P. (1988). Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 107, 1643-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen, W., Karki, S., Vaughan, K.T., Vallee, R.B., Holzbaur, E.L., Weiss, D.G., and Kuznetsov, S.A. (1997). The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell 8, 2077-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susalka, S.J., Nikulina, K., Salata, M.W., Vaughan, P.S., King, S.M., Vaughan, K.T., and Pfister, K.K. (2002). The roadblock light chain binds a novel region of the cytoplasmic dynein intermediate chain. J. Biol. Chem. 277, 32939-32946. [DOI] [PubMed] [Google Scholar]
- Tai, A.W., Chuang, J.-Z., Bode, C., Wolfrum, U., and Sung, C.-H. (1999). Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex1. Cell 97, 877-887. [DOI] [PubMed] [Google Scholar]
- Tai, A.W., Chuang, J.-Z., and Sung, C.-H. (2001). Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J. Cell Biol. 153, 1499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Grodon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Pro. Natl. Acad. Sci. USA 76, 4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti, C., Wetzel, D.M., Schrader, M., Hasbani, M.J., Gill, S.R., Kreis, T.E., and Schroer, T.A. (1999). Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell 10, 4107-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, K.T., Tynan, S.H., Faulkner, N.E., Echeverri, C.J., and Vallee, R.B. (1999). Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends. J. Cell Sci. 112, 1437-1447. [DOI] [PubMed] [Google Scholar]
- Vaughan, K.T., and Vallee, R.B. (1995). Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol. 131, 1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, P.S., Leszyk, J.D., and Vaughan, K.T. (2001). Cytoplasmic dynein intermediate chain phosphorylation regulates binding to dynactin. J. Biol. Chem. 276, 26171-26179. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., Karki, S., and Holzbaur, E.L. (1995). The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc. Natl. Acad. Sci. USA 92, 1634-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., Karki, S.B., Kuznetsov, S.A., Tabb, J.S., Weiss, D.G., Langford, G.M., and Holzbaur, E.L. (1997). The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc. Natl. Acad. Sci. USA 94, 12180-12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S., Miller, K., Hopkins, C., and Trowbridge, I.S. (1992). Monoclonal antibodies against defined epitopes of the human transferrin receptor cytoplasmic tail. Biochim. Biophys. Acta 1136, 28-34. [DOI] [PubMed] [Google Scholar]
- Wittman, T., and Hyman, A. (1999). Recombinant p50/dynamitin as a tool to examine the role of dynactin in intracellular processes. San Diego: Academic Press, 137-143. [DOI] [PubMed]