Abstract
Objective:
To measure the cognitive consequences of incident Alzheimer disease (AD) in older African American and white subjects.
Methods:
Data are from the Chicago Health and Aging Project, a longitudinal cohort study of older white and black persons residing in a geographically defined community. At 3-year intervals, the entire study population completed 4 brief cognitive tests, from which a previously established composite measure of global cognition was derived, and a subset underwent detailed clinical evaluation that supported clinical classification of mild cognitive impairment, dementia, and AD. We used mixed-effects models to examine change in cognitive function following the diagnostic evaluation.
Results:
On clinical evaluation, 614 persons were found to have no cognitive impairment, 395 had mild cognitive impairment, and 149 had AD (88.5% mild); 10 persons with other dementias were excluded from analyses. During up to 11 years of observation following the clinical evaluation (mean = 5.5, SD = 2.5), the composite measure of global cognition declined a mean of 0.042 unit per year (SE = 0.008, p < 0.001) in those with no cognitive impairment. In comparison to the no cognitive impairment group, the annual rate of decline was increased more than twofold in mild cognitive impairment (estimate = 0.086, SE = 0.011, p < 0.001) and more than fourfold in AD (estimate = 0.173, SE = 0.020, p < 0.001). Results did not reliably vary by race, sex, or age.
Conclusions:
Alzheimer disease has a devastating impact on cognition, even in its prodromal stages, with comparable effects in African American and white persons.
GLOSSARY
- AD
= Alzheimer disease;
- MCI
= mild cognitive impairment.
Progressive decline in cognitive function is the primary clinical manifestation of Alzheimer disease (AD), and the rate at which cognition declines is robustly related to development of motor dysfunction and risk of death in affected persons.1–3 Knowledge about cognitive decline in the disease is mainly based on studies of persons evaluated in clinical settings, however. In such studies, the full spectrum of the disease is unlikely to be represented in the affected group and the comparability of the unaffected group is uncertain. As a result, it has been difficult to securely determine the cognitive consequences of the disease and to test whether they vary in racial or ethnic subgroups of the population.
The aim of the present study was to quantify rates of cognitive decline in people who developed incident AD or its precursor, mild cognitive impairment (MCI). Participants are older African American and white persons residing in a geographically defined community in Chicago. At intervals of approximately 3 years, the full population completed brief tests of cognitive function and a stratified random sample underwent detailed clinical evaluation for MCI and AD. We determined rates of cognitive decline following the clinical evaluation in those diagnosed with MCI and AD and compared them to rates in persons with no cognitive impairment. Because there are limited data on cognitive function in African American persons with AD,4–6 we also examined whether diagnostic effects differed by race.
METHODS
Participants.
The Chicago Health and Aging Project is a longitudinal study of aging and AD in a geographically defined community on the south side of the city.7–10 The area was censused beginning in 1993; persons aged 65 years or older were asked to take part in an in-home interview, and subsequently persons turning 65 were invited to participate. A stratified random sample of interviewees underwent a detailed clinical evaluation. About 3 years later, the interview of the full population was repeated and a detailed clinical evaluation was done on a stratified random sample of persons deemed free of dementia in the previous wave of data collection. These 2 elements (interview of population, clinical evaluation of persons previously deemed dementia-free) were repeated at intervals of about 3 years, with the fifth wave of data collection in progress at the time of these analyses.
Eligibility for the present analyses required a completed clinical evaluation from those previously deemed dementia-free and a valid global cognitive score from the corresponding population interview and at least one subsequent population interview. If individuals were sampled for more than one clinical evaluation, we used the earliest to maximize the follow-up period. Of 2,229 persons invited to a clinical evaluation in the second, third, or fourth waves of data collection, 168 died prior to the evaluation and 1,561 (75.7% of survivors) completed it. Another 102 people died before the next population interview, leaving 1,459 eligible for follow-up, of whom 1,168 (80.1%) had follow-up cognitive data. They had a mean age of 78.7 years (SD = 5.4) and a mean education of 13.1 years (SD = 3.5); 63.3% were women and 49.3% were African American. They were followed for a mean of 5.5 years (SD = 2.5).
Standard protocol approvals, registrations, and patient consents.
The Chicago Health and Aging Project was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all participants.
Clinical evaluation.
The clinical evaluation followed a uniform protocol that has been described in this7,8,11–13 and other14–17 cohorts. It included a structured medical history, complete neurologic examination, and administration of a battery of 20 cognitive function tests. A neuropsychologist reviewed the cognitive test results and rated impairment in 5 cognitive domains (orientation, attention, memory, language, visuospatial ability). To minimize random variability in these determinations, we developed educationally adjusted cutoff scores for 11 of the tests and an algorithm for deriving impairment ratings for the 5 cognitive domains.17 The neuropsychologist reviewed these algorithmic ratings in conjunction with scores on the remaining 9 cognitive tests, education, occupation, and sensorimotor and motivational ratings before rendering final judgments about each cognitive domain. Based on these ratings, review of all clinical data, and examination of the participant, an experienced physician diagnosed dementia and AD following the guidelines of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association.18 Dementia required a history of cognitive decline and impairment in at least 2 cognitive domains, one of which had to be memory to meet AD criteria, as reported elsewhere.7,8,10–17 Those who met AD criteria and had another condition judged to be contributing to cognitive impairment were included in primary analyses.
Individuals who had impairment in 1 or 2 cognitive domains but did not meet criteria for dementia were classified as MCI. Persons meeting these MCI criteria have been shown to have intermediate levels of mortality,13,17 cognitive decline,17,19 and plaques, tangles, and cerebral infarction20 compared to no cognitive impairment and dementia subgroups.
Cognitive function assessment.
Four brief performance tests of cognition were administered as part of each population interview. Immediate and delayed recall of 12 ideas contained in the East Boston Story assessed episodic memory.21,22 Perceptual speed was assessed with the oral version of the Symbol Digit Modalities Test.23 The Mini-Mental State Examination24 assessed global cognition. In a previous factor analysis, all 4 measures loaded on a single factor that accounted for 74% of the variability in the individual tests.25 For this reason and because of the importance of minimizing floor and ceiling artifacts in longitudinal analyses, we used a composite index of global cognition based on all 4 individual measures. As previously described, raw scores on individual measures were converted to z scores, using the mean and SD in the full population at baseline, and the z scores were averaged to yield the composite.9,10,25 It was treated as missing if more than 2 component scores were missing.
Data analysis.
We used mixed-effects models26 to characterize change in the composite measure of global cognition. All models included terms to control for the potentially confounding effects of age, sex, and education, and either adjusted for or stratified by race. Analyses focused on cognitive change in persons after they underwent clinical evaluation by multiplying each variable by study time. Persons without evidence of cognitive impairment were treated as a reference group that was contrasted with MCI and AD subgroups. To test whether cognitive trajectories associated with MCI or AD differed by race, we conducted a second analysis with terms for the interaction of race with each diagnosis and multiplied by study time. We subsequently tested for interactions by age and sex. Analyses were weighted to the full Chicago Health and Aging Project population and variances were corrected for the sample design using jackknife variance estimation.
RESULTS
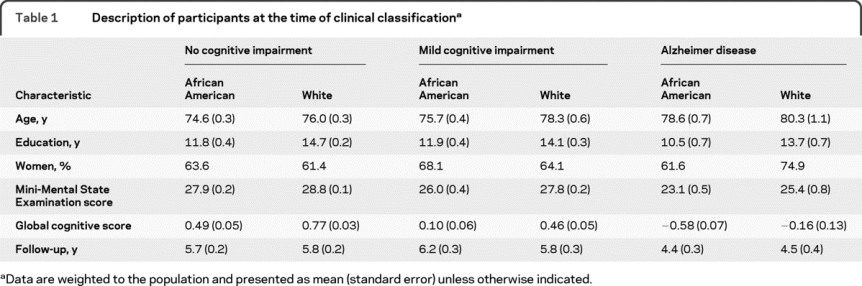
A total of 1,168 individuals completed the detailed clinical evaluation and had follow-up cognitive function data: 614 had no evidence of cognitive impairment, 395 had MCI, 149 had AD, and 10 had other forms of dementia. Because there were few other dementia cases, they were excluded from analyses. As shown in table 1, those with AD were older, less educated, and more apt to be African American than those without cognitive impairment. The level of dementia in this incident AD group was mostly mild, with 88.5% having a Mini-Mental State Examination score >17, a cutoff sometimes used to designate mild AD.27
Table 1 Description of participants at the time of clinical classification
We constructed mixed-effects models to assess change in cognitive function following the clinical evaluation. All models controlled for age, sex, education, and race. To make use of all cognitive data, the composite measure of global cognition was used in analyses. Baseline scores ranged from −3.08 to 1.48 (mean = 0.24, SD = 0.60), with higher values indicating better cognitive function.
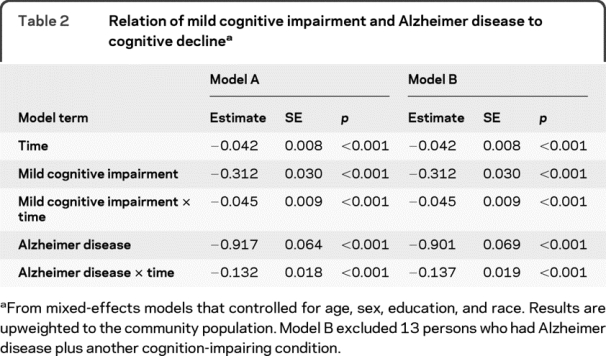
In the initial analysis, the global cognitive score declined a mean 0.042 unit per year in those without cognitive impairment, as shown by the term for time for model A in table 2. Those with MCI had a lower level of cognition at baseline than those without cognitive impairment, by definition, and they declined a mean of 0.045 unit faster per year during follow-up, a more than twofold increase. Those with AD had a much lower level of cognition at baseline than the no cognitive impairment group, and they declined a mean of 0.132 unit faster during follow-up, more than 4 times the rate of cognitive decline in the no cognitive impairment group and twice the rate in the MCI group. Thirteen people with AD had another cognition-impairing condition (11 stroke, 1 alcohol, 1 brain tumor). Excluding these individuals did not affect results (table 2, model B).
Table 2 Relation of mild cognitive impairment and Alzheimer disease to cognitive decline
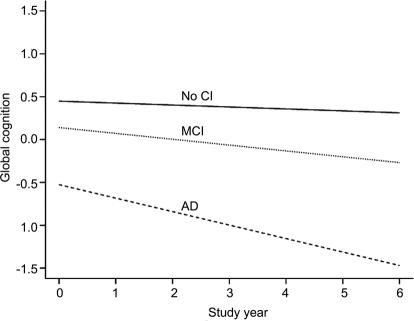
The figure shows the predicted 6-year paths of cognitive decline for each diagnostic group. Decline is negligible in the no cognitive impairment group, gradual in MCI, and substantial in AD.
Figure Cognitive decline in no cognitive impairment (CI), mild cognitive impairment (MCI), and Alzheimer disease (AD) groups
To test whether race modified the relation of diagnosis to cognitive decline, we repeated the analysis with terms added for the interactions of race with MCI and AD. The addition of these terms did not improve overall goodness of model fit, however, indicating that the cognitive consequences of MCI and AD did not reliably differ by race. In subsequent analyses, results did not vary by age or sex either.
DISCUSSION
In a biracial urban community, individuals classified as having no cognitive impairment, MCI, or AD completed brief tests of cognitive function at 3-year intervals for up to 11 years. Compared to persons found to have no cognitive impairment, rate of cognitive decline during follow-up increased approximately twofold in MCI and fourfold in AD. The differences in rate of cognitive decline between AD and MCI underscore the clinical relevance of the progression to dementia. While MCI and dementia clearly exist on a continuum, the accelerating cognitive deterioration in dementia is highly relevant to clinicians counseling patients and families with dementia.
Progressive decline of cognitive function is the primary clinical manifestation of MCI and AD, but as noted above, quantification of this key disease consequence has proven difficult. The only previous population-based study of which we are aware found, like the present study, rapid cognitive decline in AD at about twice the rate seen in a subgroup with prodromal AD, with little to no decline evident in unaffected persons.28 Similarly, in 2 previous longitudinal cohort studies, cognitive decline was increased in MCI relative to no cognitive impairment,17,19 with an approximate doubling of the rate in 1 study.17 Overall, therefore, the present results confirm in a population-based setting the deleterious cognitive consequences of AD and its precursor, MCI, and provide quantitative estimates of the size of these effects.
In this population, we found no evidence of racial disparities in the cognitive consequences of MCI or AD. This is consistent with an earlier report of comparable mortality in affected African American and white subjects from this population.13 A previous study found reduced global cognitive decline in affected black persons compared to affected white persons,5 but in 2 other studies, this effect was only observed in a subset of cognitive outcome measures.4,6 This inconsistency may be due in part to the selected groups that were studied, making it difficult to form comparable subgroups of African American and white subjects, and the small number of African American participants (<130 per study).
Age was not related to rate of cognitive decline in the incident AD group. This is consistent with previous population data.28 By contrast, studies of persons identified in clinical settings have tended to find more rapid decline in younger affected persons,22,29,30 possibly due to age-related differences in factors that bring affected people to medical attention22,30 or to the inclusion of persons under the age of 65. In addition, gender was unrelated to cognitive decline in AD, consistent with previous studies in clinical settings.31–33
This study has several strengths. Participants were sampled from a geographically defined population. Clinical classification of MCI and AD was based on a structured uniform evaluation and widely accepted criteria applied by an experienced physician. The availability of a previously established composite measure of global cognition collected at regular intervals for up to 11 years with high follow-up participation enhanced our ability to reliably quantify individual differences in cognitive decline.
Several limitations should be noted. The robust difference between black and white participants in level of cognitive function complicates racial comparisons of rate of cognitive change, making it difficult to rule out the possibility of small racial differences in cognitive decline. All analyses were based on a single global cognitive outcome. Although it was able to accommodate wide individual differences in cognitive ability, differences in measurement sensitivity across the ability spectrum could have affected results. Also, results might vary across cognitive domains, and nonlinear models might improve estimation. For these and other reasons, multiple studies will be needed to securely determine the cognitive impact of AD. Finally, including prevalent cases of MCI may have introduced error into the estimate of decline in that group because change prior to study onset is unknown.
ACKNOWLEDGMENT
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the study. They also thank Ann Marie Lane for community development and oversight of project coordination, Michelle Bos, Holly Hadden, Flavio LaMorticella, and Jennifer Tarpey for coordination of the study, Todd Beck, MS, for analytic programming, and the staff of the Rush Institute for Healthy Aging. Statistical analyses were performed by Todd Beck, MS, under supervision of Dr. Mendes de Leon and Dr. Hebert.
DISCLOSURE
Dr. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging; and receives research support from the NIH/NIA (R01AG024871 [PI], P30AG10161 [Co-I], R01AG11101 [Co-I], R01AG15819 [Co-I], R01AG021972 [Co-I], U24AG026395 [Co-I], R01AG017917 [Co-I], R01AG009966 [Co-I], and U01AG016979 [Co-I]) and from the NIH/NIEHS (ES10902 [Co-I]). Dr. Aggarwal has served on a scientific advisory board for Pfizer Inc; and receives research support from the NIH (R01AG022018 [Co-I], P30AG010161 [Clinical Core Leader], R01AG011101 [Examining Neurologist], R01AG009966 [Examining Neurologist], R01HL084209 [Co-I], and R01AG032247 [Co-I]). Dr. Barnes serves on the editorial board of the Journal of Aging and Health; and receives research support from the NIH (R01AG022018 [PI], R01NR009543 [Neuropsychologist], P30AG010161 [Co-I], R01ES010902 [Co-I], R01AG031553 [Neuropsychologist], and R01AG032247 [Co-I]), and from the Alzheimer's Association. Dr. Mendes de Leon serves as an Associate Editor of the Journals of Gerontology Social Sciences, and on the editorial boards of Psychosomatic Medicine, the Journal of Aging and Health, the International Journal of Behavioral Medicine, and Archives of Internal Medicine; and receives research support from the NIH (R01 ES010902 [PI], R01 AG032247 [PI], R01 AG011101 [Co-I], R01 HL084209 [Co-I], AG 022018 [Co-I], and NIH AG 033172 [Co-I]). Dr. Hebert receives research support from the NIH (R01 AG009966 [Biostatistician], R03 AG029652 [PI], R01 AG030544 [Biostatistician], and R01 NR010211 [PI]). Dr. Evans served on a Data Monitoring Committee for Eli Lily and Company; and receives research support from the NIH (AG11101 [PI], AG09966 [PI], AG030146 [PI], AG10161 [Co-I], AG021972 [Co-I], ES10902 [Co-I], NR009543 [Co-I], HL084209 [Co-I], AG036650 [PI], and AG12505 [Co-I]).
Address correspondence and reprint requests to Dr. Robert S. Wilson, Rush AD Center, Rush University Medical Center, 600 South Paulina Avenue, Suite 1038, Chicago, IL 60612 rwilson@rush.edu
Editorial, page 942
Study funding: Supported by the NIH (NIA R01AG 11101, NIA P30AG10161, and NIEHS ES10902), which had no role in the design and conduct of the study; in the collection management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Disclosure: Author disclosures are provided at the end of the article.
Received July 21, 2009. Accepted in final form November 16, 2009.
REFERENCES
- 1.Wilson RS, Bennett DA, Gilley DW, Beckett LA, Schneider JA, Evans DA. Progression of parkinsonism and loss of cognitive function in Alzheimer's disease. Arch Neurol 2000;57:855–860. [DOI] [PubMed] [Google Scholar]
- 2.Hui JS, Wilson RS, Bennett DA, Bienias JL, Gilley DW, Evans DA. Rate of cognitive decline and mortality in Alzheimer's disease. Neurology 2003;61:1356–1361. [DOI] [PubMed] [Google Scholar]
- 3.Wison RS, Li Y, Aggarwal NT, et al. Cognitive decline and survival in AD. Int J Geriatr Psychiatry 2006;21:356–362. [DOI] [PubMed] [Google Scholar]
- 4.Fillenbaum GG, Peterson B, Welsh-Bohmer KA, Kukull WA, Heyman A. Progression of Alzheimer's disease in black and white patients: the CERAD experience, part XVI. Consortium to Establish a Registry for Alzheimer's Disease. Neurology 1998;51:154–158. [DOI] [PubMed] [Google Scholar]
- 5.Barnes LL, Wilson RS, Li Y, et al. Racial differences in the progression of cognitive decline in Alzheimer's disease. Am J Geriatr Psychiatry 2005;13:959–967. [DOI] [PubMed] [Google Scholar]
- 6.Barnes LL, Wilson RS, Li Y, Gilley DW, Bennett DA, Evans DA. Change in cognitive function in Alzheimer's disease in African-American and white persons. Neuroepidemiology 2006;26:16–22. [DOI] [PubMed] [Google Scholar]
- 7.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer's disease in a biracial urban community: relation to apolipoprotein allele status. Arch Neurol 2003;60:185–189. [DOI] [PubMed] [Google Scholar]
- 8.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer's disease in a biracial community study. JAMA 2002;287:3230–3237. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003;61:812–816. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002;59:1910–1914. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RS, Barnes LL, Bennett DA, et al. Proneness to psychological distress and risk of Alzheimer's disease in a biracial community. Neurology 2005;64:380–383. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and AD. Arch Neurol 2009;66:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging Neuropsychol Cogn 2004;11:280–303. [Google Scholar]
- 15.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005;25:163–175. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006;27:169–176. [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer's disease and rate of cognitive decline. Neurology 2006;67:441–445. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Mild cognitive impairment is related to Alzheimer's disease pathology and cerebral infarctions. Neurology 2005;64:834–841. [DOI] [PubMed] [Google Scholar]
- 21.Albert MS, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci 1991;57:167–178. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–193. [PubMed] [Google Scholar]
- 23.Smith A. Symbol Digit Modalities Test manual–revised. Los Angeles, CA: Western Psychological Press; 1982. [Google Scholar]
- 24.Folstein MF, Folstein S, McHugh PR. Mini-Mental State: a practical method for grading the mental state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined community. J Gerontol Psychol Sci 1999;54B:P155–P160. [DOI] [PubMed] [Google Scholar]
- 26.Laird N, Ware J. Random-effects models for longitudinal data. Biometrics 1982;36:963–973. [PubMed] [Google Scholar]
- 27.Herbert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer's disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003;60:1119–1122. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer's disease. Arch Neurol 1999;56:1274–1279. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs D, Sano M, Marder K, et al. Age at onset of Alzheimer's disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology 1994;44:1215–1220. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Li Y, Aggarwal NT, et al. Education and the course of cognitive decline in Alzheimer's disease. Neurology 2004;63:1198–1202. [DOI] [PubMed] [Google Scholar]
- 31.Hebert LE, Wilson RS, Gilley DW, et al. Decline of language among women and men with Alzheimer's disease. J Gerontol: Psychol Sci 2000;55B:P1–P8. [DOI] [PubMed] [Google Scholar]
- 32.Mortimer J, Ebbit B, Jun S, Finch D. Predictors of cognitive and functional progression in patients with probable Alzheimer's disease. Neurology 1992;42:1689–1696. [DOI] [PubMed] [Google Scholar]
- 33.Stern Y, Albert M, Brandt J, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission and death in Alzheimer's disease: prospective analyses from the predictor study. Neurology 1994;44:2300–2307. [DOI] [PubMed] [Google Scholar]