Abstract
Modern acute ischemic stroke therapy is based on the premise that recanalization and subsequent reperfusion are essential for the preservation of brain tissue and favorable clinical outcomes. We outline key issues that we think underlie equipoise regarding the comparative clinical efficacy of IV recombinant tissue-type plasminogen activator (rt-PA) and intra-arterial (IA) reperfusion therapies for acute ischemic stroke. On the one hand, IV rt-PA therapy has the benefit of speed with presumed lower rates of recanalization of large artery occlusions as compared to IA methods. More recent reports of major arterial occlusions treated with IV rt-PA, as measured by transcranial Doppler and magnetic resonance angiography, demonstrate higher rates of recanalization. Conversely, IA therapies report higher recanalization rates, but are hampered by procedural delays and risks, even failing to be applied at all in occasional patients where time to reperfusion remains a critical factor. Higher rates of recanalization in IA trials using clot-removal devices have not translated into improved patient functional outcome as compared to trials of IV therapy. Combined IV-IA therapy promises to offer advantages of both, but perhaps only when applied in the timeliest of fashions, compared to IV therapy alone. Where equipoise exists, randomizing subjects to either IV rt-PA therapy or IV therapy followed by IA intervention, while incorporating new interventions into the study design, is a rational and appropriate research approach.
GLOSSARY
- IA
= intra-arterial;
- IMS
= Interventional Management of Stroke;
- MCA
= middle cerebral artery;
- rt-PA
= recombinant tissue-type plasminogen activator.
Uncertainty exists in the optimal approach to reperfusion for the treatment of acute ischemic stroke. Equipoise exists where there are 2 or more competing possible treatment paradigms and there is a lack of definitive evidence upon which to make the best choice for a particular patient. Equipoise can exist within the larger community or community of treating investigators and clinicians as well as the level of the individual clinician or investigator evaluating a given patient. Our purpose is to outline the key issues that we think underlie equipoise regarding the comparative clinical efficacy of IV recombinant tissue-type plasminogen activator (rt-PA) and intra-arterial (IA) reperfusion therapies for acute ischemic stroke.
Modern acute ischemic stroke therapy is based on the premise that recanalization and subsequent reperfusion are essential for the preservation of brain tissue and favorable clinical outcomes. This premise is not absolute. A minority of patients with robust leptomeningeal collateral circulation will have minimal damage and sustain excellent clinical recovery without recanalization. However, a large majority of ischemic stroke patients will have an improved outcome only with recanalization and reperfusion. Importantly, akin to the coronary “no-reflow” phenomenon, recanalization (restoration of flow at the site of arterial occlusion) may occur without adequate angiographic reperfusion (restoration of flow to the distal arterial bed of the arterial occlusion) or tissue reperfusion. Clear distinction between recanalization and reperfusion is lacking in some clinical trials and case series, particularly when angiographic films have not been read by a central reader with clearly defined methodology. Moreover, despite both recanalization and reperfusion, brain tissue may have already become irreversibly injured due to the duration and depth of ischemia. Reperfusion may be harmful after stroke due to a whole series of biochemical processes gathered under the rubrics of reperfusion injury and reperfusion hemorrhage. Finally, drugs, contrast agents, and procedures used to induce recanalization and reperfusion are sometimes associated with harm. Thus, a higher rate of recanalization or reperfusion of a given therapy is not proof of its clinical efficacy.
To address the issue of equipoise, we review the data regarding recanalization and reperfusion rates, risks of IA therapy, risk of IV therapy, the role of penumbral imaging in patient selection, and our conclusions based upon these data.
RECANALIZATION RATES
Small doses of IV rt-PA were used in many patients in all of the pilot dose-escalation studies which used IA angiography to assess recanalization. Mori et al.1 reported an on-the-table recanalization rate of approximately 47% for 19 arteriographically demonstrated anterior circulation occlusions after a 1-hour infusion of 40–60 mg duteplase (a dual-chain rt-PA) within 6 hours of stroke onset. The t-PA Acute Stroke Study Group evaluated patients with cerebral angiography and treated those with arterial occlusive lesion within 8 hours of acute stroke by infusing variable doses of IV duteplase (without bolus) for 1 hour. On-the-table recanalization was evaluated with a repeat angiogram after the 1-hour infusion. The recanalization rates, frequently incomplete, are listed in table 1.2,3
Table 1 Summary of recanalization rates obtained with IV administration of recombinant tissue-type plasminogen activator by time of assessment posttreatment
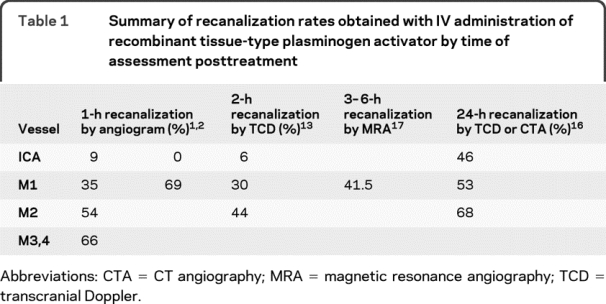
The recanalization response of middle cerebral artery (MCA) occlusions to IV alteplase (a single-chain rt-PA) in the National Institute of Neurologic Disorders dose-escalation pilot trial4,5 was likewise incomplete. Fourteen of 18 subjects in the National Institute of Neurological Disorders and Stroke pilot trial with proximal MCA occlusions (based on a hyperdense arterial sign on CT) had cerebral angiograms within 2 days of IV therapy. Nine of the 14 had evidence of an occlusion of the M1 portion of the MCA at angiography and the remaining 7 had persisting occlusion of more distal MCA branches indicating incomplete recanalization of large-vessel occlusions after IV rt-PA at varying doses.6 Caution is required when interpreting the preceding data regarding recanalization associated with IV t-PA since many patients were treated with very low doses of t-PA per the dose-escalation design and because of angiographic selection bias.
In the subsequent randomized National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial that compared 0.9 mg/kg rt-PA to placebo, routine angiography was not performed. Of the 79 patients with hyperdense middle cerebral artery sign on the baseline CT scan, a specific but insensitive diagnostic sign of middle cerebral artery occlusion, resolution of the sign at 24 hours was more common among the 37 rt-PA-treated cases (38%) than the 42 placebo cases (17%; p = 0.03). Among rt-PA-treated patients, 24-hour infarct volumes were smaller in those with resolution of the sign (p = 0.004).7
Studies utilizing TCD to monitor recanalization have reported better rates of up to 70% with standard-dose IV rt-PA.8–13 The discrepancy between angiograms obtained on the table after 1 hour of rt-PA infusion and transcranial ultrasound data obtained 2–3 hours after infusion points to a continued benefit of rt-PA bound to fibrin at the site of occlusion beyond its 5- to 8-minute serum half-life. This prolonged thrombolytic effect is also indicated by animal/preclinical studies suggesting that rt-PA exerts its biologic activity for up to 6 hours.14,15 In fact, the recanalization data from TCD ultrasound IV rt-PA studies performed at 6 hours closely parallel recanalization effects reported with IA thrombolytic therapy. One phase 4 study performed a late CT angiography or TCD follow-up examination 24 hours after IV thrombolysis in 64 patients with documented occlusion of the intracranial ICA or MCA. Complete recanalization was achieved in 36 of the 64 patients (56.3%). There was a nonsignificant trend for recanalization rates to decline with more proximal sites of occlusion: 68.4% (M2 MCA), 53.1% (M1 MCA), and 46.2% (internal carotid terminus) (p = 0.28).16 The DEFUSE Study investigators reported complete recanalization in 37.5% of 24 subjects with M1 occlusions as imaged by MRA at 3–6 hours after initiation of IV t-PA with additional partial recanalization in 4%.17 Of the 15 subjects with an ICA and M1 occlusion, 13% had complete and 28.5% had partial recanalization.
With the concern that IV rt-PA may be suboptimally effective for recanalization of moderate to severe strokes, combined reduced-dose IV rt-PA, followed by arteriography and potential IA rt-PA, has been studied.18–21 Enthusiasm for combined therapy is rooted in a local registry where subjects were treated with IV rt-PA at a median time of 126 minutes followed by IA rt-PA at median time of 210 minutes. Of the 54 subjects, 56% achieved a modified Rankin score 0–2.22
The subsequent IMS I and II multicenter IV-IA trials failed to achieve as timely IA treatment; the median time to treatment was 140 minutes in IMS I and II studies as compared to the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial treatment time of 90 minutes.23 However, in those patients treated with combined therapy where the IA component of therapy was begun within 3 hours, the combined approach resulted in better outcomes than historical subjects of similar age and severity treated with rt-PA in the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial (figure 1).22 For those subjects treated with a combined approach in which IA therapy was started at 3–5 hours from onset, the combined approach resulted in similar outcomes to comparable historical rt-PA subjects from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial who were treated within 3 hours of onset. Given the strong relationship between time to treatment and effectiveness of rt-PA,24,25 similar outcomes, despite this substantial difference in time to start of IV therapy, may argue for improved and more rapid effectiveness of the combined IV-IA approach as compared to IV rt-PA alone.

Figure 1 Rankin outcomes by time to IA therapy
mRankin 0–2 outcomes according to time to IA therapy (patterned bars) are depicted for a pre-IMS registry, IMS I, and IMS II. For subjects with NIH Stroke Scale score 10 or greater who were less than 81 years old, and who were treated intra-arterially after 3 hours, mRankin 0–2 outcomes in multicenter IV-IA trials have not differed significantly from those achieved in patients of similar age and stroke severity treated with IV rt-PA within 3 hours in the NINDS rt-PA trial (depicted as white bar). IA = intra-arterial; IMS = Interventional Management of Stroke; mRankin = modified Rankin score; NINDS = National Institute of Neurological Disorders and Stroke; rt-PA = recombinant tissue-type plasminogen activator.
It remains to be seen whether IA therapies alone started at 5 to 8 hours from onset will lead to better outcomes compared to optimized IV t-PA or combined IV-IA therapies at less than 3 hours, or even compared to placebo beyond 4.5 hours. Trials of IA devices and/or lytic agents for angiographic occlusions of the ICA and middle cerebral arteries report TIMI II/III reperfusion rates of 48%–83% depending upon the application of the definition of reperfusion and the population of subjects in whom the device was considered used.26–30 These reperfusion rates are generally higher than the recanalization rates reported in trials of IV rt-PA in subjects with similar location of occlusions. However, the proportion of subjects with a Rankin of 0–2 at 3 months is generally lower in these IA trials than the IV t-PA trials where subjects with a middle cerebral artery occlusion documented by transcranial Doppler were treated within 3 hours of onset.8 This discrepancy between recanalization rates and clinical outcome in controlled trials re-emphasizes that superior clinical efficacy and safety, not superior recanalization per se, is the goal of clinical trials and subsequent clinical practice.
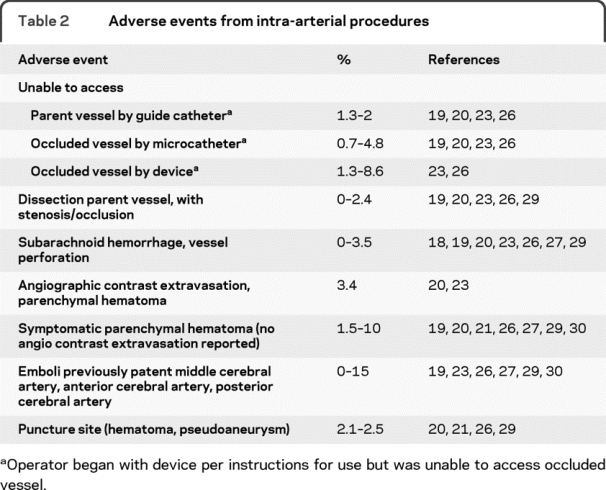
Given the clear importance of time to recanalization in the randomized IV t-PA trials and the nonrandomized IMS I and II Trials, one might argue for vascular imaging to target and treat patients with large artery occlusions by more aggressive IA methods rather than IV t-PA. It would be difficult to argue otherwise, if patients could be confidently treated at comparable time windows in the course of their disease process, and if complications of IA methods did not reduce good outcomes. These caveats are not merely theoretical, however, since delays to treatment and increased complications are inherent in IA methods (table 2). Ongoing improvements in device design may improve access to occluded arteries.
Table 2 Adverse events from intra-arterial procedures
A further consideration is that IV rt-PA may demonstrate sufficient recanalization and safety for distal occlusions such as M2 and beyond, while combined IV-IA rt-PA may be superior for more proximal occlusions as long as angiographic suite complications are limited. The Interventional Management of Stroke (IMS) III Trial will assess this through stratified analysis by location since a significant proportion of patients are undergoing baseline CT angiography prior to enrollment.31
RISKS OF IA THERAPEUTIC APPROACHES
The inability to catheterize brachiocephalic vessels has occurred in all studies, in which even very experienced angiographers encounter delays, or even failure to reach the target intracranial vascular lesion. Failure to traverse totally occluded proximal vessels due to internal carotid origin stenosis or ICA dissections, or failure to navigate proximal vessel tortuosity, occurs in all IA trials (table 2). Failures to pass guide microcatheters or recanalization devices have been encountered variably as well. Where delays occur, the likelihood of good outcome may be substantially reduced.28 Among patients who are unsuccessfully treated with IA therapy as a first step, this could lead not only to delay of an effective treatment but inability to treat with IV rt-PA within an approved time window.
Significant complications of recanalization procedures also occur with all devices (table 2). Dissection of the catheterized artery occurs more commonly with large balloon-guide catheters, and may be a limitation to procedural success. Overcoming occlusive and embolic effects of dissection with vessel stenting then adds additional hemorrhage risk associated with the antiplatelet drug requirements for stents. Perforation of occluded intracranial vessels by a guidewire or catheter or mechanical thrombectomy device may occur, though not necessarily creating catastrophic hemorrhage after IV therapy if there is no flow and perforation occurs prior to IA lytic and anticoagulant administration. Rupture of small vessels by pressure from IA fluid or contrast injection may lead to large parenchymal hematomas. Symptomatic hemorrhage rates may be slightly higher with IA therapies compared to IV alone where similar definitions of symptomatic hemorrhage have been used but there is no evidence that that IA therapy is safer than IV t-PA therapy with respect to symptomatic ICH. Parenchymal hematomas due to IV or IA reperfusion therapies are frequently symptomatic with up to a 50% mortality rate.21,24,26,27,29,30,32,33
New emboli in previously uninvolved vessels during IA procedures have received little attention. None were reported in PROACT II. Operators reported 3 instances of new embolus in the uninvolved ACA circulation during MCA revascularization in the MERCI Trial, suggesting they are uncommon with clot removal devices of that type.34 Review of angiograms by a central reading laboratory was not performed, and new emboli distal to the primary occlusion were not considered to be a previously uninvolved territory. New emboli in the A2 segment, or beyond, of the anterior cerebral artery were detected by a core reading laboratory in 3 subjects (15%) with internal carotid terminus occlusion in the IMS I and II trials, for whom opposite carotid injections verified prior intact flow with corresponding infarctions in the ACA territory in 2 of the 3 subjects.35
Contrast agents injected during IA procedures are expected to have an acceptable safety profile in general use, but their safe use in IA procedures has been questioned. Local microcatheter injections directly into or beyond occlusive thrombus following IV rt-PA are independently associated with increased ICH.36 In a second registry cohort of IV-IA and IA rt-PA alone cases, a similar relationship between microcatheter injections and both ICH and parenchymal hematoma rates has been demonstrated.37 The potential for deleterious effects of contrast on a damaged blood–brain barrier are poorly understood. Others have questioned the effects of cumulative contrast effects, including IV contrast prior to thrombolysis for baseline CT angiograms.38 Increased ICH after contrast administration in a rat MCA reperfusion model has been suggested in preliminary observations (Aigang Lu, personal communication, 2007). Less well-studied in stroke is the concept that IV contrast may negatively interact with rt-PA. Small studies in the cardiac literature suggest a possible reduced rate of thrombolysis when contrast media are combined with rt-PA.39,40
RISKS OF IV THERAPIES
IV rt-PA administration, even at reduced dose, has been associated with a symptomatic ICH rate of approximately 6% for patients treated in less than 3 hours.33 Nonrandomized trials of devices have reported similar or minimally higher rates of symptomatic ICH as randomized trials of IV rt-PA. Furthermore, thrombolytic therapy is often used to clean up residual clot or fragmented emboli in these device trials.27,34
ROLE OF PENUMBRAL IMAGING IN PATIENT SELECTION
Selection of candidates for IV or IA treatment based on imaging studies is an attractive but unproven hypothesis.41,42 The concept of MRI and CT imaging mismatch to define penumbral tissue has limitations at this time. These limitations include 1) no agreed-upon standard for perfusion imaging and marked variability in the extent of mismatch depending upon changing a key perfusion variable; 2) poor correlation with the ischemic penumbra as documented by misery-perfusion on positron emission tomographic imaging; and 3) the observation that diffusion-positive lesions do not always represent the core of irreversibly injured brain tissue.43 Testing the hypothesis that selection of patients by MRI for a revascularization procedure is associated with an improved clinical outcome is one of the primary goals of the ongoing MR Rescue Study.28
The extent of early ischemic changes, a marker of irreversible tissue injury, seen on noncontrast CT may also be critical to patient selection. Using the ASPECTS semiquantitative scale for early ischemic changes, we compared the IMS I patient cohort to historical National Institute of Neurological Disorders and Stroke tPA Stroke Study patients and demonstrated that patients with favorable baseline CT scans (ASPECTS >7) do particularly well (13% effect size) with IV-IA therapy compared to IV therapy alone, as measured by a modified Rankin score of 0–2 (figure 2).44,45 These data provide confirmatory evidence for a similar post hoc finding in the PROACT-2 study.45 This finding will be prospectively validated in the IMS III trial.
Figure 2 Rankin outcomes in IMS and NINDS trials by ASPECTS score
Ninety-day mRS in IMS I (IV-IA tPA) subjects with a baseline ASPECTS Score greater than 7. Comparison groups are NINDS rt-PA Stroke Trial subjects treated with rt-PA and placebo who also had baseline ASPECTS score >7. IA = intra-arterial; IMS = Interventional Management of Stroke; mRS = modified Rankin score; NINDS = National Institute of Neurological Disorders and Stroke; tPA = tissue-type plasminogen activator.
DISCUSSION
We must be committed to treating patients as rapidly and as safely as possible. On the one hand, IV rt-PA therapy has the benefit of speed with presumed lower rates of recanalization of large artery occlusions as compared to IA methods. Conversely, IA therapies report higher recanalization rates, but are inherently much slower in application, even failing to be applied at all in occasional patients, where time to reperfusion remains a critical factor. IA procedures are hampered by procedural delays and risks. Combined IV-IA therapy promises to offer advantages of both, but perhaps only when applied in the timeliest of fashions, compared to IV therapy alone. Even if IA approaches, with or without IV t-PA, are demonstrated to be more efficacious than IV t-PA for subgroups of acute stroke patients, the translation of this efficacy into clinical practice will likely be challenging, as seen with slow adoption of IV t-PA following its Food and Drug Administration approval.
Do current data lead to clinical equipoise regarding therapeutic decisions between IV, IA, and combined recanalization therapies for ischemic stroke patients eligible for these therapies? Or do the data clearly allow for patient-specific decision-making and therapy application at present? We think that the answer to the first question is yes and the answer to the second is no. And where clarity is lacking, equipoise exists. Where equipoise exists, randomizing subjects to either IV rt-PA therapy or IV therapy followed by IA intervention, while incorporating new interventions into the study design, is a rational and appropriate research approach.
COINVESTIGATORS
Coinvestigators who are members of the Executive Committee but are not authors: Janice A. Carrozzella, RN, University of Cincinnati; Karla J. Ryckborst, RN, University of Calgary; Edward C. Jauch, MD, Medical University of South Carolina; Yuko Y. Palesch, PhD, Medical University of South Carolina; Patrick D. Mauldin, PhD, Medical University of South Carolina; Richard W. Leinster, Medical University of South Carolina; Bernard Yan, MD, Royal Melbourne Hospital, Australia; Craig Anderson, MD, Royal Prince Alfred Hospital, Australia; Scott Janis, PhD, NIH/National Institute of Neurological Disorders and Stroke; Kevin MacDonald, Regulatory Consultant. IMS III Executive Committee members: Joseph P. Broderick, MD, Executive Committee Chair, University of Cincinnati; Thomas A. Tomsick, MD, University of Cincinnati; Pooja Khatri, MD, University of Cincinnati; Judith A. Spilker, RN, University of Cincinnati; Janice A. Carrozzella, RN, University of Cincinnati; Michael D. Hill, MD, University of Calgary; Andrew M. Demchuk, MD, University of Calgary; Karla J. Ryckborst, RN, University of Calgary; Edward C. Jauch, MD, Medical University of South Carolina; Yuko Y. Palesch, PhD, Medical University of South Carolina; Patrick D. Mauldin, PhD, Medical University of South Carolina; Richard W. Leinster, Medical University of South Carolina; Bernard Yan, MD, Royal Melbourne Hospital, Australia; Craig Anderson, MD, Royal Prince Alfred Hospital, Australia; Scott Janis, PhD, NIH/National Institute of Neurological Disorders and Stroke; Kevin MacDonald, Regulatory Consultant.
DISCLOSURE
Dr. Tomsick has received honorarium from Genentech as consultant; serves on the editorial boards of American Journal of Neuroradiology and Stroke; performs stroke treatment in office; received research funding from Boston Scientific-American Society of Neuroradiology. Dr. Khatri is the Internal Medical Monitor for the Interventional Management of Stroke (IMS) III trial; receives royalties from The Stroke Center Handbook (Taylor & Francis); received honoraria from AAN as author for Continuum; receives research funding from NIH K23 (PI). Dr. Jovin served on scientific advisory boards for Ev3 Inc., Concentric Medical Inc., and Co-Axia Inc; served as an associate editor for the Journal of Neuroimaging. Dr. Demaerschalk served as a scientific advisory board member for Genentech; served on the advisory board for Education and Lytics; received funding for travel from Otsuka, Medting, and InTouch Health; serves as the Editor-in-Chief of Journal of Brain Disease; serves on the editorial advisory board for Stroke, Hospital Practice, Open Critical Care Medicine Journal, Journal of Stroke and Cerebrovascular Diseases; serves as a section editor for The Neurologist; receives research funding from AGA Medical Corporation (RESPECT [site PI]); Vernalis (VASTT [site PI]); Neurobiological Technologies (ASP trial [site PI]); Mitsubishi (site PI); Abbott (CHOICE and ACT I [Coinvestigator]); Penumbra (PICS [Coinvestigator]); Vernalis (IN-STEP [DSMB member]); serves as an event adjudicator for Axio; serves as a medical monitor for Neuralieve; receives research funding from Arizona Department of Health Services (STRokE DOC, AZ TIME [PI], STARR [PI]); NINDS (IMS III [site PI], SAMMPRIS [site PI], SPS3 [site PI], CREST [site PI], ALIAS II [Coinvestigator]). Dr. Malisch served on the scientific advisory boards of Genentech, Inc., and Mindframe. He has significant ownership interest in Mindframe and Boston Scientific; Dr. Malisch's institution (Alexian Brothers Medical Center) was reimbursed for participation in the following clinical trials: Concentric Medical (MERCI Registry [site PI]); Penumbra (PICS Registry [site PI]), START Trial (site PI); Micrus Endovascular (VISSIT Trial [site PI]); received research funding from NIH (IMS III [site PI]). Dr. Demchuk serves on scientific advisory boards for Sanofi-Aventis and Boehringer Ingelheim; received travel funding from Sanofi-Aventis and Boehringer Ingelheim; serves on the steering committee for the Ancrod Stroke Trials (ASP-1 and ASP-2); serves on the Executive Committee of the IMS-3 trial; serves as an editorial board member for Journal of Neuroimaging and Stroke; received honoraria from Boehringer Ingelheim, Sanofi Aventis, and Astra Zeneca for speaking engagements; receives research funding from the Heart and Stroke Foundation of Alberta/NWT/Nunavut (VISION-2 study), Canadian Institutes of Health Research (VISION-1 study), and NovoNordisk Canada (PREDICT study [PI]); received research funding from NIH (IMS III [site PI and CT core lab PI]). Dr. Hill served on the scientific advisory boards of Vernalis Group Ltd. and Genentech Ltd., Protola Therapeutics, Stem Cell Therapeutics, and Sanofi-Aventis; serves on the editorial boards of Stroke, Canadian Journal of Neurological Sciences, and Stroke Rounds; has received honoraria from Boehringer Ingelheim, Hoffmann-La Roche Canada, Sanofi (Canada); serves on the Board of Directors of the Heart & Stroke Foundation Alberta/NWT/NU; received research funding from NINDS for the ALIAS trial as co-PI and for the IMS-3 trial as a member of the trial executive and for the Canadian coordinating center, CIHR for the CATCH trial, as Coinvestigator; Heart & Stroke Foundation Alberta/NWT/NU for the TWIST trial as PI and as the Heart & Stroke Professor of Stroke Research; Canadian Stroke Network for the FASTER-2 trial; received research funding from Hoffmann-La Roche Canada for the TWIST trial; Bayer Canada for personnel funding for the ALIAS trial; and Merck Canada provided drug-in-kind for the FASTER trial in which Dr. Hill was a Coinvestigator; serves on the executive committee of NIH (IMS III) trial. Dr. Jauch has served on the scientific advisory board of Genentech, Inc. (all consulting fees and honoraria are placed in an education/research fund in Dr. Jauch's department of his institution); and has received research funding from NIH/NINDS (U01 NS052220 [serves on the executive committee and is a site investigator]). Ms. Spilker has been a speaker for Genentech, Inc.; served as a consultant on advisory boards for the National Stroke Association; has received research funding from the NINDS (U01-NS052220 [Project manager]). Dr. Broderick has served on scientific advisory boards for Johnson & Johnson and Wyeth Pharmaceuticals; has received non-industry-sponsored funding for travel; holds US Patent 7,135,305, issued November 15, 2006, and a closely related patent in 2007; has received honoraria from Genentech, Hoffman-LaRoche Ltd., Boehringer-Ingelheim, and Wyeth; has received consulting fees from Novo and Genentech (all consulting fees and honoraria are placed in an education/research fund in Dr. Broderick's department of his institution); has received research support in the form of materials from Genentech, NovoNordisk, Schering Plough, Concentric, EKOS, and Johnson & Johnson; and receives research support from the NIH (NINDS U01 NS052220 [PI]; NINDS P50 NS44283 [PI]; NINDS R01 NS39512 [Coinvestigator]; and NINDS R01 NS36695 [Coinvestigator]).
Address correspondence and reprint requests to Dr. Joseph P. Broderick, Department of Neurology, UC Neuroscience Institute, University of Cincinnati Academic Health Center, 260 Stetson St, Suite 2300, PO Box 670525, Cincinnati, OH 45267-0525 joseph.broderick@uc.edu
Study funding: IMS III is funded by NINDS (U01 NS052220 and U01 NS054630). The trial receives rt-PA from Genentech, and devices from Concentric, EKOS, Penumbra, and Johnson & Johnson.
Disclosure: Author disclosures are provided at the end of the article.
Received September 29, 2009. Accepted in final form January 12, 2010.
REFERENCES
- 1.Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992;42:976–982. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo G, Poeck K, Pessin M, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–85. [DOI] [PubMed] [Google Scholar]
- 3.Wolpert S, Bruckmann H, Greenlee R, Wechsler L, Pessin M, del Zoppo G, rt-PA Acute Stroke Study Group. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 4.Brott T, Haley E, Jr., Levy D, et al. Urgent therapy for stroke: II: pilot study of tissue plasminogen activator administered within 90 minutes from onset. Stroke 1992;23:632–640. [DOI] [PubMed] [Google Scholar]
- 5.Haley E, Levy D, Brott T, et al. Urgent therapy for stroke. Part II. Pilot study of tissue plasminogen activator administered 91–180 minutes from onset. Stroke 1992;23:641–645. [DOI] [PubMed] [Google Scholar]
- 6.Tomsick T, Brott T, Barsan W, et al. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. AJNR Am J Neuroradiol 1996;17:79–85. [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols C, Khoury J, Brott T, Broderick J. Intravenous recombinant tissue plasminogen activator improves arterial recanalization rates and reduces infarct volumes in patients with hyperdense artery sign on baseline computed tomography. J Stroke Cerebrovasc Dis 2008;17:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandrov AV, Molina CA, Grotta JC, et al, CLOTBUST Investigators. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004;351:2170–2178. [DOI] [PubMed] [Google Scholar]
- 9.Demchuk AM, Burgin WS, Christou I, et al. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke 2001;32:89–93. [DOI] [PubMed] [Google Scholar]
- 10.Alexandrov AV, Demchuk AM, Felberg RA, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial Doppler monitoring. Stroke 2000;31:610–614. [DOI] [PubMed] [Google Scholar]
- 11.Molina CA, Alvarez-Sabin J, Montaner J, et al. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke 2002;33:1551–1556. [DOI] [PubMed] [Google Scholar]
- 12.von Kummer R, Meyding-Lamade U, Forsting M, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol 1994;15:9–15; discussion 16–8. [PMC free article] [PubMed]
- 13.Saqqur M, Uchino K, Demchuk AM, et al, CLOTBUST Investigators. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007;38:948–954. [DOI] [PubMed] [Google Scholar]
- 14.Maizel A, Bookstein J. Pharmacokinetics, relative advantages, and methods for maximizing rates and consistency of lysis. Cardiovasc Intervent Radiol 1986;9:236–244. [DOI] [PubMed] [Google Scholar]
- 15.Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation 1997;96:761–768. [DOI] [PubMed] [Google Scholar]
- 16.Zangerle A, Kiechl S, Spiegel M, et al. Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology 2007;68:39–44. [DOI] [PubMed] [Google Scholar]
- 17.Marks MP, Olivot JM, Kemp S, et al, DEFUSE investigators. Patients with acute stroke treated with intravenous tPA 3–6 hours after stroke onset: correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology 2008;249:614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598–2605. [DOI] [PubMed] [Google Scholar]
- 19.Flaherty ML, Woo D, Kissela B, et al. Combined IV and intra-arterial thrombolysis for acute ischemic stroke. Neurology 2005;64:386–388. [DOI] [PubMed] [Google Scholar]
- 20.IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–911. [DOI] [PubMed] [Google Scholar]
- 21.The IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke 2007;38:2127–2135. [DOI] [PubMed] [Google Scholar]
- 22.Tomsick TA. 2006: a stroke odyssey. AJNR Am J Neuroradiol 2006;27:2019–2021. [PMC free article] [PubMed] [Google Scholar]
- 23.Tomsick T, Broderick J, Carrozella J, et al, Interventional Management of Stroke II Investigators. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008;29:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hacke W, Kaste M, Bluhmki E, et al, ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 25.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–774. [DOI] [PubMed] [Google Scholar]
- 26.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke: Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 27.Smith WS, Sung G, Saver J, et al, Multi MERCI Investigators. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–1212. [DOI] [PubMed] [Google Scholar]
- 28.Broderick JP. Endovascular therapy for acute ischemic stroke. Stroke 2009;40:S103–S106. [DOI] [PubMed] [Google Scholar]
- 29.The Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–2768. [DOI] [PubMed] [Google Scholar]
- 30.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial: Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–2011. [DOI] [PubMed] [Google Scholar]
- 31.Khatri P, Hill M, Palesch Y, et al, for the IMS III Investigators. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke 2008;3:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IMS Study Investigators. Hemorrhage in the Interventional Management of Stroke study. Stroke 2006;37:847–851. [DOI] [PubMed] [Google Scholar]
- 33.NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997;28:2109–2118. [DOI] [PubMed] [Google Scholar]
- 34.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–1438. [DOI] [PubMed] [Google Scholar]
- 35.King S, Khatri P, Carrozella J, et al, IMS & IIMS II Investigators. Anterior cerebral artery emboli in combined intravenous and intra-arterial rtPA treatment of acute ischemic stroke in the IMS I and II trials. AJNR Am J Neuroradiol 2007;28:1890–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khatri P, Broderick JP, Khoury JC, Carrozzella JA, Tomsick TA, IMS I and II Investigators. Microcatheter contrast injections during intra-arterial thrombolysis may increase intracranial hemorrhage risk. Stroke 2008;39:3283–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khatri R, Khatri P, Khoury J, Broderick J, Carrozella J, Tomsick T. Microcatheter contrast injections during intraarterial thrombolysis increase parenchymal hematoma risk: registry experience. Stroke 2007;601. [DOI] [PubMed] [Google Scholar]
- 38.Dzialowski I, Puetz V, Demchuk AM, et al. Does application of radio contrast material prior to thrombolysis impact thrombolytic effect in acute ischemic stroke? Stroke 2007;38:454. [Google Scholar]
- 39.Pislaru S, Pislaru C, Szilard M, Arnout J, Van de Werf F. In vivo effects of contrast media on coronary thrombolysis. J Am Coll Cardiol 1998;32:1102–1108. [DOI] [PubMed] [Google Scholar]
- 40.Jones CI, Goodall AH. Differential effects of the iodinated contrast agents Ioxaglate, Iohexol and Iodixanol on thrombus formation and fibrinolysis. Thromb Res 2003;112:65–71. [DOI] [PubMed] [Google Scholar]
- 41.Albers GW, Thijs VN, Wechsler L, et al, DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 42.De Silva DA, Fink JN, Christensen S, et al, for the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Investigators. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). Stroke Epub 2009. [DOI] [PubMed]
- 43.Toth G, Albers GW. Use of MRI to estimate the therapeutic window in acute stroke: is perfusion-weighted imaging/diffusion-weighted imaging mismatch an EPITHET for salvageable ischemic brain tissue? Stroke 2009;40:333–335. [DOI] [PubMed] [Google Scholar]
- 44.Hill MD, Demchuk AM, Tomsick TA, Palesch YY, Broderick JP. Using the baseline CT scan to select acute stroke patients for IV-IA therapy. AJNR Am J Neuroradiol 2006;27:1612–1616. [PMC free article] [PubMed] [Google Scholar]
- 45.Hill MD, Kent DM, Hinchey J, et al, PROACT-2 Investigators. Sex-based differences in the effect of intra-arterial treatment of stroke: analysis of the PROACT-2 study. Stroke 2006;37:2322–2325. [DOI] [PubMed] [Google Scholar]



