Abstract
Background:
Vascular comorbidity adversely influences health outcomes in several chronic conditions. Vascular comorbidities are common in multiple sclerosis (MS), but their impact on disease severity is unknown. Vascular comorbidities may contribute to the poorly understood heterogeneity in MS disease severity. Treatment of vascular comorbidities may represent an avenue for treating MS.
Methods:
A total of 8,983 patients with MS enrolled in the North American Research Committee on Multiple Sclerosis Registry participated in this cohort study. Time from symptom onset or diagnosis until ambulatory disability was compared for patients with or without vascular comorbidities to determine their impact on MS severity. Multivariable proportional hazards models were adjusted for sex, race, age at symptom onset, year of symptom onset, socioeconomic status, and region of residence.
Results:
Participants reporting one or more vascular comorbidities at diagnosis had an increased risk of ambulatory disability, and risk increased with the number of vascular conditions reported (hazard ratio [HR]/condition for early gait disability 1.51; 95% confidence interval [CI] 1.41–1.61). Vascular comorbidity at any time during the disease course also increased the risk of ambulatory disability (adjusted HR for unilateral walking assistance 1.54; 95% CI 1.44–1.65). The median time between diagnosis and need for ambulatory assistance was 18.8 years in patients without and 12.8 years in patients with vascular comorbidities.
Conclusions:
Vascular comorbidity, whether present at symptom onset, diagnosis, or later in the disease course, is associated with a substantially increased risk of disability progression in multiple sclerosis. The impact of treating vascular comorbidities on disease progression deserves investigation.
GLOSSARY
- EDSS
= Expanded Disability Status Scale;
- HR
= hazard ratio;
- MS
= multiple sclerosis;
- NARCOMS
= North American Research Committee on Multiple Sclerosis;
- PDDS
= Patient Determined Disease Steps.
Multiple sclerosis (MS) is a disabling disease with considerable unexplained heterogeneity in outcomes.1,2 Previous studies of prognostic factors in MS focused on demographic and disease-related characteristics related exclusively to MS, but findings conflicted and ability to prognosticate early in the disease course remains poor.1,2
Comorbidity may potentially explain heterogeneity in outcomes. Few studies examined comorbidity and MS.3–6 Studies of autoimmune disease and MS did not identify any effect of autoimmune disease on clinical features or outcomes of MS.3–5 Reports conflict regarding the association between smoking and disability progression.6,7 Potential benefits of studying comorbidity in MS include improved prognostication; insights into the etiology and pathogenesis of MS; and the treatment of comorbidities as an avenue for improving outcomes.
Using the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, we aimed to determine the association between vascular comorbidities and disability progression, as measured using Patient Determined Disease Steps. We hypothesized that MS-affected individuals with vascular comorbidities would have more ambulatory dysfunction after accounting for disease duration, age at symptom onset, and other confounding factors.
METHODS
The methods of this study are detailed elsewhere.8 Briefly, the NARCOMS Registry is a self-report registry for patients with MS.9 Participants report disability status at enrollment and semiannually using Patient Determined Disease Steps (PDDS), a validated self-report measure which correlates well with the Expanded Disability Status Scale (EDSS).10 It is scored ordinally from 0 to 8, where a score of 3 represents early gait disability without needing an assistive device and approximates an EDSS score of 4.0 to 4.5, and scores of 4, 5, and 6 represent EDSS scores of 6 to 6.5.
In October 2006, we queried NARCOMS participants regarding physical and mental comorbidities, including the year of diagnosis, and whether the condition was currently treated.11,12 Inclusion criteria were residence in the United States, age at symptom onset between 16 and 60 years, and date of birth reported at enrollment. After initial pilot testing, the comorbidity questionnaire used in this study was tested among a convenience sample of patients attending regularly scheduled visits at the Mellen Center for Multiple Sclerosis Treatment and Research (n = 65) and at the Health Sciences Centre Multiple Sclerosis Clinic (n = 235). Trained abstractors blinded to the questionnaire responses reviewed the medical record to capture the presence and treatment status of comorbidities, where a condition was defined as present based on physician-recorded diagnosis or use of a disease-specific treatment. The reported frequency of vascular conditions among these participants was 65 (21.7%) for hypertension, 49 (16.3%) for hypercholesterolemia, 15 (5.0%) for diabetes, 13 (4.3%) for heart disease, and 2 (0.67%) for peripheral vascular disease. Agreement, as measured by a κ statistic (95% confidence interval), between the questionnaires and medical records was substantial13 for hypertension (κ = 0.83; 0.76–0.91), hypercholesterolemia (κ = 0.79; 0.69–0.88), and diabetes (κ = 0.86; 0.72–0.99), and moderate for heart disease (κ = 0.48; 0.23–0.73). Agreement could not be estimated for peripheral vascular disease because of the small number of participants affected. Agreement regarding the presence of any of these conditions was substantial (κ = 0.83; 0.76–0.90).
For analysis, we created a category for vascular comorbidity, including diabetes, hypertension, heart disease, hypercholesterolemia, and peripheral vascular disease. The first major analysis addressed the question faced by the clinician with a newly diagnosed patient with MS: “Does vascular comorbidity at diagnosis influence the risk of disability progression?” For this analysis, zero time was set as the time of MS diagnosis. The second analysis was more general: “Does the presence of vascular comorbidity at any point in the disease course influence the risk of disability progression?” To capture the entire disease course, zero time was set as the time of initial symptom onset.
To determine whether vascular comorbidity at diagnosis influences subsequent disability progression, we compared participants who reported one or more vascular comorbidities at diagnosis to those who never developed a vascular comorbidity. The outcomes of interest were time from diagnosis to early gait disability (approximately EDSS 4), unilateral assistance to walk (approximately EDSS 6), and bilateral assistance to walk (approximately EDSS 6.5); throughout the text, the term ambulatory disability is used to refer to all 3 outcomes of interest. We used the Kaplan-Meier method and log-rank tests for univariate analysis of the association of vascular comorbidity and ambulatory disability. For multivariable analysis, we constructed Cox proportional hazards models.
We also investigated whether vascular comorbidity developing at any point in the disease course affects the time between symptom onset and the same disability outcomes. For these analyses, zero time was initial symptom onset. For univariate analysis of the association of vascular comorbidity and ambulatory disability, we used the Kaplan-Meier method and log-rank tests. For multivariable analysis we constructed Cox proportional hazards models. To account for the onset of comorbidity after disease onset, comorbidity categories were included in the Cox models as time-dependent covariates, where the first occurrence of any comorbidity in the category defined onset of exposure to that category.
For all models, we considered the following potential confounders: age; sex; race; education; annual household income; health insurance status; marital status; region of residence in the United States; year of symptom onset; age at symptom onset; and treatment status. Potential effect modifiers considered were age, sex, and race. Race was included as indicator variables for white (reference group), African American, and other. Education was included as indicator variables for <12 years (reference group), high school diploma, Associate's Degree or Technical Degree, Bachelor's Degree, and postgraduate degree. Annual household income was included as indicator variables for <$15,000 (reference group), $15,000–$30,000, $30,000–$50,000, $50,000– $100,000, and >$100,000. Insurance status was included as indicator variables for private, public (reference group), and none. Region of residence was included as indicator variables for West (reference group), Midwest, South, and East as defined by the US Census bureau. Marital status was dichotomized as married/cohabiting vs single/divorced/widowed/living alone (reference group). Age at symptom onset was categorized as ≤25, 25 to 39, ≥40 years and represented by indicator variables. Year of symptom onset was categorized as <1986, 1986–1995, ≥1996. Ever treatment with any of the approved disease-modifying therapies (IFNβ-1a [Avonex], IFNβ-1b [Betaseron], IFNβ-1a [Rebif], glatiramer acetate [Copaxone], or natalizumab [Tysabri]) was dichotomized as yes or no. Ever treatment with immunosuppressive therapies was dichotomized as yes or no.
The proportional hazards assumption was tested using time-dependent covariates and graphical methods.14 We report unadjusted and adjusted hazard ratios and 95% confidence intervals as measures of association between comorbidity and disability progression. Statistical analyses were performed used SAS V9.1 (SAS Institute Inc., Cary, NC).
Standard protocol approvals, registrations, and patient consents.
The NARCOMS Registry is approved by the Western Institutional Review Board. Participants give permission for their information to be used for research purposes.
RESULTS
As reported previously,8 8,983 eligible participants completed questionnaires. We initially reported a response rate of 55.5%,8 but recent linkage to the National Death Index determined that 104 nonresponders had died in 2006, revising the response rate to 56%. Most respondents were women (75.8%) and white (94.3%), with mean (SD) age 52.7 (10.4) years. The mean (SD) ages at symptom onset (31.2 [9.0]) and diagnosis (38.2 [9.5]) are similar to those reported for the general MS population.15,16 At enrollment into the registry, 3,177 (38.5%) participants were mildly disabled, 1,415 (17.2%) were moderately disabled, and 3,656 (44.3%) were severely disabled. In October 2006, 3,178 (35.4%) participants reported mild, 1,067 (11.9%) reported moderate, and 4,738 (52.7%) reported severe disability. More than 50% of participants (4745) had a vascular comorbidity, including 3,237 (37.0%) with hypercholesterolemia, 2,664 (30.1%) with hypertension, 606 (6.9%) with heart disease, 532 (6.1%) with diabetes, and 209 (2.4%) with peripheral vascular disease. Nearly 16% of participants (1,435) had 2 vascular comorbidities, while 397 (4.4%) had 3, and 89 (0.99%) had 4 or more. The mean (SD) ages at diagnosis for these comorbidities were 47.3 (11.8) years for hypertension, 48.7 (10.3) years for hypercholesterolemia, 48.8 (12.4) years for diabetes, 50.4 (13.7) years for heart disease, and 51.7 (14.1) years for peripheral vascular disease.
Comorbidity at diagnosis and disability progression.
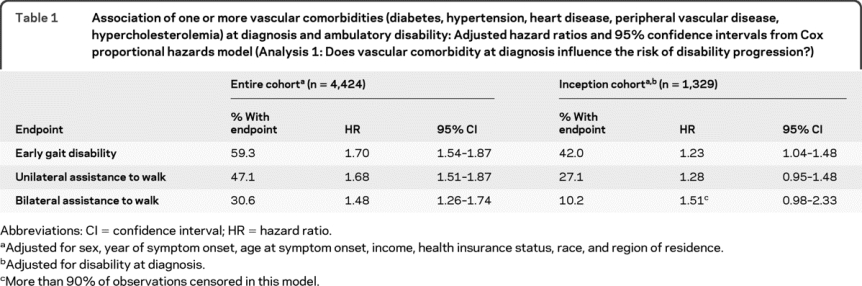
Using Kaplan-Meier analysis, participants reporting 1 or more vascular comorbidities at diagnosis had a decreased time from diagnosis to ambulatory disability compared to those without vascular comorbidity (p < 0.0001, figure). After adjusting for sex, year of symptom onset, age at symptom onset, income, health insurance status, race, and region of residence, participants reporting one or more vascular comorbidities at diagnosis had a more than 1.5-fold increased risk of ambulatory disability (table 1). Using the number of vascular comorbidities at diagnosis as a linear term in the adjusted models, we observed a dose-response relationship. The risk of early gait disability increased 50% (hazard ratio [HR] 1.51; 1.41–1.61) per comorbidity. Thus, a single vascular comorbidity at diagnosis was associated with a 51% increased risk of early gait disability, while 2 were associated with a 228% increased risk.
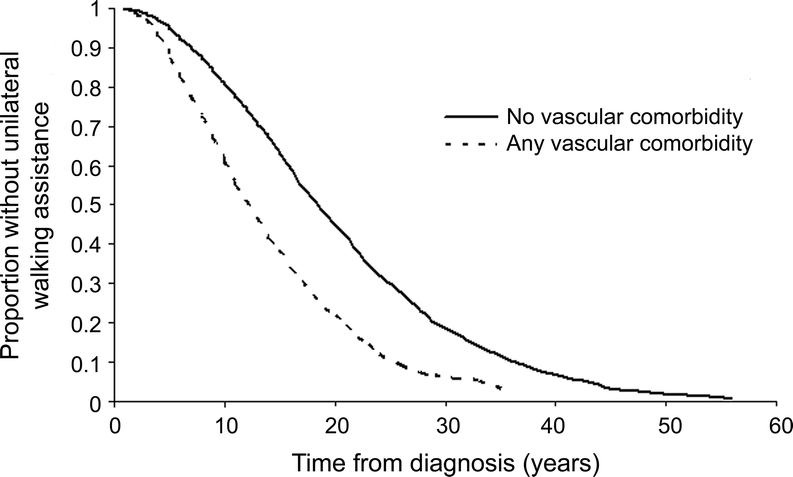
Figure Kaplan-Meier analysis
Time from diagnosis of multiple sclerosis to needing unilateral assistance to walk in North American Research Committee on Multiple Sclerosis participants with (n = 572) and without (n = 2,286) vascular comorbidity at diagnosis. Any vascular comorbidity = any of diabetes, hypertension, heart disease, hypercholesterolemia, and peripheral vascular disease.
Table 1 Association of one or more vascular comorbidities (diabetes, hypertension, heart disease, peripheral vascular disease, hypercholesterolemia) at diagnosis and ambulatory disability: Adjusted hazard ratios and 95% confidence intervals from Cox proportional hazards model (Analysis 1: Does vascular comorbidity at diagnosis influence the risk of disability progression?)
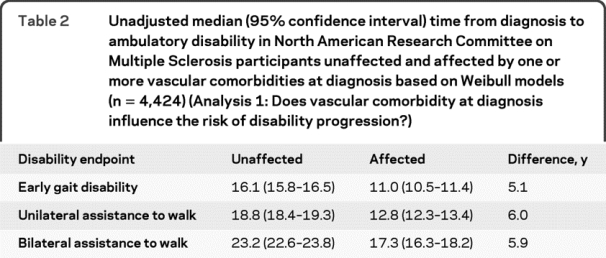
Because relative hazards are difficult to interpret clinically, we constructed univariate parametric models that predicted the median time from diagnosis to ambulatory disability in affected and unaffected participants using a Weibull distribution (table 2). Compared to unaffected participants, those with one or more vascular comorbidities at diagnosis required unilateral assistance to walk a median time of 6 years earlier.
Table 2 Unadjusted median (95% confidence interval) time from diagnosis to ambulatory disability in North American Research Committee on Multiple Sclerosis participants unaffected and affected by one or more vascular comorbidities at diagnosis based on Weibull models (n = 4,424) (Analysis 1: Does vascular comorbidity at diagnosis influence the risk of disability progression?)
Zero time for analyses of disability progression was time of diagnosis; thus the observed association of comorbidity and ambulatory disability might reflect increased disability at diagnosis, rather than effects of comorbidity on disability progression after diagnosis. To account for that possibility, we analyzed a virtual inception cohort (n = 2,375), which was defined as participants who enrolled in the NARCOMS Registry within 2 years of diagnosis, and further adjusted for the severity of disability at diagnosis. The presence of one or more vascular comorbidities remained associated with an increased risk of ambulatory disability (table 1).
Comorbidity at any point in the disease course and disability progression.
After adjusting for sex, year of symptom onset, age at symptom onset, income, health insurance status, race, and region of residence, participants reporting one or more vascular comorbidities at any point in their disease course had an increased risk of ambulatory disability as compared to participants who never developed a vascular comorbidity. The risk was similar for early gait disability (HR 1.58; 1.48–1.68), unilateral assistance to walk (HR 1.54; 1.44–1.65), and bilateral assistance to walk (HR 1.38; 1.25–1.52).
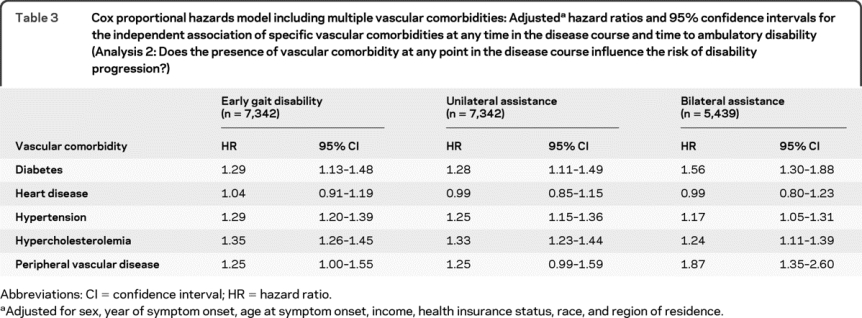
All vascular comorbidities may not have the same effect on disability progression; therefore we included individual vascular comorbidities in one adjusted model. Consistently, diabetes, hypertension, hypercholesterolemia, and peripheral vascular disease were independently associated with an increased risk of ambulatory disability regardless of the specific endpoint chosen (table 3).
Table 3 Cox proportional hazards model including multiple vascular comorbidities: Adjusted hazard ratios and 95% confidence intervals for the independent association of specific vascular comorbidities at any time in the disease course and time to ambulatory disability (Analysis 2: Does the presence of vascular comorbidity at any point in the disease course influence the risk of disability progression?)
We conducted sensitivity analyses, in which we restricted analyses to 1) persons with a relapsing course at onset; 2) persons with an age at symptom onset greater than 16 and less than 50 years; and 3) persons with at most mild disability at the time of enrollment in the NARCOMS Registry. The findings were unchanged (data not shown).
DISCUSSION
Few prior studies evaluated the influence of comorbidity on disability progression in MS. One population-based study reported no association between autoimmune disease and disability progression, but asthma was associated with a shorter time to an EDSS score of 3.3 Studies of smoking and disability in MS are conflicting.6,7 A more recent study suggested that patients recently diagnosed with MS who had musculoskeletal comorbidities experienced greater declines in physical function over 3 years.17
Using a large patient population where race and socioeconomic status are known, we examined the influence of vascular comorbidity on disability progression in MS. Vascular comorbidities were frequent but generally occurred at the rates expected for the general population.18 The risk associated with vascular comorbidities translated into a substantial difference of 6 years in the time from diagnosis to needing a unilateral assistive device for ambulation. Vascular comorbidity occurring at any time in the disease course was also associated with an increased risk of disability progression; the risk increased by more than 200% in participants with 2 comorbidities. By comparison, pivotal trials of disease-modifying therapies in MS showed effects ranging from none to 42% reduction in the probability of disability progression.19,20 Depending on the agent and study population, the effects of disease-modifying drugs translate into average delays in disability progression of less than 12 months over the course of a 2- to 3-year study, and the benefits on disability progression are less pronounced later in disease.21,22 This suggests that comorbidity has a substantial, important effect on outcome, possibly greater than the effect (in the opposite direction) of disease-modifying drugs used for MS. Furthermore, some vascular conditions are modifiable, raising the possibility that more aggressive treatment of comorbidities could improve outcomes in MS. This is a completely unexplored question, but results here suggest a rationale for aggressive management of vascular risk factors in patients with MS, and for studies to determine its effect on MS severity.
Our findings are consistent with the effects of comorbidity in other chronic diseases. Where studied, comorbidity adversely affects disability, functional status, mortality, and quality of life in conditions ranging from rheumatoid arthritis to cancer.23,24 Studies also suggest adverse effects of comorbidity on CNS disease. Comorbid diabetes, congestive heart failure, and vision impairment are associated with increased mortality in Parkinson disease.25 A growing literature suggests that vascular comorbidities and stroke accelerate the progression of Alzheimer disease.26,27
Vascular comorbidities are common in MS and are independently treatable. Several independent observations suggest that vascular comorbidity might adversely affect MS. First, even absent overt cerebrovascular disease, hypertension, hypercholesterolemia, and diabetes are associated with increased cognitive decline and brain atrophy.28–30 Second, vascular disease is associated with increased peripheral inflammation, and elevated inflammatory markers are associated with increased brain atrophy.31 Third, diabetes may increase susceptibility to oxidative stress; alter blood vessel structure and function; and increase inflammatory responses in the brain.32 Finally, abnormalities of cerebral endothelial cells are reported in MS.33 These proposed possible mechanisms assume that vascular disease influences MS at the pathophysiologic level. Alternatively, vascular disease may have independent effects on disability, which are additive to the effects of MS. For example, diabetic neuropathy could lead to gait impairment, with the combined effects of diabetes and MS leading to greater disability than either alone. Further study is needed to understand the mechanism by which vascular disease influences MS outcomes, and whether intervention directed at vascular disease influences MS disability progression.
This study had limitations. The response rate was 56%, but this compares favorably to the average response rate in published mail surveys of 60%.34 The NARCOMS Registry is a volunteer registry, but we reported previously that the NARCOMS population has similar characteristics to those reported for patients with MS from the National Health Interview Survey.15 Comorbidity data were self-reported including the dates of diagnosis, but the literature supports the accuracy of self-report for conditions which are chronic, disabling, or require ongoing care,35 including hypertension, diabetes, and heart disease. Validation work on our comorbidity questionnaire used in this study also supports the validity of the conditions reported in this study. Self-report comorbidity indices compare well to indices based on medical records or administrative data for the prediction of mortality and health care utilization.11,36 Previous work with this cohort suggests that participants accurately report the dates of MS symptom onset and diagnosis.37 The National Center for Health Statistics investigated similar event dating issues in the reporting of cancer prevention examinations and tests for women 50 and over, similar in age to a substantial part of our population.38 Although limited to screening procedures provided within the last 5 years, they found gross error to be within 3.5 months. Regardless, errors in self-report are likely to lead to nondifferential misclassification of comorbidity status, biasing our results away from finding an effect of comorbidity. Finally, this study design did not permit us to explore the reasons for our findings.
Despite its limitations, this study included a large patient population with a broad range of sociodemographic characteristics, a spectrum of disease severity, and captured patients followed in the community and in academic centers. Previous work validated diagnoses of MS in a sample of this study population, and the clinical instruments used to determine outcome.37,39 The median (interquartile range) time to needing unilateral assistance to walk—18.9 (11.9–27.4) years—is similar to that reported in a population-based study in Ontario,40 further supporting the validity of these results. Unlike other studies of comorbidity and MS, we adjusted for socioeconomic status, an important potential confounder. As demonstrated by sensitivity analyses, our results were robust to changes in our assumptions.
This study showed that vascular comorbidity was associated with greater disability progression in MS, whether present at diagnosis or later in the disease course; thus, the study suggests that vascular comorbidity explains some of the well-known heterogeneity in disease outcomes between individual patients. The magnitude of impact was surprisingly high. In addition to their clinical implications, the findings raise important research directions, including the underlying mechanisms by which vascular comorbidity influences disability progression, whether treating these comorbidities aggressively can reduce MS progression, the role of lifestyle management in MS, and whether patients with comorbidities should be managed differently than patients without comorbidities.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Ruth Ann Marrie.
DISCLOSURE
Dr. Marrie serves on the editorial board of Neurology; has received research support from BioMS Medical, sanofi-aventis, Bayer Schering Pharma (Berlex), EMD Serono, Inc., Manitoba Health Research Council, University of Manitoba (Rudy Falk Clinician Scientist), Health Sciences Centre Foundation, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, and Consortium of Multiple Sclerosis Centers. Dr. Rudick has received funding for travel from and/or served as a consultant for Biogen Idec, Genzyme Corporation, Novartis, Teva Pharmaceutical Industries Ltd., Wyeth, and Bayhill Pharmaceuticals; receives royalties from the publication of Multiple Sclerosis Therapeutics, Third Edition (Informa Healthcare, 2007); has received speaker honoraria from Teva Pharmaceutical Industries Ltd and Biogen Idec; and receives research support from the NIH (PO1NS38667 [Director, Project 4], UL1RR024989 [Co-PI], and KL2RR024990 [PI]). Dr. Horwitz serves on the editorial board of the European Journal of Clinical Investigation; has received research support from the NIH/NINDS (R01NS044876 [Co-I]); and serves on the Advisory Committee to the Director, NIH. Dr. Cutter has served on scientific advisory boards for and/or received funding for travel from Millenium Pharmaceuticals, Inc., Klein Buendel, Inc., Alexion Pharmaceuticals, Inc., Androclus Therapeutics, Inc., University of Illinois, Amgen, New York University, and Somnus Therapeutics, Inc.; receives royalties from publishing Evaluation of Health Promotion and Disease Prevention (McGraw Hill Companies, 1984); has received honoraria from GlaxoSmithKline, Biogen Idec, Novartis, Advanced Health Media Inc., Biogen Idec, EMD Serono Inc., EDJ Associates, Inc., the National Heart, Lung, and Blood Institute, National Institute of Neurological Diseases and Stroke, National Marrow Donor Program, Consortium of Multiple Sclerosis Centers; serves as a consultant to Peptimmune Inc., Aegis Creative Marketing, Novartis, National Industrial Sand Association, Bayer Pharmaceuticals, and Teva Pharmaceuticals Industries Ltd.; has served on independent data and safety monitoring committees for Antisense Therapeutics Limited, sanofi-aventis, Bayhill Pharmaceuticals, BioMS Medical Corp, Daiichi-Sankyo Co. Inc, Glaxo Smith Klein, Genmab, Medivation Inc., PTC Therapeutics Inc., Teva Pharmaceutical Industries Ltd., Vivus Inc., NHLBI, NINDS, NMSS; has received research support from ApopLogic Pharmaceuticals, LLC; receives research support from the NIH (NINDS 5U01NS042685-02 [PI], NINDS U01 NS45719-01A1 [PI, Coordinating Center], NIAID Contract HHSN266200400068C [Co-I], NHLBI 5R01 HL06991-02 [PI, Coordinating Center], NIAID N01AI30025 [Director, Coordinating Center], NIDR 3R01DE016684-03S109 [Co-I], NHLBI 5P50HL084923-030001 [Director, Coordinating Center], NIDDK 1R01DK078826 [Co-I], NIAID P30AI27767 [Co-Director, Biostatistics Core], NIDDK 1P30DK079337 [Director, Biostatistics Core]) and from the Consortium of Multiple Sclerosis Centers (Director NARCOMS Data Center) and the National Multiple Sclerosis Society; and serves as President of Pythagoras, Inc. Dr. Tyry has served as a consultant to EMD Serono, Inc. Dr. Campagnolo has served on a scientific advisory board for and received funding for travel from Biogen Idec; serves as an Associate Editor of the American Journal of Physical Medicine and Rehabilitation and as Chief Editor of eMedicine; has received speaker honoraria from EMD Serono, Inc.; receives royalties from the publication of Spinal Cord Medicine: Principles and Practice (Demos Medical Publishing, LLC, 2003); and has received research support from the NIH (NINDS 1UO1NS45719-02 A1 [Co-I], and K24 HD043819-01A1 [PI]), Novartis, PDL BioPharma Inc., EMD Serono, Inc. Pfizer Inc, Teva Pharmaceutical Industries Ltd., Genentech, Inc. and Barrow Neurological Foundation. Dr. Vollmer has served on scientific advisory boards for Teva Pharmaceuticals Industries Ltd., Novartis, GlaxoSmithKline, EMD Serono, Inc., Biogen Idec, Abbott, Accorda Therapeutics, Bayhill Therapeutics, Metabolic Solutions Development Co., and Genentech, Inc.; has received speaker honoraria from EMD Serono, Inc., Teva Pharmaceuticals Industries Ltd., Biogen Idec, and the National Multiple Sclerosis Society; has served on speakers' bureaus for Biogen Idec, Teva Pharmaceuticals Industries Ltd., and Athena Diagnostics, Inc.; has received research support from Teva Pharmaceuticals Industries Ltd., Daiichi Sankyo, Genzyme Corporation, Ono Pharmaceutical Co., Ltd., Eli Lilly and Company, sanofi-aventis, BioMS Medical, Novartis, PDL BioPharma, Inc., Pfizer Inc, Merck Serono S.A., Accorda Therapeutics, Genentech, Inc., the NIH (NIAID/ITN NO1-AL015416/CFDA 93ZZZ [Co-I], and NINDS 1UO1NS45719-02 A1 [Co-I]) and from the Barrow Neurological Foundation, Translational Genomics Research Institute, the Rocky Mountain Multiple Sclerosis Society, and the National Multiple Sclerosis Society; and gave expert testimony in a trial re: Genentech, Inc. VS Biogen Idec.
Address correspondence and reprint requests to Dr. Ruth Ann Marrie, Health Sciences Center, GF-533, 820 Sherbrook Street, Winnipeg, MB R3A 1R9, Canada rmarrie@hsc.mb.ca.
Study funding: Supported in part by the NIH, National Institute of Child Health and Human Development, and Multidisciplinary Clinical Research Career Development Program Grant K12 HD04909. The NARCOMS Registry is supported by the Consortium of Multiple Sclerosis Centers.
Disclosure: Author disclosures are provided at the end of the article.
Received September 17, 2009. Accepted in final form January 6, 2010.
REFERENCES
- 1.Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study: 3: multivariate analysis of predictive factors and models of outcome. Brain 1991;114:1045–1056. [DOI] [PubMed] [Google Scholar]
- 2.Langer-Gould A, Popat RA, Huang SM, et al. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: a systematic review. Arch Neurol 2006;63:1686–1691. [DOI] [PubMed] [Google Scholar]
- 3.Kirby S, Brown MG, Murray TJ, et al. Progression of multiple sclerosis in patients with other autoimmune diseases. Mult Scler 2005;11:S28–S29. [Google Scholar]
- 4.Tourbah A, Clapin A, Gout O, et al. Systemic autoimmune features and multiple sclerosis: a 5-year follow-up study. Arch Neurol 1998;55:517–521. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt S, Wessels L, Augustin A, Klockgether T. Patients with multiple sclerosis and concomitant uveitis/periphlebitis retinae are not distinct from those without intraocular inflammation. J Neurol Sci 2001;187:49–53. [DOI] [PubMed] [Google Scholar]
- 6.Hernan MA, Jick SS, Logroscino G, Olek MJ, Ascherio A, Jick H. Cigarette smoking and the progression of multiple sclerosis. Brain 2005;128:1461–1465. [DOI] [PubMed] [Google Scholar]
- 7.Koch M, van Harten A, Uyttenboogaart M, De Keyser J. Cigarette smoking and progression in multiple sclerosis. Neurology 2007;69:1515–1520. [DOI] [PubMed] [Google Scholar]
- 8.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status, and multiple sclerosis. Mult Scler 2008;14:1091–1098. [DOI] [PubMed] [Google Scholar]
- 9.Consortium of Multiple Sclerosis Centers. NARCOMS Multiple Sclerosis Registry. Available at: http://www.mscare.org/cmsc/CMSC-NARCOMS-Information.html. Accessed January 5, 2008.
- 10.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a longitudinal study comparing disease steps and EDSS to evaluate disease progression. Mult Scler 1999;5:349–354. [DOI] [PubMed] [Google Scholar]
- 11.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–163. [DOI] [PubMed] [Google Scholar]
- 12.Marrie RA. The influence of comorbid diseases and health behaviors on clinical characteristics, disability at diagnosis, and disability progression in multiple sclerosis. Dissertation: Case Western Reserve University; 2007. [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 14.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text, 1st ed. New York, NY: Springer-Verlag New York, Inc.; 1997. [Google Scholar]
- 15.Collins JG. Prevalence of selected chronic conditions, United States, 1986–88: National Center for Health Statistics. Vital Health Statistics 1993;10:1–87. [PubMed] [Google Scholar]
- 16.Jacobs LD, Wende KE, Brownscheidle CM, et al. A profile of multiple sclerosis: The New York State Multiple Sclerosis Consortium. Mult Scler 1999;5:369–376. [DOI] [PubMed] [Google Scholar]
- 17.Dallmeijer AJ, Beckerman H, Groot VD, Port IGLVD, Lankhorst GJ, Dekker J. Long-term effect of comorbidity on the course of physical functioning in patients after stroke and with multiple sclerosis. J Rehabil Med 2009;41:322–326. [DOI] [PubMed] [Google Scholar]
- 18.Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Comorbid conditions are common in multiple sclerosis. Int J MS Care 2007;9:69. [Google Scholar]
- 19.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 20.The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995;45:1277–1285. [PubMed] [Google Scholar]
- 21.PRISMS Study Group. Randomized double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998;352:1498–1504. [PubMed] [Google Scholar]
- 22.European Study Group on interferon beta-1b in secondary progressive MS. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998;352:1491–1497. [PubMed] [Google Scholar]
- 23.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL, Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441–2447. [DOI] [PubMed] [Google Scholar]
- 24.Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005;32:1013–1019. [PubMed] [Google Scholar]
- 25.Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson's disease. Med Sci Monitor 2002;8:CR241–CR246. [PubMed] [Google Scholar]
- 26.Regan C, Katona C, Walker Z, Hooper J, Donovan J, Livingston G. Relationship of vascular risk to the progression of Alzheimer disease. Neurology 2006;67:1357–1362. [DOI] [PubMed] [Google Scholar]
- 27.Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology 2007;69:1850–1858. [DOI] [PubMed] [Google Scholar]
- 28.Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 2005;64:1704–1711. [DOI] [PubMed] [Google Scholar]
- 29.Knopman DS, Mosley TH, Catellier DJ, Sharrett AR. Atherosclerosis Risk in Communities (ARIC) Study: Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology 2005;65:876–881. [DOI] [PubMed] [Google Scholar]
- 30.Elkins JS, O'Meara ES, Longstreth WT, Jr., Carlson MC, Manolio TA, Johnston SC. Stroke risk factors and loss of high cognitive function. Neurology 2004;63:793–799. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume: The Framingham Heart Study. Neurology 2007;68:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Launer LJ. Diabetes and brain aging: epidemiologic evidence. Curr Diabetes Rep 2005;5:59–63. [DOI] [PubMed] [Google Scholar]
- 33.Minagar A, Jy W, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res 2006;28:230–235. [DOI] [PubMed] [Google Scholar]
- 34.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol 1997;50:1129–1136. [DOI] [PubMed] [Google Scholar]
- 35.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care 2005;43:607–615. [DOI] [PubMed] [Google Scholar]
- 37.Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Validation of the NARCOMS Registry: diagnosis. Mult Scler 2007;13:770–775. [DOI] [PubMed] [Google Scholar]
- 38.Sudman S, Warnacke R, Johnson T, O'Rourke D, Davis AM. Cognitive aspects of reporting cancer prevention examinations and tests. Vital Health Statistics 1994;series 6:1–161. [Google Scholar]
- 39.Marrie RA, Goldman MD. Validity of Performance Scales for disability assessment in multiple sclerosis. Mult Scler 2007;13:1176–1182. [DOI] [PubMed] [Google Scholar]
- 40.Weinshenker BG, Bass B, Rice PA, et al. The natural history of multiple sclerosis: a geographically based study: I: clinical course and disability. Brain 1989;112:133–146. [DOI] [PubMed] [Google Scholar]


