Abstract
Protein interactions with the integrin β-subunit cytoplasmic domain (β-tail) are essential for adhesion-dependent processes, including cell spreading and the connection of integrins with actin filaments at adhesion sites. Talin-1 binds to the conserved membrane-proximal NPxY motif of β-tails (NPIY in β1 integrin) promoting the inside-out activation of integrins and providing a linkage between integrins and the actin cytoskeleton. Here, we characterize the role of interactions between talin-1 and β-tail downstream of integrin activation, in the context of recombinant integrins containing either the wild type (WT) or the (YA) mutant β1A tail, with a tyrosine to alanine substitution in the NPIY motif. In addition to inhibiting integrin activation, the YA mutation suppresses cell spreading, integrin signaling, focal adhesion and stress-fiber formation, as well as microtubule assembly. Constitutive activation of the mutant integrin restores these integrin-dependent processes, bringing into question the importance of the NPIY motif downstream of integrin activation. Depletion of talin-1 using TLN1 siRNA demonstrated that talin-1 is required for cell spreading, focal adhesion and stress-fiber formation, as well as microtubule assembly, even when cells are adhered by constitutively activated WT integrins. Depletion of talin-1 does not inhibit these processes when cells are adhered by constitutively activated mutant integrins, suggesting that the binding of an inhibitory protein to the NPIY motif negatively regulates integrin function when talin-1 is depleted. We identified filamin A (FLNa) as this inhibitory protein; it binds to the β1A tail in an NPIY-dependent manner and inhibition of FLNa expression in talin-1-depleted cells restores integrin function when cells are adhered by constitutively activated WT integrins. FLNa binds FilGAP, which is a negative regulator of Rac activation. Expression of the dominant inhibitory mutant, FilGAPΔGAP, which lacks GAP activity restores spreading in cells adhered by constitutively activated integrins containing the β1A tail, but not by integrins containing the β1D tail, which is known to bind poorly to FLNa. Together, these results suggest that the binding of talin-1 to the NPIY motif is required downstream of integrin activation to promote cell spreading by preventing the inappropriate recruitment of FLNa and FilGAP to the β1A tail. Our studies emphasize the importance of understanding the mechanisms that regulate the differential binding FLNa and talin-1 to the β1 tail downstream of integrin activation in promoting integrin function.
Keywords: Talin, Integrin, Cytoplasmic domain, Microtubule, FLNa
Introduction
Integrins comprise a large family of α/β-heterodimeric receptors that mediate cell adhesion to components of the extracellular matrix. Integrins form transmembrane links with the actin cytoskeleton and activate cytoplasmic signaling cascades to promote cell adhesion, migration, proliferation, survival and differentiation (Gahmberg et al., 2009; Hynes, 2002). By regulating these cellular events, integrins contribute to many normal and pathological processes including tissue morphogenesis, wound healing and tumor metastasis (Gahmberg et al., 2009; Larsen et al., 2006).
The activity of integrins is conformationally regulated (Askari et al., 2009). Interactions between the transmembrane and cytoplasmic domains (tails) of the α- and β-subunits stabilize the inactive conformation of integrins (Hughes et al., 1996; Kim et al., 2003; Li et al., 2003; Luo et al., 2005; Partridge et al., 2005). Current evidence indicates that the ubiquitously expressed cytoskeletal protein talin-1 promotes integrin activation by binding to the conserved membrane-proximal NPxY motif present in β-tails, resulting in changes in the interactions between the α- and β-subunits (Calderwood et al., 1999; Wegener et al., 2007). Talin-1 is composed of an N-terminal globular head domain and a large C-terminal rod domain (Critchley, 2004). The binding of the talin-1 head to the β-tail is thought to be the final step in integrin activation (Calderwood et al., 1999; Tadokoro et al., 2003). Inhibition of talin-1 expression or substitution of tyrosine with alanine in the membrane-proximal NPxY is sufficient to inhibit integrin activation (O'Toole et al., 1995; Tadokoro et al., 2003).
Talin-1 also provides a connection between the β-tail and the actin cytoskeleton (Critchley, 2004). The rod domain links integrins to the actin cytoskeleton by binding to F-actin, or through vinculin, which binds to both talin-1 and F-actin (Critchley, 2004). Other proteins can also connect integrins with the actin cytoskeleton, including α-actinin, kindlin, tensin and filamin (FLN) (Larjava et al., 2008; Legate and Fassler, 2009; Otey and Carpen, 2004). Talin-1, tensin and FLN have overlapping binding sites on the β-tail. Although all three interactions are inhibited by a tyrosine to alanine substitution in the membrane-proximal NPxY motif, only talin-1 promotes integrin activation (Calderwood et al., 2003; Pfaff et al., 1998). Interestingly, interactions of FLN with the β-tail can inhibit integrin activation (Kiema et al., 2006). Thus, the mechanisms that regulate the binding of these proteins to the β-tail have important implications in controlling integrin function.
Although there are three isoforms of filamin: FLNa, FLNb and FLNc (Stossel et al., 2001; van der Flier and Sonnenberg, 2001), most studies on the interaction of FLNa and the β-tail have focused on FLNa, which is the most abundant ubiquitously expressed isoform. In addition to crosslinking cortical actin filaments, FLNa serves as a scaffolding protein for Rho family members, as well as their regulatory proteins. For example, FLNa binds to Rac and its inhibitor FilGAP, which has recently been shown to inhibit Rac activation, lamellapodia formation and cell spreading (Ohta et al., 2006), suggesting that FLNa binding to the β-tail is an important regulator of cell morphology.
Our recent studies demonstrated that a tyrosine-to-alanine substitution in the membrane-proximal NPIY motif in the β1A tail inhibited microtubule assembly during interphase and mitosis, and suppressed cell proliferation by preventing cytokinesis (Reverte et al., 2006). We also showed that constitutively activating the YA (Y783A) mutant integrin rescued these cellular processes, suggesting that a tyrosine-to-alanine substitution in the NPIY motif is tolerated when integrins are constitutively activated, at least for the regulation of the microtubule cytoskeleton. In the current study, we examined the requirement for talin-1 and the NPIY motif in the regulation of other outside-in integrin-signaling-dependent events, including cell spreading, adhesion-triggered tyrosine phosphorylation, and the formation of focal adhesions and stress fibers. For these studies, we used CHO K1 cells, which express recombinant αIIb-5/β3-1A chimeric integrin receptors, containing either a wild type β1A tail (WT) or the Y783A mutant β1A tail (YA). To constitutively activate the recombinant integrins, we used previously characterized activating mutations in either the β-tail or the β-integrin transmembrane domain (O'Toole et al., 1994; Wegener et al., 2007).
Our results indicate that constitutive activation of the YA mutant restored adhesion signaling, cell spreading, focal adhesion and stress fiber formation, in addition to the assembly of the microtubule cytoskeleton, bringing into question the importance of the NPIY motif downstream of integrin activation. Additionally, we demonstrate that talin-1 is required downstream of integrin activation, when cells are adhered by WT integrins, but not by the YA mutant. Thus, talin-1 is not required downstream of integrin activation when its NPIY binding motif is mutated. Taken together, these results suggest that in the absence of talin-1, an inhibitory protein binds to the β1A tail by an NPIY-dependent mechanism. We demonstrate that this inhibitory protein is FLNa, whose interaction with the β-tail is also inhibited by the YA mutation (Pfaff et al., 1998). We propose that the GTPase-activating protein FilGAP is involved in the inhibitory role of FLNa on integrin function. Thus, our results suggest a novel role for talin-1-NPIY interactions in promoting integrin function by preventing the inappropriate binding of FLNa and recruitment of FilGAP to the β1A tail downstream of integrin activation.
Results
A tyrosine-to-alanine substitution in the membrane-proximal NPIY motif of the β1A tail inhibits cell spreading, integrin signaling, focal adhesions and stress fibers
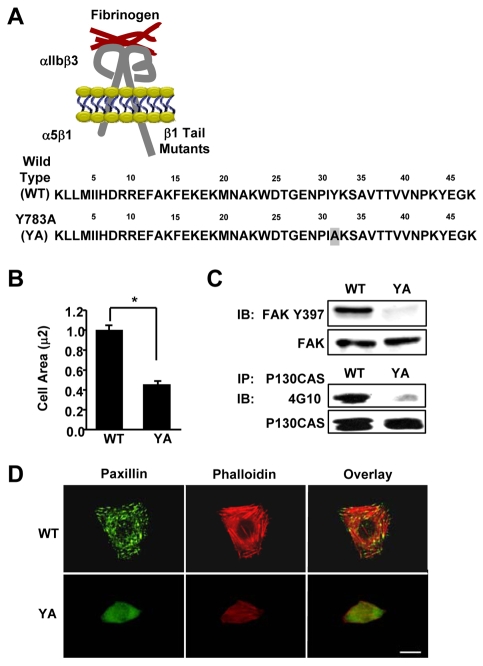
To gain insight into the mechanism by which the YA mutation inhibited the proper formation of the microtubule cytoskeleton (Reverte et al., 2006), we characterized the affect of the YA mutation on other integrin-regulated processes. For these studies, we used CHO K1 cells stably expressing the αIIb-5/β3-1A (WT) or αIIb-5/β3-1AY783A (YA) chimeric integrins (Fig. 1A), and isolated their function by adhering cells to fibrinogen in the serum-free, growth-promoting medium CCM1 as previously described (Reverte et al., 2006). Consistent with previous studies (Kaapa et al., 1999), we showed that the YA mutation inhibited cell spreading and the formation of focal adhesions (Fig. 1B,D). Furthermore, the mutant integrin was unable to trigger the tyrosine phosphorylation of p130CAS and the autophosphorylation of FAK at Tyr397 (Fig. 1C), indicating that Src signaling and FAK activation are inhibited by the mutant integrin. The formation of actin stress fibers was also inhibited (Fig. 1D). Thus, these results demonstrate that an intact NPIY motif within the β1A tail is required for cell spreading, integrin signalling and formation of focal adhesion and stress fibers.
Fig. 1.
A tyrosine-to-alanine substitution in the NPIY motif of the β1A tail inhibits cell spreading, integrin signaling, and the formation of focal adhesions and stress fibers. (A) Schematic representation of the αIIlb-5/β3-1A heterodimeric chimeric integrins containing either the wild type (WT) or the Y783A (YA) mutant β1A tail with a tyrosine to alanine substitution within the membrane-proximal NPIY motif. These chimeras contain the extracellular and transmembrane domains of the αIIbβ3 fibrinogen receptor connected to the tails of the α5β1 fibronectin receptor. (B-D) CHO K1 cells stably expressing the WT or YA mutant integrin were adhered to fibrinogen for 1 hour in CCM1. (B) Cell area was measured and average cell area ± s.d. from three independent experiments is plotted, n=150 (*P<0.05). (C) Phosphorylation of FAK at Y397 was assayed by western blotting using antibodies against phosphorylated Y397 (upper panel). Blots were then stripped and reprobed for total FAK as a loading control (lower panel). p130CAS was immunoprecipitated and assayed for tyrosine phosphorylation by western blotting using monoclonal antibody 4G10. Blots were then stripped and reprobed for total immunoprecipitated p130CAS as a control. (D) Focal adhesions were visualized with antibodies to paxillin (green) and stress fibers with phalloidin (red). Scale bar: 20 μm.
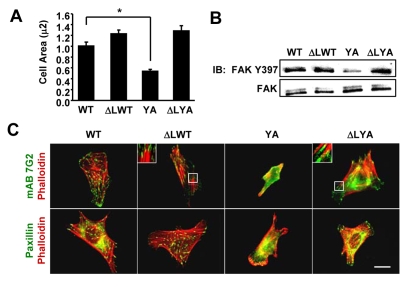
Our previous studies indicated that constitutive activation of the mutant integrin restored microtubule assembly (Reverte et al., 2006), suggesting that the YA mutation did not affect signaling events required for microtubule assembly downstream of integrin activation. Therefore, we questioned whether the constitutive activation of the mutant integrin would be sufficient to rescue other outside-in integrin-signaling-dependent processes. To constitutively activate the YA mutant, we used the α subunit, αIIb-ΔL, which contains a deletion of the membrane-proximal GFFKR sequence, resulting in constitutive integrin activation (O'Toole et al., 1994). CHO K1 cells were co-transfected with the αIIlb-ΔL subunit, together with either the β3-1A (ΔLWT) or β3-1AYA (ΔLYA) subunits and then adhered to fibrinogen. This approach allowed us to determine the effects of the YA mutation independently of its effects on integrin activation. The data indicate that experimentally activating the mutant integrin rescues cell spreading (Fig. 2A), adhesion-triggered FAK activation (Fig. 2B), as well as the formation of focal adhesions and stress fibers (Fig. 2C). Importantly, the rescue of outside-in integrin-signaling-dependent processes was not specific for the αIIb-ΔL mutant. Adhesion of cells to fibrinogen by αIIb-5/β3L712R-1AYA, which contains an activating mutation within the β3 transmembrane domain (Wegener et al., 2007) also restored integrin function (supplementary material Fig. S1). These results demonstrate that an intact NPIY motif is not required downstream of integrin activation for events dependent on outside-in signaling, and that a tyrosine-to-alanine substitution in this motif is tolerated once integrins are activated.
Fig. 2.
Constitutively activating the mutant integrin rescued cell spreading, integrin signaling, and the formation of focal adhesions and stress fibers. CHO K1 cells were transiently transfected with expression vectors for the indicated integrin subunits: WT (αIIb-5 and β3-1AWT); ΔLWT (αllb-ΔL and β3-1AWT), YA (αIIb-5 and β3-1AYA) or ΔLYA (αllb-ΔL and β3-1AYA). Transfected cells were adhered to fibrinogen for 1 hour in CCM1. (A) Plotted is the average cell area ± s.d. from three independent experiments, n=150 (*P<0.05). (B) FAK activation was assayed by western blotting with antibodies against phosphorylated Y397. Blots were reprobed for total FAK. (C) Localization of the recombinant integrins visualized by staining with monoclonal antibody 7G2 against the extracellular domain of the human β3 subunit (green) together with phalloidin for actin filaments (red). Focal adhesions are visualized by staining for paxillin (green) along with phalloidin for actin filaments (red). Scale bar: 20 μm.
Talin-1 is required downstream of integrin activation when cells are adhered by integrins containing an intact NPIY motif
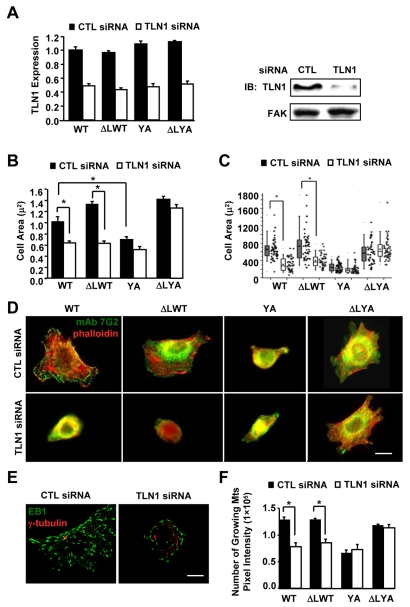
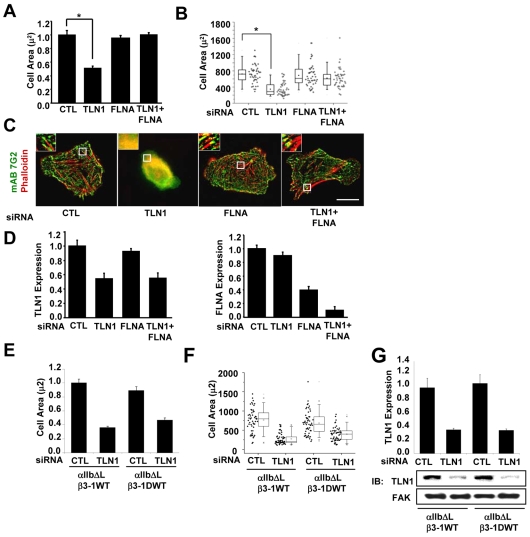
In addition to regulating the inside-out activation of integrins, talin-1 provides a mechanism to link integrins with the actin cytoskeleton downstream of integrin engagement or activation (Critchley, 2004). Data from the constitutively activated YA mutant suggest that binding of talin-1 to the NPIY motif is not strictly required for many outside-in integrin-signaling-dependent processes. Since talin-1 can also bind to the membrane-proximal region of β-tails (Gingras et al., 2009; Wegener et al., 2007), we wanted to further investigate the role of talin-1 in cells adhered by the constitutively activated YA mutant integrin. Thus, we assayed the effects of TLN1 siRNA on cell spreading, focal adhesion, stress fibers, and microtubule assembly in the presence or absence of constitutive integrin activation. Integrins were activated by co-expression of the αIIb/ΔL subunit, which activates integrins independently of talin-1 protein expression (O'Toole et al., 1995; Tadokoro et al., 2003), and talin-1 depletion was confirmed by western blotting (Fig. 3A). Consistent with published studies (Priddle et al., 1998; Zhang et al., 2008), we demonstrated that talin-1 depletion inhibits sustained spreading when cells were adhered by WT integrins (Fig. 3B,C). Surprisingly, constitutively activating the WT integrin (ΔLWT) did not restore cell spreading (Fig. 3B,C). By contrast, the constitutively activated mutant integrin (ΔLYA) supported cell spreading, and promoted the formation of focal adhesions and stress fibers in talin-1-depleted cells (Fig. 3B-D). The inhibition of talin-1 expression suppressed integrin activation, as expected, without affecting surface expression, as previously shown by others (O'Toole et al., 1995; Tadokoro et al., 2003) (supplementary material Fig. S2). Furthermore, similar results were obtained with a second siRNA against TLN1 (supplementary material Fig. S3). Thus, the observed results were not likely to be due to off-target affects.
Fig. 3.
Talin-1 is required downstream of integrin activation for outside-in integrin signaling-dependent events when cells are adhered by WT, but not mutant integrins. CHO K1 cells were transiently transfected with either control siRNA or TLN1 siRNA#1 for 48 hours, and then co-transfected for 24 hours with expression vectors for the indicated integrin subunits: WT (αIIb-5 and β3-1AWT); YA (αIIb-5 and β3-1AYA); ΔLWT (αllb-ΔL and β3-1AWT); and ΔLYA (αllb-ΔL and β3-1AYA). Aliquots of transfected cells were used to confirm knockdown of talin-1 and to assay cell spreading, focal adhesions and stress fibers, as well as microtubule growth. For these experiments, cells were replated on fibrinogen for 1 hour in CCM1. (A) The efficiency of talin-1 knockdown was determined by western blotting. Plotted is the average expression level of talin-1 from three independent experiments, together with a representative blot. (B) Plotted is the average cell area ± s.d. from the same three independent experiments in A, n=150. (C) The areas of individual cells from one of these experiments are depicted in a box and whiskers plot. (D) Localization of the recombinant integrins visualized by staining for the human β3 subunit (green) together with phalloidin for actin filaments (red). (E,F) Cells were stained for α-tubulin to label the centrosome (red) and for EB1 to label the plus-ends of growing microtubules (green). Images of individual cells were acquired as a Z-series with the identical acquisition parameters, deconvolved, and then used to generate a maximum projection. (E) Representative images of cells adhered to fibrinogen by the WT integrin with or without TLN1 siRNA. (F) The pixel intensity for EB1 staining was measured for individual cells. Plotted is the average pixel intensity ± s.d. from three independent experiments, n=15 (*P<0.05). Scale bars: 20 μm.
We also asked whether talin-1 was required downstream of integrin activation for the regulation of microtubule assembly, since our published studies demonstrated that constitutively activating the YA mutant rescued this process. For these studies, cells were transfected with control or TLN1 siRNA, and then the various subunits to express WT, YA, ΔLWT and ΔLYA integrins, as above. Transfected cells were adhered to fibrinogen, and microtubule growth was quantified by measuring EB1, a protein that binds to the plus-ends of growing microtubules (Akhmanova and Steinmetz, 2008). The results indicated that cells depleted of talin-1 and adhered by WT or constitutively activated WT integrins were significantly inhibited in microtubule growth (Fig. 3E,F). By contrast, cells adhered by constitutively activated YA integrins supported microtubule growth even when talin-1 was depleted. The results suggest that β-tail binding proteins that promote or inhibit cell spreading and focal adhesion formation similarly regulate microtubule growth.
The requirement for talin-1 downstream of integrin activation is independent of the mechanism of activation
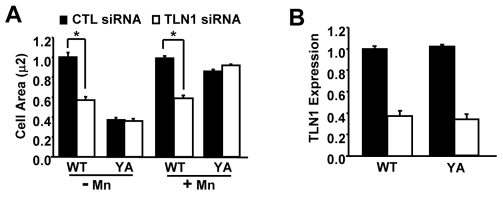
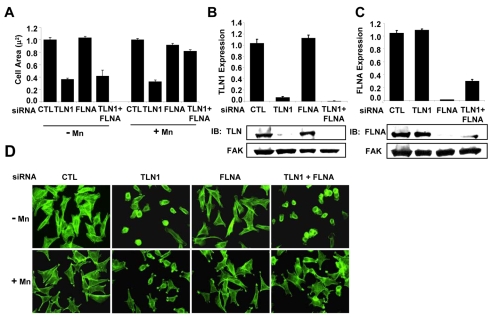
To test whether the effects of talin-1 knockdown are independent of the mechanism used to promote integrin activation, we assayed cells treated with manganese, an extracellular activator of β1A integrins. Similarly to our activating mutations, we found that manganese promoted spreading when cells were adhered by the YA mutant integrin, even when talin-1 was depleted (Fig. 4). However, manganese was unable to restore spreading in talin-1-depleted cells adhered by WT integrins (Fig. 4). Thus, the effects of the talin-1 knockdown are independent of the mechanism used to promote integrin activation. In addition, locking integrins in a constitutively activated state by either mechanism cannot circumvent the absence of talin-1 if there is an intact NPIY motif. Taken together, our results indicate that talin-1-NPIY interactions are important downstream of integrin activation in a variety of adhesion-dependent processes.
Fig. 4.
The requirement for talin-1 downstream of integrin activation independent of the mechanism of activation. (A,B) Cells stably expressing the WT or the YA mutant integrin were transfected with control or TLN1 siRNA#1 for 72 hours. Aliquots of transfected cells were used to confirm talin-1 knockdown and to assay cell spreading following adhesion to fibrinogen for 1 hour in CCM1 with or without manganese (Mn2+). (A) Plotted is the average cell area ± s.d. from three independent experiments, n=150 (*P<0.05). (B) The efficiency of talin-1 knockdown was determined by western blotting. Plotted is the average level of talin-1 expression ± s.d. from the same three independent experiments shown in A.
Filamin A (FLNa) inhibits outside-in signaling-dependent events when talin-1-depleted cells are adhered by constitutively activated WT integrins
The constitutively activated YA mutant integrin supported cell spreading, focal adhesion and stress fiber formation, as well as microtubule growth, even when talin-1 was depleted. By contrast, the constitutively activated WT integrin was unable to support these processes in talin-1-depleted cells. Taken together, these results suggest that when talin-1 is depleted, a protein binds to the β1A tail in an NPIY-dependent manner to inhibit these processes. A likely candidate is FLNa, because its binding to the β1A tail is inhibited by the YA mutation (Pfaff et al., 1998). Although FLNa has been shown to inhibit integrin activation (Kiema et al., 2006), its role as an inhibitor of outside-in integrin signaling dependent events has not been analyzed in the context of constitutively activated integrins. Therefore, we investigated the inhibitory role of FLNa downstream of integrin activation. For these studies, cells were depleted of talin-1 or FLNa individually or together using siRNA. The results show that knocking down talin-1 expression inhibited spreading and focal adhesion formation as expected, whereas inhibiting expression of FLNa had no obvious affect on these processes (Fig. 5A-C). Importantly, co-depletion of talin-1 and FLNa restored spreading and the formation of focal adhesions and stress fibers when cells were adhered by constitutively activated WT integrins (Fig. 5A-C). Depletion of talin-1 and FLNa was confirmed by western blotting (Fig. 5D). Similar results were obtained using a second FLNA siRNA to inhibit the expression of FLNa, indicating that the results are not due to off-target effects (Fig. S4). Additional controls showed that a high percentage (>90%) of the cells adhered to fibrinogen expressed the chimeric integrins and did so at similar levels with or without knockdown of talin-1 and FLNa (supplementary material Fig. S5).
Fig. 5.
FLNa inhibits outside-in signaling-dependent events when talin-1-depleted cells are adhered by constitutively activated WT integrins. CHO K1 cells were transiently transfected with control siRNA, TLN1 siRNA#1 or FLNA siRNA#1, or both TLN1 and FLNA siRNA for 48 hours (for details see Materials and Methods), and then co-transfected for 24 hours with expression vectors for αllb-ΔL along with the β3-1A WT or β3-1D WT integrin subunits. Aliquots of transfected cells were used to confirm knockdown and to assay cell spreading and stress fibers following adhesion to fibrinogen for 1 hour in CCM1. (A) Plotted is the average cell area ± s.d. from three independent experiments (n=150). (B) Areas of individual cells from one of these experiments are depicted in a box and whisker plot (*P<0.05). (C) Localization of the recombinant integrins visualized by staining with monoclonal antibody 7G2 against human β3 subunit (green) together with phalloidin for actin filaments (red). (D) The efficiency of talin-1 (left) and FLNa (right) knockdown was determined by western blotting. Plotted is the average expression level of the indicated proteins ± s.d. from three independent experiments shown in A-C. (E) Plotted is the average cell area ± s.d. of cells expressing αIIb-ΔL/β3-1A WT or αIIb-ΔL/β3-1D WT (n=150). (F) Areas of individual cells from one of these experiments are depicted in a box and whiskers plot (*P<0.05). (G) The efficiency of talin-1 knockdown was determined by western blotting. Plotted is the average expression level of the indicated proteins.
The ability of constitutively activated YA integrins to support cell spreading in talin-depleted cells suggests that alternative β1A-tail–cytoskeletal linkages are able to support cell spreading (Fig. 3). Our data suggest that FLNa binds to wild-type β1A tails, thereby inhibiting these alternative cytoskeletal linkages in talin-depleted cells. To examine this possibility, we made use of constitutively activated integrins containing the alternatively spliced β1D tail, which binds to talin, but binds poorly to FLNa (Calderwood et al., 2001; Pfaff et al., 1998). The results indicate that the β1D tail cannot promote spreading in talin-depleted cells (Fig. 5E-G), even though cells adhered to fibrinogen express similar levels of integrins containing the β1D tail whether or not talin-1 expression is knocked down (supplementary material Fig. S6). These results suggest that inhibition of the ability of FLNa to bind to the β-tail is not sufficient to restore spreading and that specific amino acids present in the β1A tail and not in the alternatively spliced β1D tail also contribute to this phenotype. Proteins that bind to the β1A tail to promote spreading in talin-depleted CHO cells might not bind to the β1D tail.
The requirement for talin-1 downstream of integrin activation is not specific to recombinant chimeric integrins
Published studies indicate that adhesion of CHO cells to fibronectin is dependent upon endogenous α5β1 integrins (Brown and Juliano, 1985). To confirm that the effects of talin-1 knockdown were not specific to the WT chimeric integrins, we examined cells adhered to fibronectin by their endogenous α5β1 integrins. Talin-1 and FLNa expression was inhibited individually and together using siRNA. Integrins were then activated with manganese as above and the effects on spreading were analyzed. Similarly to what was observed for cells adhered by WT chimeric integrins, the depletion of talin-1 inhibited spreading on fibronectin mediated by endogenous α5β1 integrins (Fig. 6A-C). Activating endogenous integrins with manganese failed to restore cell spreading in talin-1-depleted cells (Fig. 6A-C). Importantly, depleting cells of FLNa together with talin-1 restored cell spreading by manganese-activated α5β1 integrins (Fig. 6A-C). Thus, the inhibitory effects of FLNa in talin-1-depleted cells are not specific to the chimeric integrin, because similar effects were observed for endogenous α5β1 integrins.
Fig. 6.
Talin-1 is required downstream of integrin activation for spreading when cells are adhered by endogenous integrins. CHO K1 cells were transiently transfected with control, TLN1 siRNA#1, FLNA siRNA#1 or both TLN1 and FLNA siRNAs for 48 hours. Aliquots of transfected cells were used to confirm knockdown and to assay cell spreading, following adhesion to fibronectin for 1 hour in CCM1 with or without Mn2+. (A) Plotted is the average cell area ± s.d. from three independent experiments, n=150. (B) The efficiency of talin-1 (left) and FLNa (right) knockdown was determined by western blotting. Plotted is the average expression level of the indicated proteins ± s.d. (C) Representative images of cells adhered to fibronectin by endogenous integrins with or without Mn2+ are depicted by staining with monoclonal antibody 7G2 against human β3 subunit (green).
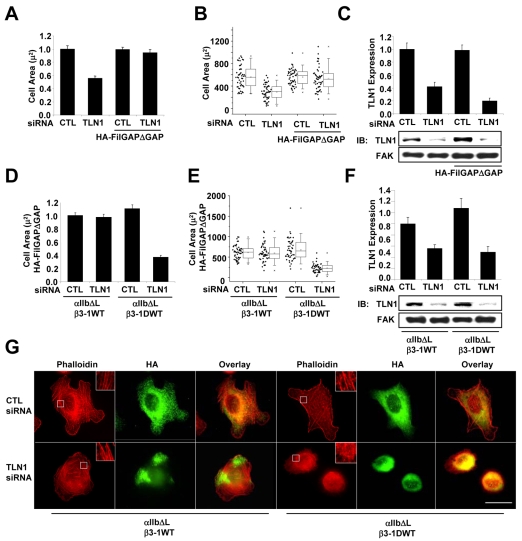
Expression of FilGAPΔGAP rescues spreading in talin-depleted cells adhered by integrins containing the β1A tail, but not the β1D tail
Our data suggest that when cells are depleted of talin-1, β1A-tail-FLNa interactions inhibit spreading downstream of integrin activation. Studies from several laboratories, including our own, demonstrated that Rac activity is required for cell spreading (Berrier et al., 2000; Price et al., 1998). We previously reported that the integrin β-tail is both required and sufficient to activate Rac, indicating a central role for β-tail protein interactions in regulating Rac activity (Berrier et al., 2002). Interestingly, a novel FLNa-specific binding protein, FilGAP, contains GTPase-activating protein (GAP) activity, which specifically antagonizes Rac activity, cell spreading and lamellipodia formation (Ohta et al., 2006). Therefore, we investigated the possibility that the β1A tail recruits FLNa and FilGAP to inhibit spreading when talin-1 is depleted. To test this hypothesis, we suppressed the activity of endogenous FilGAP by expressing the HA-FilGAPΔGAP mutant, which binds FLNa, but lacks the GAP domain (Ohta et al., 2006). In addition, we inhibited FLNa-β-tail interactions by adhering cells by constitutively activated integrins containing the β1D tail. The results showed that expression of HA-FilGAPΔGAP restored spreading in talin-1-depleted cells adhered by constitutively activated integrins containing the β1A tail (Fig. 7G and Fig. 7A-C). Interestingly, expression of HA-FilGAPΔGAP failed to restore spreading in cells adhered by constitutively activated integrins containing the β1D tail (Fig. 7D-F). As expected, control experiments demonstrated that >85% of the cells adhered to fibrinogen expressed similar levels of the chimeric integrin and approximately 60% of these cells expressed HA-FILGAPΔGAP (supplementary material Fig. S7). A majority of these adherent cells were also positively transfected with the indicated siRNAs, because talin-1 expression was significantly inhibited in cells adhered to fibrinogen (supplementary material Fig. S7). Taken together, these results support the idea that FilGAP regulates spreading by a mechanism requiring FLNa-β-tail interactions.
Fig. 7.
Expression of FilGAPΔGAP restores spreading and stress fiber formation in cells adhered by integrins containing the β1A tail, but not the β1D tail. CHO K1 cells were transiently transfected with control or TLN1 siRNA#1 for 48 hours, and then co-transfected for 24 hours with expression vectors for αIIlb-ΔL together with β3-1A WT integrin subunits with or without HA-FilGAPΔGAP. Aliquots of transfected cells were used to confirm knockdown and to assay spreading following adhesion to fibrinogen for 1 hour in CCM1. (A) Plotted is the average cell area ± s.d. from three independent experiments, n=150. (B) Areas of individual cells from one of these experiments depicted in a box and whisker plot (*P<0.05). (C) The efficiency of talin-1 knockdown was determined by western blotting. Plotted is the mean expression level of the indicated proteins ± s.d. (D) CHO K1 cells were transiently transfected with control or TLN1 siRNA #1 for 48 hours, and then co-transfected for 24 hours with expression vectors for αIIlb-ΔL together with either the β3-1A WT or β3-1D integrin subunits with or without HA-FilGAPΔGAP. Aliquots of transfected cells were used to confirm knockdown and to assay spreading and stress fiber formation following adhesion to fibrinogen for 1 hour in CCM1. Plotted is the average cell area ± s.d. from three independent experiments, n=150 of cells transfected with HA-FilGAPΔGAP. (E) Areas of individual cells from one of these experiments depicted in a box and whisker plot (*P<0.05). (F) The efficiency of talin-1 knockdown was determined by western blotting. Plotted is the average expression level of the indicated proteins ± s.d. (G) Representative images of cells stained with monoclonal antibody HA to visualize FilGAPΔGAP positive cells (green) together with phalloidin for actin filaments (red). Scale bar: 20 μm.
Discussion
Our current study has three main findings: (1) Constitutively activated YA integrins are able to promote spreading and the formation of focal adhesions and stress fibers, in addition to the assembly of the microtubule cytoskeleton, even when talin-1 is depleted. These results indicate that talin-1 binding to the NPIY motif or other regions of the β1A tail is not strictly required to link integrins to actin filaments at adhesion sites or to promote several other outside-in integrin signaling-dependent processes, and suggest that other proteins that do not require the NPIY motif to bind to the β1A tail can replace talin-1 in these processes. (2) Constitutively activated WT integrins are inhibited in their ability to promote these processes when talin-1 is depleted, unless FLNa is also depleted. These results provide strong evidence that the binding of talin-1 to the NPIY motif promotes these processes, at least in part, by preventing the inhibitory affects of FLNa, presumably by blocking its ability to bind to the β1A tail. (3) The expression of FilGAPΔGAP rescues spreading and the formation of focal adhesions and stress fibers in talin-depleted cells adhered by constitutively activated integrins containing the β1A tail, but not the β1D tail. These results suggest that FLNa and FilGAP regulate these integrin-mediated processes by binding to the β1A tail.
Our findings are consistent with previous studies demonstrating that the depletion of talin-1 inhibits integrin activation, cell spreading, focal adhesions and stress fibers (Priddle et al., 1998; Tadokoro et al., 2003; Zhang et al., 2008). Zhang and colleagues (Zhang et al., 2008) showed that treating talin-depleted mouse embryonic fibroblasts with manganese restored cell spreading. However, we did not find that constitutively activating WT integrins with manganese or with activating mutations was able to restore cell spreading when talin-1 was depleted. Importantly, we demonstrated that manganese, similarly to our activating mutation, promoted spreading when cells were adhered by the YA integrin even when talin-1 was depleted. The conflicting results in the two studies could be due to differences in cell types. For example, the expression of FLNa or its β-tail binding activity could be greater in CHO cells compared with mouse fibroblasts.
Although we focused on FLNa because it is the most abundant isoform expressed in non-muscle cells (Stossel et al., 2001; van der Flier and Sonnenberg, 2001), cells can express all three isoforms (see Baldassarre et al., 2009). FLNb can also bind to the integrin β1 tail, and the abilities of FLNa and FLNb to bind to the tail can be regulated by alternative splicing (van der Flier et al., 2002). We do not know whether CHO cells express significant levels of FLNb and/or FLNc. FLNa is likely to be the only isoform relevant to the inhibition of cell spreading when talin-1-depleted CHO cells are adhered by constitutively activated integrins containing the WTβ1A tail, because both siRNAs used to inhibit FLNa expression are specific for the FLNA gene and do not have significant homology to the genes encoding FLNb or FLNc. Additionally, FilGAP binds to FLNa and not to FLNb (Nakamura et al., 2009). Thus, our observation that FilGAPΔGAP rescues spreading in talin-1-depleted cells adhered by integrins containing the β1A tail, and not the β1D tail, further supports the idea that FLNa and its binding to the β1A tail contributes to the inhibition of spreading in talin-1-depleted cells. A recent study identified a role for FLN proteins in regulating early stages of cell spreading in HT1080 fibrosarcoma cells, and suggested that FLNb can compensate for the depletion FLNa in regulating these early stages of spreading (Baldassarre et al., 2009). We assayed cell spreading only after 1 hour, which is more a readout for sustained cell spreading than initial events during cell spreading. Our data suggest that if FLNb is expressed in CHO cells, it cannot compensate for the function of FLNa in our assays.
How might the β1A tail connect to actin filaments at adhesion sites independently of talin-1 and the NPIY motif? There are several candidates for linking β-tails with actin filaments independently of this motif, including kindlin, α-actinin and skelemin (Otey et al., 1993; Rajfur et al., 2002; Reddy et al., 1998; Shi et al., 2007; Harburger et al., 2009). The binding sites for α-actinin and skelemin are present in the membrane-proximal regions of both the β1A tail and the β1D tail. Interestingly, kindlin binds amino acids between the two NPxY motifs of the β1A tail; these amino acids are not present in the β1D tail (Harburger et al., 2009; Legate and Fassler, 2009). Thus, kindlin may be the best candidate to link the β1A tail to actin filaments in talin-depleted cells. The integrin-linked kinase (ILK) could also mediate this linkage; however, the binding site for ILK has not yet been characterized (Hannigan et al., 1996; Legate and Fassler, 2009).
Since FLNa and talin-1 have overlapping binding sites, including the membrane-proximal NPxY motif, they compete for binding β-tails (Kiema et al., 2006). Interestingly, individual β-tails differ in their abilities to bind talin-1 and FLNa. For example, the β1D tail binds strongly to talin, but poorly to FLNa; by contrast, the β7-tail binds strongly to FLNa and not as well to talin-1 (Calderwood et al., 2001). Importantly, talin-β-tail and FLNa-β-tail interactions differentially affect cell behavior. The binding of talin-1 to the β-tail promotes integrin activation, cell spreading and the formation of focal adhesions and stress fibers, whereas FLNa-β-tail interactions inhibit integrin activation, cell adhesion and migration (Kiema et al., 2006; Takala et al., 2008). Our studies suggest that in CHO cells talin-1 preferentially binds to β1A tails, because the inhibitory effects of FLNa are only observed after talin-1 depletion.
Although our studies examine the effects of FLNa-β-tail interactions when talin-1 expression is inhibited experimentally, these results are physiologically important, because the abilities of talin-1 and FLNa to bind to β-tails can be regulated by intracellular signaling. Phosphorylation of threonine residues or the corresponding phosphomimetic mutations in the β2- and β7-tails inhibits their interaction with FLNa (Kiema et al., 2006; Takala et al., 2008). Importantly, interactions between talin-1 and the β-tail are not inhibited by these same phosphorylation events. Interestingly, tyrosine phosphorylation of the membrane-proximal NPLY motif in the β3-tail inhibits talin-1 binding (Calderwood et al., 2003). It will be important to determine whether similar signaling events regulate the interaction of FLNa and talin-1 with the β1A tail.
The abilities of talin-1 and FLNa to bind to β-tails are also conformationally regulated (Goksoy et al., 2008; Lad et al., 2007; Martel et al., 2001; Yan et al., 2001). The binding of talin-1 to phosphoinositol 4,5-bisphosphate (PIP2) relieves the autoinhibitory interactions of talin-1 to promote its association with integrin β-tails; the cleavage of talin-1 by calpain also unmasks the integrin-binding site (Goksoy et al., 2008; Martel et al., 2001; Yan et al., 2001). Specific signaling pathways that impact the conformation of FLNa to promote its interaction with the β-tail have not yet been identified; however, it has been suggested that unfolding of FLNa induced by mechanical force unmasks its integrin-binding domain (Pentikainen and Ylanne, 2009).
Our studies provide novel insight into the mechanism by which FLNa-β-tail interactions alter cell behavior downstream of integrin activation. Our data suggest that when talin-1 is depleted, FLNa binds to the β1A tail and recruits FilGAP to locally inhibit Rac activation needed for cell spreading. Recent studies demonstrated that β1A-integrins can recruit FLNa and FilGAP to collagen-coated beads to inhibit lamellipodia formation in response to applied force to these adhesion sites (Shifrin et al., 2009). Thus, force applied to integrin-mediated adhesions signals FLNa recruitment, which presumably replaces talin-1 on the β-tail, although this has not yet been directly tested. In a related study, FLNa-β1A tail interactions were found to be required for collagen remodeling and tissue morphogenesis in collagen gels (Gehler et al., 2009). Morphogenesis usually occurs in compliant gels and not in rigid matrices; however, increasing FLNa expression and FLNa-β1A tail interactions restored morphogenesis in stiffer matrices (Gehler et al., 2009). It is not known whether FilGAP has a role in this process. It will be interesting to determine the effects of force and/or matrix rigidity on talin-β-tail interactions in these contexts.
There is increasing evidence that supports the crosstalk between microtubules, actin stress fibers and focal adhesions (Kaverina et al., 1999; Kaverina et al., 1998; Small et al., 2002; Small and Kaverina, 2003). The growth of microtubules is induced by increased stress in the actin cytoskeleton and some studies suggest that microtubules target focal adhesions by tracking along actin filaments (Small et al., 2002; Small and Kaverina, 2003). Our current findings demonstrate that the ability of constitutively activated YA mutant integrins to restore microtubule assembly correlates with their ability to promote cell spreading, and formation of focal adhesions and actin stress fibers, suggesting that these processes are functionally linked. Since Rho-family GTPases have central roles in regulating both the actin and microtubule cytoskeletons (Jaffe and Hall, 2005), it will be interesting to determine whether the misregulation of these
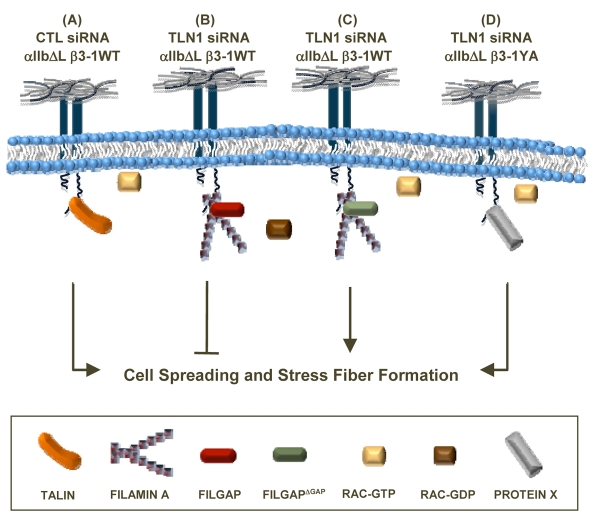
Fig. 8.
Model showing how talin-1 promotes outside-in integrin-signaling dependent events by inhibiting the binding of FLNa and the recruitment of FilGAP. Talin-1 is required downstream of integrin activation to prevent the binding of FLNa to the NPIY motif and the recruitment of FilGAP to the β1 tail, thereby promoting cell spreading and the subsequent formation of stress fibers. (A) Talin-1 binds to the NPIY motif of activated integrins to promote these processes. (B) When WT integrins are activated in the absence of talin-1, FLNa binds to the NPIY motif and recruits FilGAP to inhibit cell spreading. (C) However, when FilGAPΔGAP, which lacks the GAP domain, is expressed in talin-1-depleted cells adhered by the constitutively activated WT integrins, spreading and stress fiber formation are restored because the activation of Rac is not inhibited. (D) In talin-1-depleted cells adhered by the constitutively activated YA mutant integrin, a protein (X) that does not require the NPIY motif binds to the β1A tail to promote cell spreading and stress fiber formation.
GTPases contributes to both cytoskeletal phenotypes in cells adhered by the mutant integrin.
In summary, we propose that many outside-in integrin signaling-dependent processes require the binding of talin-1 to the membrane-proximal NPIY motif downstream of integrin activation to prevent the inhibitory effects of FLNa–β1-tail interactions and the recruitment of FilGAP to inhibit Rac activation. The mechanisms that regulate the differential binding of talin-1 and FLNa to the β-tails of matrix-engaged integrins and how they contribute to the regulation of adhesion-dependent processes is an important direction for future investigation. These findings ultimately provide mechanistic insight into how differential protein-β-tail interactions regulate specific adhesion-mediated processes.
Materials and Methods
Cell culture and transfection
Chinese hamster ovary (CHO K1) cell lines stably expressing αIIb-5/β3-1A recombinant chimeric integrins containing the WT or the Y783A (YA) β1A tail were previously described (Reverte et al., 2006). Parental CHO K1 and WT and YA cell lines were maintained in Ham's F12 medium (Invitrogen) supplemented with 10% FBS, and cultured in the serum-free growth promoting medium CCM1 (Hyclone) as indicated. For many experiments, CHO K1 cells were transiently co-transfected with expression vectors for various integrin subunits, as indicated, using TransIT-CHO reagent (Mirus Bio Corporation). The following integrin subunits were used: αIIb-5, αIIb-ΔL (O'Toole et al., 1994), β3-1WT or β3-1YA (Reverte et al., 2006), β3L712R-1AWT and β3L712R-1AYA (see below).
Generation of β3L712R-1A subunits
Vectors encoding the WT and YA mutant β3-1 subunits containing the L712R activating mutation were generated by ligating three fragments: (1) the BamHI-XhoI restriction fragment containing the pcDNA3.1(+)/Zeo backbone and the extracellular domain of the β3 subunit isolated from the previously described β3-1A WT expression vector (Reverte et al., 2006); (2) the BamHI-HindIII fragment containing the L712R activating mutation in the transmembrane domain, which was generated by PCR using the following primers and PCR products and activated by digestion with the BamHI and HindIII restriction enzymes: 5′-TGGCTGGCTGGGATCCCAGTGTGAG-3′ and 5′-ATTAAAAGCTTCCAGATGAGCCGGGCGGCAAGG-3′; and (3) the HindIII-XhoI fragments containing the WT or YA β1A tail isolated from the Tac-β1A WT and Tac-β1A YA expression vectors (Bodeau et al., 2001). The generation of these mutant subunits was confirmed by DNA sequence analysis.
Generation of β3-1D subunit
The pcDM8 expression vector containing the β3-1D WT construct was kindly provided by Mark Ginsberg (University of California, San Diego, La Jolla, CA). The β1D tail was excised with HindIII and XhoI restriction endonucleases (New England Biolabs, Ispwich MA) and subsequently ligated to the BamHI-XhoI restriction fragment containing the pcDNA3.1(+)/Zeo backbone and the extracellular domain of the β3 subunit along with the BamHI-HindIII transmembrane fragment, as described above.
Signaling assays
To assay integrin-triggered tyrosine phosphorylation of FAK and p130CAS, 1×106 cells were plated in CCM1 on 100 mm dishes precoated with 15 μg/ml fibrinogen and incubated at 37°C for 1 hour. Cells were lysed with modified RIPA buffer. Phosphorylation of FAK at Tyr397 was assayed directly by western blotting using phosphospecific polyclonal antibodies (Biosource). p130CAS was immunoprecipitated and its phosphorylation was assayed by western blotting using the phosphotyrosine-specific monoclonal antibody 4G10 (BD Transduction Laboratories) as previously described (Berrier et al., 2008).
Immunofluorescence microscopy
Immunofluorescence microscopy was performed using a Nikon TE200-E inverted microscope equipped with phase-contrast and epifluorescence, a Roper coolsnap HQ digital camera, Ludl rotary encoded stage, and Metavue and Autoquant deconvolution software.
Cell spreading, focal adhesions, stress fibers and microtubule growth
For all these assays, cells were replated onto fibrinogen-coated coverslips in CCM1 and incubated at 37°C for 1 hour. To examine focal adhesions and actin stress fibers, cells were fixed with 4% paraformaldehyde, permeabilized with 0.4% Triton-X-100, blocked with 2% BSA and then stained with monoclonal antibody to paxillin (BD Transduction Laboratories) or 7G2 against human integrin β3 and phalloidin-AF594 (Invitrogen). Recombinant human β3 integrins were stained prior to permeabilization. To assay cell spreading, the area of individual cells was defined by thresholding phalloidin-AF594 staining and then measured with Metavue software. In each of three individual experiments the data were normalized to WT. We also present the data from a representative experiment using a box and whisker plot showing the distribution individual cell areas. To assay microtubule growth, cells were fixed with methanol (20°C), blocked with 2% BSA and stained with polyclonal antibodies for γ-tubulin (Sigma, AK15) and monoclonal antibody to EB1 (clone 5, BD Transduction Laboratories). Staining was visualized with Alexa Fluor 594 and Alexa Fluor 488 (Invitrogen). Images were acquired and analyzed as described in the legend to Fig. 3.
siRNA
To inhibit the expression of talin-1 and FLNa, targeting sequences were identified that are identical in human, mouse and rat. Two siRNAs to knock down the expression of each gene were purchased from Dharmacon: TLN1 siRNA#1 (ACAAGAUGGAUGAAUCAAAUU), TLN1 siRNA#2 (AGCAGAAGGGAGAG CGUAAUU), FLNA siRNA#1 (ACTTCAAGGTGTACACAAA) and FLNA siRNA#2 (CTTTGAGCCT GCAGAGTTT). The control siRNA (siRNA CONTROL Non-Targeting no. 2) was also purchased from Dharmacon.
CHO cells were transfected with 200 μM of the indicated siRNA(s) using Oligofectamine (Invitrogen) following the manufacturer's protocol. For co-knockdown experiments 400 μM of siRNA was used for each transfection: 400 μM control; 200 μM control + 200 μM TLN1 ; 200 μM control + 200 μM FLNA; or 200 μM TLN1 + 200 μM FLNA. Cells were assayed 72 hours after transfection. Knockdown efficiency was determined by western blotting using monoclonal antibody 8D4 (Sigma) that recognizes both talin-1 and talin-2 (Zhang et al., 2008) and monoclonal antibody PM6/317 to FLNa (Chemicon). The blots were stripped and reprobed with the indicated antibodies as loading controls.
Integrin-activation assay
To assay integrin activation, cells expressing the WT or the YA integrin were transfected with a GFP expression vector alone or together with siRNA against a control or talin-1 sequence. Cells were harvested and incubated in suspension in Tyrodes Buffer with monoclonal antibody PAC1 (BD Biosciences) ±10 mM EDTA or 2 mM MnCl2 for 30 minutes at room temperature. PAC1 binding was detected with Alexa Fluor 647 IgM antibody (BD Biosciences) by flow cytometry gating on the GFP-positive cell population. The mean fluorescence intensity was measured in the absence of treatment (F), in the presence of 10 mM EDTA (F0), or in the presence of 2 mM MnCl2 (Fmax). The activation index was calculated as AI=100*(F–F0)/(Fmax–F0) (Wegener et al., 2007).
Supplementary Material
Acknowledgments
The authors thank Jane Sottile and Diane Colello Borges for critically reading this manuscript, Mark Ginsberg for expression vectors for various chimeric integrins, Eric Brown for monoclonal antibody 7G2 against human β3 integrin. This work was supported by NIH grants GM51540 and T32-HL07194. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/??/????/DC1
References
- Akhmanova A., Steinmetz M. O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309-322 [DOI] [PubMed] [Google Scholar]
- Askari J. A., Buckley P. A., Mould A. P., Humphries M. J. (2009). Linking integrin conformation to function. J. Cell Sci. 122, 165-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre M., Razinia Z., Burande C. F., Lamsoul I., Lutz P. G., Calderwood D. A. (2009). Filamins regulate cell spreading and initiation of cell migration. PLoS One 4, e7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier A. L., Mastrangelo A. M., Downward J., Ginsberg M., LaFlamme S. E. (2000). Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J. Cell Biol. 151, 1549-1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier A. L., Martinez R., Bokoch G. M., LaFlamme S. E. (2002). The integrin beta tail is required and sufficient to regulate adhesion signaling to Rac1. J. Cell Sci. 115, 4285-4291 [DOI] [PubMed] [Google Scholar]
- Berrier A. L., Jones C. W., LaFlamme S. E. (2008). Tac-beta1 inhibits FAK activation and Src signaling. Biochem. Biophys. Res. Commun. 368, 62-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodeau A. L., Berrier A. L., Mastrangelo A. M., Martinez R., LaFlamme S. E. (2001). A functional comparison of mutations in integrin beta cytoplasmic domains: effects on the regulation of tyrosine phosphorylation, cell spreading, cell attachment and beta1 integrin conformation. J. Cell Sci. 114, 2795-2807 [DOI] [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. (1985). Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science 228, 1448-1451 [DOI] [PubMed] [Google Scholar]
- Calderwood D. A., Zent R., Grant R., Rees D. J., Hynes R. O., Ginsberg M. H. (1999). The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274, 28071-28074 [DOI] [PubMed] [Google Scholar]
- Calderwood D. A., Huttenlocher A., Kiosses W. B., Rose D. M., Woodside D. G., Schwartz M. A., Ginsberg M. H. (2001). Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat. Cell Biol. 3, 1060-1068 [DOI] [PubMed] [Google Scholar]
- Calderwood D. A., Fujioka Y., de Pereda J. M., Garcia-Alvarez B., Nakamoto T., Margolis B., McGlade C. J., Liddington R. C., Ginsberg M. H. (2003). Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 100, 2272-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley D. R. (2004). Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem. Soc. Trans. 32, 831-836 [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Fagerholm S. C., Nurmi S. M., Chavakis T., Marchesan S., Gronholm M. (2009). Regulation of integrin activity and signalling. Biochim. Biophys. Acta 1790, 431-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehler S., Baldassarre M., Lad Y., Leight J. L., Wozniak M. A., Riching K. M., Eliceiri K. W., Weaver V. M., Calderwood D. A., Keely P. J. (2009). Filamin A-beta1 integrin complex tunes epithelial cell response to matrix tension. Mol. Biol. Cell 20, 3224-3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A. R., Ziegler W. H., Bobkov A. A., Joyce M. G., Fasci D., Himmel M., Rothemund S., Ritter A., Grossmann J. G., Patel B., et al. (2009). Structural determinants of integrin binding to the talin rod. J. Biol. Chem 284, 8866-8876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksoy E., Ma Y. Q., Wang X., Kong X., Perera D., Plow E. F., Qin J. (2008). Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G. E., Leung-Hagesteijn C., Fitz-Gibbon L., Coppolino M. G., Radeva G., Filmus J., Bell J. C., Dedhar S. (1996). Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379, 91-96 [DOI] [PubMed] [Google Scholar]
- Harburger D. S., Bouaouina M., Calderwood D. A. (2009). Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 284, 11485-11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. E., Diaz-Gonzalez F., Leong L., Wu C., McDonald J. A., Shattil S. J., Ginsberg M. H. (1996). Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Biol. Chem. 271, 6571-6574 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687 [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. (2005). Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247-269 [DOI] [PubMed] [Google Scholar]
- Kaapa A., Peter K., Ylanne J. (1999). Effects of mutations in the cytoplasmic domain of integrin beta(1) to talin binding and cell spreading. Exp. Cell Res. 250, 524-234 [DOI] [PubMed] [Google Scholar]
- Kaverina I., Rottner K., Small J. V. (1998). Targeting, capture, and stabilization of microtubules at early focal adhesions. J. Cell Biol. 142, 181-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J. V. (1999). Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146, 1033-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiema T., Lad Y., Jiang P., Oxley C. L., Baldassarre M., Wegener K. L., Campbell I. D., Ylanne J., Calderwood D. A. (2006). The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337-347 [DOI] [PubMed] [Google Scholar]
- Kim M., Carman C. V., Springer T. A. (2003). Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720-1725 [DOI] [PubMed] [Google Scholar]
- Lad Y., Kiema T., Jiang P., Pentikainen O. T., Coles C. H., Campbell I. D., Calderwood D. A., Ylanne J. (2007). Structure of three tandem filamin domains reveals auto-inhibition of ligand binding. EMBO J. 26, 3993-4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Plow E. F., Wu C. (2008). Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9, 1203-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M., Artym V. V., Green J. A., Yamada K. M. (2006). The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463-471 [DOI] [PubMed] [Google Scholar]
- Legate K. R., Fassler R. (2009). Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J. Cell Sci. 122, 187-198 [DOI] [PubMed] [Google Scholar]
- Li R., Mitra N., Gratkowski H., Vilaire G., Litvinov R., Nagasami C., Weisel J. W., Lear J. D., DeGrado W. F., Bennett J. S. (2003). Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science 300, 795-798 [DOI] [PubMed] [Google Scholar]
- Luo B. H., Carman C. V., Takagi J., Springer T. A. (2005). Disrupting integrin transmembrane domain heterodimerization increases ligand binding affinity, not valency or clustering. Proc. Natl. Acad. Sci. USA 102, 3679-3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel V., Racaud-Sultan C., Dupe S., Marie C., Paulhe F., Galmiche A., Block M. R., Albiges-Rizo C. (2001). Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 276, 21217-21227 [DOI] [PubMed] [Google Scholar]
- Nakamura F., Heikkinen O., Pentikainen O. T., Osborn T. M., Kasza K. E., Weitz D. A., Kupiainen O., Permi P., Kilpelainen I., Ylanne J., et al. (2009). Molecular basis of filamin A-FilGAP interaction and its impairment in congenital disorders associated with filamin A mutations. PLoS One 4, e4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Hartwig J. H., Stossel T. P. (2006). FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat. Cell Biol. 8, 803-814 [DOI] [PubMed] [Google Scholar]
- Otey C. A., Carpen O. (2004). Alpha-actinin revisited: a fresh look at an old player. Cell Motil. Cytoskeleton 58, 104-111 [DOI] [PubMed] [Google Scholar]
- Otey C. A., Vasquez G. B., Burridge K., Erickson B. W. (1993). Mapping of the alpha-actinin binding site within the beta 1 integrin cytoplasmic domain. J. Biol. Chem. 268, 21193-21197 [PubMed] [Google Scholar]
- O'Toole T. E., Katagiri Y., Faull R. J., Peter K., Tamura R., Quaranta V., Loftus J. C., Shattil S. J., Ginsberg M. H. (1994). Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 124, 1047-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Ylanne J., Culley B. M. (1995). Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J. Biol. Chem. 270, 8553-8558 [DOI] [PubMed] [Google Scholar]
- Partridge A. W., Liu S., Kim S., Bowie J. U., Ginsberg M. H. (2005). Transmembrane domain helix packing stabilizes integrin alphaIIbbeta3 in the low affinity state. J. Biol. Chem. 280, 7294-7300 [DOI] [PubMed] [Google Scholar]
- Pentikainen U., Ylanne J. (2009). The regulation mechanism for the auto-inhibition of binding of human filamin A to integrin. J. Mol. Biol. 393, 644-657 [DOI] [PubMed] [Google Scholar]
- Pfaff M., Liu S., Erle D. J., Ginsberg M. H. (1998). Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 273, 6104-6109 [DOI] [PubMed] [Google Scholar]
- Price L. S., Leng J., Schwartz M. A., Bokoch G. M. (1998). Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9, 1863-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddle H., Hemmings L., Monkley S., Woods A., Patel B., Sutton D., Dunn G. A., Zicha D., Critchley D. R. (1998). Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J. Cell Biol. 142, 1121-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfur Z., Roy P., Otey C., Romer L., Jacobson K. (2002). Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat. Cell Biol. 4, 286-293 [DOI] [PubMed] [Google Scholar]
- Reddy K. B., Gascard P., Price M. G., Negrescu E. V., Fox J. E. (1998). Identification of an interaction between the m-band protein skelemin and beta-integrin subunits. Colocalization of a skelemin-like protein with beta1- and beta3-integrins in non-muscle cells. J. Biol. Chem. 273, 35039-35047 [DOI] [PubMed] [Google Scholar]
- Reverte C. G., Benware A., Jones C. W., LaFlamme S. E. (2006). Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 174, 491-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Ma Y. Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F., Wu C. (2007). The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455-20466 [DOI] [PubMed] [Google Scholar]
- Shifrin Y., Arora P. D., Ohta Y., Calderwood D. A., McCulloch C. A. (2009). The role of FilGAP-filamin A interactions in mechanoprotection. Mol. Biol. Cell 20, 1269-1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Kaverina I. (2003). Microtubules meet substrate adhesions to arrange cell polarity. Curr. Opin. Cell Biol. 15, 40-47 [DOI] [PubMed] [Google Scholar]
- Small J. V., Geiger B., Kaverina I., Bershadsky A. (2002). How do microtubules guide migrating cells? Nat. Rev. Mol. Cell. Biol. 3, 957-964 [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Condeelis J., Cooley L., Hartwig J. H., Noegel A., Schleicher M., Shapiro S. S. (2001). Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell. Biol. 2, 138-145 [DOI] [PubMed] [Google Scholar]
- Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. (2003). Talin binding to integrin beta tails: a final common step in integrin activation. Science 302, 103-106 [DOI] [PubMed] [Google Scholar]
- Takala H., Nurminen E., Nurmi S. M., Aatonen M., Strandin T., Takatalo M., Kiema T., Gahmberg C. G., Ylanne J., Fagerholm S. C. (2008). Beta2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood 112, 1853-1862 [DOI] [PubMed] [Google Scholar]
- van der Flier A., Sonnenberg A. (2001). Structural and functional aspects of filamins. Biochim. Biophys. Acta 1538, 99-117 [DOI] [PubMed] [Google Scholar]
- van der Flier A., Kuikman I., Kramer D., Geerts D., Kreft M., Takafuta T., Shapiro S. S., Sonnenberg A. (2002). Different splice variants of filamin-B affect myogenesis, subcellular distribution, and determine binding to integrin [beta] subunits. J. Cell Biol. 156, 361-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener K. L., Partridge A. W., Han J., Pickford A. R., Liddington R. C., Ginsberg M. H., Campbell I. D. (2007). Structural basis of integrin activation by talin. Cell 128, 171-182 [DOI] [PubMed] [Google Scholar]
- Yan B., Calderwood D. A., Yaspan B., Ginsberg M. H. (2001). Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J. Biol. Chem. 276, 28164-28170 [DOI] [PubMed] [Google Scholar]
- Zhang X., Jiang G., Cai Y., Monkley S. J., Critchley D. R., Sheetz M. P. (2008). Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 10, 1062-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.