Abstract
The transmembrane protein ephrin-B2 regulates angiogenesis, i.e. the formation of new blood vessels through endothelial sprouting, proliferation and remodeling processes. In addition to essential roles in the embryonic vasculature, ephrin-B2 expression is upregulated in the adult at sites of neovascularization, such as tumors and wounds. Ephrins are known to bind Eph receptor family tyrosine kinases on neighboring cells and trigger bidirectional signal transduction downstream of both interacting molecules. Here we show that ephrin-B2 dynamically modulates the motility and cellular morphology of isolated endothelial cells. Even in the absence of Eph-receptor binding, ephrin-B2 stimulates repeated cycling between actomyosin-dependent cell contraction and spreading episodes, which requires the presence of the C-terminal PDZ motif. Our results show that ephrin-B2 is a potent regulator of endothelial cell behavior, and indicate that the control of cell migration and angiogenesis by ephrins might involve both receptor-dependent and receptor-independent activities.
Keywords: Eph-ephrin signaling, Cell contraction, Membrane blebbing, Cell migration, Endothelial cell
Introduction
Ephrins are membrane-tethered ligands for Eph family receptor tyrosine kinases (RTKs). Interactions between ephrins and Eph receptors present on contacting cells triggers bidirectional signaling events that have been shown to modulate the behavior of both cells (Pasquale, 2005). Eph-ephrin signaling regulates cell morphology and movements, primarily by stimulating downstream effectors that regulate actin cytoskeletal rearrangements. By driving the positioning of cells relative to their neighbors, the Eph-ephrin system directs key cell guidance events during embryonic development and in adult tissues.
Although Eph-ephrin interactions are generally repulsive in nature, they can be attractive too. The mechanisms underlying these opposite responses are poorly understood; nevertheless, there is some evidence that the cellular Eph:ephrin ratio regulates the nature of these interactions and ultimately the intensity of the activated signaling pathways (Hansen et al., 2004; Huynh-Do et al., 1999; McLaughlin et al., 2003).
Ephrins, like Eph receptors, are categorized into two subclasses. GPI-anchored ephrin-A ligands bind preferentially to EphA receptors, while ephrin-B ligands are transmembrane proteins that interact mainly with B-class Eph RTKs. Three ephrin-B ligands have been identified, among which ephrin-B2 (encoded by the gene EFNB2 in humans) has been shown to be involved in vascular remodeling in normal and tumor tissues. Ephrin-B2 is highly expressed in arteries, while one of its cognate receptors, EphB4, is a marker for veins in many vertebrate species. Both molecules are essential for angiogenesis in the mouse embryo (Adams et al., 1999; Gerety et al., 1999; Wang et al., 1998). Ephrin-B2 expression is controlled by VEGF and hypoxia and, accordingly, the ligand is upregulated at sites of physiological as well as pathological neovascularization in the adult (Gale et al., 2001; Vihanto et al., 2005). Recently, deregulation of ephrin-B2 expression has been linked to tumor progression and is therefore hypothesized to play a role in cancer progression and metastasis (Surawska et al., 2004).
How ephrin-B reverse signaling impinges on the actin cytoskeleton and cellular behavior is incompletely understood. Studies in cell lines expressing exogenous ephrin-B1 indicate that the ligand can induce cell detachment and rounding, either by a tyrosine phosphorylation-dependent pathway involving recruitment of the SH2 adaptor protein Grb4 or by a tyrosine phosphorylation-independent pathway, which is mediated by the c-Jun N-terminal kinase (JNK). Eph-receptor-binding-mediated clustering and activation of ephrin-Bs, induced the phosphorylation of five conserved tyrosines and the recruitment of adaptor proteins to the cytoplasmic domain (Cowan and Henkemeyer, 2001). The best studied of these adaptors is the SH2- and SH3-domain-containing protein Grb4, which binds to a region of ephrin-B1, that is identical in ephrin-B2. Furthermore, ephrin-B1 activation leads to increased activity of focal adhesion kinase (FAK), redistribution of the adapter protein paxillin, loss of focal adhesions, cell rounding and disassembly of F-actin stress fibers (Cowan and Henkemeyer, 2001). All B-class ephrins carry a C-terminal YYKV PDZ-binding motif, which allows the recruitment of the protein phosphatase PTP-BL and, subsequently, dephosphorylation of the conserved tyrosine residues in the ephrin-B intracellular region (Palmer et al., 2002). Several other PDZ domain-containing proteins can be recruited to ephrin-Bs upon phosphorylation (Bruckner et al., 1999; Lin et al., 1999; Lu et al., 2001; Torres et al., 1998). For example, in cerebellar granule cells, which express the G-protein-coupled receptor CXCR4 and therefore migrate towards the chemoattractant SDF-1, EphB-mediated activation of ephrin-B1 induces binding and activation of PDZ-RGS3 (Lu et al., 2004; Lu et al., 2001). This in turn, negatively regulates G-protein-coupled signaling by hydrolyzing GTP to GDP and thereby inhibits SDF-1-induced chemotaxis.
Conventionally, Ephs and ephrins require cell-cell contact for interaction (in trans). However, EphA receptors exhibit higher tyrosine phosphorylation in retinal ganglion cells expressing higher levels of ephrin-As, which impairs repulsion in response to exogenous ephrin (Hornberger et al., 1999). An explanation for this effect could be the masking of bindings sites for trans interactions by co-expression of EphA and ephrin-As in the same cell (Yin et al., 2004). By contrast, EphA receptors and ephrin-As are segregated to distinct membrane compartments in spinal cord motor neurons and can be independently activated with antagonistic effects on cell behavior. This suggests that Eph receptors and ephrins might signal independently even when co-expressed in the same cell (Marquardt et al., 2005).
More recently, Foo and colleagues (Foo et al., 2006) suggested that ephrin-B2 might have some contact-independent, i.e. cell-autonomous, functions. The loss of ephrin-B2 expression led to morphological changes even in single, isolated cells, which appeared elongated, insufficiently spread and with numerous but unstable lamellipodial protrusions. These defects were accompanied by more random migration with frequent changes of direction of the ephrin-B2-deficient cells (Foo et al., 2006).
To investigate how ephrin-B2 ‘reverse’ signaling pathways direct changes during angiogenesis and how the expression level of ephrin ligands influences changes in cell behavior and cell motility, we have used microinjected HUVECs (human umbilical vein endothelial cells) as a model system. The key advantage of this approach is that it allows the immediate observation of dynamic changes in cell morphology and migration. We observe that overexpression of ephrin-B2 alone can increase motility and trigger repeated cycles of cell contraction and respreading in isolated cells. Activation of endogenous ephrin-B2 with soluble, recombinant EphB4 leads to a similar but non-repetitive cycle of cell shape change, which is terminated by ligand internalization. Our findings suggest that ephrin-B2 is capable of differential signaling responses depending on expression levels and the presence or absence of cognate receptors on adjacent cells.
Results
Constitutive ephrin-B2 expression in HUVECs triggers repeated contraction-expansion episodes
Ephrin-B2 is upregulated by hypoxia and is highly expressed in many cancer cells (Brantley-Sieders and Chen, 2004; Heroult et al., 2006; Noren and Pasquale, 2007; Vogt et al., 1998). Genetic inactivation of ephrin-B2 in isolated, cultured vascular smooth muscle cells alters their morphology and motility properties, which suggests that ephrins might act in a cell-autonomous fashion (Foo et al., 2006). To examine whether ephrin-B2 overexpression can also stimulate changes in cell shape and/or cell behavior, isolated HUVECs and human umbilical artery endothelial cells (HUAECs) that endogenously express both ephrin-B2 and EphB4 in culture (supplementary material Fig. S1C) (see also Fuller et al., 2003), were microinjected with an expression construct containing the full-length open reading frame of the murine EFNB2. Induced expression of ephrin-B2, associated with the Golgi complex, was detected after 30 minutes by immunostaining of permeabilized cells and surface expression was detected after 60 minutes by staining of non-permeabilized cells (Fig. 1A). Expression of ephrin-B2, increased with time, and 3 hours after microinjection total ephrin-B2 levels were approximately 40-fold that of endogenous ephrin-B2 (supplementary material Fig. S1A,B). In order to assess the role of ephrin-B2 on cell dynamics, HUVECEfnB2 cells were imaged by phase-contrast time-lapse microscopy 5-10 minutes after microinjection. Ephrin-B2 protein expression was confirmed by antibody staining at the end of recording experiments.
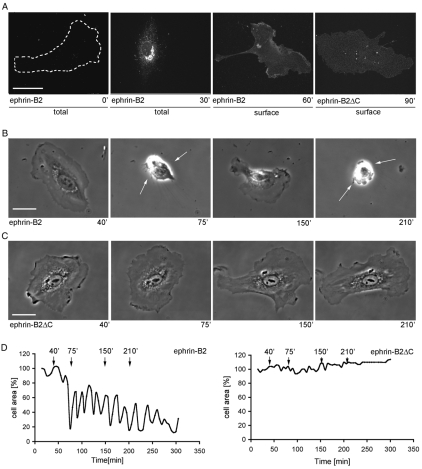
Fig. 1.
Constitutive ephrin-B2 expression stimulates repeated morphology switches. (A) Subconfluent cells were injected with pRK5-ephrin-B2 or pRK5-ephrin-B2ΔC (200 μg/ml; n=10), fixed and stained for ephrin-B2 total (with cell permeabilization) or surface expression (without cell permeabilization). (B,C) Stills taken from time-lapse movies (collected for 5 hours at 1 frame every 30 seconds) of (B) ephrin-B2- and (C) ephrin-B2ΔC-injected HUVECs, at different time-points. (D) Using Volocity software, changes in cell area, at 5-minute intervals, were measured and graphed. Measurements (at the times indicated) that correspond to movie stills in B and C are marked with arrows in D. HUVECsEfnB2 showed continuous switches between a retracted and a spread state. Scale bars: 20 μm.
Consistently, around 60 minutes after injection, HUVECsEfnB2 contracted and became round; they left behind retraction fibers and lost all lamellipodia (Fig. 1B,D). This was a transient response, as ephrin-B2 expression triggered repeated episodes of cell contraction (often associated with membrane blebbing) followed by extension of lamellae and membrane ruffling (Fig. 1B; supplementary material Movie 1). The periodicity of the expansion-contraction cycles was approximately 20 minutes and several cells underwent 12-15 cycles of contraction with constant periodicity (Fig. 1D). Expression of a truncated version of the ligand lacking the entire cytoplasmic domain (ephrin-B2ΔC) did not trigger cell contraction, indicating an important role for the association of effector molecules with the intracellular tail of ephrin-B2 (Fig. 1C,D).
Contraction-expansion cycling is ephrin-B and cell type specific
To test whether these effects of ephrin-B2 overexpression were specific for the particular ligand, we microinjected cells with expression constructs for ephrin-B1 or ephrin-A2 (Fig. 2). Dynamic cell shape changes and repeated expansion-contraction cycles were seen after expression of ephrin-B1 but not the A-class ligand ephrin-A2 (Fig. 2A,D). Activation of Eph receptors has been previously shown to induce ligand-independent cell retraction through modulation of integrin-mediated adhesion (Noren et al., 2009). Consistent with these previous observations, expression of EphB4, in isolated HUVECs did stimulate cell contraction, which, however, occurred only after 3-4 hours, despite detection of surface EphB4 by 1 hour (data not shown). However, EphB4-microinjected cells did not show repeated cycles of cell contraction and expansion even over a 5-hour observation period (Fig. 2A).
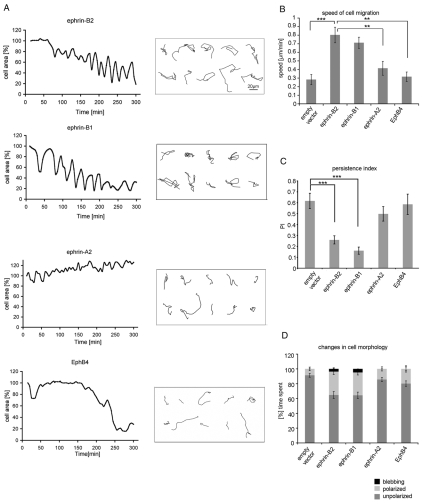
Fig. 2.
Cell contraction-expansion cycling and increased migration is ephrin-B specific. The changes in area, morphology and migration tracks of 10 injected cells were analyzed using Volocity software, between 10 minutes and 5 hours after cell microinjection. (A) Injected cells were manually traced at 5-minute intervals and the area of the ‘foot-print’ of the cells was measured and graphed. (B) By following the nuclei of cells, migration tracks (boxed area in A) were made between 10 minutes and 3 hours and migration speed calculated. (C) Persistence index (PI) is a measure of how directed migration is, and was calculated from the distance between the first and the last point of the tracked cell's nucleus divided by the total distance traveled. (D) The time the injected cells spend in three different morphological states: unpolarized (without polarized lamellae), polarized (with a distinct leading edge and a trailing tail) and blebbing (showing membrane blebbing) was quantified from the time-lapse movies and graphed as a percentage of the total time of analysis. Error bars indicate ± s.e.m. Statistical analysis was performed using the Student's t-test (***P<0.001; **P<0.01). Scale bar: 20 μm.
Our time-lapse movies of ephrin-B2- and ephrin-B1-expressing cells indicate that cell migration speed is increased nearly threefold, but their persistence of directional migration is reduced compared to cells injected with empty vector or ephrin-A2 or EphB4 expression constructs (Fig. 2B,C). Furthermore, the effects of ephrin-B2 and ephrin-B1 on cell morphology and motility were also observed in a second endothelial cell type HUAECs (data not shown). By contrast, overexpression of ephrin-B2 did not induce cell morphology changes (supplementary material Fig. S2A,B) or increase the speed of cell migration (supplementary material Fig. S2C) in NIH3T3 or MDCK cells. Ephrin-B2 and its specific receptor EphB4 are known to play a crucial role in vascular development in vivo (Adams et al., 1999; Gerety and Anderson, 2002), which suggests that endothelial cells are physiologically a more relevant cell type for ephrin-B2 signaling than are NIH3T3 fibroblasts or MDCK cells.
Expression of ephrin-B2 stimulates increased membrane ruffling
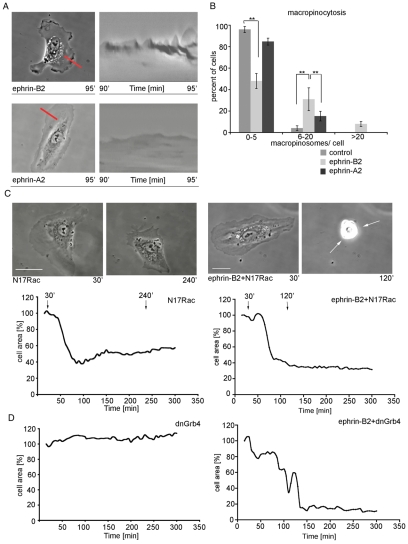
Our time-lapse movies suggested that HUVECsEfnB2 exhibit increased membrane ruffling, which was observed both during cell expansion and the fully spread phases. To examine this further, kymographic analysis and macropinocytosis assays were performed, both of which provide a measure of the extent of membrane ruffling (Fig. 3A,B). At 90 minutes after microinjection with ephrin-B2, the frequency of the kymograph peaks was increased and cells contained more dextran-labeled macropinosomes, compared with control and ephrin-A2-injected cells (Fig. 3A,B). Furthermore, we found that ephrin-B2-dependent respreading after the first cell contraction episode is Grb4 and also Rac dependent (Fig. 3C,D). Coexpression of ephrin-B2 with dominant-negative versions of these two proteins (dnGrb4 or N17Rac, respectively) revealed that cell respreading from a contracted state was inhibited, compared with ephrin-B2 expression alone. Cells exhibited membrane blebbing for approximately a further 3 hours. The speed of cell migration was decreased during this period (supplementary material Movie 2). Thus Grb4 or Rac are required for respreading but not retraction or membrane blebbing in HUVECsEfnB2.
Fig. 3.
HUVECsEfnB2 exhibit increased Rac- and Grb4-dependent membrane ruffling. For kymographic analysis (A) time-lapse movies were taken before and after expression of injected constructs, for 5 minutes at 1 frame per second (n=2) and four different regions of the cell periphery were analyzed for each cell. For the macropinocytosis assay (B), isolated HUVECs were either uninjected or injected with ephrin-B2 (200 μg/ml) or ephrin-A2 (200 μg/ml) and left for 3 hours, after which the culture medium was replaced with medium containing 2 mg/ml RITC-dextran for 5 minutes before the cells were fixed. (C) Stills taken from time-lapse movies and (D) cell area changes of subconfluent cells injected with either dominant negative Rac (N17Rac; 300 μg/ml) or dominant negative Grb4 (dnGrb4; 300 μg/ml) and those that were also co-injected with ephrin-B2 (200 μg/ml). Error bars indicate ± s.e.m. Statistical analysis was performed using the Student's t-test (**P<0.01). Scale bars: 20 μm.
Ephrin-B2 controls cell morphology through its PDZ-binding motif
As mentioned above, truncated ephrin-B2 (ephrin-B2ΔC) failed to induce dynamic cell shape changes. This observation implies a role for reverse signaling through the intracellular region of ephrin-B2, which contains functionally important and highly conserved tyrosine residues and the C-terminal PDZ-binding motif. Two mutants were used to identify the relevant amino acid residues: ephrin-B2ΔV, which lacks Val at the C-terminus and is unable to bind PDZ proteins (Lu et al., 2001) and a 5Y-mutant, in which all five tyrosine phosphorylation sites are mutated (Fig. 4). Eliminating phosphorylation-dependent signaling in the 5Y-mutant did not affect its ability to induce cell contraction episodes (Fig. 4C). By contrast, ephrin-B2ΔV was significantly less able to elicit membrane blebbing and cell contraction (Fig. 4A). Makinen and colleagues (Makinen et al., 2005) proposed that PDZ-RGS3, a PDZ-binding protein capable of binding to ephrin-B2 may be the key candidate for ephrin-B2-dependent remodeling of lymphatic vessels in the developing mouse (Makinen et al., 2005). Coexpression of ephrin-B2 with the PDZ region of PDZ-RGS3 protein (Fig. 4B) diminished contraction responses similarly to those seen for ephrin-B2ΔV, and thereby confirmed the importance of PDZ-mediated interactions in this process.
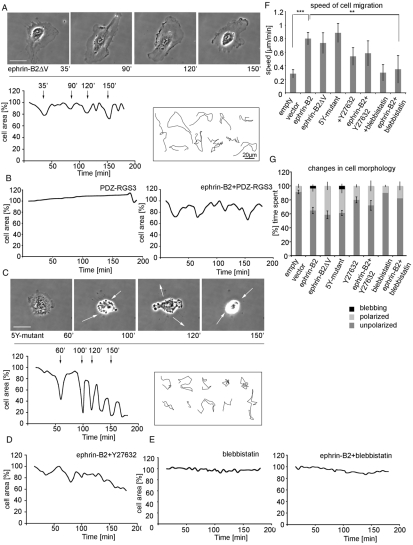
Fig. 4.
The PDZ-binding domain is necessary for cell retraction and membrane blebbing. (A) Subconfluent HUVECs were injected with ephrin-B2ΔV (200 μg/ml), (B) co-injected with ephrin-B2 and PDZ-RGS3 (200 μg/ml and 300 μg/ml, respectively, n=5) or (C) injected with ephrin-B2-5Y-mutant (200 μg/ml). (D,E) HUVECsEfnB2 were treated with either Y27632 (20 μM; D) or with blebbistatin (100 μM; E). Time-lapse movies were collected for 3 hours at a rate of one frame every 30 seconds. (F) Using Volocity software changes in cell area and cell migration tracks for each treatment were analyzed and migration speed calculated. (G) The time the injected cells spent in three different morphological states (unpolarized, polarized and blebbing) was quantified from time-lapse movies and graphed as a percentage of the total time of analysis. This revealed that cells expressing the 5Y-mutant also had retraction-protrusion oscillations like HUVECsEfnB2 (C,G). Episodes of severe retraction and membrane blebbing were not observed in ephrin-B2ΔV-injected cells (A) or in the cells co-injected with ephrin-B2 and PDZ-RGS3 (B). Retraction and membrane blebbing also requires ROCK (D) and actomyosin contraction (E). Error bars indicate ± s.e.m. Statistical analysis was performed using the Student's t-test (***P<0.001; **P<0.01). Scale bars: 20 μm.
Next, we investigated which signaling pathways downstream of the PDZ-binding domain of ephrin-B2 might regulate cell retraction and membrane blebbing. We found that blocking Rho kinase (ROCK) with the specific inhibitor Y27632 (Ishizaki et al., 2000) eliminated cell contraction and membrane blebbing (Fig. 4D), although both the speed of cell migration and membrane ruffling (cells spent more time in the polarized state) appeared to be increased (Fig. 4F,G). However, treatment of non-injected HUVECs with Y27632 also stimulated membrane ruffling and accelerated cell migration (Fig. 4F), which suggests that Rho and Rac-mediated processes are linked (Machacek et al., 2009). In addition to ROCK we investigated the potential role of myosin II activation in the contraction process of HUVECsEfnB2. The myosin II ATPase inhibitor, blebbistatin, was used to block actomyosin contraction. Upon ephrin-B2 expression, blebbistatin-treated cells did not retract and remained well spread (P<0.007; Fig. 4E,F,G). These data indicate that ephrin-B2-regulated cell retraction is actomyosin dependent.
JNK activity has been previously implicated in the rounding of HEK293 cells upon ephrin-B1 overexpression. This effect was independent of ephrin-B1 phosphorylation and Grb4 and was blocked by the JNK inhibitor SP600125 (Xu et al., 2003). Consistent with these findings, we also found that inhibition of JNK activity blocked cell shape changes and decreased the motility of HUVECsEfnB2 (supplementary material Fig. S3).
Cell shape oscillations triggered by ephrin-B2 expression are independent of Eph binding
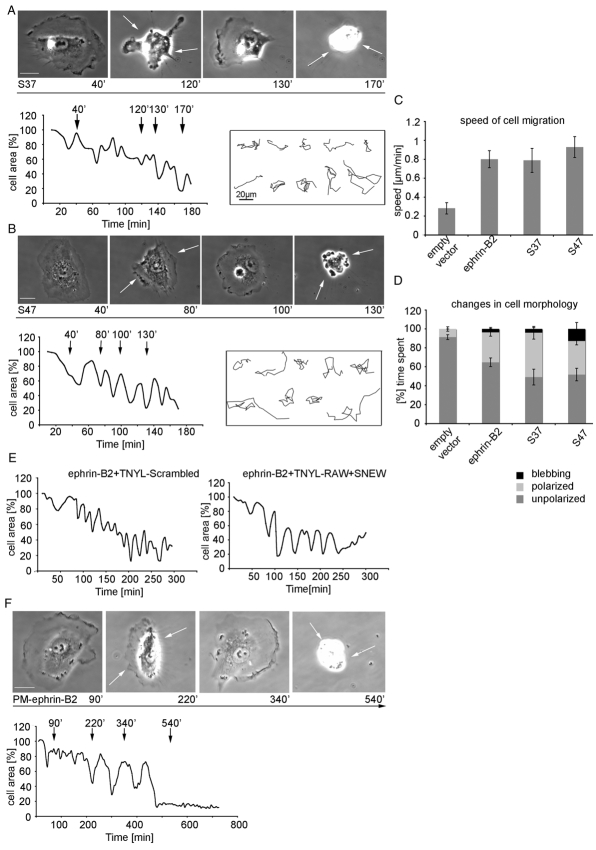
A cis interaction between ephrin ligands and Eph receptors was previously shown in retinal ganglion cells, where the responsiveness of the axons of EphA receptor-expressing neurons is negatively modulated by co-expressed ephrin-As (Hornberger et al., 1999; Yin et al., 2004). More recently, it was shown that the binding of ephrin-A ligand to the fibronectin type III domains of EphA receptors in the same cell can suppress forward signaling by the RTK (Carvalho et al., 2006). However, upon ephrin-B2 overexpression in isolated HUVECs no activation of Eph receptors was detected (supplementary material Fig. S1D) although phosphorylated-Eph was detected where ephrin-B2-expressing cells contacted a neighbor that expressed endogenous EphB4 (supplementary material Fig. S1D). To investigate in more detail whether the morphological changes observed in isolated HUVECsEfnB2 occur via Eph-ephrin interaction in cis or in an Eph-receptor-independent manner, we expressed mutant forms of ephrin-B2 in which residues with a known critical role in EphB binding (Himanen et al., 2001) were mutated. Surprisingly, the exchange of residues in the extracellular G-H loop and the important FSPNL motif (mutants S37 and S47, see supplementary material Fig. S4A), which abolishes interactions with EphB4 almost completely (supplementary material Fig. S4B,C) did not impair dynamic contraction and expansion morphology switching in microinjected HUVECs (Fig. 5A,B,D). Further analysis also revealed no difference in speed of cell migration of S37- or S47-injected cells in comparison to HUVECsEfnB2 (Fig. 5C).
Fig. 5.
Cell morphology oscillations and increased migration speed of HUVECEfnB2 are independent of EphB-receptor binding. (A,B,E,F) Subconfluent cells were injected with either ephrin-B2 binding mutants S37 (200 μg/ml; A) or S47 (200 μg/ml; B), or with ephrin-B2 (200 μg/ml; E) and treated with TNYL-RAW (50 μM) or a combination of TNYL-RAW with SNEW (75 μM each) or injected with PM-ephrin-B2 (400 μg/ml; F). Time-lapse movies were collected either for 3 hours (S37, S47, ephrin-B2 treated with peptide) or for 12 hours (PM-ephrin-B2 mutant) at a rate of 1 frame every 30 seconds. Using Volocity software the cell area was measured and graphed. (C) By following the nuclei of injected cells their migration tracks were recorded and migration speed was quantified. (D) The time the injected cells spend in three different morphological states (unpolarized, polarized and blebbing) was quantified from time-lapse movies and graphed as a percentage of the total time of analysis. Error bars indicate ± s.e.m. Scale bars: 20 μm.
In an independent approach, microinjected cells were treated with two peptide antagonists (TNYL-RAW and SNEW), which were previously shown to block ephrin-B-EphB interactions (Chrencik et al., 2006). Treatment with 50 μM TNYL-RAW or SNEW (supplementary material Fig. S4D) or a TNYL-RAW/SNEW combination (1:1; 75 μM each; data not shown) inhibited retraction of cells seen after stimulation with exogenous, soluble ephrin-B-Fc fusion proteins, whereas scrambled control peptides failed to do so even at high concentrations (supplementary material Fig. S4D). Time-lapse microscopy revealed that HUVECsEfnB2 treated with either TNYL-RAW or combined TNYL-RAW/SNEW still showed cell retraction and/or blebbing followed by membrane protrusion cycles, which argues against a role of Eph receptors in this process (Fig. 5E).
To completely eliminate any possibility of ephrin-B2–EphB interactions, we microinjected cells with a truncated ephrin-B2 mutant consisting only of the 80 C-terminal amino acids residues. This cytoplasmic fragment was anchored to the membrane with an N-terminal sequence of the tyrosine kinase Lyn (Pyenta et al., 2001), termed a PM anchor, which contains myristoylation and palmitoylation sites (supplementary material Fig. S4A). Interestingly, microinjection of this truncated ephrin-B2 mutant induced cell contraction and membrane protrusion oscillations even though the initiation of this response was delayed (Fig. 5F). This lag phase might be caused by the time required for protein expression, transport to the cell membrane and subsequent localization of the PM-anchored protein. Taken together, our data strongly suggest that ephrin-B2 can induce cell morphology switches and also increase the speed of cell migration in an Eph-receptor-independent fashion.
Cell shape regulation by activation of endogenous ephrin-B2
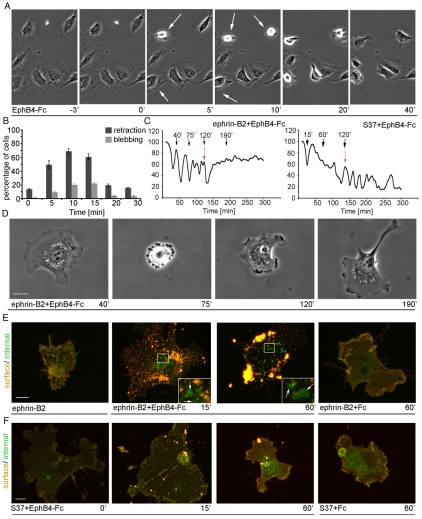
Human arterial endothelial cells (HUAECs) express ephrin-B2 (supplementary material Fig. S1C) and have a spread morphology and ruffled lamellipodia in culture (supplementary material Fig. S5A). In order to characterize the effects of endogenous ephrin-B2 activation on cell morphology, HUAECs were treated with pre-clustered EphB4-Fc, which binds ephrin-B2 with high affinity but not other ephrin ligands (Kim et al., 2002). The dynamic responses observed were recorded by time-lapse microscopy (Fig. 6A) and cell shape changes were quantified after fixing and staining cells for actin structures (Fig. 6B; supplementary material Fig. S5A). EphB4-Fc treatment stimulated clustering of ephrin-B2 (not shown) and cells showed a rapid cell contraction response by retracting their lamellipodia (Fig. 6A; supplementary material S5A and Movie 3). Subsequently, after 20-30 minutes and despite the continued presence of EphB4-Fc, the cells reversed this morphological response and expanded lamellipodia along the retraction fibers similarly to microinjected HUVECEfnB2 (Fig. 6A; supplementary material Fig. S5A). Not all cells rounded up completely, which may reflect heterogenous expression levels of endogenous ephrin-B2 in cultured endothelial cells (Fuller et al., 2003); however those that did contract then exhibited dynamic membrane blebbing for 10 minutes before recovering to a spread morphology (Fig. 6B; supplementary material Fig. S5A, Movie 3). EphB4-treated cells lacked detectable actin stress fibers for up to an hour (data not shown). Treatment of HUAECs with pre-clustered Fc molecules as a control did not trigger retraction or membrane blebbing (data not shown). Taken together, the phenotypic changes following the activation of endogenous ephrin-B2 with recombinant, soluble EphB4 mimicked the behavior of ephrin-B2-injected HUVECs. However, stimulation of endogenous ephrin-B2 only resulted in a single cycle of cell retraction and respreading.
Fig. 6.
Internalization of ephrin-B2 inhibits morphology switches. (A) Phase-contrast time-lapse stills of HUAECs stimulated with pre-clustered EphB4-Fc (5 μg/ml). Following stimulation the cells can be seen to retract (5 minutes and 10 minutes; indicated by white arrows). Within 20 minutes, cell retraction is rapidly reversed. (B) The number of retracted and blebbing cells upon stimulation with EphB4-Fc, was scored, at the indicated time-points, and graphed. (C,E,F) Subconfluent HUVECs were microinjected with ephrin-B2 (200 μg/ml; C,E) or with S37 (200 μg/ml; C and F) and allowed to express for 2 hours. The cells were stimulated with EphB4-Fc (1 μg/ml, preclustered with 10 μg/ml IgG) and fixed after 0, 15 or 60 minutes, as indicated. Ephrin-B2 was detected using goat anti-ephrin-B2 antibodies. Cy3-labeled donkey anti-goat was used to detect both external and internal ephrin-B2. FITC-labeled donkey anti-goat was used to detect external ephrin-B2 after permeabilization (E,F). (D) Stills from time-lapse movies collected for 5 hours at 1 frame every 30 seconds. 2 hours post-injection cells were treated with pre-clustered EphB4-Fc (1 μg/ml; C,D) at the times indicated by red arrows in C). Using Volocity software the cell area of a microinjected and EphB4-Fc treated cells was measured and graphed. Error bars indicate ±s.e.m. Scale bars: 10 μm.
Internalization of ephrin-B2 blocks retraction-expansion oscillations
Stimulation of endogenous ephrin-B2 in HUAECs with EphB4-Fc induced a single cell contraction-expansion episode but failed to trigger oscillations. We reasoned that this might be due to the internalization of receptor-bound ephrin-B2 and its removal from the cell surface. To directly address this possibility, HUVECsEfnB2 were treated with EphB4-Fc and the internalization of ephrin-B2 complexes was monitored by antibody staining (Fig. 6E). While most of the ephrin-B2 was present at the cell surface (red staining) 2 hours after microinjection, treatment with EphB4-Fc induced ephrin-B2 surface clustering and internalization (green staining) after only 15 minutes (Fig. 6E). One hour after stimulation, larger intracellular clusters had formed indicating efficient internalization of ephrin-B2. By contrast, treatment of HUVECsEfnB2 with a control Fc did not lead to ephrin-B2 clustering or internalization nor did it affect ephrin-B2-regulated cell contraction-expansion episodes (Fig. 6E; supplementary material Fig. S5B,C).
Time-lapse microscopy of EphB4-Fc-stimulated HUVECsEfnB2 showed a rapid termination of the contraction and respreading cycles with the same time-course as the ephrin-B2 internalization described above (Fig. 6C,D). By contrast, the S37 mutant with strongly reduced receptor binding was not internalized after EphB4-Fc treatment and, concomitantly, cell shape oscillations were also not abrogated (Fig. 6C,F). These data indicate that ephrin-B2 on the cell surface is capable of inducing repeated cell contraction-expansion cycling, whereas ephrin-B2 internalization terminates this process.
How does the morphology switching behavior of HUVECsEfnB2 affect migration and invasion?
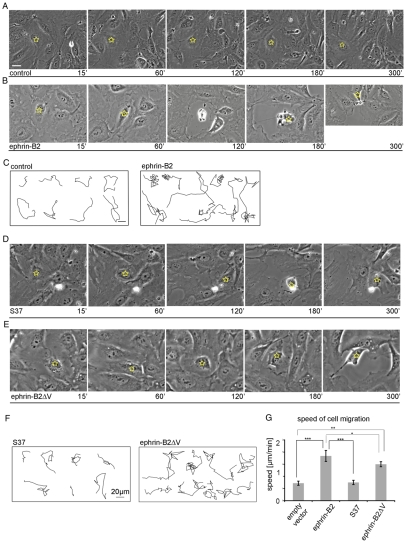
Next, we examined how upregulated ephrin-B2 expression might affect cell behavior in the context of neighboring (uninjected) cells, which might provide insight into the motile and invasive behavior of ephrin-B2-expressing cells in cancers and during angiogenesis. HUVECs were cultured to form a confluent monolayer and an individual cell within the cell monolayer was selected for microinjection with ephrin-B2 (Fig. 7B; indicated with yellow star). Time-lapse movies revealed that HUVECsEfnB2 within a monolayer went through repeated episodes of cell contraction and membrane protrusion just as they do in isolation (Fig. 7B; supplementary material Movie 4). Strikingly, the immediate neighbors of HUVECsEfnB2, which endogenously express EphB4 receptors (supplementary material Fig. S1C) showed repulsive responses to the ephrin-B2-injected cell – they retracted and thereby provided free areas for the movement of HUVECsEfnB2. As a result, HUVECsEfnB2 migrated an average of two-cell diameters within the monolayer over the 5-hour period, which was significantly further and faster than control-injected cells (Fig. 7A,C,G). Interestingly, similar injections of the S37 mutant, which has reduced binding to EphB receptors but is able to induce retraction-protrusion cycling, did not trigger repulsion responses in the uninjected neighboring cells (Fig. 7D). Accordingly, migration within the monolayer was slower than for HUVECsEfnB2 (P<0.001; Fig. 7F,G). Furthermore, to examine if the retraction-protrusion cycles are required for enhanced migration of densely packed HUVECs, individual cells were injected with ephrin-B2ΔV. The injected cells did not show retraction-protrusion cycles and although the speed of migration was decreased compared with ephrin-B2-expressing cells (P<0.048; Fig. 7G), ephrin-B2ΔV cells did migrate further and faster than control injected cells (P<0.0046; Fig. 7G). Since ephrin-B2ΔV stimulates forward signaling of EphB4-expressing adjacent cells, which regulates their retraction (Groeger and Nobes, 2007; Marston et al., 2003), these data indicate that the retraction-protrusion cycles of HUVECsEfnB2 together with the repulsion of adjacent, uninjected HUVECs enhances invasive behavior within a cell monolayer.
Fig. 7.
Increased migration of HUVECsEfnB2 within a cell monolayer. Stills taken from time-lapse movies of either (A) control-injected cells, (B) HUVECsEfnB2, (D) S37-expressing cells or (E) ephrin-B2ΔV-expressing cells. HUVECsEfnB2 underwent changes in cell shape retracting (B, 120 minutes) and respreading (B, 180 minutes). Time-lapse movies were collected for 5 hours at a rate of 1 frame per 30 seconds. At the end of each movie the cells were fixed and the expression of ephrin-B2 was examined by immunocytochemistry. (C,F,G) Using Volocity software cell migration tracks and speed were quantified. HUVECsEfnB2 and HUVECs expressing ephrin-B2ΔV migrated more within the monolayer than did control injected cells, over 5 hours. Error bars indicate ±s.e.m. Statistical analysis was performed using the Student's t-test (***P<0.001; **P<0.01; *P<0.05). Scale bars: 20 μm.
Discussion
In this study, we have shown that microinjection of ephrin-B2 and ephrin-B1 trigger dynamic changes in cell morphology, which involves switching between cell contraction and respreading, as well as an increase in cell motility and a decrease in persistence of directional migration. This response was specifically observed in endothelial cells and did not occur in microinjected NIH3T3 or MDCK cells. Our examination of ephrin-B2 downstream signaling indicates that multiple downstream regulatory mechanisms are contributing to the ephrin-B2-induced dynamic cell shape changes. Some of our data suggest also that ephrin-B2, when expressed at high levels, might signal in a cell-autonomous fashion and therefore independently of Eph receptor interaction. Nevertheless, even this signaling response can be modulated by interactions with Eph receptors in trans, which lead to the internalization of ligand-receptor complexes and thereby terminate the ephrin-B2-induced morphological changes.
Ephrin-B2 regulation of the actin cytoskeleton through Rho family small GTPases
Activation of endogenous ephrin-B2 or overexpression of ephrin-B2 in endothelial cells revealed that cells undergo retraction and membrane blebbing followed by membrane protrusion. Overexpressed ephrin-B2 appears not to be visibly clustered at the cell surface, nor is it concentrated in vesicles inside the expressing cells. This might suggest that overexpressed ephrin-B2 is only weakly clustered at the cell surface and that this is sufficient for its autoactivation and subsequent stimulation of cycles of membrane retraction and cell expansion. How ephrin-B2 becomes activated is unclear, but EphB receptors have been shown to become autoactivated and autophosphorylated by overexpression (Tanaka et al., 2003). The difference in cell behavior between endothelial cells on the one hand and MDCKs and NIH3T3s on the other, after increased ephrin-B activation, might be due to expression of different complements of downstream effectors involved in ephrin-B2 signaling, expressed by these cells. It has been demonstrated that focal adhesion formation and membrane ruffling can be controlled, in smooth muscle cells (Foo et al., 2006) or in human aortic endothelial cells (Nagashima et al., 2002), via Eph-ephrin signaling, through the Crk-p130 (CAS)-Rac pathway. This pathway, for example, may not be activated in NIH3T3 and MDCK cells by upregulated ephrin-B2 expression.
We show that ephrin-B2 expression stimulates repeated episodes of actomyosin-dependent cell contraction, which requires the C-terminal PDZ motif. Numerous PDZ-domain proteins have been reported to interact with the PDZ-binding motif of ephrin-Bs, such as GRIP-1, GRIP-2, syntenin, PTP-BL or PDZ-RGS3, but the biological function of these interactions remains incompletely understood. Studies in Xenopus have suggested that Rho and ROCK are activated downstream of ephrin-B1 (Tanaka et al., 2003). This activation is mediated by Dishevelled and necessary for the sorting of EphB2- and ephrin-B1-expressing cell populations in an in vitro re-aggregation assay (Tanaka et al., 2003). We have also found that cell retraction and membrane blebbing responses downstream of ephrin-B2 involve ROCK activation. Furthermore, the cell contraction in HUVECsEfnB2 appeared to be independent of tyrosine phosphorylation and was unaffected in the ephrin-B2 5Y-mutant, in which all five conserved cytoplasmic tyrosine residues have been mutated. However, recent studies have shown that ephrin-B2 can regulate PDZ-interacting proteins, such as GRIP, in a tyrosine-phosphorylation-independent fashion (Essmann et al., 2008), via phosphorylation of a single serine residue that is located close to the PDZ-binding motif of ephrin-B.
Ephrin-B2 expression regulates cell motility
Both Eph receptors and ephrins have been implicated as important factors in guiding cell migration. For example, ephrin-B1 and ephrin-B2 can act as repulsive guidance cues for EphB-receptor-expressing migratory neural crest cells (Davy and Soriano, 2005) and EphB–ephrin-B signaling in the adult central nervous system is also required for normal neuroblast chain migration from the subventricular zone (Conover et al., 2000). Here, we have shown that ephrin-B2- and ephrin-B1-overexpressing HUVECs become more motile and migrate faster but more randomly than control cells, in ways that are reminiscent of the invasive phenotype of cancer cells in vitro and in vivo (Sahai and Marshall, 2003). That signaling downstream of ephrin-B2 may play an important role in endothelial cell migration was suggested by Noren et al. (Noren et al., 2004). Endothelial cells treated with soluble EphB4 fusion protein migrated more efficiently through a three-dimensional environment, and tumor cells expressing EphB4 attracted endothelial cells significantly more efficiently than control cells (Noren et al., 2004). In this context, it is noteworthy that angiogenic endothelial cells express higher levels of ephrin-B2 (Gale et al., 2001; Shin et al., 2001). Ephrin-B2 upregulation has been also linked to wound healing, promoting more rapid closure of scratch wounds in cultured endothelial and epithelial cell monolayers (Hafner et al., 2005; Hafner et al., 2004; Huynh-Do et al., 2002), which complements the enhanced motility that we observed for HUVECsEfnB2 (Fig. 7B). Additionally, recent data have implicated a role for EphB-ephrin-B signaling in the assembly of endothelial and mural cells into vascular structures (Salvucci et al., 2009) and also in the adhesion and migration of monocytes on endothelial cells (Pfaff et al., 2008).
The PDZ motif in B-class ephrins has been demonstrated to play an important function in ephrin-B signaling. Ephrin-B1 regulates axon guidance in a phosphorylation-independent fashion through its PDZ-binding motif in mice (Bush and Soriano, 2009). Similarly, genetic replacement of endogenous ephrin-B2 with a mutant version lacking the PDZ motif led to severe lymphangiogenesis defects, whereas removal of the cytoplasmic tyrosine residues had no effect (Makinen et al., 2005). Our data now attributes specific functional roles to the PDZ motif. Although ephrin-B2ΔV mutants or coexpression of ephrin-B2 and the (dominant negative) PDZ region of PDZ-RGS3 did not inhibit increased cell motility, cell contraction and membrane blebbing strictly depended on intact PDZ interactions. This indicates that PDZ-interacting proteins are required for certain signaling responses downstream of ephrin-B ligands, but are not needed for others.
Ephrin-B2 function in pathological situations
In addition to the many known important roles of Eph receptors and ephrins in developmental processes, there is mounting evidence for an involvement in tumorigenesis and tumor angiogenesis (Noren and Pasquale, 2007; Surawska et al., 2004). High expression levels of ephrin-B2 in malignant melanoma correlate with increased tumor vascularization and the tendency to metastasize (Vogt et al., 1998). Upregulated ephrin-B2 expression has been also correlated with the metastatic potential and progression of oesophageal squamous carcinoma (Tachibana et al., 2007). Furthermore, new evidence indicates that higher ephrin-B2 activation is correlated with increased migration and invasion of glioma cells (Nakada et al., 2009). Our results with microinjected cells suggest that ephrin-B2 upregulation might induce dynamic changes in cell morphology and invasive behavior that parallel those described for cancer cells during tumor invasion (Friedl et al., 2004; Sahai and Marshall, 2003). Future work will need to address the precise role of ephrin-B2 and downstream signaling interactions in tumor cell motility and metastasis.
How HUVECsEfnB2 repeatedly cycle between ROCK-dependent contraction and respreading of lamellae is unclear. One possibility is that expressed ephrin-B2 is internalized and recycled back to the cell surface to regulate another wave of cell contraction. However, as yet we have no direct evidence for ephrin-B2 association with intracellular vesicles in HUVECsEfnB2. Also arguing against this is our observation that treatment of HUVECsEfnB2 with EphB4-Fc triggers ephrin-B2 internalization and stops the cycles of cell contraction and respreading. Alternatively, constitutive activation of antagonistic Rho and Rac signaling pathways might result in the dual cycling of retraction and expansion episodes we observe, although again we have no direct evidence in support of this possibility.
In conclusion, our results show that ephrin-B2 is a potent inducer of changes in cell morphology and motility in endothelial cells. An examination of ephrin-B2 downstream signaling pathways indicates that the PDZ binding domain of ephrin-B2 is responsible for cell contraction and membrane blebbing responses. In addition, we have provided new evidence that ephrin-B2 can function in a cell-autonomous manner and independently of Eph receptor interaction when expression levels are raised. This mode of signaling might be of particular relevance in tissues and cells that express high levels of ephrin-B2 but little or no Eph receptors, such as arterial endothelial cells or certain tumors. Given the important roles of Eph and ephrin molecules in almost every tissue and cell type, future studies are needed to further distinguish the biological relevance of receptor-dependent and -independent functions of ephrins as well as the exact contributions of forward and reverse signaling in various physiological scenarios.
Materials and Methods
Mammalian cell culture and microinjection
HUVECs and HUAECs were routinely cultured on 10 cm dishes coated with attachment factor (TCS-Cellworks), in endothelial cell medium (SFM, Gibco) containing: 10% FBS, EGF, FGF, gentamicin (25 μg/ml), amphotericinB (50 μg/ml; TCS-Cellworks), at 37°C and 5% CO2. NIH3T3 fibroblasts and MDCK cells were cultured in DMEM (Gibco), containing 10% FBS, at 37°C, 5% CO2.
Cells were plated for microinjection at a density of 0.6×105cells/6 cm dish or at 3×105 cells for monolayer experiments. The culture medium was replaced with fresh medium every day. Cells were injected with expression constructs at 200-400 μg/ml as indicated.
Sub-confluent HUAECs were stimulated with pre-clustered EphB4-Fc (5 μg/ml; R&D Systems) for up to 60 minutes. EphB4-Fc was pre-clustered with anti-human IgG (1.78 μg/ml) at 37°C for 20 minutes prior to cell stimulation. Cells treated with EphB4-Fc were fixed and stained for F-actin using TRITC-conjugated phalloidin.
Treatment of the cells with peptides, pharmacological inhibitors and EphB4-Fc
Two peptides, were prepared that prevent ephrin-B–EphB interactions (Protein & Peptide Chemistry Department at CRUK, London). TNYL-RAW (TNYLFSPNGPIARAW), TNYL-Scrambled (TYLFNSPNGPIAARW), SNEW (SNEWIQPRIPQH) and Scrambled-SNEW (EPQNHSWPIRQL) were used at a concentration of 50 and 100 μM, or when combined, 75 μM each.
To examine downstream signaling pathways of ephrin-B2 pharmacological inhibitors were added to the cell medium 1 hour (Y27632; 20 μM, blebbistatin; 100 μM) and 15 minutes (SP600125; 30 μM) prior to cell microinjection. To drive internalization of expressed ephrin-B2, HUVECsEfnB2 were stimulated with 1 μg/ml EphB4-Fc (pre-clustered for 20 minutes with 10 μg/ml anti-human IgG).
Western blotting
HUAECs and HUVECs were washed twice in cold PBS, lysed in 180 μl of RIPA buffer and analyzed by western blotting for EphB4 (R&D Systems; 1:1000), ephrin-B2 (Sigma; 1:1000) and for tubulin (Serotec; 1:3000), to determine endogenous expression of EphB4, ephrin-B2, and loading respectively. These primary antibodies were then detected using HRP-conjugated secondary antibodies (Jackson; 1:3000).
Preparation of ephrin-B2 mutants
Ephrin-B2ΔV, lacking Val at the C-terminus of ephrin-B2, was prepared using site-directed mutagenesis (Stratagene); the following primers were used: 5′-CTGCCAACATTTACTACAAGTGAGGATCCTCTAGAGTACG-3′ and 5′-CGTACTCTAGAGGATCCTCACTTGTAGTAAATGTTGGCAG-3′. The PDZ-domain of PDZ-RGS3 (Lu et al., 2001; Su et al., 2004) was obtained from John Flanagan (MIT, Boston, USA) and consists of the N-terminal region of the PDZ-RGS3 protein with an additional myc-tag.
The PM-ephrin-B2 mutant was constructed using PCR and consisted of an N-terminal sequence for Lyn that encodes for the myristoyl (M) and palmitoyl (P) fatty acylation sites (Pyenta et al., 2001) and the intracellular region (C-terminal 80 amino acids) of mouse ephrin-B2.
Immunofluorescence
Cells were fixed for 10 minutes in 4% formaldehyde, in cytoskeletal buffer (10 mM MES, 3 mM MgCl2, 138 mM KCl, 2 mM EGTA, pH 6.1, 0.32 M sucrose). After fixation, cells were permeabilized using 0.2% Triton X-100 in PBS at room temperature for 5 minutes, and quenched for 10 minutes with NaBH4 (0.5 mg/ml in PBS; Sigma). Cells were incubated with primary antibodies (goat anti-ephrin-B2, 1:100; R&D Systems) for 45 minutes and bound antibodies were revealed with FITC- or TRITC-conjugated secondary antibodies as appropriate. To reveal surface ephrin-B2, cell permeabilization with Triton X-100 was omitted. F-actin was stained with TRITC-conjugated phalloidin (0.1 μg/ml; Sigma-Aldrich). Alexa-Fluor–Streptavidin (100 μg/ml; Invitrogen) was used to detect biotin-dextran co-injected with DNA expression constructs.
To demonstrate internalization of ephrin-B2 following stimulation of cells with pre-clustered EphB4-Fc (1 μg/ml; R&D Systems; pre-clustered with goat or mouse anti-human IgG, in a 1:10 ratio) a double fixation protocol was used. Routinely fixed cells were initially immunostained prior to permeabilization to detect EphB4-Fc bound to the cell external surface, using fluorescently labeled secondary antibodies to human Fc. After washing with PBS, coverslips were post-fixed with 4% formaldehyde in cytoskeletal buffer for 10 minutes to prevent dissociation of the EphB4-Fc–antibody complex. Following cell permeabilization, internalized EphB4-Fc was detected using secondary antibodies with a different color fluorophore. The internalized EphB4-Fc will be detected by the second fluorophore only, whereas externally bound EphB4 should show co-localization of both fluorophores.
Confocal and time-lapse microscopy and image analysis
Images were acquired using Leica confocal imaging software. Phase-contrast time-lapse images were collected from 10 minutes after injection, for between 3 and 12 hours at a rate of 1 frame per 30 seconds, using an inverted Zeiss microscope, with a ×40 phase-contrast objective, an Orca-ER camera (Hamamatsu) and Improvision software. Cells were maintained at 37°C with 5% CO2 during recording. After recording, cells were routinely fixed and stained for exogenously expressed proteins, to confirm that the cell that was recorded had been successfully microinjected.
Using Volocity Software (Improvision) the changes in cell ‘foot-print’ area and speed of cell migration were measured. Injected cells were manually traced and the area of foot-print was measured at 5-minute (real-time) intervals. Data were normalized to the foot-print of the cell measured at the first time point, and graphs were drawn to show changes in cell morphology over time. For speed of migration, the nucleus of the analyzed cell was tracked at 5-minute intervals. The total distance the cell migrated was measured (in μm) and divided by the total time the cell was traced for (in minutes). To calculate persistence index (PI), a measure of the persistence of directional migration, a straight line was drawn from the first to the last position of the tracked nucleus. This distance divided by the total distance traveled is the persistence index of migration. The time the injected cells spent in three different morphological states: unpolarized (without polarized lamellae), polarized (with a distinct leading edge and a trailing tail) and blebbing (showing membrane blebbing) was quantified from the time-lapse movies and is shown as a percentage of the total time of analysis.
Supplementary Material
Acknowledgments
We would like to thank Sanne Kuijper (Cancer Research UK, London Research Institute) for preparing the S37 and S47 ephrin-B2 binding mutants and for performing the AP-binding assay. The PDZ-RGS3 construct and the dnGrb4 construct were kind gifts from John Flanagan (MIT, Boston) and Mark Henkemeyer (University of Texas, Southwestern Medical Centre), respectively. We thank Paul Martin for comments on the manuscript and the Cell Imaging Facility at the University of Bristol for technical support. This work was supported by a University of Bristol Postgraduate Scholarship (M.L.B.), the Medical Research Council (C.D.N.) and Cancer Research UK (R.H.A.). Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/8/1235/DC1
References
- Adams R. H., Wilkinson G. A., Weiss C., Diella F., Gale N. W., Deutsch U., Risau W., Klein R. (1999). Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13, 295-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders D. M., Chen J. (2004). Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis 7, 17-28 [DOI] [PubMed] [Google Scholar]
- Bruckner K., Pablo Labrador J., Scheiffele P., Herb A., Seeburg P. H., Klein R. (1999). EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22, 511-524 [DOI] [PubMed] [Google Scholar]
- Bush J. O., Soriano P. (2009). Ephrin-B1 regulates axon guidance by reverse signaling through a PDZ-dependent mechanism. Genes Dev. 23, 1586-1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R. F., Beutler M., Marler K. J., Knoll B., Becker-Barroso E., Heintzmann R., Ng T., Drescher U. (2006). Silencing of EphA3 through a cis interaction with ephrinA5. Nat. Neurosci. 9, 322-330 [DOI] [PubMed] [Google Scholar]
- Chrencik J. E., Brooun A., Kraus M. L., Recht M. I., Kolatkar A. R., Han G. W., Seifert J. M., Widmer H., Auer M., Kuhn P. (2006). Structural and biophysical characterization of the EphB4*ephrinB2 protein-protein interaction and receptor specificity. J. Biol. Chem. 281, 28185-28192 [DOI] [PubMed] [Google Scholar]
- Conover J. C., Doetsch F., Garcia-Verdugo J. M., Gale N. W., Yancopoulos G. D., Alvarez-Buylla A. (2000). Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 3, 1091-1097 [DOI] [PubMed] [Google Scholar]
- Cowan C. A., Henkemeyer M. (2001). The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 413, 174-179 [DOI] [PubMed] [Google Scholar]
- Davy A., Soriano P. (2005). Ephrin signaling in vivo: look both ways. Dev. Dyn. 232, 1-10 [DOI] [PubMed] [Google Scholar]
- Essmann C. L., Martinez E., Geiger J. C., Zimmer M., Traut M. H., Stein V., Klein R., Acker-Palmer A. (2008). Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat. Neurosci. 11, 1035-1043 [DOI] [PubMed] [Google Scholar]
- Foo S. S., Turner C. J., Adams S., Compagni A., Aubyn D., Kogata N., Lindblom P., Shani M., Zicha D., Adams R. H. (2006). Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161-173 [DOI] [PubMed] [Google Scholar]
- Friedl P., Hegerfeldt Y., Tusch M. (2004). Collective cell migration in morphogenesis and cancer. Int. J. Dev. Biol. 48, 441-449 [DOI] [PubMed] [Google Scholar]
- Fuller T., Korff T., Kilian A., Dandekar G., Augustin H. G. (2003). Forward EphB4 signaling in endothelial cells controls cellular repulsion and segregation from ephrinB2 positive cells. J. Cell Sci. 116, 2461-2470 [DOI] [PubMed] [Google Scholar]
- Gale N. W., Baluk P., Pan L., Kwan M., Holash J., DeChiara T. M., McDonald D. M., Yancopoulos G. D. (2001). Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 230, 151-160 [DOI] [PubMed] [Google Scholar]
- Gerety S. S., Anderson D. J. (2002). Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development 129, 1397-1410 [DOI] [PubMed] [Google Scholar]
- Gerety S. S., Wang H. U., Chen Z. F., Anderson D. J. (1999). Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell 4, 403-414 [DOI] [PubMed] [Google Scholar]
- Groeger G., Nobes C. D. (2007). Co-operative Cdc42 and Rho signalling mediates ephrinB-triggered endothelial cell retraction. Biochem. J. 404, 23-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner C., Schmitz G., Meyer S., Bataille F., Hau P., Langmann T., Dietmaier W., Landthaler M., Vogt T. (2004). Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin. Chem. 50, 490-499 [DOI] [PubMed] [Google Scholar]
- Hafner C., Meyer S., Langmann T., Schmitz G., Bataille F., Hagen I., Becker B., Roesch A., Rogler G., Landthaler M., et al. (2005). Ephrin-B2 is differentially expressed in the intestinal epithelium in Crohn's disease and contributes to accelerated epithelial wound healing in vitro. World J. Gastroenterol. 11, 4024-4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. J., Dallal G. E., Flanagan J. G. (2004). Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron 42, 717-730 [DOI] [PubMed] [Google Scholar]
- Heroult M., Schaffner F., Augustin H. G. (2006). Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp. Cell Res. 312, 642-650 [DOI] [PubMed] [Google Scholar]
- Himanen J. P., Rajashankar K. R., Lackmann M., Cowan C. A., Henkemeyer M., Nikolov D. B. (2001). Crystal structure of an Eph receptor-ephrin complex. Nature 414, 933-938 [DOI] [PubMed] [Google Scholar]
- Hornberger M. R., Dutting D., Ciossek T., Yamada T., Handwerker C., Lang S., Weth F., Huf J., Wessel R., Logan C., et al. (1999). Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22, 731-742 [DOI] [PubMed] [Google Scholar]
- Huynh-Do U., Stein E., Lane A. A., Liu H., Cerretti D. P., Daniel T. O. (1999). Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J. 18, 2165-2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh-Do U., Vindis C., Liu H., Cerretti D. P., McGrew J. T., Enriquez M., Chen J., Daniel T. O. (2002). Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J. Cell Sci. 115, 3073-3081 [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Uehata M., Tamechika I., Keel J., Nonomura K., Maekawa M., Narumiya S. (2000). Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 57, 976-983 [PubMed] [Google Scholar]
- Kim I., Ryu Y. S., Kwak H. J., Ahn S. Y., Oh J. L., Yancopoulos G. D., Gale N. W., Koh G. Y. (2002). EphB ligand, ephrinB2, suppresses the VEGF- and angiopoietin 1-induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. FASEB J. 16, 1126-1128 [DOI] [PubMed] [Google Scholar]
- Lin D., Gish G. D., Songyang Z., Pawson T. (1999). The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J. Biol. Chem. 274, 3726-3733 [DOI] [PubMed] [Google Scholar]
- Lu Q., Sun E. E., Klein R. S., Flanagan J. G. (2001). Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 105, 69-79 [DOI] [PubMed] [Google Scholar]
- Lu Q., Sun E. E., Flanagan J. G. (2004). Analysis of PDZ-RGS3 function in ephrin-B reverse signaling. Methods Enzymol. 390, 120-128 [DOI] [PubMed] [Google Scholar]
- Machacek M., Hodgson L., Welch C., Elliott H., Pertz O., Nalbant P., Abell A., Johnson G. L., Hahn K. M., Danuser G. (2009). Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T., Adams R. H., Bailey J., Lu Q., Ziemiecki A., Alitalo K., Klein R., Wilkinson G. A. (2005). PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 19, 397-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T., Shirasaki R., Ghosh S., Andrews S. E., Carter N., Hunter T., Pfaff S. L. (2005). Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell 121, 127-139 [DOI] [PubMed] [Google Scholar]
- Marston D. J., Dickinson S., Nobes C. D. (2003). Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat. Cell Biol. 5, 879-888 [DOI] [PubMed] [Google Scholar]
- McLaughlin T., Hindges R., Yates P. A., O'Leary D. D. (2003). Bifunctional action of ephrin-B1 as a repellent and attractant to control bidirectional branch extension in dorsal-ventral retinotopic mapping. Development 130, 2407-2418 [DOI] [PubMed] [Google Scholar]
- Nagashima K., Endo A., Ogita H., Kawana A., Yamagishi A., Kitabatake A., Matsuda M., Mochizuki N. (2002). Adaptor protein Crk is required for ephrin-B1-induced membrane ruffling and focal complex assembly of human aortic endothelial cells. Mol. Biol. Cell 13, 4231-4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M., Anderson E. M., Demuth T., Nakada S., Reavie L. B., Drake K. L., Hoelzinger D. B., Berens M. E. (2009). The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int. J. Cancer 126, 1155-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren N. K., Pasquale E. B. (2007). Paradoxes of the EphB4 receptor in cancer. Cancer Res. 67, 3994-3997 [DOI] [PubMed] [Google Scholar]
- Noren N. K., Lu M., Freeman A. L., Koolpe M., Pasquale E. B. (2004). Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc. Natl. Acad. Sci. USA 101, 5583-5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren N. K., Yang N. Y., Silldorff M., Mutyala R., Pasquale E. B. (2009). Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem. J. 422, 433-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A., Zimmer M., Erdmann K. S., Eulenburg V., Porthin A., Heumann R., Deutsch U., Klein R. (2002). EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol. Cell 9, 725-737 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. (2005). Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell. Biol. 6, 462-475 [DOI] [PubMed] [Google Scholar]
- Pfaff D., Heroult M., Riedel M., Reiss Y., Kirmse R., Ludwig T., Korff T., Hecker M., Augustin H. G. (2008). Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J. Cell Sci. 121, 3842-3850 [DOI] [PubMed] [Google Scholar]
- Pyenta P. S., Holowka D., Baird B. (2001). Cross-correlation analysis of inner-leaflet-anchored green fluorescent protein co-redistributed with IgE receptors and outer leaflet lipid raft components. Biophys. J. 80, 2120-2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E., Marshall C. J. (2003). Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell. Biol. 5, 711-719 [DOI] [PubMed] [Google Scholar]
- Salvucci O., Maric D., Economopoulou M., Sakakibara S., Merlin S., Follenzi A., Tosato G. (2009). EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood 114, 1707-1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Garcia-Cardena G., Hayashi S., Gerety S., Asahara T., Stavrakis G., Isner J., Folkman J., Gimbrone M. A., Jr, Anderson D. J. (2001). Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev. Biol. 230, 139-150 [DOI] [PubMed] [Google Scholar]
- Su Z., Xu P., Ni F. (2004). Single phosphorylation of Tyr304 in the cytoplasmic tail of ephrin B2 confers high-affinity and bifunctional binding to both the SH2 domain of Grb4 and the PDZ domain of the PDZ-RGS3 protein. Eur. J. Biochem. 271, 1725-1736 [DOI] [PubMed] [Google Scholar]
- Surawska H., Ma P. C., Salgia R. (2004). The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 15, 419-433 [DOI] [PubMed] [Google Scholar]
- Tachibana M., Tonomoto Y., Hyakudomi R., Hyakudomi M., Hattori S., Ueda S., Kinugasa S., Yoshimura H. (2007). Expression and prognostic significance of EFNB2 and EphB4 genes in patients with oesophageal squamous cell carcinoma. Dig. Liver Dis. 39, 725-732 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kamo T., Ota S., Sugimura H. (2003). Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 22, 847-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R., Firestein B. L., Dong H., Staudinger J., Olson E. N., Huganir R. L., Bredt D. S., Gale N. W., Yancopoulos G. D. (1998). PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron 21, 1453-1463 [DOI] [PubMed] [Google Scholar]
- Vihanto M. M., Plock J., Erni D., Frey B. M., Frey F. J., Huynh-Do U. (2005). Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J. 19, 1689-1691 [DOI] [PubMed] [Google Scholar]
- Vogt T., Stolz W., Welsh J., Jung B., Kerbel R. S., Kobayashi H., Landthaler M., McClelland M. (1998). Overexpression of Lerk-5/Eplg5 messenger RNA: a novel marker for increased tumorigenicity and metastatic potential in human malignant melanomas. Clin. Cancer Res. 4, 791-797 [PubMed] [Google Scholar]
- Wang H. U., Chen Z. F., Anderson D. J. (1998). Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741-753 [DOI] [PubMed] [Google Scholar]
- Xu Z., Lai K. O., Zhou H. M., Lin S. C., Ip N. Y. (2003). Ephrin-B1 reverse signaling activates JNK through a novel mechanism that is independent of tyrosine phosphorylation. J. Biol. Chem. 278, 24767-24775 [DOI] [PubMed] [Google Scholar]
- Yin Y., Yamashita Y., Noda H., Okafuji T., Go M. J., Tanaka H. (2004). EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci. Res. 48, 285-296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.