Abstract
Actin-filament disassembly is crucial for actin-based motility, to control filament network architecture and to regenerate subunits for assembly. Here, we examined the roles of three actin cytoskeletal proteins, coronin, cofilin and Aip1, which have been suggested to combine in various ways to control actin dynamics by promoting or regulating disassembly. We studied their functions during the endocytosis process in budding yeast, where actin-filament dynamics at the cortical actin ‘patch’ contribute to the formation and movement of endocytic vesicles. We found that all three proteins were recruited during the late phase of the life of the actin patch. They all arrived at the same time, when actin and other actin-associated proteins were leaving the patch. Cofilin point mutations influenced the localization of coronin and Aip1, but the complete loss of coronin had no effect on localization of cofilin or Aip1. Using quantitative patch motion analysis and comparing mutant alleles, the phenotypes for mutations of the three genes showed some commonalities, but also some striking differences. Cofilin was clearly the most important; it displayed the most severe mutant phenotypes affecting actin-patch assembly and movement. Together, the results suggest that all three proteins work together to promote actin disassembly, but not in a simple way, and not with equal importance.
Keywords: Actin, Cofilin, Coronin, Aip1, Endocytosis
Introduction
Actin filaments are involved in a broad range of cell activities, including morphological changes, cell migration, cell division and endocytosis. Networks of actin filaments in cells undergo rapid changes in response to a variety of intracellular and extracellular cues. Remodeling of filament networks occurs rapidly, on a time scale of seconds, and depolymerization of filaments is crucial to provide an adequate supply of actin monomers for the assembly of new actin-filament structures.
Coronin, cofilin and Aip1 have been implicated, singly and together, as important molecules for actin-filament remodeling and disassembly. Cofilin and the related protein actin-depolymerizing factor (ADF) are involved in depolymerizing actin filaments; they sever actin filaments, and have a preference for older (ADP-containing) actin filaments (Ono, 2007). The activity of ADF/cofilin can be regulated by various mechanisms, including phosphorylation, pH and polyphosphoinositides (Bernstein et al., 2000; Van Troys et al., 2008). Other molecules, including the proteins Aip1 and coronin, appear to act in concert with cofilin to optimize the in vivo flux of actin turnover (Bamburg, 1999; Ono, 2007). In vitro, the combination of cofilin, Aip1 and coronin depolymerizes actin filaments at a remarkably high speed, by a phenomenon called ‘bursting’ (Kueh et al., 2008). Given this wealth of information about the biochemical activities of cofilin, a current challenge for the field is to define the action of cofilin in cells.
Aip1 interacts with cofilin genetically and biochemically (Clark et al., 2006; Ono, 2001; Ren et al., 2007). Aip1 promotes cofilin-mediated actin turnover by enhancing its severing activity and by capping the barbed ends of cofilin-severed actin filaments, to block reannealing and elongation (Balcer et al., 2003; Okada et al., 2002; Ono et al., 2004). These functions are proposed to depend on interactions of Aip1 with cofilin and the actin filament (Clark and Amberg, 2007; Clark et al., 2006; Mohri et al., 2004). Recently, Aip1 was found to colocalize with cofilin at the leading edge of migrating fibroblasts, where it appears to cap barbed ends generated by cofilin, but it dissociates rapidly, to promote polymerization rather than depolymerization (Tsuji et al., 2009).
For coronin, a variety of roles in cofilin-mediated actin depolymerization have been proposed, based on different types of experiments in different systems. In yeast, coronin interacts genetically with cofilin (Goode et al., 1999). In a biochemical study, mammalian coronin-1A decorated Listeria actin comet tails, which facilitated the binding of cofilin and enhanced its actin depolymerization activity (Brieher et al., 2006). By contrast, another study found that purified coronin-1B inhibited cofilin-mediated actin disassembly (Cai et al., 2007a). This paradox was recently addressed in a study by Gandhi and colleagues, who concluded that the actin nucleotide is a crucial variable for coronin-cofilin interaction (Gandhi et al., 2009). In this new model, the binding of coronin to the ADP-actin subunits of older actin filaments facilitates severing of cofilin and thereby disassembly. However, the binding of coronin to ATP/ADP+Pi-actin in new actin filaments, protects the filaments from the severing action of cofilin. Again, the relevance of these differential biochemical activities in cells is uncertain.
To address the physiological relevance of these observations and hypotheses, we examined the roles of cofilin, coronin and Aip1 in vivo, using endocytosis in budding yeast as a model system for actin assembly, remodeling and disassembly. Coronin, cofilin and Aip1 are highly conserved across species, and the budding yeast genome contains single genes encoding homologues of each protein. The cofilin gene COF1 is essential for viability, and several viable cof1 mutants display abnormal aggregates of actin (Lappalainen and Drubin, 1997; Lappalainen et al., 1997; Moon et al., 1993). Coronin (CRN1) and Aip1 (AIP1) null mutants are viable; however, they do display synthetic phenotypes when combined with certain cof1 alleles (Balcer et al., 2003; Clark et al., 2006; Goode et al., 1999; Heil-Chapdelaine et al., 1998; Rodal et al., 1999). Budding yeast cofilin, coronin and Aip1 proteins all localize to the cortical actin patches, which contribute to the endocytic machinery and which undergo rapid dynamics and turnover (Kaksonen et al., 2003; Kaksonen et al., 2005; Moseley and Goode, 2006).
The lifespan of actin patches can be considered in three broad phases based on their molecular composition and motion features (Galletta and Cooper, 2009; Kaksonen et al., 2006). During Phase I, actin patches assemble at the cortex and display a limited amount of motion. Endocytic adaptors and activators of actin-nucleating factors are recruited during this phase. Near the end of Phase I, the actin-filament network begins to assemble, as indicated by the arrival of the Arp2/3 complex and other actin-binding proteins. Phase II patches are characterized by a slow and short inward movement. The assembly of Arp2/3-mediated actin networks in this stage is proposed to generate force to push on endocytic coats and cause membrane invagination (Galletta et al., 2008; Kaksonen et al., 2003; Winter et al., 1997). During Phase III, the endocytic vesicles pinch off from the membrane and then undergo rapid and lengthy movements into and around the cell. The actin networks on the internalized patches ultimately disappear, presumably because of filament depolymerization.
Here, we asked how coronin, cofilin and Aip1 localize with respect to each other and the actin cytoskeleton, in time and space, using real-time fluorescence analysis of living cells. We determined their molecular stoichiometries, in absolute terms, which place constraints on molecular models. We asked how mutations affecting one protein affect the localization dynamics of the other proteins, and how a variety of mutations affect the assembly and motion of actin patches. We learned that the proteins do interact and work together during actin disassembly, but not in a simple way, and certainly not as a single functional complex. In particular, the mildness of the Aip1- and coronin-null mutant phenotypes suggests that the ‘bursting’ phenomenon defined in vitro is unlikely to have a major role in this setting.
Results
Construction of functional fluorescent-protein-tagged cofilin
Yeast cofilin (Cof1) has long been known to localize to cortical actin patches (Lappalainen and Drubin, 1997; Moon et al., 1993). However, neither N-nor C-terminus-tagged cofilin proteins are functional, and the temporal dynamics of cofilin in actin patches was recently examined with an internal green fluorescent protein (GFP) fusion protein (Okreglak and Drubin, 2007). In that study, the GFP-Cof1 fusion needed to be expressed at a high level to rescue the lethality of a cof1Δ mutant, suggesting that the fusion might not be fully functional. Therefore, we constructed a novel fluorescent-protein-tagged Cof1.
We sought to identify the interior residues of Cof1 that we could modify without interfering with its functions. Mammalian ADF/cofilin can rescue the lethality of the yeast cof1Δ mutant (Iida et al., 1993; Moriyama et al., 1996). We performed a structural alignment of yeast (pdb 1CFY) and human (pdb 1Q8G) cofilin-1 based on the central core of the protein. We observed that human cofilin-1 has inserts in two positions on the surface of the protein (supplementary material Fig. S1A), corresponding to the G22K23 and E43Y50 regions of yeast Cof1, respectively (supplementary material Fig. S1B). We inserted monomeric forms of GFP (S65T) or red fluorescent protein (RFP) from pKT vectors with modified linkers AGAGDGAGL and LGAGDGAGA (Sheff and Thorn, 2004) into the G22K23 or E43T44 sites, using the COF1 gene. The COF1-GFP or COF1-RFP fragments, along with non-coding genomic regions of COF1, were cloned into low-copy-number centromere vectors (Sikorski and Hieter, 1989).
First, we examined the localization pattern of the Cof1-GFP and Cof1-RFP fusions in wild-type backgrounds. Insertions at the G22K23 site (insert-1) showed completely diffuse cytosolic distributions (supplementary material Fig. S1C, left), indicating a loss of function. By contrast, the inserts at the E43T44 site (insert-2) gave a cortical-patch localization with low cytosolic background (supplementary material Fig. S1C, right; Fig. 1A; supplementary material Movie 1). Cof1-RFP showed less diffuse background than did Cof1-GFP (data not shown).
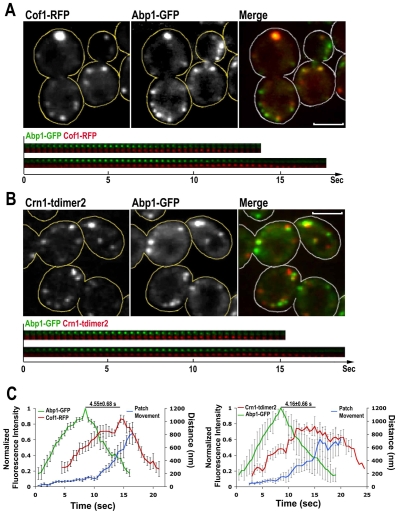
Fig. 1.
Timing of localization of cofilin, coronin and Abp1 to actin patches. (A,B) Single-frame images from a confocal time-lapse movie of cells expressing Cof1-RFP Abp1-GFP (A) or Abp1-GFP and Crn1-tdimer2 (B) are shown in the upper panels. Two isolated actin patches are shown in the montages on the bottom. Scale bars: 3 μm. (C) Mean fluorescence intensity (green and red lines) and average distance (blue line) for nine actin patches over time. Left, Cof1-RFP Abp1-GFP cells. Right, Crn1-tdimer2 Abp1-GFP cells. Error bars are s.e.m. Images were collected at room temperature.
The Cof1-RFP insert-2 plasmid could rescue the lethality of a cof1Δ null mutation at all temperatures tested (supplementary material Table S2; supplementary material Fig. S1D). The cortical patch localization of Cof1-RFP in the wild-type or cof1Δ cells showed no difference (supplementary material Fig. S1C). The endogenous Cof1 could also be replaced by this Cof1-RFP fusion. However, the COF1-RFP cells showed growth defects at 34°C (supplementary material Fig. S1E), and a few cells showed some large cytosolic aggregates of fluorescence (data not shown). We conclude that our novel Cof1-RFP fusion localizes to cortical actin patches in a manner similar to that of wild-type Cof1, and that the fusion functions well, but not perfectly. For convenience, we used the CEN plasmid form of COF1-RFP in wild-type or cof1Δ backgrounds throughout this study.
One reason why our Cof1-RFP functions better than previous Cof1 constructs could be because the insertion site (E43T44) we used to fuse RFP is not crucial for protein function. This is supported by a previous alanine-scanning study, in which two COF1 alleles, cof1-10 and cof1-11, adjacent to this region showed wild-type phenotypes (Lappalainen et al., 1997). The N74G75 site for the previous GFP-Cof1 fusion is located in the F-actin interaction domain for Cof1 and several point mutants in this region were shown to be lethal (Okreglak and Drubin, 2007; Lappalainen et al., 1997).
Localization of cofilin and Abp1
To gain insight into how Cof1 is involved in actin-patch movement in yeast cells, we first characterized the temporal incorporation of Cof1-RFP, compared with Abp1-GFP, which is a marker for actin filaments (Kaksonen et al., 2003; Kim et al., 2006). The still images of Cof1-RFP, Abp1-GFP cells showed partial colocalization of Cof1 and Abp1, and two-color movies showed that Cof1 appeared at cortical actin patches relatively late in their lifetime (Fig. 1A; supplementary material Movie 1, arrowheads).
We compared the timing of recruitment by quantifying fluorescence intensity over time. Cof1-RFP also labeled some large particles with little or no Abp1-GFP (supplementary material Movie 1, arrows). These particles did not behave like endocytic actin patches; therefore, we avoided them in the following analyses and selected only the cortical patches that were visible for the full length of the movie. The results showed a time delay in the appearance and peak intensity of Cof1 compared with Abp1 (Fig. 1C, left), and this is similar to the previous report (Okreglak and Drubin, 2007). We measured the temporal difference of peak molecular levels of Abp1 and Cof1 in individual patches, and we found that Cof1 reached its peak intensity 4.6±0.7 seconds after the highest Abp1 value; at this point in time, Abp1 fluorescence was almost undetectable. This observation fits with the notion that cofilin is involved in the disassembly of F-actin structures (Ono, 2003; Ono, 2007). In this analysis, we also measured the motion of the patches (Fig. 1C, left, blue line). Comparison of patch motion with fluorescence intensity shows that Cof1 assembled at a time when the initial short-distance movement of the cortical patches, characteristic of Phase II motion, had already begun. After a few seconds, fast movement (indicated by the steep segment of the blue line) of the endocytic patches occurred, which is characteristic of Phase III motion. The Cof1 level reached its mid point during this phase. In addition, we discovered that the patches kept moving as they accumulated Cof1 and lost Abp1 (Fig. 1A,C, left), suggesting that relatively less actin is required for the final portions of the movement observed here. This was not found in previous work (Okreglak and Drubin, 2007), indicating that our functional Cof1-RFP fusion, reveals more details of the behavior of endogenous Cof1.
Localization of cofilin, coronin, and Aip1
Coronin and Aip1 have been localized to cortical actin patches (Heil-Chapdelaine et al., 1998; Rodal et al., 1999); however, we do not know when these two molecules are recruited. If the cell uses a combination of cofilin, Aip1 and coronin to depolymerize actin networks in late actin patches, the three proteins should co-localize over time.
The temporal dynamics of coronin in actin patches was examined with a tandem dimer of DsRed (tdimer2) fusion (Campbell et al., 2002). Two-color movies of Abp1-GFP Crn1-tdimer2 cells showed that coronin localized to actin patches in a time frame that was similar to that observed for cofilin above (Fig. 1B; supplementary material Movie 2, arrowheads). Crn1-tdimer2 also labeled a few large particles that were similar to those observed in Cof1-RFP cells (supplementary material Movie 2, arrows). Coronin arrived at actin patches after Abp1, and similarly to Cof1, persisted in the internalized patches as Abp1 became undetectable (Fig. 1B, bottom). The fluorescence intensity of isolated patches over time showed a delay of 4.2±0.7 seconds between the peaks of Abp1-GFP and Crn1-tdimer2 (Fig. 1C, right), which is similar to the gap between the peaks for Abp1 and cofilin. The results all suggest that the behavior of coronin in the actin patches is similar to that of cofilin.
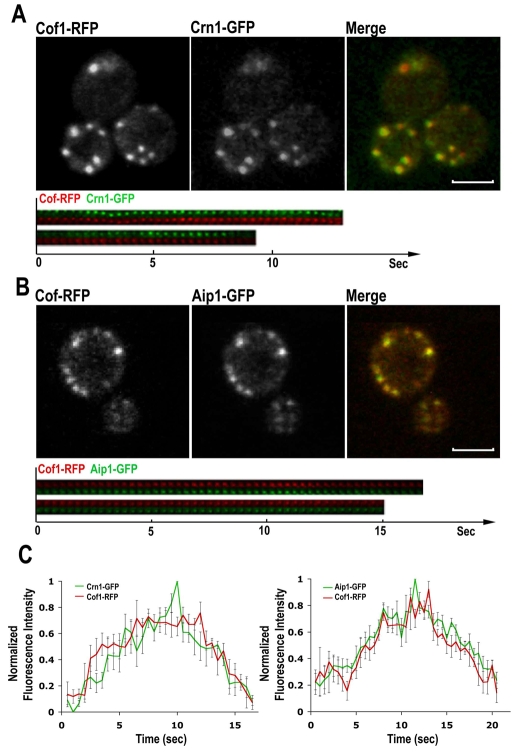
We then compared the timing of recruitment for cofilin, coronin and Aip1 at actin patches, to see if they overlapped. We transformed Cof1-RFP plasmids into Crn1-GFP or Aip1-GFP cells. Two-color movies of patches from these cells showed that cofilin, coronin and Aip1 co-localize to actin patches within the same period of time (Fig. 2A,B; supplementary material Movies 3 and 4). Aip1 was also recruited to the unknown organelles (supplementary material Movie 4, arrows). Note that our figures were captured from live cells and the partial offset between green and red images was because they were taken sequentially. The fluorescence intensity data of isolated patches from the two strains also show that cofilin, coronin and Aip1 behave in the same manner in the cortical patches (Fig. 2C).
Fig. 2.
Timing of cofilin, coronin and Aip1 localization to actin patches. (A,B) Single-frame images from a confocal time-lapse movie of cells expressing Cof1-RFP and Crn1-GFP (A) or Aip1-GFP and Cof1-RFP (B). Scale bars: 3 μm. (C) Mean fluorescence intensity (green and red lines) of cortical actin patches from cells in A (n=5) or B (n=10) were plotted over time. Error bars indicate s.e.m. Images were collected at room temperature.
A recent study concluded that coronin in yeast can inhibit actin filaments from recruiting cofilin, based on the spatial analysis of membrane-associated actin aggregates in a sla2Δ mutant (Gandhi et al., 2009). Here, we tested this hypothesis by analyzing the temporal recruitment of cofilin to actin patches undergoing assembly and disassembly in a SLA2 crn1Δ background. We found that cofilin localized to the patch few seconds after Abp1 (supplementary material Fig. S3), and this was similar to results obtained in a wild-type strain. Thus, coronin does not inhibit cofilin recruitment to actin patches in this setting.
Molecular stoichiometry of coronin, cofilin and Aip1
To determine whether the three proteins act as a simple heterotrimeric complex, and to serve as a guide for biochemical experiments, we examined their molecular stoichiometries. In operational terms, we asked how many molecules of each protein were located on the patch, at the peak of recruitment and over time? We quantified GFP fluorescence and compared it with an internal standard of Cse4-GFP, following the method developed by Joglekar and colleagues (Joglekar et al., 2006). The comparison with Cse4-GFP allows one to calculate an absolute number of molecules per patch. In addition, the method uses a superior approach for correction of background fluorescence (see Materials and Methods). We determined the peak number of molecules per patch as the mode of the distribution of values, not the mean, to avoid influence from high-value outliers that might skew the distribution (see Materials and Methods). The value for coronin was 93 (range 86-107, 95% confidence interval, n=384), and that of Aip1 was 45 (range 38-52, 95% confidence interval, n=331). For cofilin, our fusion used RFP, not GFP, so we needed to create an RFP-based internal standard. RFP-Cse4 cells were not healthy, so we constructed a fusion of RFP with Spc105, which is present in 80 copies per anaphase kinetochore cluster (Joglekar et al., 2006). We found 930 molecules of cofilin per patch (range 740-1155, 95% confidence interval, n=160). In this experiment, we used cof1Δ cells transformed with our pCOF1-RFP CEN plasmid (supplementary material Fig. S1D). We considered that cofilin might be overexpressed by a certain amount because of using the CEN plasmid. We performed immunostaining for cofilin and compared the level of cofilin per actin patch in wild-type and cof1Δ pCOF1-RFP cells. The cortical patch cofilin intensity in the cof1Δ pCOF1-RFP cells was 1.76-fold higher than that in wild-type cells, therefore we normalized the value of cofilin molecules per patch to 530 from 930. Therefore, the molecular stoichiometry is not a simple 1:1:1 relationship. If the ‘bursting’ phenomenon were to exist in vivo in this setting, then the activity would be limited by the molecular quantity of the limiting protein, Aip1. As discussed below, the aip1Δ null mutant phenotype is extremely mild; therefore, bursting is not likely to be a major contributor to actin depolymerization here.
We then determined how much F-actin was present per patch. GFP-actin fusions are not functional, but we were able to estimate the amount of actin in a patch by another approach. In a previous EM study of partially purified actin patches, we found 145 nm of actin filament per Arp2/3-mediated branch point, on average (Young et al., 2004). In this study, to obtain a value for the number of Arp2/3 complex molecules per patch in living cells, we labeled either the ArpC5-Arc15 or the ArpC2-Arc35 subunit. Previous studies have established that these fusion proteins are functional (Kaksonen et al., 2003; Schaerer-Brodbeck and Riezman, 2000). Both probes gave us similar results, with the number of Arp2/3 molecules per patch of ~95. For ARPC5-Arc15, the result was 102 (range 97-108, 95% confidence interval, n=473), and for ARPC2-Arc35, the result was 90 (range 80-96, 95% confidence interval, n=477). Together, these values yield a value of 5100 subunits of F-actin per patch, based on 370 actin subunits per micrometer of filament (Hanson and Lowy, 1963). Thus, the ratio of cofilin to F-actin subunits is 530 to 5100, or ~1:10. If every molecule of cofilin does sever an actin filament, then the quantity of cofilin should be ample to enable a substantial degree of depolymerization. In addition, the value for Arp2/3 complex (95) is similar to the value for coronin (93), which is consistent with the hypothesis that coronin directly interacts with and inhibits Arp2/3 complex (Cai et al., 2007b; Humphries et al., 2002)
Co-dependence of cofilin, coronin, and Aip1 localization
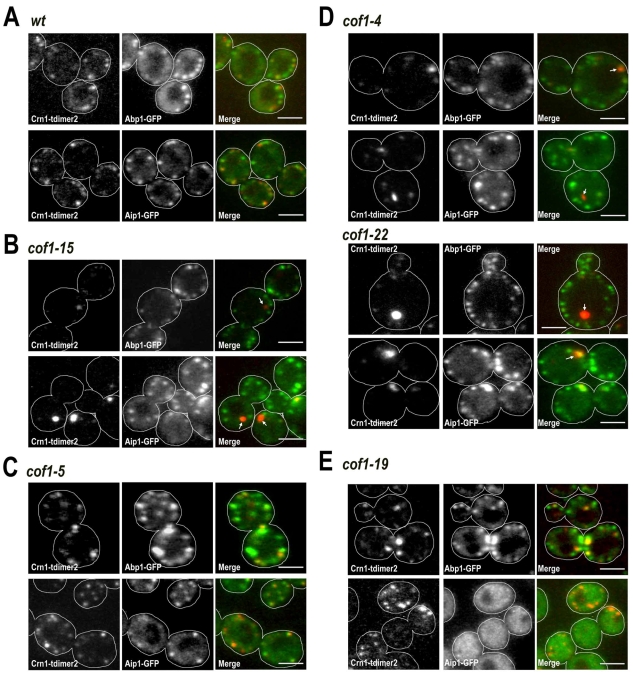
To determine the extent to which these three proteins might depend on each other and interact in the cell. We first asked whether cofilin is required for the proper localization of coronin and Aip1. Cofilin cof1Δ null mutations are lethal, so we examined, with live images, the distribution of Crn1-tdimer2 (and Crn1-GFP, data not shown) or Aip1-GFP in a collection of cof1 point-mutant strains (Lappalainen et al., 1997). We also examined the morphology of the cells and their actin cytoskeleton. The restrictive temperature for cof1-22 and cof1-5 alleles is 34°C, and we used this temperature for all strains we tested and compared their phenotypes. We grouped the cof1 alleles into five classes, listed in supplementary material Table S3 along with summaries of coronin and Aip1 distribution, growth phenotype, cell morphology and actin structure. Fig. 3 illustrates the localization of coronin and Aip1, along with cell morphology and the localization of Abp1, which should reflect the distribution of F-actin.
Fig. 3.
Localization of coronin and Aip1 in cof1 mutants of different classes. (A-E) Class 1 to Class 5 cof1 mutants are shown in panels A-E, respectively. Wild-type cells illustrate the Class 1 phenotype (A). Crn1-tdimer2 Abp1-GFP or Crn1-tdimer2 Aip1-GFP were expressed in wild-type and cof1 cells. Cells were cultured at 34°C, and two-color confocal images were captured sequentially on equatorial cell planes. Arrows in D indicate abnormal Crn1 aggregates. Scale bars: 3 μm.
Class 1 cells were pseudo-wild-type, with normal actin patch, coronin and Aip1 localization (Fig. 3A). Class 1 included cof1-6, cof1-11, cof1-12, cof1-13 and cof1-21 (supplementary material Table S3). Class 2 contained only one mutant, cof1-15. The cof1-15 cells showed normal morphology of actin-patch structures and Aip1 distribution. However, the coronin levels in the actin patches were greatly reduced. Instead, coronin aggregated into one or two cytosolic foci per cell without any obvious diffuse cytoplasmic component (Fig. 3B, cof1-15, arrows; supplementary material Movie 5). Cells of class 3 strains, consisting of only cof1-5, a previously identified temperature-sensitive allele (Lappalainen et al., 1997), showed enlarged actin patches, with coronin and Aip1 localized to patches. Some cells also showed abnormal coronin aggregates, but the percentage was not as high as in classes 2 and 4. Class 4 had the strongest phenotypes and included cof1-4 and cof1-22. Cells displayed several different abnormalities, including enlarged cell shape, accumulation of abnormal actin structures, and cytosolic coronin aggregates (Fig. 3D). However, Aip1 was still recruited to the cortical patches of these two mutants. cof1-22 is temperature sensitive for growth, similarly to cof1-5 (Lappalainen et al., 1997). cof1-4 did not show a severe phenotype in a previous study; however, its actin-binding activity was slightly reduced when compared with the wild type (Lappalainen et al., 1997). We categorized cof1-19 into class 5 (supplementary material Table S3). This mutant, cof1-19, was the only one that showed abnormal Aip1 localization. Aip1 was diffuse in the cytosol without any cortical patch localization (Fig. 3E, right panel), which is consistent with previous studies (Clark et al., 2006; Rodal et al., 1999). cof1-19 also displayed slightly increased actin structures and a normal coronin distribution (Fig. 3E).
Together, the localization defects of coronin and Aip1 in different cof1 mutants indicate that cofilin is important for the cortical patch localization of these two other proteins. Cofilin and Aip1 have been proposed to interact physically (Clark et al., 2006; Rodal et al., 1999). However, the interaction between cofilin and coronin was suggested to be indirect (Brieher et al., 2006; Gandhi et al., 2009). Our results for cof1-15, namely normal actin distribution with abnormal coronin, suggest that the interaction between cofilin and coronin is not simply via actin and might therefore be direct.
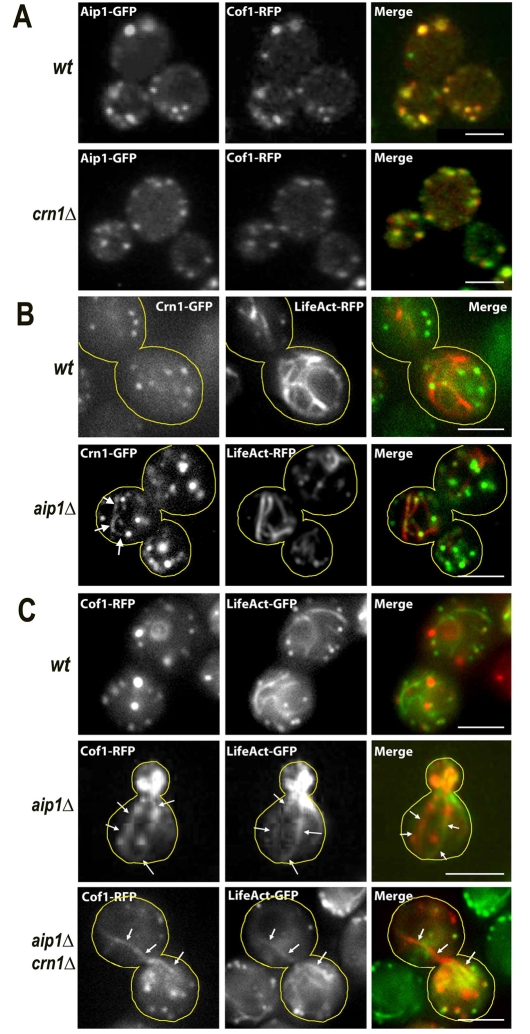
To test whether coronin was important for the localization of cofilin and Aip1 in live cells, we examined the localization of both Cof1-RFP and Aip1-GFP in crn1Δ null cells and found the localization of both proteins was essentially normal (Fig. 4A). This result argues against an important role for coronin in recruiting cofilin or Aip1.
Fig. 4.
Localization of cofilin, coronin and Aip1 in crn1Δ and aip1Δ null mutant live cells. (A) Wild-type and crn1Δ cells expressing Aip1-GFP and Cof1-RFP. Two-color images were collected with a wide-field fluorescence microscope at room temperature. (B) Wild-type and aip1Δ cells expressing Crn1-GFP and LifeAct-RFP, to label actin cables and patches. (C) Wild-type, CRN1 aip1Δ and crn1Δ aip1Δ cells expressing Cof1-RFP and LifeAct-GFP. Cells were cultured at 30°C. Arrows in B and C indicate Crn1-GFP-, Cof1-RFP- or LifeAct-GFP-labeled actin cables. Scale bars: 3 μm.
We examined the localization of cofilin and coronin in aip1Δ null mutants. Cof1 has been reported to localize ectopically to actin cables, in an aip1Δ background, in addition to its normal patch localization (Okada et al., 2006; Rodal et al., 1999). To test this phenotype in live cells, we used the recently reported LifeAct to label both actin patches and actin cables (Riedl et al., 2008). Cof1-RFP was localized to actin patches and cables in the aip1Δ cells (Fig. 4C), as expected. In addition, we found that coronin was also ectopically recruited to actin cables, while still remaining at patches, in the aip1Δ background (Fig. 4B). Finally, we examined the localization of cofilin in a crn1Δ aip1Δ double mutant. Cofilin localized to actin patches and cables (Fig. 4C), as seen in the aip1Δ single mutant.
Together, our results show that the localization of cofilin, coronin and Aip1 to actin structures are interdependent, but not in a simple way. Cofilin is required for the localization of coronin and Aip1 to actin patches. Aip1 is necessary to prevent localization of cofilin and coronin to actin cables. However, coronin is not important for the localization of the other two proteins.
Actin patch dynamics: crn1Δ and cof1-15 display similar phenotypes
The co-localization of cofilin, coronin and Aip1 to actin patches implies that these three molecules might have cooperative functions. Yeast actin patches are sets of complex machines that show rapid motions and molecular turnover as they contribute to endocytosis. We developed computer-assisted analysis of the assembly and movement of yeast actin patches (Carlsson et al., 2002), and we can use those routines to identify and quantify potentially subtle defects of actin patch assembly and movement (Galletta et al., 2008). We examined patch phenotypes in mutants defective for one or more of the three proteins.
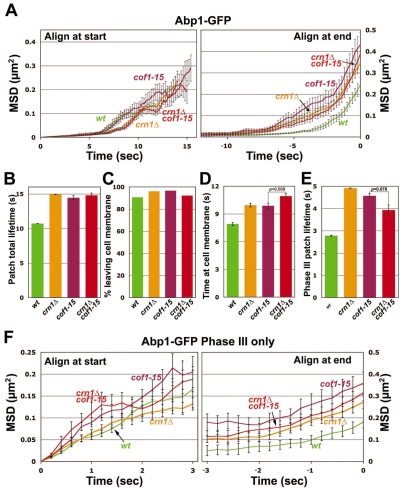
First, by using Abp1-GFP as a marker for mid- to late-stage patches (Galletta et al., 2008), we compared patch motion phenotypes of crn1Δ mutants with those of cof1-15 mutants. As discussed above, cof1-15 cells show greatly decreased coronin localization to the actin patch, without observable morphological and actin patch abnormalities; thus we hypothesized that the cof1-15 mutation was only defective in coronin recruitments to the patch and we predicted that the patch motion defects in cof1-15 and crn1Δ cells would be similar. For crn1Δ, mean squared displacement (MSD) plots of patch position, aligned either at their start or end point (Fig. 5A) showed increased movements compared with the wild type (Fig. 5A), in agreement with our previous findings (Galletta et al., 2008). For the cof1-15 mutant, the MSD plots showed similar but slightly larger shifts, compared with the wild type and crn1Δ (Fig. 5A). We also analyzed patch movements in the cof1-15 crn1Δ double mutant. The MSD plots for the double mutant were not significantly different from those for the single mutants (Fig. 5A), suggesting that any mis-localized coronin protein in the cof1-15 mutant does not function in patch dynamics.
Fig. 5.
Quantitative motion analysis of Abp1-GFP-labeled actin patches in wild-type, cof1-15, crn1Δ and cof1-15 crn1Δ cells. (A) Mean-squared displacement (MSD) plots for patches aligned at the start (left) or end (right) of their lifetimes. On the left, the curves are truncated at the median lifetime. (B) Average total lifetime of patches, defined as the time from the appearance of a patch until its disappearance. (C) Percentage of patches that leave the membrane, defined as traveling >200 nm from their point of origin. (D) Average time spent by patches within 200 nm from their point of origin. (E) Average lifetime of patches after they travel 200 nm from their point of origin until the time they disappear (Phase III lifetime). (F) MSD plot of Phase III patch movement. For each patch, only data for travel more than 200 nm from the point of origin were included. Means ± s.e. of three segregants are shown. Student's t-tests for statistical significance were performed in D and E, as indicated. Strains and numbers of patches were as follows: wild type: YJC6537 (n=41), YJC6538 (n=43) and YJC6543 (n=29); crn1Δ: YJC6779 (n=33), YJC6780 (n=42) and YJC6781 (n=31); cof1-15: YJC6536 (n=19), YJC6541 (n=29) and YJC6542 (n=23); crn1Δ cof1-15: YJC6920 (n=60), YJC6921(n=30) and YJC6922 (n=65).
We analyzed the patch movement data in several other ways. First, we found that the total lifetimes of the patches in the three mutants increased to a similar extent (Fig. 5B). We analyzed how patch motions were altered in different patch phases. The percentage of patches able to leave the membrane was essentially unaffected, with only a slight increase in the mutants compared with the wild type (Fig. 5B).
An operational cut-off distance of 200 nm was used to separate the slow inward movement that presumably precedes or accompanies fission of endocytic vesicles from the more rapid movement that presumably occurs after fission (Galletta et al., 2008; Kaksonen et al., 2003). We measured the lifetimes for Phases I and II compared with Phase III based on this 200 nm cut-off (Fig. 5D,E). The patches in the crn1Δ and cof1-15 cells showed similar lengthening of time span before and after the 200 nm point. The double mutant, compared with the single mutants, showed a similar Phase III lifetime and a small but significant increase in time localized on the membrane (Fig. 5D,E).
To examine Phase I and Phase II patch motions in cof1-15 more directly, we also examined patches labeled with Sla2-GFP (supplementary material Fig. S2). Sla2 localizes to membrane-tethered endocytic vesicles, and it has been used to characterize early actin patch behavior (Galletta et al., 2008). Both Phases I and II were prolonged compared with the wild type (supplementary material Fig. S2A,D,E), which was similar to but stronger than the phenotypes we previously reported for crn1Δ (Galletta et al., 2008).
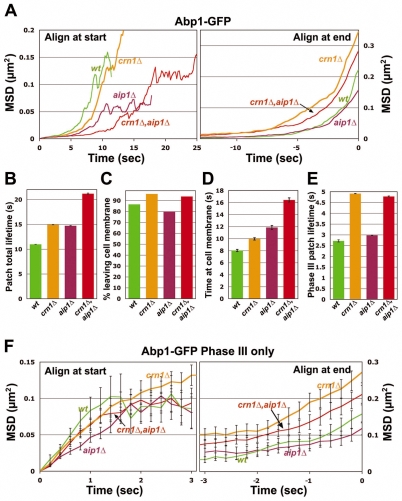
Finally, we examined Phase III movement (i.e. movement after 200 nm) directly by MSD plots with Abp1-GFP (Fig. 5F). The cof1-15, crn1Δ and double mutants were rather similar to the wild type during the early part of the movement [when tracks are aligned at the start (Fig. 5F, left)], but during the later part of the movement, cof1-15 showed a larger travel distance and a slightly higher travel speed, compared with crn1Δ, which showed no difference compared with the wild type (Fig. 5F, right; Table 1).
Table 1.
Phase III patch movements in different mutants*
Thus, overall, the cof1-15, crn1Δ and double mutant had similar defects, when compared with the wild type, showing that the patch motion defects in these three mutants might result from a loss of coronin in the patch. The most striking defects were increases in the time and distance traveled in the cytoplasm, which is consistent with the hypothesis that coronin works to disassemble the actin filaments associated with moving patches.
Actin-patch dynamics: cofilin is more important than Aip1
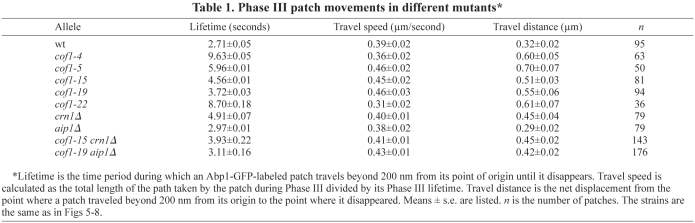
Next, we compared the mutant phenotypes of aip1Δ cells with cof1-19 cells. As discussed above, patch localization of Aip1 was essentially lost in the cof1-19 mutant. We hypothesized that actin patch movement in cof1-19 cells might resemble that of aip1Δ. The ABP-GFP total MSD data for both mutants showed delayed internalization; however, the movement of cof1-19 patches was dramatically increased, compared with the wild type, whereas that of the aip1Δ cells was slightly reduced (Fig. 6A,B). We also found that the percentage of patch internalization, as defined by movement 200 nm away from the origin site of the patch, in the aip1Δ mutant was slightly decreased, whereas that of the cof1-19 mutant was slightly increased (Fig. 6C). The average total lifetimes of the patches in the two mutants showed a similar increase (Fig. 6D). However, patches in the aip1Δ mutant had shorter lifespans near the membrane (before 200 nm), with a slightly longer lifespan after leaving the membrane (after 200 nm) compared with the wild type. Patches in cof1-19 cells showed increased lifespans in both cases (Fig. 6D,E). The Phase III MSD plots also showed that the cof1-19 defect was much stronger than that of aip1Δ, and that aip1Δ was very similar to the wild type (Fig. 6F, Table 1).
Fig. 6.
Quantitative motion analysis of Abp1-GFP-labeled actin patches in wild-type, cof1-19, aip1Δ and cof1-19 aip1Δ cells. (A-F) Panels are as described for Fig. 5. Student's t-tests for statistical significance were performed in E, as indicated. Strains and numbers of patches as follows: wild type: YJC6537 (n=53), YJC6538 (n=43) and YJC6543 (n=51); cof1-19: YJC6785 (n=35), YJC6786 (n=63) and YJC6787 (n=40); aip1Δ: YJC6782 (n=60), YJC6783 (n=56) and YJC6784 (n=58); aip1Δ cof1-19: YJC9623 (n=85), YJC9624 (n=61) and YJC9625 (n=51).
Aip1 localized diffusely in the cytosol in cof1-19 cells (Fig. 3E). Therefore, we analyzed patch motion in a cof1-19 aip1Δ double mutant to determine whether and how the cytosolic pool of Aip1 affected actin assembly. Surprisingly, in comparison with cof1-19, we found milder phenotypes in the double mutant, especially in the later stages of patch movement, as seen by MSD analysis (Fig. 6A,F). Accordingly, the Phase III travel distances were significantly different (P=0.03, Table 1). Thus, the diffuse cytosolic pool of Aip1 in cof1-19 cells provides a positive contribution to actin turnover in patches traveling through the cytosol.
We further dissected the patch activities on the membrane into Phase I and II with the Sla2-GFP marker. The Sla2 MSD plots showed cof1-19 and aip1Δ patches were defective in similar ways. However, cof1-19 had stronger phenotypes, including patch motions (supplementary material Fig. S2A,E) and lengthened Phase I and II patch lifetimes (supplementary material Fig. S2B,D,E). Our localization analysis suggested that cofilin, coronin and Aip1 are recruited to the cortical patches after mid-Phase II (Figs 1 and 2). However, we observed patch motion phenotypes in cells defective in these three proteins as early as Phase I. It is possible that in cells with disrupted actin-depolymerization machinery, actin subunits are not efficiently replenished to the nascent endocytic sites, and this hampers the invagination of cortical vesicles.
Aip1 was required for bursting in vitro (Kueh et al., 2008). However, the complete loss of Aip1 caused only minor defects in motion of actin patches. Thus, this argued against a role for the bursting phenomenon in this case. Furthermore, the cof1-19 mutation, which affects Aip1 localization, had more severe defects than did the aip1Δ mutation: mainly prolonged and extended movement of patches in the late phases. cof1-19 has been proposed to be defective in G-actin interactions. In addition, the cof1-19 srv2 mutation is lethal. Srv2 functions as a mediator to transfer actin subunits from cofilin to profilin and facilitates actin recycling in cells (Balcer et al., 2003; Bertling et al., 2007). Thus, cof1-19 must affect more than the association of Aip1 with actin patches. Together, our results argue that cofilin is more essential than Aip1 for actin depolymerization.The severe patch motion phenotypes of cof1-19 might result from actin-monomer cycling.
Actin-patch dynamics: distinct roles for coronin and Aip1
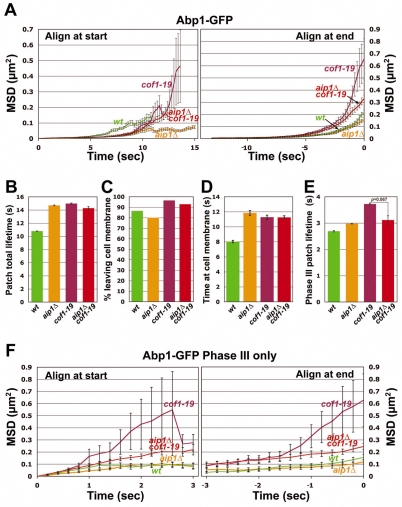
To test for functional overlap between coronin and Aip1, we analyzed patch movement in a crn1Δ aip1Δ double mutant. Depending on the assay, the phenotype of the double mutant was either worse than that of either single mutant or similar to that of the worst single mutant. Total patch lifetime, for example, was greater for the double mutant than for either single mutant (Fig. 7A, left; Fig. 7B). This enhanced effect in the double mutant is seen in the lifetime of patches during Phase I and II (before 200 nm; Fig. 7D) but not in Phase III (after 200 nm; Fig. 7E). Thus, the results do provide some evidence that Aip1 and coronin cooperate in actin disassembly, but the simple notion that the two proteins work together in one function is not supported by the complexity of the results.
Fig. 7.
Quantitative motion analysis of Abp1-GFP-labeled actin patches in wild-type, crn1Δ, aip1Δ and crn1Δ aip1Δ cells. (A-F) Panels are as described for Fig. 5. The data for crn1Δ and aip1Δ cells are the same as those in Figs 5 and 6, respectively, reproduced here for comparison. Strains and numbers of patches as follows: wild-type: YJC6537 (n=80), YJC6538 (n=88) and YJC6543 (n=67); crn1Δ, aip1Δ: YJC6816 (n=17), YJC6817 (n=27) and YJC6818 (n=28).
Cofilin has the most important role in actin-patch dynamics
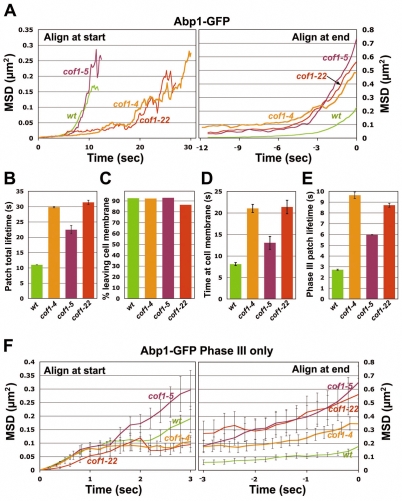
Because cofilin is the most abundant of the three proteins in the cortical patch, and because null mutations of cof1 are lethal, we examined its relative importance in patch dynamics by analyzing other viable cof1 alleles. First, to clarify the functional differences between cofilin and coronin, we examined patch dynamics in two other alleles of cof1 that show decreased levels of coronin in the patch, cof1-22 and cof1-4 (Fig. 3D; supplementary material Table S3). cof1-22 is defective in F-actin binding and severing activities, and cof1-4 has minor actin-binding defects (Fan et al., 2008; Lappalainen et al., 1997). The patch assembly and motion phenotypes of these two alleles were more severe than those observed for crn1Δ (Figs 5 and 8; Table 1); both showed dramatically lengthened patch lifetimes and travel distances. In addition, cof1-22, compared with crn1Δ, displayed stronger Phase I and II motion defects (supplementary material Fig. S2). Thus, the phenotypes of cof1-22 and cof1-4 did not result from the loss of coronin at the patch, indicating that cofilin is far more important than coronin for actin assembly. Together, the results comparing mutations affecting the three proteins show that cofilin has the most important role, which also argues against the relevance of the bursting phenomenon here.
Fig. 8.
Quantitative motion analysis of Abp1-GFP-labeled actin patches in wild-type, cof1-4, cof1-5 and cof1-22 cells. (A-F) Panels are as described for Fig. 5. Strains and numbers of patches are as follows: wild-type: YJC6516 (n=85), YJC6521 (n=85) and YJC6522 (n=77). cof1-4: YJC6515 (n=17), YJC6519 (n=27) and YJC6520 (n=28); cof1-5: YJC4439 (n=33), YJC4440 (n=11) and YJC4441 (n=9); cof1-22: YJC4427 (n=25), YJC4428 (n=9) and YJC4429 (n=11).
Cofilin alleles: distinct functional defects and patch phenotypes
We noticed that the patch assembly and motion phenotypes of cof1-22 and cof1-4 were different from those of cof1-19 (Figs 6 and 8). We suspected that the phenotypic diversity resulted from the different biochemical and functional properties of the mutant cofilins. To test this hypothesis, we analyzed patch assembly and motion in the cof1-5 mutant, which is reported to have Srv2-interaction defects (Quintero-Monzon et al., 2009) that might disrupt actin-monomer cycling. As expected, the MSD results for cof1-5 were similar to those for cof1-19. The total and Phase III MSD plots of cof1-5 and cof1-19 patches were steeper than those of cof1-4 and cof1-22, indicating faster movement of the patches (Fig. 8A,F; Table 1). By contrast, in cof1-5 and cof1-19 cells, the total patch lifetime, the time before patches move 200 nm from the membrane, and the Phase III patch lifetime were shorter than those of cof1-4 and cof1-22, and closer to, albeit still greater than, those of wild-type cells (Fig. 8B,D,E). Taken together, our results show that the motion of patches is affected differently in the different cof1 mutants, implying the potential for multiple functions of Cof1 during and after the process of internalization. Most notably, however, all four alleles showed increased patch life and moving distance, consistent with the action of cofilin in disassembly of actin networks.
Discussion
Actin patches are a set of highly dynamic modules that contribute to the endocytic machinery of budding yeast, driving the actin-based motility of endocytic vesicles (Galletta and Cooper, 2009; Kaksonen et al., 2006). The molecular composition of actin patches changes continuously and rapidly throughout their lifespan. Here, we examined the in vivo functions of coronin, cofilin and Aip1 – a set of actin-patch proteins linked by a variety of biochemical and cell biological studies.
One important discovery was that the three proteins co-localize to the late phase of the life of an actin patch, when actin filaments are disassembling. Second, their localization depends on each other, albeit not in a simple way. Third, their mutant phenotypes with respect to actin-patch assembly, movement and lifespan have some commonalities, but also some key differences. Loss-of-function phenotypes showed an increased patch lifetime or travel distance, which is consistent with the notion that all three proteins contribute to filament disassembly.
In particular, our data argued against the in vivo relevance of the bursting activity described for cofilin, coronin and Aip1 in the turnover of actin patches (Kueh et al., 2008). The study that discovered bursting, found that all three proteins needed to be present for the phenomenon to occur. We did find that all three proteins were present at the actin patch at the same time, during actin disassembly. In addition, actin patches in all three mutants required a longer time to leave the membrane. However, this could be caused either by decreased actin-monomer flux or by decreased bursting. Moreover, we found that the mutant phenotypes of the three genes were not identical; in particular, coronin and Aip1 were not nearly as important as cofilin, and the number of molecules of Aip1 and coronin were substantially fewer than the number of cofilin molecules. Therefore, if bursting does require coronin and Aip1, as well as cofilin, then bursting cannot have a major functional effect in this in vivo setting.
Dependence of cofilin and coronin on each other for recruitment
To investigate potential interactions among the proteins, one question we asked was whether coronin recruitment depended on cofilin. Coronin has been shown to interact directly with actin filaments (Cai et al., 2007a; Galkin et al., 2008; Goode et al., 1999), but not with cofilin (Gandhi et al., 2009). We found that the patch localization of coronin was severely disrupted in three cofilin mutants, cof1-4, cof1-15 and cof1-22. On one hand, cof1-4 and cof1-22 have F-actin-binding defects (Lappalainen and Drubin, 1997; Lappalainen et al., 1997), so the reduced binding affinity of these mutant cofilins to actin might account for the loss of localization of coronin. On the other hand, coronin localization was selectively affected in the cof1-15 mutant, which did not display effects on actin. Therefore, the mutated region in this cof1 allele could be responsible for the binding of coronin, suggesting the possibility of a direct interaction between cofilin and coronin. An alternative possibility is that the residues mutated in cof1-15 are crucial for another function of cofilin, which when mutated changes the actin filament, or its associated proteins in such a fashion to prevent binding of coronin to the filament.
Conversely, we asked whether coronin influenced cofilin recruitment in vivo, a question raised by previous in vitro studies. For example, purified mammalian coronin1A facilitated the binding of cofilin to the Listeria actin comet tail (Brieher et al., 2006). Also, when yeast coronin was bound to older (i.e. ADP) actin filaments, then the presence of coronin facilitated actin severing by cofilin (Gandhi et al., 2009). Here, in cells, the complete loss of coronin had no obvious effect on the recruitment of cofilin to actin patches. Thus, we conclude that these in vitro results do not adequately describe the behavior of the proteins in cells, at least in this one particular setting.
In a previous study in yeast cells, coronin was found to influence the distribution of cofilin in some distinctive actin-containing comet-like structures that form in a sla2Δ background (Gandhi et al., 2009). However, several pieces of evidence in our work do not support a significant role for this mechanism. First, coronin does not appear to bind actin filaments before cofilin does; the timing of recruitment of coronin and cofilin is essentially identical. Second, the results of our temporal recruitment analysis of cofilin to actin patches in cells, in wild-type SLA2 backgrounds, with or without coronin showed no significant difference. Therefore, our results do not support the conclusion that coronin inhibits recruitment of cofilin to actin, in the context of an otherwise normal actin patch.
Interactions of Aip1 with cofilin and coronin based on localization
Cofilin is not normally a component of actin cables, but cofilin was found by immunofluorescence analysis to decorate actin cables in aip1Δ cells (Clark and Amberg, 2007; Okada et al., 2006; Rodal et al., 1999). Here, we confirmed this finding with real-time images and novel fusions in living cells. Coronin is also not normally a component of actin cables; strikingly, we found that coronin also displayed cable localization in aip1Δ mutant cells. Perhaps coronin is recruited to the actin cable as a result of the recruitment of cofilin, which would support the hypothesis that cofilin promotes the interaction of coronin with actin in cells. In addition, the results support the notion that coronin is involved in the disassembly of cables (Gandhi et al., 2009).
Aip1 localization to the actin patch depends on cofilin, based on the lack of immunofluorescence localization of Aip1 in the cofilin mutant cof1-19 (Clark et al., 2006; Rodal et al., 1999). Here, we confirmed that result with real-time images of live cells. In addition, we found that coronin is not necessary for recruitment of Aip1 to actin patches.
The essential and diverse roles of cofilin in yeast actin-patch turnover
The relatively small actin-patch motion defects seen in aip1Δ and crn1Δ null cells compared with those of cof1 point mutants imply that cofilin, of the three proteins, has a more important role in actin turnover in vivo. Cofilin is proposed to disassemble actin by severing actin filaments (Bamburg, 1999; Ono, 2003; Ono, 2007). Biochemically, cof1-22 has defects in both F-actin-binding and actin-filament-severing activity (Fan et al., 2008; Lappalainen et al., 1997). Here, cof1-22 was the most severe cofilin mutant tested. During Phase III, patch motions were decreased and patch lifetimes were increased, compared with the wild type and other cof1 mutants. Therefore, the severing activity of cofilin might be crucial to actin-patch assembly and movement.
Another biochemical function for cofilin is in actin-monomer recycling, which might also be crucial for patch assembly and movement. The cof1-5 mutant protein is biochemically defective in Srv2 protein interaction (Quintero-Monzon et al., 2009). Srv2 mediates transfer of actin monomers produced by cofilin depolymerization to profilin, to facilitate actin cycling in cells (Balcer et al., 2003; Bertling et al., 2007; Mattila et al., 2004; Quintero-Monzon et al., 2009). In addition, cof1-19 mutant protein has been shown, biochemically, to have G-actin-binding defects (Ojala et al., 2001), and cof1-19 has a genetic interaction with SRV2 and the gene encoding profilin (Balcer et al., 2003). Here, during Phase III, patch travel speed increased for both cof1-5 and cof1-19. How such a phenotype might result from actin-monomer cycling defects is not clear at this point and will require further study.
Furthermore, the Sla2-GFP results showed that Phase I and Phase II patch motions were decreased and lifetimes were lengthened in all cof1 alleles tested. We speculate that defects in actin-filament turnover in cofilin mutants cause a decrease in the pool of actin subunits and thus a longer time to recruit actin to assembly sites. The phenotypes of cof1-22 cells were much more severe than those of cof1-19 cells, suggesting that the actin-filament-severing activity of Cof1 is more important than its actin-monomer-cycling activity in assembling actin networks during Phase II. In migrating animal cells, barbed ends generated by cofilin-mediated actin severing have been proposed to be crucial for actin nucleation at the leading edge (DesMarais et al., 2005; Ghosh et al., 2004). Cofilin might have a similar role in promoting assembly here, for yeast actin patches.
Molecular stoichiometry at the patch
To provide a framework for molecular models of actin patch assembly and function, we determined the absolute number of molecules per patch. We quantified fluorescence, and then, using an internal standard, calculated the number of molecules over time during patch assembly and disassembly. At the peak of fluorescence intensity, we found ~45 molecules of Aip1, ~90 of coronin and ~500 of cofilin. These values argue against a simple stoichiometric model for the function of the three proteins.
In addition, we were able to estimate the amount of F-actin in the patch. In a previous study, we found, by electron microscopy, a length of 145 nm of actin filament per Arp2/3 branch point (Young et al., 2004). Here, we found ~95 molecules of Arp2/3 per patch at peak height, leading to an estimate of ~5100 molecules of actin per patch. The fact that the numbers of Arp2/3 and coronin molecules are similar is consistent with coronin functioning as an inhibitor of Arp2/3 (Cai et al., 2007a; Galletta et al., 2008; Humphries et al., 2002; Rodal et al., 2005). Note that these numbers of molecules are values at peak height. During the maturation of a typical patch, the values for actin and Arp2/3 will be falling, whereas those of coronin, cofilin and Aip1 will be rising. One would imagine that a ratio of cofilin to F-actin of 1:10 or higher would be sufficient to depolymerize the filaments.
Overall, these values might be useful for the design of biochemical experiments in vitro, with purified proteins or cell extracts. Obviously, one would be interested in the biochemical properties of the system at molar ratios and concentrations that are similar to those in vivo.
Materials and Methods
Construction of yeast strains and plasmids
Yeast strains used in this study are listed in supplementary material Table S1. CRN1-tdimer2::KanRMX6 fragments were PCR-amplified from pKT178 with primer sets that complemented the C-terminus of the yeast CRN1 coding region using previously described methods (Sheff and Thorn, 2004). PCR products were transformed into wild-type (wt) or ABP1-GFP cells. Following selection with G418, transformants were verified with PCR, and tdimer2 cortical patch localization was observed with fluorescence microscopy.
crn1 and aip1 deletion PCR fragments were amplified with primers complementing the TEF promoter and terminator regions in the pKT selection marker cassettes (Sheff and Thorn, 2004). PCR products were transformed into wt or ABP1-GFP cells with proper selection. The deletions of CRN1 or AIP1 were verified by PCR. The crn1Δ aip1Δ double mutant was constructed by sequential transformation.
Three COF1 PCR products were prepared: fragment 1 consisted of 652 bp upstream of the COF1 coding region, the 5′ end of the COF1 coding region (including the intron), and a linker sequence. Fragment 2 was amplified from the pKT178 vector to contain a single copy of RFP with linkers at both ends. Fragment 3 contained a linker, the COF1 3′ coding region, and 283 bp downstream of COF1. The three fragments were fused with two sequential PCR reactions, sequence of COF1-RFP was confirmed and then cloned into pRS413, pRS415 and pRS416 vectors at BamHI and NotI sites (Sikorski and Hieter, 1989).
The SpHIS5 marker cassette was PCR-amplified from the pKT128 vector (Sheff and Thorn, 2004), and placed into a site 286 nucleotides downstream of the COF1 coding region with the fusion PCR method to generate a fragment containing COF1-RFP, the SpHIS5 cassette, and 552bp of COF1 3′ non-coding sequence. This fragment was transformed into diploid wt cells. HIS+ transformants were tetrad-dissected to isolate haploid HIS+ progeny, which were confirmed for COF1-RFP knock-in by DNA sequencing. The Cof1-RFP patch localization in these cells was confirmed by fluorescence microscopy.
PCR fragments consisted of LifeAct (Riedl et al., 2008), GFP or tdimer2, and the Ttef cassette were amplified from the pKT vectors. The fragments were then cloned into the p415- or p416-Pcyc1 vectors at BamHI and HindIII sites (Mumberg et al., 1995).
To construct SPC105-RFP cells, the RFP::SpHIS5 construct was inserted at the C-terminus of the endogenous SPC105. A single-copy RFP sequence with the SpHIS5 cassette was PCR amplified from the pKT146 vector with primers complementing the 3′ end of SPC105. The RFP coding sequence was confirmed, and PCR fragments were transformed into wt cells. SPC105-RFP knock-in strains were identified by fluorescence microscopy.
Microscopy
Yeast strains were grown at 30°C to OD600=0.3-0.5, unless otherwise indicated. Cells were recovered, concentrated in SD medium, placed on 2% agarose pads with SD, and covered with a number 1 coverslip. Cells carrying plasmids were grown and resuspended in medium with proper selection. Confocal time-lapse images were collected at room temperature, unless otherwise indicated, with a spinning-disc confocal microscope system, as described earlier (Galletta et al., 2008). Two-color images were taken sequentially at 2 frames/second with QED InVivo software (Media Cybernetics). Collected images were processed with ImageJ software (NIH, rsbweb.nih.gov/ij). Wide-field fluorescence images were acquired with an Olympus IX70 inverted microscope equipped with a CoolsnapHQ camera (Photonics), with images taken at 1 frame/second with QED InVivo software.
Fluorescence intensity measurements
Confocal two-color time-lapse images were collected from GFP-RFP double-labeled cells at room temperature. Actin patch images showing green-red co-localization were isolated, and their mean GFP and RFP intensities within a 7×7 pixel area from each frame were measured with ImageJ. The average fluorescence intensities from dark central regions of the cells were also taken from each frame and used as the background values. The background intensities were subtracted from the GFP and RFP intensities, and these adjusted values were normalized into percentages, which were aligned at the time point when the patches reached their peak green fluorescence intensity. The percentages from different patches at the same time points were averaged and plotted over time. The moving distances of the Abp1-GFP-labeled actin patches in this assay were obtained by measuring their linear displacements from their points of origin at different time points. The distances of different patches at the same time points were then averaged and plotted over time.
Computer-assisted patch tracking and quantitative motion analysis
Cells were kept at 34°C on the microscope because this was the restrictive temperature of the cofilin alleles. Abp1-GFP images were collected with Piper software (Agile Automation) at 5 frames/second, and Sla2-GFP images were collected with QED InVivo software at 2 frames/second. Actin patches were tracked and MSD plots were created as previously described (Carlsson et al., 2002; Galletta et al., 2008; Kim et al., 2006). The method is based on smoothing and thresholding of the fluorescence intensity, elimination of very small putative patches, and subsequent centroid calculation to obtain patch coordinates. In addition to MSD plots, Abp1-GFP patch motion data were analyzed in other ways. The total patch lifetime was the time from the emergence of a patch to its disappearance. The time on the cell membrane was the average time spent by patches within 200 nm from their point of origin. The Phase III patch lifetime and the Phase III MSD were calculated from the portion of the data collected after the time point at which a patch was greater than 200 nm from its point of origin.
Molecular stoichiometry
Overall, the approach was based on one described by Joglekar and colleagues (Joglekar et al., 2006). Cells were grown and mounted as described (Galletta et al., 2008). Cells were imaged on an inverted wide-field fluorescence microscope (Olympus IX-70) with an EM-CCD camera (Hamamatsu). Illumination was achieved using a 488 nm or 543 nm laser (Melles Griot). Time-lapse movies were acquired with an acquisition rate of 1 frame/second for 60 seconds. Three independent seggregants per genotype were analyzed. Actin patches were tracked and movies edited as described (Galletta et al., 2008). The integrated intensity for each patch, in each frame, within a 9×9 pixel box was collected and background subtraction was done as described (Hoffman et al., 2001). On the same slide, movies of 15 kinetochore clusters of Cse4-GFP, for Aip1-GFP and Crn1-GFP movies, or Spc105-DsRed, for Cof1-RFP movies, were also collected, using the same acquisition parameters. The intensity values of these kinetochore clusters were then averaged to give a reference intensity for each time point of a 60 second movie. Each reference intensity value was then divided by 32 for Cse4 or 80 for Spc105 to determine the intensity corresponding to one molecule of the protein (Joglekar et al., 2006). The intensity value for an actin patch at a given time point was then divided by the reference intensity value for that that time point, resulting in the number of molecules of the fluorescently labeled actin patch protein at that time point in the movie, while simultaneously correcting for the effects of photobleaching. For example, the intensity of an actin patch acquired 14 seconds into a movie was divided by the reference intensity for the 14 second time point. The molecular number versus time data for all patches from a given seggregant was then aligned at the time point when they had reached their peaks. The mode was calculated as the mean of the bootstrapped half-range modes (M-HRMB), and the s.e. of the mode was a bootstrap s.e., calculated by taking the s.d. of the bootstrapped mode estimates (Hedges and Shah, 2003). Standard error of the mode was generated by bootstrapping the calculation 100 times. The 95% confidence interval range accounts for both symmetric and asymmetric distribution, as it specifies the range between the 2.5% and 97.5% bootstrap values generated.
Immunostaining for cofilin
Full-length COF1 coding sequence, without the intron, was cloned into the pRSFDuet-1 vector (Novagen) to generate a His-Cof1 fusion expression construct. pRSFDuet-COF1 was transformed into BL21(DE3) E. coli cells. Fresh transformants were used for expression. Cells were grown at 37°C to OD600=0.6 in LB, induced with 1 mM IPTG, and incubated at 25°C for another 4 hours. The cells were harvested at 4°C, resuspended in cold lysis buffer [50 mM sodium phosphate, pH 8.0, 300 mM NaCl, 0.2 μM PMSF, Complete Protease Inhibitor (Roche)] and sonicated. The cell lysate was cleared by centrifugation and applied to Talon beads (Clontech) following the standard protocol from the manufacturer. Eluted His-Cof1 was dialyzed into Tris-HCl buffer (10 mM Tris-HCl, pH 8.0, 50 mM KCl, 0.2 μM PMSF) and concentrated. To produce anti-Cof1 antibodies, purified His-Cof1 was used to immunize rabbits at Cocalico Biologicals (Reamstown, PA).
Immunostaining method was based on a described protocol (Amberg et al., 2005). Briefly, wt and cof1Δ pCOF1-RFP cells were fixed, treated with Zymolyase 100T (BioMol) and attached to microscope slides. The cells were blocked with BSA, stained with 1:1000 rabbit anti-Cof1 serum, and 1:500 Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen). Images were collected with a wide-field fluorescence microscope. The fluorescence intensities of 100 and 146 patches from wt and cof1Δ pCOF1-RFP cells, respectively, were collected, and background subtraction was done as described (Hoffman et al., 2001). The modes of the Cof1-RFP intensity data were calculated with the same method as in the molecular stoichiometry section above.
Supplementary Material
Acknowledgments
This research was supported by NIH grants GM67246 to D.S. and GM38542 to J.C. We are grateful to Jarod DuVall for technical support and for editorial suggestions. We thank David Drubin for providing his set of cof1 mutant alleles. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/8/1329/DC1
References
- Amberg D. C., Burke D., Strathern J. N. (2005). Methods in Yeast Genetics Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Balcer H. I., Goodman A. L., Rodal A. A., Smith E., Kugler J., Heuser J. E., Goode B. L. (2003). Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13, 2159-2169 [DOI] [PubMed] [Google Scholar]
- Bamburg J. R. (1999). Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15, 185-230 [DOI] [PubMed] [Google Scholar]
- Bernstein B. W., Painter W. B., Chen H., Minamide L. S., Abe H., Bamburg J. R. (2000). Intracellular pH modulation of ADF/cofilin proteins. Cell Motil. Cytoskeleton 47, 319-336 [DOI] [PubMed] [Google Scholar]
- Bertling E., Quintero-Monzon O., Mattila P. K., Goode B. L., Lappalainen P. (2007). Mechanism and biological role of profilin-Srv2/CAP interaction. J. Cell Sci. 120, 1225-1234 [DOI] [PubMed] [Google Scholar]
- Brieher W. M., Kueh H. Y., Ballif B. A., Mitchison T. J. (2006). Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J. Cell Biol. 175, 315-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Makhov A. M., Bear J. E. (2007a). F-actin binding is essential for coronin 1B function in vivo. J. Cell Sci. 120, 1779-1790 [DOI] [PubMed] [Google Scholar]
- Cai L., Marshall T. W., Uetrecht A. C., Schafer D. A., Bear J. E. (2007b). Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell 128, 915-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. E., Tour O., Palmer A. E., Steinbach P. A., Baird G. S., Zacharias D. A., Tsien R. Y. (2002). A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877-7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. E., Shah A. D., Elking D., Karpova T. S., Cooper J. A. (2002). Quantitative analysis of actin patch movement in yeast. Biophys. J. 82, 2333-2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. G., Amberg D. C. (2007). Biochemical and genetic analyses provide insight into the structural and mechanistic properties of actin filament disassembly by the Aip1p cofilin complex in Saccharomyces cerevisiae. Genetics 176, 1527-1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. G., Teply J., Haarer B. K., Viggiano S. C., Sept D., Amberg D. C. (2006). A genetic dissection of Aip1p's interactions leads to a model for Aip1p-cofilin cooperative activities. Mol. Biol. Cell 17, 1971-1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesMarais V., Ghosh M., Eddy R., Condeelis J. (2005). Cofilin takes the lead. J. Cell Sci. 118, 19-26 [DOI] [PubMed] [Google Scholar]
- Fan X., Martin-Brown S., Florens L., Li R. (2008). Intrinsic capability of budding yeast cofilin to promote turnover of tropomyosin-bound actin filaments. PLoS ONE 3, e3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin V. E., Orlova A., Brieher W., Kueh H. Y., Mitchison T. J., Egelman E. H. (2008). Coronin-1A stabilizes F-actin by bridging adjacent actin protomers and stapling opposite strands of the actin filament. J. Mol. Biol. 376, 607-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletta B. J., Cooper J. A. (2009). Actin and endocytosis: mechanisms and phylogeny. Curr. Opin. Cell Biol. 21, 20-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletta B. J., Chuang D. Y., Cooper J. A. (2008). Distinct roles for Arp2/3 regulators in actin assembly and endocytosis. PLoS Biol. 6, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M., Achard V., Blanchoin L., Goode B. L. (2009). Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Mol. Cell 34, 364-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M., Song X., Mouneimne G., Sidani M., Lawrence D. S., Condeelis J. S. (2004). Cofilin promotes actin polymerization and defines the direction of cell motility. Science 304, 743-746 [DOI] [PubMed] [Google Scholar]
- Goode B. L., Wong J. J., Butty A. C., Peter M., McCormack A. L., Yates J. R., Drubin D. G., Barnes G. (1999). Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J. Cell Biol. 144, 83-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J., Lowy J. (1963). The structure of F-actin and of actin filaments isolated from muscle. J. Mol. Biol. 6, 46-60 [Google Scholar]
- Hedges S. B., Shah P. (2003). Comparison of mode estimation methods and application in molecular clock analysis. BMC Bioinformatics 4, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil-Chapdelaine R. A., Tran N. K., Cooper J. A. (1998). The role of Saccharomyces cerevisiae coronin in the actin and microtubule cytoskeletons. Curr. Biol. 8, 1281-1284 [DOI] [PubMed] [Google Scholar]
- Hoffman D. B., Pearson C. G., Yen T. J., Howell B. J., Salmon E. D. (2001). Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell 12, 1995-2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C. L., Balcer H. I., D'Agostino J. L., Winsor B., Drubin D. G., Barnes G., Andrews B. J., Goode B. L. (2002). Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 159, 993-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Moriyama K., Matsumoto S., Kawasaki H., Nishida E., Yahara I. (1993). Isolation of a yeast essential gene, COF1, that encodes a homologue of mammalian cofilin, a low-M(r) actin-binding and depolymerizing protein. Gene 124, 115-120 [DOI] [PubMed] [Google Scholar]
- Joglekar A. P., Bouck D. C., Molk J. N., Bloom K. S., Salmon E. D. (2006). Molecular architecture of a kinetochore-microtubule attachment site. Nat. Cell Biol. 8, 581-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., Drubin D. G. (2003). A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115, 475-487 [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. (2005). A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123, 305-320 [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. (2006). Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 6, 404-414 [DOI] [PubMed] [Google Scholar]
- Kim K., Galletta B. J., Schmidt K. O., Chang F. S., Blumer K. J., Cooper J. A. (2006). Actin-based motility during endocytosis in budding yeast. Mol. Biol. Cell 17, 1354-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh H. Y., Charras G. T., Mitchison T. J., Brieher W. M. (2008). Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers. J. Cell Biol. 182, 341-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen P., Drubin D. G. (1997). Cofilin promotes rapid actin filament turnover in vivo. Nature 388, 78-82 [DOI] [PubMed] [Google Scholar]
- Lappalainen P., Fedorov E. V., Fedorov A. A., Almo S. C., Drubin D. G. (1997). Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 16, 5520-5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila P. K., Quintero-Monzon O., Kugler J., Moseley J. B., Almo S. C., Lappalainen P., Goode B. L. (2004). A high-affinity interaction with ADP-actin monomers underlies the mechanism and in vivo function of Srv2/cyclase-associated protein. Mol. Biol. Cell 15, 5158-5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri K., Vorobiev S., Fedorov A. A., Almo S. C., Ono S. (2004). Identification of functional residues on Caenorhabditis elegans actin-interacting protein 1 (UNC-78) for disassembly of actin depolymerizing factor/cofilin-bound actin filaments. J. Biol. Chem. 279, 31697-31707 [DOI] [PubMed] [Google Scholar]
- Moon A. L., Janmey P. A., Louie K. A., Drubin D. G. (1993). Cofilin is an essential component of the yeast cortical cytoskeleton. J. Cell Biol. 120, 421-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K., Iida K., Yahara I. (1996). Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1, 73-86 [DOI] [PubMed] [Google Scholar]
- Moseley J. B., Goode B. L. (2006). The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70, 605-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Müller R., Funk M. (1995). Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119-122 [DOI] [PubMed] [Google Scholar]
- Ojala P. J., Paavilainen V., Lappalainen P. (2001). Identification of yeast cofilin residues specific for actin monomer and PIP2 binding. Biochemistry 40, 15562-15569 [DOI] [PubMed] [Google Scholar]
- Okada K., Blanchoin L., Abe H., Chen H., Pollard T. D., Bamburg J. R. (2002). Xenopus actin-interacting protein 1 (XAip1) enhances cofilin fragmentation of filaments by capping filament ends. J. Biol. Chem. 277, 43011-43016 [DOI] [PubMed] [Google Scholar]
- Okada K., Ravi H., Smith E. M., Goode B. L. (2006). Aip1 and cofilin promote rapid turnover of yeast actin patches and cables: a coordinated mechanism for severing and capping filaments. Mol Biol. Cell 17, 2855-2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okreglak V., Drubin D. G. (2007). Cofilin recruitment and function during actin-mediated endocytosis dictated by actin nucleotide state. J. Cell Biol. 178, 1251-1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. (2001). The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments. J. Cell Biol. 152, 1313-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. (2003). Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1, new blades for twisted filaments. Biochemistry 42, 13363-13370 [DOI] [PubMed] [Google Scholar]
- Ono S. (2007). Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int. Rev. Cytol. 258, 1-82 [DOI] [PubMed] [Google Scholar]
- Ono S., Mohri K., Ono K. (2004). Microscopic evidence that actin-interacting protein 1 actively disassembles actin-depolymerizing factor/Cofilin-bound actin filaments. J. Biol. Chem. 279, 14207-14212 [DOI] [PubMed] [Google Scholar]
- Quintero-Monzon O., Jonasson E. M., Bertling E., Talarico L., Chaudhry F., Sihvo M., Lappalainen P., Goode B. L. (2009). Reconstitution and dissection of the 600-kDa Srv2/CAP complex: roles for oligomerization and cofilin-actin binding in driving actin turnover. J. Biol. Chem. 284, 10923-10934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N., Charlton J., Adler P. N. (2007). The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics 176, 2223-2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J., Crevenna A. H., Kessenbrock K., Yu J. H., Neukirchen D., Bista M., Bradke F., Jenne D., Holak T. A., Werb Z., Sixt M., Wedlich-Soldner R. (2008). Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal A. A., Tetreault J. W., Lappalainen P., Drubin D. G., Amberg D. C. (1999). Aip1p interacts with cofilin to disassemble actin filaments. J. Cell Biol. 145, 1251-1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal A. A., Sokolova O., Robins D. B., Daugherty K. M., Hippenmeyer S., Riezman H., Grigorieff N., Goode B. L. (2005). Conformational changes in the Arp2/3 complex leading to actin nucleation. Nat. Struct. Mol. Biol. 12, 26-31 [DOI] [PubMed] [Google Scholar]
- Schaerer-Brodbeck C., Riezman H. (2000). Functional interactions between the p35 subunit of the Arp2/3 complex and calmodulin in yeast. Mol. Biol. Cell 11, 1113-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff M. A., Thorn K. S. (2004). Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 21, 661-670 [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Miyoshi T., Higashida C., Narumiya S., Watanabe N. (2009). An order of magnitude faster AIP1-associated actin disruption than nucleation by the Arp2/3 complex in lamellipodia. PLoS ONE 4, e4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M., Huyck L., Leyman S., Dhaese S., Vandekerkhove J., Ampe C. (2008). Ins and outs of ADF/cofilin activity and regulation. Eur. J. Cell Biol. 87, 649-667 [DOI] [PubMed] [Google Scholar]
- Winter D., Podtelejnikov A. V., Mann M., Li R. (1997). The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches. Curr. Biol. 7, 519-529 [DOI] [PubMed] [Google Scholar]
- Young M. E., Cooper J. A., Bridgman P. C. (2004). Yeast actin patches are networks of branched actin filaments. J. Cell Biol. 166, 629-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.