Abstract
Previous studies indicated that ethanol-induced neurodegeneration in postnatal day 7 (P7) mice, widely used as a model for the fetal alcohol spectrum disorders, was accompanied by glycogen synthase kinase-3β (GSK-3β) and caspase-3 activation. Presently, we examined whether tau, a microtubule associated protein, is modified by GSK-3β and caspase-3 in ethanol-treated P7 mouse forebrains. We found that ethanol increased phosphorylated tau recognized by the paired helical filament (PHF)-1 antibody and by the antibody against tau phosphorylated at Ser199. Ethanol also generated tau fragments recognized by an antibody against caspase-cleaved tau (C-tau). C-tau was localized in neurons bearing activated caspase-3 and fragmented nuclei. Over time, cell debris and degenerated projections containing C-tau appeared to be engulfed by activated microglia. A caspase-3 inhibitor partially blocked C-tau formation. Lithium, a GSK-3β inhibitor, blocked ethanol-induced caspase-3 activation, phosphorylated tau elevation, C-tau formation, and microglial activation. These results indicate that tau is phosphorylated by GSK-3β and cleaved by caspase-3 during ethanol-induced neurodegeneration in the developing brain.
Keywords: ethanol, caspase-cleaved tau, glycogen synthase kinase-3β, neurodegeneration, developing brain, microglia
Introduction
Ethanol triggers wide-spread apoptotic neurodegeneration in P7 mice, which are in the middle of the brain growth spurt and in a period of brain development corresponding to the human third trimester (Ikonomidou et al., 2000; Olney et al., 2002b). The P7 rodent model of fetal alcohol spectrum disorders has been widely used for elucidating mechanisms of ethanol-induced toxicity in the developing brain (Young et al., 2003; Carloni et al., 2004; Han et al., 2006). Ethanol-induced neurodegeneration in the P7 mouse brain is preceded by caspase-3 activation (Olney et al., 2002b; Saito et al., 2007) and by decreases in phosphorylation levels of Akt and GSK-3β (Chakraborty et al., 2008). GSK-3β and caspase-3 thus activated by ethanol may affect the downstream effector molecule tau.
Phosphorylation of tau by GSK-3β decreases the microtubule-binding capacity of tau and disrupts microtubule stability (Utton et al., 1997; Sang et al., 2001). When the majority of tau is phosphorylated at Ser396/Ser404 (the PHF-1 antibody epitope) by GSK-3β activation, tau aggregation is favored (Chun et al., 2007). In addition to hyper-phosphorylation, tau can be cleaved at Asp421/422 by caspases-3, 6, 7, and 8 (Gamblin et al., 2003). The caspase-cleaved tau (C-tau) assembles more rapidly into filaments than full-length tau (Gamblin et al., 2003), and has been detected in Alzheimer brains (Rissman et al., 2004). C-tau is also detected in brains injured by kainic acid (Zemlan et al., 2003) and trauma (Zemlan et al., 2002; Gabbita et al., 2005), and C-tau is pro-apoptotic in cultured neurons (Chung et al., 2001; Fasulo et al., 2005). Lithium, a GSK-3β inhibitor, which blocks ethanol-induced caspase-3 activation (Zhong et al., 2006; Chakraborty et al., 2008), has been shown to reduce phosphorylation and aggregation of tau in a mouse model of Alzheimer’s disease (Noble et al., 2005).
From these studies, we hypothesized that ethanol-induced GSK-3β and caspase-3 activation modify tau as a downstream target, which may result in neurodegeneration. We further hypothesized that lithium inhibits tau modification as well as subsequent neurodegeneration. Although recent studies have shown that ethanol induces tau accumulation in neuroblastoma cells (Gendron et al., 2008) and generates c-Tau in P7 mouse brains (Zhang et al., 2009), the effects of ethanol on tau are largely unknown. It is expected that the majority of tau proteins at P7 display a fetal form. This form is highly phosphorylated and recognized by PHF-1 antibody (Goedert and Jakes, 1990), although it is less phosphorylated compared to the paired helical filament tau observed in tauopathies and retains a low but significant level of activity for promoting tubulin assembly (Morishima-Kawashima et al., 1995). Here, we examined tau modifications during ethanol-induced neurodegeneration in the P7 mouse brain.
Experimental Procedure
Animals and treatment
C57BL/6By mice were maintained at the Animal Facility of the Nathan S. Kline Institute for Psychiatric Research. All procedures followed guidelines consistent with those developed by the National Institute of Health and the Institutional Animal Care and Use Committee of the Nathan S. Kline Institute. An ethanol treatment paradigm shown to induce robust neurodegeneration in P7 C57BL/6 mice (Olney et al., 2002b) was followed using P7 C57BL/6By mice as described (Saito et al., 2007). Each mouse in a litter of more than 8 pups was assigned to a saline, a lithium, an ethanol, or an “ethanol + lithium” group, and the experiment was repeated 3 to 5 times using different litters. The mice were injected subcutaneously with saline or ethanol (2.5 g/kg, 20% solution in sterile saline) twice at 0 h and 2 h. Lithium chloride (LiCl, 0.6M, 10 μl/g) or saline was injected intraperitoneally 15 min after the first ethanol injection as described previously (Zhong et al., 2006). Four to 48 h after the first ethanol injection, brains were removed and processed for immunoblotting and immunohistochemical staining. For experiments using a caspase 3/7 inhibitor, N-benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethyl ketone (Z-DEVD-FMK) (BioVision, Mountain View, CA), the inhibitor was administered by intracerebroventricular (icv) injections as described (Sadakata et al., 2007). Z-DEVD-FMK (1 μg in 2 μl of 1.5% DMSO in saline) or vehicle was given twice 1 h before and 4 h after the ethanol injection, which was administered once at a concentration of 5.0 g/kg. Ethanol administration once at 5.0 g/kg induces neurodegeneration similar to that elicited by two time injections of 2.5 g/kg ethanol (Ieraci and Herrera, 2006). Eight hours after the ethanol injection, brains were removed and processed for immunoblotting.
Western blot analysis
Western blot analyses for forebrain samples were performed as described (Chakraborty et al., 2008). Primary antibodies used under manufactures’ instructions were: rabbit polyclonal anti-cleaved caspase-3 (Asp175) antibody, rabbit polyclonal anti-caspase-3 antibody, rabbit polyclonal anti-phospho (Ser9)-GSK-3β antibody, rabbit polyclonal anti-cleaved poly (ADP-ribose) polymerase (PARP) antibody, rabbit monoclonal anti-GSK-3β antibody (all from Cell Signaling Technology, Danvers, MA), mouse monoclonal anti C-tau antibody (clone C3), mouse monoclonal Tau-5 antibody, mouse monoclonal Tau-1 antibody (all from Millipore, Billerica, MA), rabbit polyclonal anti-Tau (phospho-Ser199) (PS199) antibody, rabbit polyclonal anti-Tau (phospho-Thr231) (PT231) antibody, rabbit polyclonal anti-Tau (phospho-Ser422 ) (PS422) antibody (all from GenScript, Piscataway, NJ), mouse monoclonal anti-cleaved caspase-6 antibody (BioVision), and mouse monoclonal PHF-1 antibody (a gift from Dr. Peter Davies at Albert Einstein College of Medicine, New York, NY). Mouse monoclonal anti-β-actin antibody (loading control, Abcam Inc. Cambridge, MA) or rabbit monoclonal anti-β-actin antibody (Cell Signaling Technology) was always included. Antigens were detected by the Odyssey infrared imaging system (LI-COR Inc. Nebraska, NE) using fluorescence-labeled secondary antibodies, goat anti-rabbit IgG-680 (Invitrogen, Carlsbad, CA) and goat anti-mouse IgG-800 (Rockland Immunochemicals, Gilbertsville, PA). Intensities of desired bands were quantified using Fuji film MultiGauge V3.2 software. The intensities of protein bands were normalized to the intensities of corresponding actin, except for cleaved caspase-3 intensities which were normalized to corresponding uncleaved caspase-3 band intensity. Finally, treatment to saline ratios were calculated. The amount of protein was measured by a BCA method (Pierce, Rockford, IL).
Immunohistochemistry
Immunohistochemistry was performed as described previously (Saito et al., 2007). Briefly, brains from perfused mice were sectioned with a vibratome into 50 μm thick sections, and the free-floating sections were blocked with Mouse Ig Blocking Reagent (Vector) and incubated with rabbit polyclonal anti-cleaved caspase-3 (Asp175) antibody (diluted 1:1500), mouse monoclonal anti-C-tau (clone C3) antibody (diluted 1:500), and rabbit anti-Iba1 antibody (Wako Chemicals, Richmond, VA, diluted 1:500). Then the sections were incubated with Alexa Fluor 488 goat anti-rabbit IgG and/or Alexa Fluor 594 goat anti-mouse IgG (Molecular Probes). The first antibodies were omitted from reactions as a control. Also, a blocking peptide for anti-C-tau antibody (CSSTGSEDMVD, GenScript) was used to assess specificity of the antibody. Specificity of anti-cleaved caspase-3 was confirmed previously (Saito et al., 2007) using a blocking peptide (Cell Signaling). Fluorescence images were obtained with a Nikon Eclipse TE2000 fluorescent microscope attached to a digital camera, DXM1200F.
Preparation of cultured cortical neurons
Cultured cortical neurons were prepared from embryonic day 17 (E17) C57BL/6 embryos. Cerebral cortices were removed and digested with 10 units/ml of papain for 15 min at 37°C. After stopping the digestion by adding serum, cells were mechanically dissociated with a pipette in DMEM/F12 medium supplemented with 5% fetal calf serum and 5% horse serum. The cell suspension was passed through a 40 μm cell strainer, and plated on poly-d-lysine-coated dish at a density of 2 × 105 cells/cm2. After 1.5 h, the medium was changed to Neurobasal medium supplemented with B27. Three days after plating, cultures were treated with or without 100 mM ethanol for 24 h to 48 h. Cell viability was assessed using a mixture of fluorescein diacetate and propidium iodide as previously described (Saito et al., 1999). For immunocytochemistry, cells were fixed with 4% paraformaldehyde in PBS, permeabilized with PBS containing 0.3% Triton X-100 and 1% BSA, blocked with Mouse Ig Blocking Reagent, and stained with primary antibodies (anti-CC3 and anti-C-tau antibodies) and secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 594 goat anti-mouse IgG) as described above for immunohistochemisty.
Statistics
The values are expressed as mean ± Standard Error of Mean (SEM) obtained from 4-6 different mice. Statistical analysis of the data was performed by ANOVA with Bonferroni’s post hoc test using the SPSS 11.0 program.
Results
Ethanol enhances tau phosphorylation
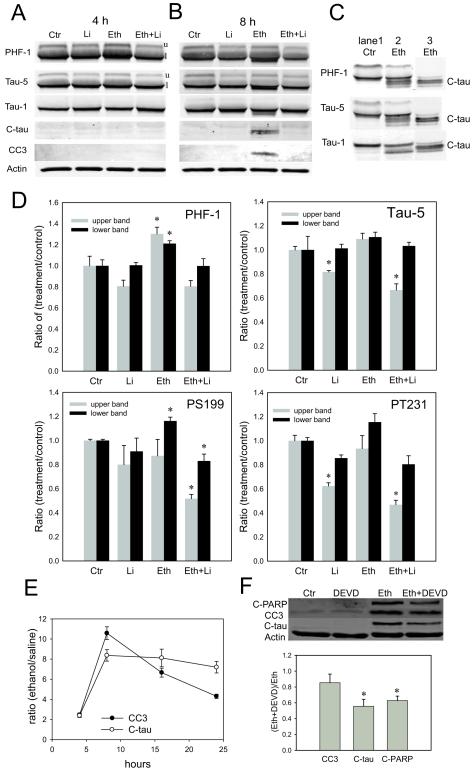
In agreement with our previous studies (Chakraborty et al., 2008), the level of GSK-3β phosphorylated at Ser9 was significantly reduced in the forebrain of ethanol-treated P7 mice while the level of GSK-3β was unchanged (data not shown). Because the reduction in phosphorylation at Ser9 suggested that ethanol activated GSK-3β, we tested if ethanol augmented tau phosphorylation at Ser396/Ser404 (substrates of GSK-3β) using the PHF-1 antibody. The intensities of both the upper and the lower bands (marked as u and l in Fig. 1A) recognized by the PHF-1 antibody significantly increased 4 h after ethanol administration, and lithium treatment blocked the increase (Fig. 1A, D). We further used antibodies against tau phosphorylated at Ser199 (PS199) and tau phophorylated at Thr231 (PT231), because these sites are known to be phosphorylated by GSK-3β (Ishiguro et al., 1995). As shown in Fig. 1D, the intensity of the lower band recognized by anti-Tau PS199 antibody significantly increased 4 h after ethanol administration, and lithium treatment blocked the increase. The intensity of the lower band recognized by anti-Tau PT231 also increased although it did not reach the significant level, and the intensity of the upper band significantly decreased by lithium and “lithium + ethanol” treatments compared to controls (Fig. 1D). The band intensities recognized by anti-Tau PS422 antibody were not significantly altered by ethanol treatment (data not shown). It has been reported that tau phosphorylation at Ser422 is not mediated by GSK-3β (Ishiguro et al., 1995). Ethanol did not change the intensities of bands recognized by phosphorylation-independent Tau-5 antibody, while the upper band intensity was reduced by lithium and “lithium + ethanol” treatments compared to controls (Fig. 1A, D). Also, ethanol did not significantly change the band intensity recognized by Tau-1 antibody, which binds to tau with unphosphorylated Ser199/Ser202 (Fig. 1A). Thus, GSK-3β activation by ethanol and its inhibition by lithium correlate with increased tau phosphorylation at GSK-3β sites by ethanol and its prevention by lithium.
Fig. 1.
Ethanol increases tau phosphorylation and induces C-tau formation. A-D: Forebrain samples were taken from P7 mice 4 h (A, D) and 8 h (B, C) after the first ethanol/saline injection for immunoblot analyses of tau and CC3. Tau proteins were analyzed using PHF-1, Tau-1, Tau-5, anti-Tau PS199, anti-Tau PT231, and anti-c-Tau antibodies, and CC3 was analyzed using anti-CC3 antibody as described in the Experimental Procedure. A and B show representative immunoblots of saline, lithium (Li), ethanol (Eth), and “ethanol + lithium” (Eth+Li) groups 4 h (A) and 8 h (B) after the ethanol/saline injection. C shows that tau fragments in ethanol-treated samples detected by PHF-1, Tau-5, and Tau-1 antibodies (lane 2) were also recognized by anti-C-tau antibody (lane 3). Lanes 1, 2, and 3 were run in the same gel, and a part of the blot containing lane 1 and 2 were incubated with PHF-1, Tau-5, or Tau-1 antibodies, and a part of the blot containing lane 3 was incubated with anti-C-tau antibody. D shows quantitative analyses of immunoblots for tau detected by PHF-1, Tau-1, Tau-5, anti-Tau PS199, and anti-Tau PT231 antibodies 4 h after ethanol/saline injection. Data (mean ± SEM, n=4) are expressed as ratios of protein band intensities in treatment groups to those in the control (saline) group after normalization to β-actin. E: Amounts of C-tau and CC3 (expressed as a ratio of treatment to saline control) were examined 4 h, 8 h, 16 h, and 24 h after ethanol treatment and expressed as mean ± SEM (n=4). F: The effects of a caspase inhibitor, Z-DEVD-FMK (DEVD), on ethanol-induced C-PARP, CC3 and C-tau formation in P7 mouse forebrains were examined as described in the Experimental Procedure. A representative gel picture and a result of quantitative analyses of immunoblots are shown. Data (mean ± SEM, n=4) are expressed as ratios of protein band intensities in the “Eth + DEVD” group to those in the Eth group after normalization to β-actin. *Significantly (P< 0.05) different from control groups by ANOVA with Bonferroni’s post hoc test.
Ethanol induces tau cleavage
As shown in Fig. 1B and C, shorter forms of tau (~50kDa) were detected by PHF-1, Tau-5, and Tau-1 antibody 8 h after ethanol exposure. These bands were recognized also with anti-C-tau antibody, which reacts with tau cleaved at Asp421/422. The tau cleavage was detected when cleaved (activated) caspase-3 (CC3) was produced 8 h after ethanol administration (Fig. 1B), although caspase-3 activation declined thereafter while C-tau levels remained constant between 8 h and 24 h (Fig. 1E). Administration of Z-DEVD-FMK (a caspase-3/7 inhibitor) by icv injections partially but significantly reduced ethanol-induced C-tau and cleaved PARP (C-PARP) formation (Fig. 1F). The similar reduction in C-tau and C-PARP by Z-DEVD-FMK supports the notion that C-tau is produced by DEVDases, such as caspase-3 and caspase-7. Lithium treatment effectively blocked tau cleavage as well as caspase-3 cleavage (Fig. 1B). C-tau fragments with smaller molecular weight, such as a 17-kDa fragment reported to be generated by calpain (Canu et al., 1998; Park and Ferreira, 2005), were not detected under our experimental conditions.
Localization of C-tau expression
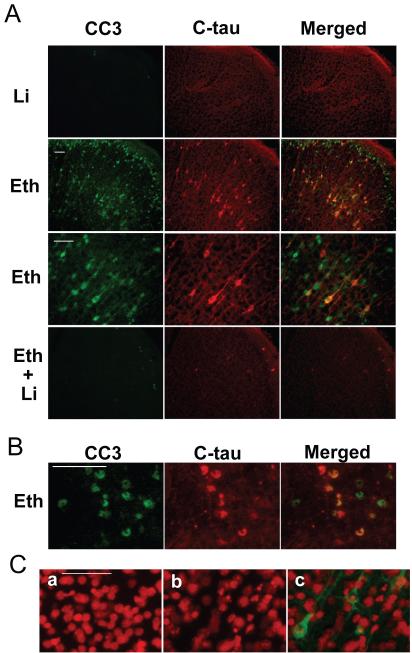
C-tau expression was detected generally in CC3-positive cells in the regions of strong CC3 expression, such as the frontal, parietal, cingulate, occipital and retrosplenial cortices, thalamus, and caudate nucleus. Figs. 2A and 2B show the cingulate cortex and caudate nucleus regions, respectively. C-tau expression in the cortex (Fig. 2A) was observed not only in the cell soma, but also in the neurites. CC3-positive but C-tau negative cells appeared to be losing or missing neurites. Lithium treatment effectively blocked ethanol-induced CC3 and C-tau formation.
Fig. 2.
Localization of C-tau expression in ethanol-treated P7 mouse forebrains. A: Mice were treated with lithium (Li), ethanol (Eth), and “ethanol + lithium” (Eth+Li) as described in the Experimental Procedure, the brains were removed 8 h after the ethanol/saline injection, and brain sections were dual-labeled with anti-CC3 and anti-C-tau antibodies. Representative images (CC3-labeled, C-tau-labeled, and the overlapped images) show the cingulate cortex region. B: Brain sections from mice taken 8 h after ethanol injection were stained with anti-CC3 and anti-C-tau antibodies. Representative images (CC3 labeled, C-tau-labeled, and overlapped images) show the caudate nucleus region. C: Brains were taken 8 h after saline or ethanol injection, and sections were stained first with anti-C-tau antibody and then with propidium iodide (5 μg/ml) in PBS. a shows a representative image of saline-treated samples stained with propidium iodide. b and c are representative images of ethanol-treated samples stained with propidium iodide and anti-C-tau antibody. b shows propidium iodide staining, and c is an overlaid image of propidium iodide staining and anti-C-tau antibody labeling. The bar indicates 50 μm.
In order to examine if C-tau positive neurons were actually dying, we assessed the nuclear fragmentation of C-tau positive neurons using propidium iodide staining. As shown in Fig. 2C, C-tau positive neurons showed picnotic or fragmented nuclei, indicating that those cells were in the process of apoptosis. Also, CC3 positive and C-tau negative cells showed picnotic or fragmented nuclei (data not shown).
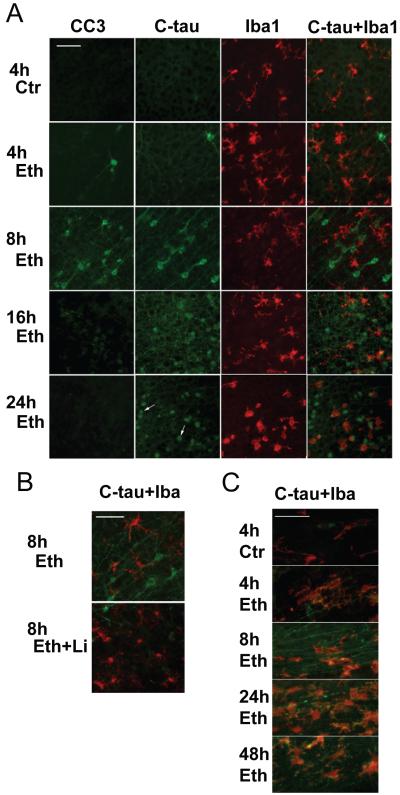
The time course studies in the cingulate cortex region (Fig. 3A) indicated that the number of CC3-positive neurons peaked 8 h after ethanol injection and decreased thereafter, while C-tau expression was more prolonged than CC3 expression specifically in fragmented dendrites/axons. These results collaborate with the immunoblotting data in Fig. 1E. As shown by arrows in Fig.3A, small spherical cells which appear to be dead neurons/apoptotic bodies stained non-specifically with anti-mouse IgG antibody. Aside from these cells, the specificity of anti-C-tau antibody was confirmed using blocking peptides.
Fig. 3.
Time course of CC3 and C-tau expression and microglial activation after ethanol exposure. A: Mice were treated as described in Experimental Procedure, and brains were taken 4, 8, 16, and 24 h after ethanol injection. Brain sections were stained with anti-CC3 and anti-C-tau antibodies. The same brain sections stained with anti-C-tau antibody were dual-labeled with anti-Iba1 antibody to examine changes in microglial morphology. B: C-tau expression and microglial activation were compared between an ethanol sample (Eth) and an “ethanol + lithium” (Eth+Li) sample from brains taken 8 h after ethanol treatment. Both A and B show the cingulate cortex region. Arrows in a panel of C-tau staining 24 h after ethanol exposure in A indicate examples of spherical cells stained with anti-mouse IgG antibody as described in the text. C: Brain sections obtained from mice 4 h, 8 h, 24 h, and 48 h after the saline/ethanol injection were dual-labeled with anti-C-tau antibody (green) and anti-Iba-1 antibody (red). The pictures show the corpus callosum regions. The bar indicates 50 μm.
Fig. 3A also shows that ethanol caused changes in the morphology of microglia detected by anti-Iba1 antibody. In control brains, these cells had small cell bodies and resembled the primitive ramified microglia reported previously (Kadowaki et al., 2007). Four hours after ethanol exposure, cells exhibited thicker processes and resembled activated microglia, and 16-24 h after ethanol exposure, cells became brain macrophage-like with a few short processes. These macrophage-like cells appeared to engulf spherical cells recognized with anti-mouse IgG antibody and/or anti-C-tau antibody (Fig. 3A). Morphological changes in microglia were less apparent under the ethanol+lithium conditions (Fig. 3B). Changes in morphology of microglia were observed not only in the cingulate cortex but also in other brain regions (data not shown), reflecting widespread ethanol-induced neurodegeneration. C-tau expression was also observed in axon bundles in the corpus callosum (Fig. 3C), and other areas such as dorsal fornix, alveus hippocampus, and caudate nucleus (data not shown). C-tau staining in the corpus callosum was already detected 4 h after ethanol exposure and still detected 48 h after ethanol exposure in some fragmented projections (Fig. 3C). Microglia in the corpus callosum in ethanol-treated mice appeared to engulf degenerating projections (Fig. 3C).
Ethanol induced tau cleavage in cultured cortical neurons
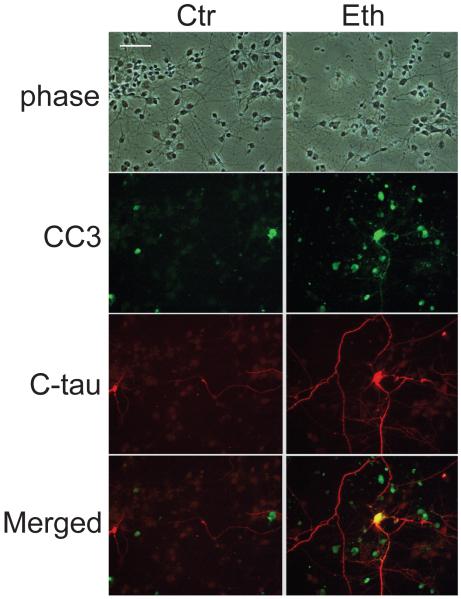
Ethanol also produced C-tau in cultured cortical neurons. Cultured neurons were treated with 100 mM ethanol for 48 h as described in the Experimental Procedure. The dose was chosen because previous studies have indicated that 50-100 mM ethanol causes highly significant apoptosis in cultured neurons (Saito et al., 1999; Takadera and Ohyashiki, 2004). The percentage of dead cells in the control culture was 18.9 ± 0.6% (mean ± SD, n=6), and that of cells treated with ethanol was 66.6 ± 8.9% (mean ± SD, n=6). Fig. 4 shows that some of the cortical neurons expressed CC3 and C-tau after incubation with 100 mM ethanol for 24 h. C-tau was expressed both in cell bodies and neurites as shown in the ethanol-treated P7 mouse brain. Also, similar to the in vivo study, neurites were scarce in CC3-positive but C-tau-negative neurons.
Fig. 4.
C-tau and CC3 staining in cultured cortical neurons. Cortical neurons were prepared as described in Experimental Procedure and treated with or without 100 mM ethanol for 24 h. Then the cells were fixed and double-stained with anti-CC3 antibody and anti-C-tau antibody. The bar indicates 50 μm.
Discussion
In the present study, we examined whether ethanol-induced GSK-3β and caspase-3 activation in the P7 mouse brain lead to tau phosphorylation and cleavage. Our results demonstrated that ethanol increased phosphorylated tau (phospho-tau) recognized by PHF-1 antibody and by anti-Tau PS199 antibody, and that lithium blocked such phospho-tau elevation (Fig. 1A, D). Because the major epitopes of these antibodies are the sites phosphorylated by GSK-3β (Ishiguro et al., 1995), it is highly probable that ethanol increases tau phosphorylation through GSK-3β activation although involvement of other protein kinases and/or phosphatases cannot be ruled out. Lithium itself did not induce a significant decrease in the level of phospho-tau recognized by PHF-1 antibody while lithium partially decreased the level of phospho-tau recognized by anti-Tau PT231 antibody. It is possible that phosphorylation/dephosphorylation of tau is differentially regulated depending on the phosphorylation site.
It has been shown that GSK-3β is expressed primarily in neurons and especially in neurites until P21 in the rat brain (Takahashi et al., 2000), suggesting that GSK-3β activated by ethanol could phosphorylate tau in neurites. Although fetal tau is already highly phosphorylated, further phosphorylation by kinases such as PKA and GSK-3β has been shown to reduce its assembly promoting activity (Morishima-Kawashima et al., 1995). While fetal tau’s low efficiency in promoting microtubule assembly may be important for the dynamic microtubule state during periods of high plasticity (Brion et al., 1994), further phosphorylation of fetal tau may excessively destabilize developing axons.
Ethanol also produced a group of approximately 50-kDa C-tau proteins with slightly different mobility on Western blots (Fig. 1B, C), probably due to varied degrees of phosphorylation. Considering the previous studies showing that caspase-3 cleaves tau at Asp421/422 and produces truncated tau with a molecular weight of ~50kDa (Gamblin et al., 2003; Park et al., 2007), it is likely that ethanol-induced caspase-3 activation leads to C-tau formation in the developing brain. The notion is supported by the present results showing that C-tau formation is partially inhibited by a caspase-3/7 inhibitor (Z-DEVD-FMK) (Fig. 1F) and that C-tau is expressed in CC3-positive neurons both in the brain (Fig. 2A, B) and in the cultured cortical neurons (Fig.4). Previous studies have indicated that axon degeneration may be regulated by caspase-6 while cell body apoptosis is regulated by caspase-3 (Guo et al., 2004; Nikolaev et al., 2009). Although caspase-6 may cleave tau at Asp421/422 (Gamblin et al., 2003), we were unable to detect ethanol-induced caspase-6 activation in P7 forebrains by either immunoblotting or immunohistochemical analyses (data not shown). Likewise, it has been reported that caspase-7 and -8, which may also generate C-tau (Gamblin et al., 2003), are not activated in the P7 mouse brain exposed to ethanol under the conditions which induce robust caspase-3 activation (Young et al., 2003; Olney et al., 2002a). These studies strongly indicate that ethanol-induced caspase-3 activation results in tau cleavage.
C-tau and CC3 were expressed in cell soma and neurites, although C-tau staining was stronger than the CC3 staining in the neurites. As early as 4 h after ethanol exposure, C-tau expression was observed in the neuronal cell soma, neurites, and axon bundles (Fig. 3A, C). These results suggest that ethanol induces caspase-3 activation simultaneously in the cell soma, dendrites, and axons in the P7 brain, and the activated caspase-3 cleaves fetal tau, which has been reported to be localized in the cell soma, dendrites, and axons (Tashiro et al., 1997). However, the possibility that CC3 or C-tau diffuses from cell soma to dendrites and proximal axons cannot be ruled out. The expression of C-tau in cultured cortical neurons was very similar to that of the P7 brain (Fig.4). In both P7 brains and cultured neurons, CC3-positive but C-tau-negative neurons lacked neurites while many fragmented neurites only expressed C-tau. Unlike CC3, C-tau was still detected 48 h after ethanol exposure in some processes in the brain, which makes C-tau staining a useful tool to examine ethanol-induced dendrite/axon degeneration. It is possible that the turnover rate of C-tau in neurites is slower than that of CC3, while the turnover rate of C-tau in the cell body is faster than that of CC3.
Localization of activated caspase-3 in axons has been demonstrated under several pathological conditions. For example, CC3 and caspase cleaved β-actin are expressed in degenerating axons in dopaminergic neurons after axotomy (El-Khodor and Burke, 2002), and post-ischemic activation of caspase-3 occurs in axons and dendrites in the rat hippocampus (Rami et al., 2003). Because previous studies have indicated a role of C-tau in dendritic damage (Kim et al., 1999) and apoptosis (Chung et al., 2001; Fasulo et al., 2005), C-tau production by ethanol may promote neuronal cell and axon degeneration. In accordance with the notion that CC3 and C-tau formation are important in ethanol-induced neurodegeneration, neurons expressing C-tau showed nuclear fragmentation (Fig. 3C), and lithium blocked ethanol-induced CC3 and C-tau elevation and neurodegeneration (Fig.3A). It has been shown that lithium inhibits tau phosphorylation and aggregation in the mouse model of Alzheimer’s disease (Noble et al., 2005).
We found that ethanol-induced neurodegeneration was accompanied by microglial activation (Fig.3A). Although abundant resting microglial cells were detected in the control brain, morphological changes in microglia occurred within 4 h after ethanol treatment and continued until microglia changed to phagocytic macrophage-like cells. These phagocytic microglial cells appeared to engulf degraded cell bodies and processes containing C-tau. Also, microglia in the corpus callosum in ethanol-treated mice appeared to engulf degenerating projections (Fig. 3C). However, morphology of such microglia in the corpus callosum was similar to that of amoeboid microglial cells (the nascent form of ramified microglia, Kaur et al., 2009) found in the subventricular white matter in both saline and ethanol-treated mouse brains (data not shown). Activation of microglia was not apparent in ethanol+lithium treated mice (Fig. 3B), probably because lithium inhibited initiation of the apoptotic pathway that induces activation of microglia. Collectively, our results indicate that ethanol-induced neurodegeneration is associated with tau modifications by GSK-3β and caspase-3 in the neuronal cell soma, dendrites, and axons. Tau may play an important role in ethanol-induced neurodegeneration in the developing brain, as observed in other pathological conditions. The possible causal relationship between tau modifications and neurodegeneration remains to be explored.
Acknowledgements
We thank Dr. Peter Davies (Albert Einstein College of Medicine, New York, NY) for providing the PHF-1 antibody. This work was supported by National Institute on Alcohol Abuse and Alcoholism grant AA015355.
References
- Brion JP, Octave JN, Couck AM. Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience. 1994;63:895–909. doi: 10.1016/0306-4522(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A, Calissano P. Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J. Neurosci. 1998;18:7061–7074. doi: 10.1523/JNEUROSCI.18-18-07061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, Mazzoni E, Balduini W. Caspase-3 and calpain activities after acute and repeated ethanol administration during the rat brain growth spurt. J. Neurochem. 2004;89:197–203. doi: 10.1111/j.1471-4159.2004.02341.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Saito M, Mao RF, Wang R, Vadasz C, Saito M. Lithium blocks ethanol-induced modulation of protein kinases in the developing brain. Biochem. Biophys. Res. Commun. 2008;367:597–602. doi: 10.1016/j.bbrc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun W, Waldo GS, Johnson GV. Split GFP complementation assay: a novel approach to quantitatively measure aggregation of tau in situ: effects of GSK3beta activation and caspase 3 cleavage. J. Neurochem. 2007;103:2529–2539. doi: 10.1111/j.1471-4159.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol. Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- El-Khodor BF, Burke RE. Medial forebrain bundle axotomy during development induces apoptosis in dopamine neurons of the substantia nigra and activation of caspases in their degenerating axons. J. Comp. Neurol. 2002;452:65–79. doi: 10.1002/cne.10367. [DOI] [PubMed] [Google Scholar]
- Fasulo L, Ugolini G, Cattaneo A. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. J Alzheimers Dis. 2005;7:3–13. doi: 10.3233/jad-2005-7102. [DOI] [PubMed] [Google Scholar]
- Gabbita SP, Scheff SW, Menard RM, Roberts K, Fugaccia I, Zemlan FP. Cleaved-tau: a biomarker of neuronal damage after traumatic brain injury. J. Neurotrauma. 2005;22:83–94. doi: 10.1089/neu.2005.22.83. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. U S A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, McCartney S, Causevic E, Ko LW, Yen SH. Ethanol enhances tau accumulation in neuroblastoma cells that inducibly express tau. Neurosci. Lett. 2008;443:67–71. doi: 10.1016/j.neulet.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. Embo. J. 1990;9:4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC. Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am. J. Pathol. 2004;165:523–531. doi: 10.1016/S0002-9440(10)63317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Jeong JY, Lee YK, Roh GS, Kim HJ, Kang SS, Cho GJ, Choi WS. Suppression of survival kinases and activation of JNK mediate ethanol-induced cell death in the developing rat brain. Neurosci. Lett. 2006;398:113–117. doi: 10.1016/j.neulet.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Sato K, Takamatsu M, Park J, Uchida T, Imahori K. Analysis of phosphorylation of tau with antibodies specific for phosphorylation sites. Neurosci. Lett. 1995;202:81–84. doi: 10.1016/0304-3940(95)12206-0. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicontinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain. PLoS Med. 2006;3(4):e101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Dheen ST, Ling EA. From blood to brain: amoeboid microglial cell, a nascent macrophage and its functions in developing brain. Acta. Pharmacol. Sin. 2007;28:10087–1096. doi: 10.1111/j.1745-7254.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Nakadate K, Sakakibara S, Hirata K, Ueda S. Expression of Iba1 protein in microglial cells of zitter mutant rat. Neurosci. Lett. 2007;411:26–31. doi: 10.1016/j.neulet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- Kim D, Su J, Cotman CW. Sequence of neurodegeneration and accumulation of phosphorylated tau in cultured neurons after okadaic acid treatment. Brain Res. 1999;839:253–262. doi: 10.1016/s0006-8993(99)01724-2. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Watanabe A, Titani K, Ihara Y. Hyperphosphorylation of tau in PHF. Neurobiol. Aging. 1995;16:365–371. doi: 10.1016/0197-4580(95)00027-c. discussion 371-380. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DDM, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. U S A. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D’Sa C, Roth KA. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol. Dis. 2002a;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res. Dev. Brain Res. 2002b;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Park SY, Ferreira A. The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J. Neurosci. 2005;25:5365–5375. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Tournell C, Sinjoanu RC, Ferreira A. Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience. 2007;144:119–127. doi: 10.1016/j.neuroscience.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami A, Jansen S, Giesser I, Winckler J. Post-ischemic activation of caspase-3 in the rat hippocampus: evidence of an axonal and dendritic localisation. Neurochem. Int. 2003;43:211–223. doi: 10.1016/s0197-0186(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, Nakamura K, Iwata Y, Tsuchiya KJ, Mori N, Detera-Wadleigh SD, Ichikawa H, Itohara S, Yoshikawa T, Furuichi T. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J. Clin. Invest. 2007;117(4):931–43. doi: 10.1172/JCI29031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Saito M, Berg MJ, Guidotti A, Marks N. Gangliosides attenuate ethanol-induced apoptosis in rat cerebellar granule neurons. Neurochem. Res. 1999;24:1107–1115. doi: 10.1023/a:1020704218574. [DOI] [PubMed] [Google Scholar]
- Saito M, Mao RF, Wang R, Vadasz C, Saito M. Effects of gangliosides on ethanol-induced neurodegeneration in the developing mouse brain. Alcohol Clin. Exp. Res. 2007;31:665–674. doi: 10.1111/j.1530-0277.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Sang H, Lu Z, Li Y, Ru B, Wang W, Chen J. Phosphorylation of tau by glycogen synthase kinase 3beta in intact mammalian cells influences the stability of microtubules. Neurosci. Lett. 2001;312:141–144. doi: 10.1016/s0304-3940(01)02206-6. [DOI] [PubMed] [Google Scholar]
- Takadera T, Ohyashiki T. Glycogen synthase kinase-3 inhibitors prevent caspase-dependent apoptosis induced by ethanol in cultured rat cortical neurons. Eur. J. Pharmacol. 2004;499:239–245. doi: 10.1016/j.ejphar.2004.07.115. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Tomizawa K, Ishiguro K. Distribution of tau protein kinase I/glycogen synthase kinase-3beta, phosphatases 2A and 2B, and phosphorylated tau in the developing rat brain. Brain Res. 2000;857:193–206. doi: 10.1016/s0006-8993(99)02424-5. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Hasegawa M, Ihara Y, Iwatsubo T. Somatodendritic localization of phosphorylated tau in neonatal and adult rat cerebral cortex. Neuroreport. 1997;8:2797–2801. doi: 10.1097/00001756-199708180-00029. [DOI] [PubMed] [Google Scholar]
- Utton MA, Vandecandelaere A, Wagner U, Reynolds CH, Gibb GM, Miller CC, Bayley PM, Anderton BH. Phosphorylation of tau by glycogen synthase kinase 3beta affects the ability of tau to promote microtubule self-assembly. Biochem. J. 1997;323(Pt 3):741–747. doi: 10.1042/bj3230741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Klocke BJ, Tenkova T, Choi J, Labruyere J, Qin YQ, Holtzman DM, Roth KA, Olney JW. Ethanol-induced neuronal apoptosis in vivo requires BAX in the developing mouse brain. Cell Death Differ. 2003;10:1148–1155. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Mulchahey JJ, Gudelsky GA. Quantification and localization of kainic acid-induced neurotoxicity employing a new biomarker of cell death: cleaved microtubule-associated protein-tau (C-tau) Neuroscience. 2003;121:399–409. doi: 10.1016/s0306-4522(03)00459-7. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Jauch EC, Mulchahey JJ, Gabbita SP, Rosenberg WS, Speciale SG, Zuccarello M. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;947:131–139. doi: 10.1016/s0006-8993(02)02920-7. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang X, Jie Chen, Miao Y, Sun A. Role of caspase-3 in tau truncation at D421 is restricted in transgenic mouse model s for tauopathies. J. Neurochem. 2009;109:476–484. doi: 10.1111/j.1471-4159.2009.05959.x. [DOI] [PubMed] [Google Scholar]
- Zhong J, Yang X, Yao W, Lee W. Lithium protects ethanol-induced neuronal apoptosis. Biochem. Biophys. Res. Commun. 2006;350:905–910. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]