Abstract
Purpose
To investigate whether long-term intraocular pressure (IOP) fluctuations are a risk factor for conversion from ocular hypertension to glaucoma.
Design
Observational cohort study.
Participants
The study included 252 eyes of 126 patients with ocular hypertension observed untreated as part of the Diagnostic Innovations in Glaucoma Study. At baseline, ocular hypertensive eyes had elevated IOP, normal visual fields (VFs) on standard automated perimetry, and normal optic discs as evaluated by stereophotograph assessment.
Methods
Glaucoma conversion was defined as development of reproducible VF loss or optic disc damage. Analyses included all IOP measurements from the baseline visit to time of progression (for converters) and last follow-up (for nonconverters). Mean IOP and IOP fluctuation were calculated as the arithmetic mean and standard deviation (SD), respectively, of all available IOP measurements per eye.
Main Outcome Measures
Univariable and multivariable Cox regression analyses were used to evaluate the association between IOP fluctuation and time to progression. Multivariable models adjusted for age, mean IOP, central corneal thickness, vertical cup-to-disc ratio, and pattern SD.
Results
Forty eyes of 31 subjects developed glaucoma during follow-up. Mean IOPs during follow-up were 25.4±4.2 mmHg for the eyes that converted to glaucoma and 24.1±3.5 mmHg for the eyes that did not. Corresponding values for IOP fluctuation were 3.16±1.35 mmHg and 2.77±1.11 mmHg, respectively. Intraocular pressure fluctuation was not a risk factor for conversion to glaucoma both in univariable analysis (hazard ratio [HR], 1.30 per 1 mmHg higher; 95% confidence interval [CI], 0.76–1.96; P = 0.092) and in multivariable analysis (adjusted HR, 1.08 per 1 mmHg higher; 95% CI, 0.79–1.48; P = 0.620). Mean IOP during follow-up was a significant risk factor for progression both in univariable analysis (HR = 1.16 per 1 mmHg higher; 95% CI, 1.04–1.31; P = 0.010) and in multivariable analysis (adjusted HR, 1.20 per 1 mmHg higher; 95% CI, 1.06–1.36; P = 0.005).
Conclusion
Long-term IOP fluctuations do not appear to be significantly associated with the risk of developing glaucoma in untreated ocular hypertensive subjects.
Although elevated intraocular pressure (IOP) has been unquestionably demonstrated to be a risk factor for development and progression of glaucoma,1–5 there has been controversy with regard to the IOP features that are the most relevant. Long-term (i.e., visit to visit) IOP fluctuation, in particular, has been proposed as an independent predictive factor for progression of glaucoma.6–9 Results from the Advanced Glaucoma Intervention Study (AGIS) indicated that larger long-term IOP fluctuations were associated with progressive visual field (VF) deterioration.7 In contrast, a recent report from the Early Manifest Glaucoma Trial (EMGT) did not find any relationship between long-term IOP fluctuations and risk of glaucoma progression.10
Both the AGIS and EMGT included only patients with definite glaucoma diagnosis at baseline. It is possible that the role of long-term IOP fluctuation as a risk factor for glaucoma development could be different than that for glaucoma progression. Two recent multicenter prospective randomized clinical trials reported on the predictive factors for conversion from ocular hypertension to glaucoma. In both the Ocular Hypertension Treatment Study11 and the European Glaucoma Prevention Study,12 higher IOP levels at the baseline visits were associated with a higher likelihood of developing VF loss and/or optic disc changes over time. However, neither of these studies has reported yet whether fluctuation of IOP over multiple follow-up visits also is a risk factor for development of glaucoma.
The purpose of the current study was to investigate whether long-term IOP fluctuation is a risk factor for conversion from ocular hypertension to glaucoma.
Materials and Methods
Participants
This observational cohort study included 252 eyes of 126 ocular hypertensive patients who were observed without receiving ocular hypotensive treatment during follow-up. All patients were observed at the Hamilton Glaucoma Center, University of California San Diego as part of an ongoing prospective longitudinal study (Diagnostic Innovations in Glaucoma Study) designed to evaluate optic nerve structure, visual function, and risk factors in glaucoma. Patients in the Diagnostic Innovations in Glaucoma Study were longitudinally evaluated according to a preestablished protocol that includes annual follow-up visits in which they underwent clinical examination and several other imaging and functional tests, including VFs and optic disc photographs. All the data were entered into a computer database. For this particular study, we have retrospectively selected a cohort of ocular hypertensive subjects from the Diagnostic Innovations in Glaucoma Study population, and clinical information was obtained from our research database. All patients who met the inclusion criteria described below were enrolled in the current study. Informed consent was obtained from all participants. The University of California San Diego Human Subjects Committee approved all protocols, and the methods described adhered to the tenets of the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations.
Baseline and follow-up examinations consisted of a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, dilated fundoscopic examination using a 78-diopter (D) lens, stereoscopic optic disc photography, and VF examination with standard automated perimetry (SAP). At baseline, SAP testing was performed using program 24-2 (Carl Zeiss Meditec, Dublin, CA), full-threshold strategy. During follow-up, SAP testing was performed using either full-threshold or Swedish Interactive Threshold Algorithm strategies. All patients also had central corneal thickness (CCT) measurements obtained during follow-up by a trained technician who was masked to the status and other examinations of the patients. For each patient, CCT was calculated as the average of 3 measurements obtained during the same visit using ultrasound pachymetry (Pachette DGH 500, DGH Technology, Inc., Philadelphia, PA).
To be included, subjects had to have BCVA of 20/40 or better, spherical refraction within ±5.0 D and cylinder correction within ±3.0 D, and open angles on gonioscopy. Patients with secondary causes of high IOP (e.g., pseudoexfoliation, pigment dispersion syndrome, iridocyclitis, trauma) or other diseases possibly affecting VF (e.g., demyelinating diseases, pituitary lesions) were excluded. Patients with a history of refractive surgery also were excluded.
Evaluation of structural damage to the optic disc was based on assessment of simultaneous stereoscopic optic disc photographs (TRC-SS, Topcon Instrument Corp. of America, Paramus, NJ). Stereoscopic sets of slides were examined using a stereoscopic viewer (Pentax, Asahi, Japan). The photographs were evaluated by 2 experienced graders, and each was masked to the subject's identity and to the other test results. Each grader had been previously trained using a set of standard reference photographs used in the Optic Disc Reading Center of the Hamilton Glaucoma Center at the University of California San Diego. This set of photographs includes multiple examples of normal and definite glaucomatous optic discs. After training, each grader was certified after completing a test to evaluate grading skills. For inclusion, photographs needed to be graded adequate quality or better. The graders visually estimated the horizontal and vertical cup-to-disc (C/D) ratios based on the contour of the cup.
Ocular hypertensive patients met the following criteria: baseline IOP ≥ 24 mmHg in one eye and ≥ 21 mmHg in the other eye, but not higher than 32 mmHg; normal-appearing optic discs and retinal nerve fiber layer (RNFL) on baseline stereophotographs of both eyes (no diffuse or focal rim thinning, hemorrhage, cupping, or nerve fiber layer defects indicative of glaucoma or other ocular pathologies); and normal VF test results. A normal VF was defined as a mean deviation and pattern standard deviation (PSD) within 95% confidence limits and a glaucoma hemifield test within normal limits.
Follow-up and Determination of Primary Open-angle Glaucoma End Points
Conversion from OHT to glaucoma was considered as the development of a reproducible VF defect or glaucomatous change in the appearance of the optic disc in at least one eye. The time of the first abnormal SAP VF or change in optic disc (whichever came first) in the eye that developed primary open-angle glaucoma (POAG) was defined as the end point time for patients showing conversion.
Glaucomatous change in the appearance of the optic disc was defined as development of focal or diffuse thinning of the neuro-retinal rim, increased excavation, or appearance of RNFL defects. Changes in rim color, presence of disc hemorrhage, or progressive parapapillary atrophy were not sufficient for characterization of progression. When grading photographs for progression, the examiner received a pair of photographs containing a baseline and a follow-up photograph. Examiners were masked as to the order of photographs (i.e., dates) and to the patient's identification. Discrepancies between the 2 graders were resolved by either consensus or adjudication of a third experienced grader.
Abnormality on SAP was defined as the presence of a glaucoma hemifield test result outside normal limits and/or PSD with P<0.05. Based on previous results from the OHTS, a confirmed VF defect required 3 consecutive abnormal VFs.13 The VFs were also evaluated by a glaucoma specialist who excluded other causes of nonglaucomatous VF loss or presence of VF artifacts as possible causes of VF abnormality. Only reliable VFs were included in the analysis. This was defined as 33% or fewer false-positive results, false-negative results, and fixation losses.
None of the patients was receiving any ocular hypotensive medication at baseline, and they also were left untreated during follow-up. Fifteen of the 126 patients (12%) were assigned to treatment during follow-up for causes other than development of glaucoma, such as unacceptably high IOP (based on the attending ophthalmologist's decision). For these patients, only the period without treatment was evaluated in the study. No evidence for informative censoring was found when the analyses were repeated after exclusion of these patients or after assigning them to the conversion group. Therefore, we report only the results of the analyses considering these patients as censored when they first received treatment.
Data Analysis
Analyses included all IOP measurements from baseline to time of progression or last follow-up visit. An average of 23.6 (median, 22; first quartile, 19; third quartile, 29) IOP measurements were available per eye included in the study. Mean IOP and IOP fluctuation were calculated as the arithmetic mean and standard deviation (SD), respectively, of all available IOP measurements per eye.
The primary purpose of the study was to determine whether mean IOP and IOP fluctuation were predictive of glaucoma development. Other variables analyzed as predictive factors were age, CCT, vertical C/D ratio, and the baseline SAP VF index PSD. These variables have been reported to be significantly associated with the risk of development of glaucoma among patients with ocular hypertension or glaucoma suspects.11,12,14 Due to the initial OHTS results reporting diabetes mellitus as a significant protective factor for development of glaucoma,11 we also tested models with and without diabetes mellitus included as a covariate. Hazard ratios (HRs) for the association between potential predictive factors and development of glaucoma were obtained by Cox proportional hazards models. We report HRs from univariate models, which do not adjust for the presence of other factors, as well as adjusted HRs from multivariable Cox proportional hazards models. To adjust for potential intrasubject correlation in the Cox models, the robust sandwich variance estimate of Lin and Wei15 was used.
Statistical analyses were performed using SPSS 15.0 software (SPSS, Chicago, IL) and Stata 9.0 (StataCorp, College Station, TX). The α level (type I error) was set at 0.05.
Results
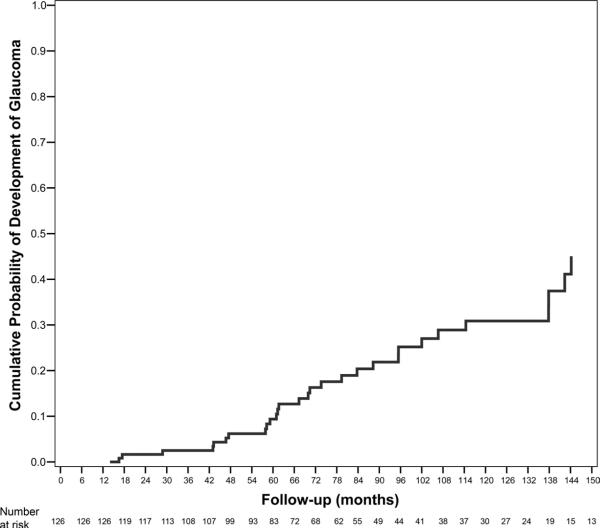
Demographic and clinical characteristics of the 252 eyes of 126 ocular hypertensive patients included in the study are shown in Table 1. Forty eyes of 31 subjects developed POAG during follow-up. Figure 1 shows the cumulative probability of developing glaucoma in at least one eye during the study. Mean follow-up time until conversion to glaucoma was 82.8 months (median, 70.4; first quartile, 56.9; third quartile, 106.7). Mean follow-up time for nonconverters was 86.3 months (median, 78.9; first quartile, 52.3; third quartile, 120.5). There was no statistically significant difference in mean follow-up time between converters and nonconverters (P = 0.718). From the 40 eyes that developed glaucoma, 22 (55%) developed damage to the optic disc, 13 (33%) developed repeatable VF abnormalities, and 5 (13%) developed both.
Table 1.
Clinical Factors for Ocular Hypertensive Eyes That Developed and Did Not Develop Glaucoma
| Developed Glaucoma | Did Not Develop Glaucoma | |
|---|---|---|
| Age at baseline* | 59.5±11.3 | 55.2±13.6 |
| Race* | ||
| White, non-Hispanic | 29 (94) | 89 (94) |
| Black | 2 (6) | 2 (2) |
| Hispanic | 0 | 2 (2) |
| Asian | 0 | 2 (2) |
| Gender (% female)* | 52 | 60 |
| Diabetes (yes)* | 5 (16) | 9 (10) |
| CCT (μm)† | 555.4±39.5 | 580.9±35.4 |
| Vertical cup-to-disc ratio at baseline† | 0.45±0.15 | 0.42±0.17 |
| PSD at baseline (dB)† | 1.98±0.62 | 1.74±0.41 |
| Mean IOP during follow-up (mmHg)† | 25.4±4.2 | 24.1±3.5 |
| Long-term IOP fluctuation (mmHg)† | 3.16±1.35 | 2.77±1.11 |
CCT = central corneal thickness; dB = decibels; IOP = intraocular pressure; PSD = pattern standard deviation.
Values are given as mean ± standard deviation unless otherwise noted.
Numbers in parentheses refer to percentages of patients.
Data refer to 31 patients who converted to glaucoma and 95 who did not.
Data refer to 40 eyes that converted to glaucoma and 212 that did not.
Figure 1.
Kaplan-Meier curve showing the cumulative probability of development of glaucoma in at least one eye during follow-up.
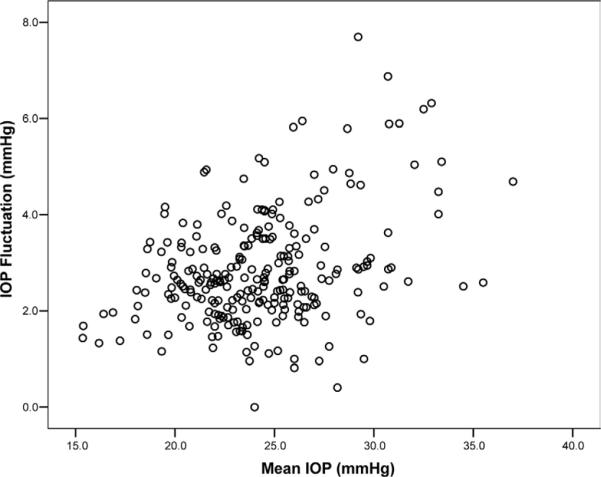
Table 1 also shows clinical characteristics of the eyes that developed and did not develop glaucoma. Mean IOPs during follow-up were 25.4±4.2 mmHg for the eyes that converted to glaucoma and 24.1±3.5 mmHg for the eyes that did not. Corresponding values for IOP fluctuation were 3.16±1.35 mmHg and 2.77±1.11 mmHg, respectively. There was a significant correlation between mean IOP and IOP fluctuation (r = 0.351, P<0.001; Pearson correlation coefficient). Patients with higher mean IOP values during follow-up tended to have higher IOP fluctuations. Figure 2 shows a scatterplot of IOP fluctuation versus mean IOP values.
Figure 2.
Scatterplot of long-term intraocular pressure (IOP) fluctuation values versus mean IOP.
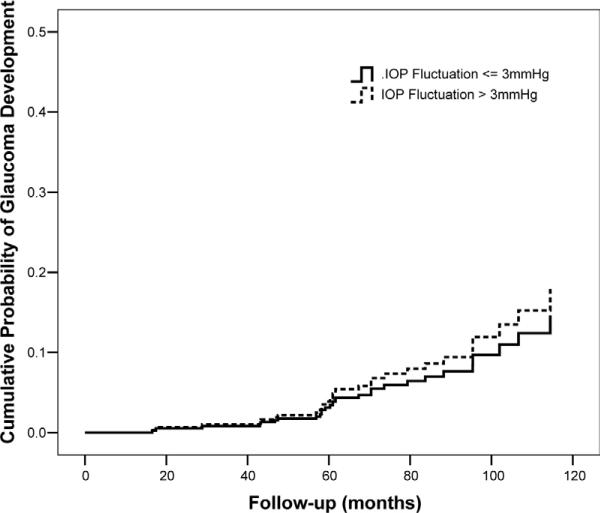
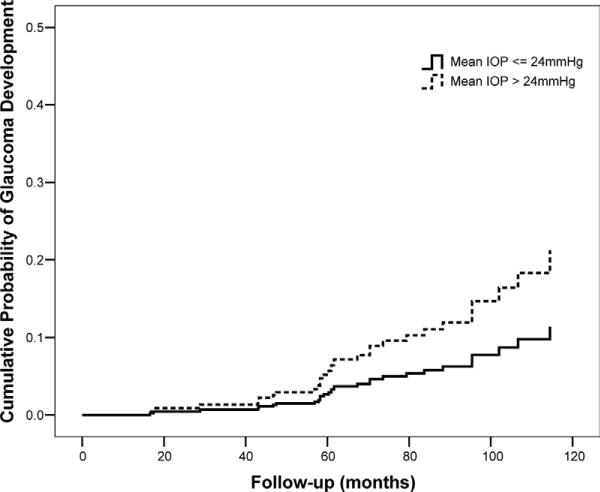
Table 2 shows univariate and multivariate HRs for each putative predictive factor for development of glaucoma. Mean IOP was a significant predictive factor in univariate analysis. Each 1-mmHg increase in mean IOP was associated with a 16% higher chance of developing glaucoma during follow-up (HR, 1.16; 95% confidence interval [CI], 1.04–1.31; P = 0.010). Intraocular pressure fluctuation was not a significant predictive factor in univariate analysis. A multivariable model was then constructed including mean IOP and IOP fluctuation and adjusting for age, CCT, baseline vertical C/D ratio, and baseline SAP PSD. In this model, mean IOP was significantly predictive of conversion (adjusted HR, 1.20 per 1 mmHg higher; 95% CI, 1.06–1.36; P = 0.005), whereas IOP fluctuation was not significantly associated with the outcome (adjusted HR, 1.08 per 1 mmHg higher; 95% CI, 0.79–1.48; P = 0.620). We also tested the model including an interaction term between mean IOP and IOP fluctuation, but it was also not significant. Figure 3 shows covariate-adjusted survivorship functions comparing the cumulative probability of glaucoma development for eyes with IOP fluctuation ≤ 3 mmHg with that of eyes with IOP fluctuation > 3 mmHg. Figure 4 shows covariate-adjusted survivorship functions for eyes with mean IOP ≤ 24 mmHg compared with eyes with mean IOP > 24 mmHg. The inclusion or exclusion of diabetes mellitus as a predictor variable in these models did not change the significance of the results.
Table 2.
Hazard Ratios (HRs) with 95% Confidence Intervals (CIs) for Risk Factors Associated with Development of Glaucoma
| Univariable HR (95% CI) | P Value | Adjusted HR (95% CI)* | P Value | |
|---|---|---|---|---|
| Age (per decade older) | 1.52 (1.13–2.04) | 0.005 | 1.63 (1.17–2.26) | 0.004 |
| CCT (per 40μm thinner) | 2.43 (1.64–3.62) | <0.001 | 2.30 (1.57–3.36) | <0.001 |
| Vertical C/D ratio (per 0.1 larger) | 1.07 (0.89–1.28) | 0.478 | 1.20 (1.02–1.42) | 0.028 |
| PSD (per 0.2 dB higher) | 1.20 (1.07–1.34) | 0.002 | 1.12 (0.97–1.30) | 0.119 |
| Mean IOP (per 1 mmHg higher) | 1.16 (1.04–1.31) | 0.010 | 1.20 (1.06–1.36) | 0.005 |
| IOP fluctuation (per 1 mmHg higher) | 1.30 (0.96–1.76) | 0.092 | 1.08 (0.79–1.48) | 0.620 |
CCT = central corneal thickness; C/D = cup-to-disc; dB = decibels; IOP = intraocular pressure; PSD = pattern standard deviation.
Multivariable model includes all variables listed in the table.
Figure 3.
Covariate-adjusted survivorship functions for eyes with long-term intraocular pressure (IOP) fluctuation ≤ 3 mmHg and for eyes with long-term IOP fluctuation > 3 mmHg. Survivorship functions are reported at mean levels of the covariates.
Figure 4.
Covariate-adjusted survivorship functions for eyes with mean intraocular pressure (IOP) ≤ 24 mmHg and for eyes with mean IOP > 24 mmHg. Survivorship functions are reported at mean levels of the covariates.
Discussion
In the current study, we investigated the association between long-term IOP fluctuation and mean IOP with risk of developing glaucoma among ocular hypertensive eyes. Although higher mean IOP levels were consistently associated with increased likelihood of developing glaucoma, IOP fluctuation was not associated with the study end points, in both univariable and multivariable models that adjusted for other clinically important variables.
The results of our study agree with those reported with the EMGT for patients with already existing glaucoma; only mean IOP, but not IOP fluctuation, was associated with glaucoma progression.10 In both studies, IOP fluctuation was calculated as the SD of IOP measurements obtained during different visits over the follow-up period. Long-term (or intervisit) IOP fluctuation is distinct from diurnal IOP fluctuation, which refers to the change of IOP occurring only during the 24-hour period. The EMGT results found that long-term IOP fluctuation was not a significant risk factor for glaucoma progression in both treated and untreated patients. In contrast, mean IOP levels were consistently associated with progressive glaucoma, with HRs of approximately 1.11 per 1 mmHg higher mean IOP in both situations. When both mean IOP and IOP fluctuation were entered into the same multivariable model, EMGT results showed that IOP fluctuation had an insignificant contribution to the risk of glaucoma progression. For untreated subjects, the HR for IOP fluctuation was 0.94 (95% CI, 0.73–1.21; P = 0.6378) in a model containing mean IOP and other clinically significant variables. In our study, which included only untreated subjects, the adjusted HR for long-term IOP fluctuation in the model containing mean IOP and other clinically significant variables was 1.08 (95% CI, 0.79–1.48; P = 0.620), similar to the EMGT result. The adjusted HR for mean IOP in this model was 1.20 per 1 mmHg higher (95% CI, 1.08–1.36; P = 0.001)—that is, each 1 mmHg higher mean IOP was associated with a 20% increase in the risk of developing glaucoma.
Although the results from both our study and the EMGT suggest that IOP fluctuation is not significantly associated with risk of glaucoma development or progression, respectively, a recent report by AGIS investigators found that each 1 mmHg higher long-term IOP fluctuation was associated with 31% higher odds of progressive VF loss (P = 0.0013 in a multivariable logistic regression model).7 Although all 3 studies have calculated long-term IOP fluctuation in a similar way, several other factors could explain the different findings between our study and the EMGT when compared with the AGIS. Besides differences in inclusion and exclusion criteria, as well as in study end points, the most important difference among these studies is likely the fact that AGIS calculations of IOP fluctuation included measurements obtained after progression had occurred, whereas in both the EMGT and our study measurements were obtained only up to the study end point. After progression occurred, it is possible that treatment would have been intensified and resulted in further IOP lowering and a consequent increase in IOP fluctuation. This could have resulted in an artifacticiously positive relationship between IOP fluctuation and risk of progression in the AGIS investigation. It is also possible that issues related to compliance with treatment could have affected the association between IOP and progression in treated patients.
The results of our study also agree with those reported by Bengtsson and Heijl16 as part of the Malmö Ocular Hypertension Study. In their investigation, high-risk ocular hypertensive patients were observed for 10 years as part of a prospective investigation to compare the rates of development of glaucomatous VF loss in patients treated with timolol versus a placebo. The patients were observed every 3 months with Goldmann tonometry measurements obtained at 8 AM, 11:30 AM, and 3:30 PM. No association was found between parameters measuring variation of IOP during the follow-up period and risk of glaucoma development. Although the inclusion criteria and definition of end points in our study differed considerably from theirs, neither study found an association of long-term IOP fluctuation and glaucoma development in multivariable models adjusting for mean IOP level.
Our study evaluated only long-term IOP fluctuations. Although it is possible that these fluctuations are correlated with diurnal fluctuations, magnitudes of these changes are likely to differ. Therefore, it is possible that the predictive ability of diurnal IOP fluctuations would differ from that of long-term IOP fluctuations. Previous studies have evaluated the role of diurnal IOP fluctuations as a risk factor for glaucoma progression.6,8,9 Most of these studies were limited by their retrospective analysis and lack of control for potentially confounding factors. Asrani et al6 found that diurnal IOP fluctuation, as measured by home self-tonometry, was a significant risk factor for progression. In their study, home tonometry measurements were obtained at baseline and their association with risk of progression over time was investigated. The authors found a significant HR for diurnal IOP fluctuation in a model adjusting for office IOP (mean of 2 measurements at baseline), age, race, gender, and severity of VF loss at baseline. It is important to note, however, that the predictive effect of IOP measurements obtained during follow-up was not taken into account. This study was also limited by the lack of well-defined criteria for VF progression and large number of patients excluded due to loss of follow-up. In contrast, a recent report by Liu et al17 performing IOP measurements at a sleep laboratory over the 24-hour period in untreated glaucomatous patients and healthy subjects did not find any significant difference in 24-hour IOP fluctuations between these two groups, suggesting that diurnal IOP fluctuations may not be a significant risk factor for glaucoma. However, longitudinal studies evaluating the predictive ability of 24-hour IOP measurements for development or progression of glaucoma are still necessary to evaluate this hypothesis.
It is important to emphasize that, although long-term IOP fluctuations were not a predictive factor for glaucoma development in our study, IOP measurements obtained during follow-up still are important when evaluating the likelihood of ocular hypertensive patients developing glaucoma over time, as indicated by the strong predictive ability of mean IOP. Current predictive models for glaucoma development have used only baseline IOP (i.e., values at the beginning of follow-up) as a predictive factor, along with other clinically significant variables.18–20 It is possible that models developed to incorporate updated values of IOP and other risk factors over time would provide an improved risk assessment.21,22 Development of these models, however, requires assessment of the optimal frequency of updating of risk factor measurements and other complex issues.21
In conclusion, our results suggest that long-term IOP fluctuations are not a significant risk factor for glaucoma development in untreated patients with ocular hypertension observed over time.
Acknowledgments
Supported in part by an independent research grant from Pfizer, Inc., New York, New York (FAM), and the National Eye Institute, Bethesda, Maryland (grant nos. EY11008 [LMZ], EY08208 [PAS]).
References
- 1.Leske MC, Heijl A, Hussein M, et al. Early Manifest Glaucoma Trial Group Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 3.Lichter PR, Musch DC, Gillespie BW, et al. CIGTS Study Group Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 4.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7 The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–40. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 6.Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–42. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–35. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Bergea B, Bodin L, Svedbergh B. Impact of intraocular pressure regulation on visual fields in open-angle glaucoma. Ophthalmology. 1999;106:997–1004. doi: 10.1016/S0161-6420(99)00523-0. discussion 1004–5. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez I, Pablo LE, Pueyo M, et al. Assessment of diurnal tensional curve in early glaucoma damage. Int Ophthalmol. 1996–1997;20:113–5. doi: 10.1007/BF00212956. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson B, Leske MC, Hyman L, Heijl A, Early Manifest Glaucoma Trial Group Fluctuation of intraocular pressure and glaucoma progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:205–9. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 11.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 12.European Glaucoma Prevention Study (EGPS) Group Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 13.Keltner JL, Johnson CA, Quigg JM, et al. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2000;118:1187–94. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–13. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 16.Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2005;243:513–8. doi: 10.1007/s00417-004-1103-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–90. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 19.Ocular Hypertension Treatment Study Group. European Glaucoma Prevention Study Group Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansberger SL. A risk calculator to determine the probability of glaucoma. J Glaucoma. 2004;13:345–7. doi: 10.1097/00061198-200408000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Karp I, Abrahamowicz M, Bartlett G, Pilote L. Updated risk factor values and the ability of the multivariable risk score to predict coronary heart disease. Am J Epidemiol. 2004;160:707–16. doi: 10.1093/aje/kwh258. [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000;139:272–81. doi: 10.1067/mhj.2000.96469. [DOI] [PubMed] [Google Scholar]