Abstract
Purpose
To evaluate the relationship between intraocular pressure (IOP) and progressive retinal nerve fiber layer (RNFL) loss, as measured by scanning laser polarimetry with enhanced corneal compensation (GDx ECC), in a cohort of glaucoma patients and individuals suspected of having the disease followed over time.
Design
Observational cohort study.
Participants
The study included 344 eyes of 204 patients recruited from the Diagnostic Innovations in Glaucoma Study (DIGS). There were 98 eyes (28%) with a diagnosis of glaucoma and 246 (72%) were considered glaucoma suspects at baseline.
Methods
Images were obtained annually with the GDx ECC scanning laser polarimeter, along with stereophotographs and SAP. The study included a total of 1211 GDx ECC visits with an average of 3.5 visits per eye. Progression was determined by the Guided Progression Analysis software for SAP and by masked assessment of stereophotographs performed by expert graders.
Main Outcome Measures
Random coefficient models were used to evaluate the relationship between IOP and RNFL thickness measurements over time in progressors and nonprogressors. Models were adjusted for baseline diagnosis and central corneal thickness.
Results
For all 344 eyes, the overall rate of change for the GDx ECC average thickness at an average IOP of 17 mmHg was −0.25 μm per year (P = 0.002). Each 1-mmHg higher IOP was associated with an additional loss of 0.05 μm per year of RNFL (P = 0.001). Twenty-nine eyes (8%) showed progression on SAP and/or optic disc stereophotographs. These eyes had a significantly higher rate of RNFL change (−0.95μm/year) than nonprogressors (−0.17 μm/year; P = 0.001). For progressors, each 1-mmHg higher IOP was associated with an additional loss of 0.13 μm per year of RNFL.
Conclusions
Higher levels of IOP during follow-up were significantly related to higher rates of progressive RNFL loss detected by the GDx ECC. These findings suggest that the GDx ECC may be helpful in monitoring progression and estimating rates of change in patients with glaucoma or suspected of having the disease. Also, they may contribute to a better understanding of the relationship between IOP and structural deterioration in glaucoma.
Several prospective clinical trials have provided compelling evidence for the role of intraocular pressure (IOP) as the main risk factor for development and progression of glaucoma.1-6 In the Ocular Hypertension Treatment Study (OHTS), IOP-lowering therapy was associated with a 54% relative reduction in the risk of developing primary open-angle glaucoma during follow-up.7 For disease progression, the Early Manifest Glaucoma Trial showed that each 1-mmHg higher mean IOP during follow-up was associated with a 12% increase in the chance of developing progressive visual field loss over time in patients who had already been diagnosed with glaucoma at the baseline visit.5 In another multicenter clinical trial of patients with advanced disease, the Advanced Glaucoma Intervention Study, eyes that had lower IOP during follow-up also had lower changes in visual field scores, indicating less progression.4
Most of the studies evaluating the role of IOP in glaucoma have used visual fields as the sole end point for estimating disease development or progression. Although automated perimetry has been the standard method for detecting progressive disease, it is known that many patients can have progressive structural damage that precedes detectable associated changes in the visual field.2,3 In the OHTS, changes to the optic nerve were seen in more than half of the patients before development of visual field defects.3 Although structural evaluation of the optic nerve was used as an end point in the OHTS, it was performed by subjective assessment of color stereophotographs and did not include an evaluation of changes to the retinal nerve fiber layer (RNFL).
Changes in the RNFL may be the earliest and even the only sign of glaucoma development and progression in many patients. In a longitudinal study of ocular hypertensive patients, Sommer et al8 found RNFL defects on red-free photographs up to 6 years before the development of visual field loss. In another longitudinal study, Quigley et al9 demonstrated that serial red-free nerve fiber layer examinations were more sensitive than color optic disc evaluation in the detection of progressive glaucoma damage. However, owing to the difficulties associated with obtaining and assessing RNFL red-free photographs, this method has not been widely used in clinical practice.
The development of imaging technologies has facilitated and improved our ability to evaluate the RNFL. One of these technologies, scanning laser polarimetry (SLP), provides quantitative estimates of the thickness of the RNFL with potential use for diagnosis and follow-up of glaucoma patients.10-14 The most recent commercially available version, the SLP with enhanced corneal compensation (GDx ECC; Carl Zeiss Meditec, Inc., Dublin, CA), provides objective and reproducible evaluation of the RNFL, with improved diagnostic accuracy compared with earlier versions of this instrument.10,15-17
The purpose of this study was to evaluate the relationship between IOP and progressive RNFL loss, as measured by the GDx ECC scanning laser polarimeter, in a cohort of glaucoma patients, and individuals suspected of having the disease followed over time. Longitudinal changes in the RNFL were also compared with changes detected by conventional methods, including standard automated perimetry (SAP) and color stereophotographs of the optic disc.
Methods
This was an observational cohort study. Participants from this study were included in a prospective, longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma (Diagnostic Innovations in Glaucoma Study) conducted at the Hamilton Glaucoma Center, University of California, San Diego. Participants in this study were longitudinally evaluated according to a preestablished protocol that included regular follow-up visits in which patients underwent clinical examination and several other imaging and functional tests. All data were entered in a computer database. All participants from the Diagnostic Innovations in Glaucoma Study who met the inclusion criteria described were enrolled in the current study. Informed consent was obtained from all participants. The University of California San Diego Human Subjects Committee approved all protocols and the methods described adhered to the tenets of the Declaration of Helsinki.
At each visit during follow-up, subjects underwent a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, IOP measurement using Goldmann applanation tonometry, gonios-copy, dilated fundoscopic examination, stereoscopic optic disc photography, and automated perimetry using either 24-2 Full-threshold or Swedish Interactive Threshold Algorithm (SITA). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented best-corrected visual acuity less than 20/40, spherical refraction outside ±5.0 diopters, and/or cylinder correction outside 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
The study included patients diagnosed with glaucoma, as well as patients suspected of having the disease, as determined at the baseline visit. Eyes were classified as glaucomatous if they had repeatable (2 consecutive) abnormal visual field test results on the baseline visits, defined as a pattern standard deviation outside of the 95% normal confidence limits, or a Glaucoma Hemifield Test result outside normal limits, regardless of the appearance of the optic disc. Eyes were classified as glaucoma suspects if they had a history of elevated IOP (>21 mmHg) and/or suspicious or glaucomatous appearance of the optic nerve, but normal and reliable visual field results on the baseline visits. If both eyes from the same patient were eligible for the study, both eyes were included in the analysis and statistical procedures were used to take into account the correlation between measurements within the same patient (see below).
A minimum follow-up period of 1 year with GDx ECC and a minimum of 2 separate visits were required for inclusion in this study. The GDx ECC images were obtained annually during follow-up. The study included a total of 1211 GDx ECC visits, with an average of 3.5 visits per eye. The number of visits per eye ranged from 2 to 7, with 85% of the eyes having ≥3 visits and 51% having ≥4 visits during follow-up. Eligible subjects were required to have had a visual field examination and optic disc stereophotographs taken close in time to the GDx ECC scans. During the follow-up period, each patient was treated at the discretion of the attending ophthalmologist.
Scanning Laser Polarimetry with Enhanced Corneal Compensation
Patients were imaged using a commercially available GDx ECC (Carl Zeiss Meditec). The instrument uses a near infrared laser beam with a wavelength of 785 nm to scan the ocular fundus, within a field of 40° horizontally × 20° vertically and a density of 256 × 128 pixels. Because corneal polarization axis and magnitude affect SLP measurements and are not similar in all eyes,18 the GDx is equipped with 2 adjustable linear retarders in rotating mounts that allow eye-specific compensation of anterior segment birefringence based on the macular retardation profile.19 The enhanced corneal compensator algorithm is implemented in the GDx with variable corneal compensation (VCC) by a software modification, without requiring hardware changes.15 In the ECC, a known birefringence bias is introduced into the measurement beam path to shift the measurement of total retardation into a more sensitive region of the curve of detection of polarization of the instrument. The bias retarder is formed by the combination of the variable corneal compensator and cornea. However, instead of completely canceling corneal birefringence, the retarder is adjusted so that the combination has retardance close to 55 nm and slow axis of polarization close to vertical. After image acquisition, the birefringent bias is removed mathematically, point-by-point, to yield the RNFL retardation values that are converted to RNFL thickness (in micrometers) using a fixed conversion factor.
Assessment of GDx ECC image quality was performed by an experienced examiner masked to the subject’s identity and results of the other tests. The assessment was based on the appearance of the reflectance image, presence of residual anterior segment retardation, and presence of an atypical pattern of retardation. To be classified as good quality, an image required a focused and evenly illuminated reflectance image with a centered optic disc. All images had typical scan scores >80, indicating minimal or no atypical patterns of retardation.20
The RNFL retardation measurements were obtained on a 3.2-mm diameter calculation circle around the optic nerve head. Three parameters were calculated from RNFL measures obtained within this calculation circle and used in this study: temporal–superior–nasal–inferior–temporal (TSNIT) average (average of RNFL measurements obtained on the 360° around the optic nerve), inferior average, and superior average. Superior average measurements extend from 10 to 2 o’clock and inferior average measurements extend from 4 to 8 o’clock. These parameters are provided on the standard GDx ECC printout. To evaluate changes in GDx ECC RNFL measurements in localized sectors, the calculation circle was also divided in 16 sectors (22.5° each) and the average retardation was recorded for each of these sectors. There were 8 sectors for the superior hemiretina and 8 sectors for the inferior hemiretina, with sectors numbered in a clockwise fashion and sector S1 corresponding to the most temporal sector of the superior hemiretina and S16 to the most temporal sector of the inferior hemiretina.
Standard Automated Perimetry
Standard automated perimetry visual fields were obtained using either 24-2 Full Threshold or SITA (Humphrey Field Analyzer; Carl Zeiss Meditec) strategies during follow-up. Only reliable tests (≤33% fixation losses and false negatives, and <15% false positives) were included. Glaucomatous visual field progression was assessed using the Humphrey Field Analyzer Guided Progression Analysis (GPA) software. Each eye had a minimum of 5 visual fields available to run the Humphrey Field Analyzer GPA. For each individual point on the visual field, the GPA compares the sensitivity on a follow-up test with the sensitivity for the same location obtained from averaging 2 baseline tests. It flags points that show change greater than the expected variability (at the 95% significance level). If significant change is detected in ≥3 points, and repeated in the same points in 2 consecutive follow-up tests, the GPA software flags the last examination as Possible Progression. If the same ≥3 points have significant change detected and repeated in 3 consecutive follow-up tests, the GPA software flags the last examination as Likely Progression. For the purpose of this study, only the GPA classification Likely Progression was considered as indicating visual field progression.
The GPA accepts either a pair of Full-Threshold or a pair of SITA tests to be included as baseline. Baseline tests should be reliable and similar, to establish a consistent baseline point with which every follow-up test (SITA only) will be compared. In the present study, the baseline tests were chosen as those closest to the baseline GDx ECC date and the last visual field test date was also the one closest to the last available GDx ECC examination.
Stereophotograph Grading
Simultaneous stereoscopic optic disc photographs (TRC-SS; Topcon Instrument Corp of America, Paramus, NJ) were reviewed using a stereoscopic viewer (Asahi Pentax Stereo Viewer II; Asahi Optical Co., Tokyo, Japan). Baseline stereophotographs were evaluated by 2 masked, experienced graders and classified as glaucomatous or normal. Glaucomatous optic disc appearance was defined based on the presence of neuroretinal rim thinning, excavation, notching, or characteristic RNFL defects.
For progression assessment, each patient’s most recent stereophotograph was compared with baseline. Each grader was masked to the temporal sequence of the photographs. Definition of change was based on focal or diffuse thinning of the neuroretinal rim, increased excavation, appearance, or enlargement of RNFL defects. Changes in rim color, presence of disc hemorrhage, or progressive parapapillary atrophy were not sufficient for characterization of progression. Discrepancies between the 2 graders were either resolved by consensus or by adjudication of a third experienced grader. Only photographs with adequate quality were included.
Statistical Analysis
Random coefficient models were used to evaluate the relationship between IOP and RNFL thickness measurements over time. We have previously used these models to investigate the rate of RNFL change in glaucoma using SLP with VCC.21 In brief, these models are a type of linear mixed model that involve both random intercepts and random slopes and take into account the clustered structure of the data, allowing the residuals associated with the longitudinal measures on the same unit of analysis to be correlated. Because of these properties, these models are ideally suitable for evaluating change over time. Further information on the statistical modeling principles can be found in the literature.22,23
An initial model was built to evaluate the relationship between IOP and GDx ECC RNFL thickness measurements over time, without considering other explanatory variables. For this model, GDx ECC RNFL thickness measurements were considered as the dependent variable, and IOP (variable IOP) was included as a time-varying predictor. Time (variable TIME) was included as a continuous predictor. The significance of the coefficient associated with the variable TIME indicated whether there was a significant trend in GDx measurements over time, that is, whether GDx measurements tended to decrease or increase significantly over time. The 2-way interaction between IOP and TIME was included in the model to evaluate whether there was a significant influence of IOP on the slope of RNFL loss over time. The following random components were added to the model: random patient-specific effects associated with both the intercept and slope (i.e., the effect of time) for each patient and random specific effects associated with both the intercept and slope for each eye nested within patient. The inclusion of random intercepts allows for the variation in baseline RNFL, whereas the random slopes allow for the variation in the rate of progressive RNFL loss among eyes and patients.
The general form of the model for an individual GDx measurement t (t represents visit during follow-up) on eye i nested within patient j (denoted by GDxtij) was as follows:
The parameters β0through β3represented the fixed effects associated with the intercept, time, IOP values and the 2-way interaction between time and IOP; ζ0j and ζ1j were random patient effects associated with the intercept and time slope, respectively; ζ0ij and ζ1ij were the random effects (intercept and slope, respectively) associated with eye nested within patient; and ∊tij represented the residual.
Subsequent models were built taking into account other possible predictors as fixed effects, including central corneal thickness (CCT), diagnosis at baseline (glaucoma vs suspect), progression as assessed by stereophotographs and visual fields, as well as their interactions with time. Progression as assessed by stereophotographs and SAP was included as a fixed-effect covariate with a value of 1 if the eye progressed by stereophotographs and/or SAP and a value of 0 if the eye did not show progression with any of these methods. The 2-way interaction between time and progression was included in the model to evaluate whether there was a significant difference in longitudinal GDx measurements over time between progressors and nonprogressors. The significance of the predictors was evaluated using Wald tests and deviance statistics to reach the most parsimonious final model. After the final model was built, estimates of rates of change for individual eyes were obtained by best linear unbiased prediction.
Statistical Analyses were performed using STATA v. 10.0 (StataCorp, College Station, TX) and SPSS v.16.0 (SPSS Inc., Chicago, IL). The α level (type I error) was set at 0.05.
Results
This study included 344 eyes of 204 patients with a mean ± standard deviation age at baseline of 62±13 years. One hundred nineteen patients were female (58%). One hundred twenty-nine patients were Caucasian (63%), 70 were African American (34%), and 5 were Asian (3%). Of the 344 eyes included in the study, 98 (28%) had a diagnosis of glaucoma and 246 (72%) were considered as glaucoma suspects at baseline. Median (first quartile, third quartile) mean deviation (MD) and pattern standard deviation of the visual field closest to the baseline imaging test date in glaucomatous eyes were −3.98 dB (−6.33, −2.14) and 3.78 dB (2.57, 7.18). Corresponding values for glaucoma suspect eyes were −0.75 dB (−1.56, −0.04) and 1.60 dB (1.39, 1.87). Table 1 shows average values of the RNFL thickness parameters in glaucoma eyes and eyes suspected of having the disease at baseline.
Table 1.
Baseline Scanning Laser Polarimetry with Enhanced Corneal Compensation Retinal Nerve Fiber Layer Measurements in Glaucoma and Glaucoma Suspect Eyes
| Glaucoma Suspect (n = 238) | Glaucoma (n = 106) | P | |
|---|---|---|---|
| TSNIT average | 48.8±5.8 | 44.3±7.5 | <0.001 |
| Superior average | 61.1±8.6 | 54.8±11.9 | <0.001 |
| Inferior average | 60.2±7.9 | 56.6±10.3 | <0.001 |
TSNIT = temporal–superior–nasal–inferior–temporal.
Average IOP was 17.2±4.9 mmHg for all eyes during all visits. However, there was a large variation among eyes in the levels of IOP during follow-up. Figure 1 shows a distribution of the average IOPs per eye during follow-up.
Figure 1.
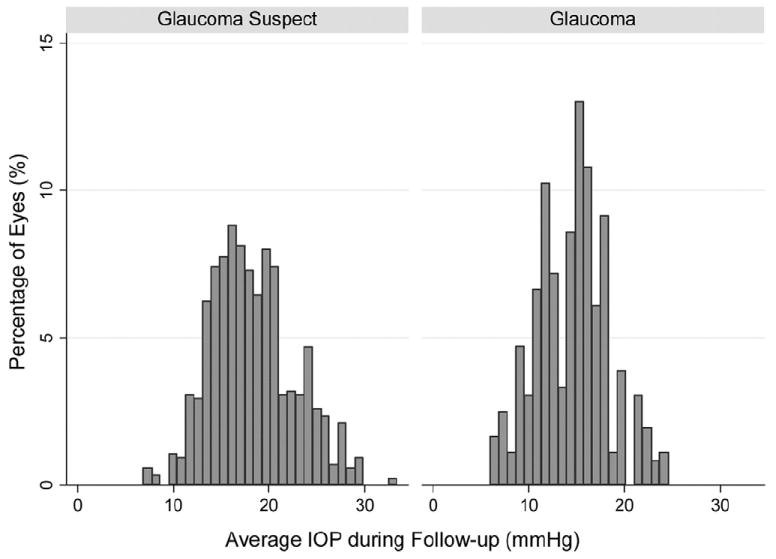
Distribution of average intraocular pressure (IOP) measurements during follow-up for all 344 eyes included in the study.
Table 2 shows results of the random coefficients model when applied to investigate the relationship between IOP and changes in the GDx ECC TSNIT average parameter over time for all eyes. The statistically significant coefficient associated with TIME indicates that GDx ECC measurements significantly decreased over time (P = 0.002). The overall rate of RNFL change for all 344 eyes was −0.25 μm per year, considering an average IOP during follow-up of 17 mmHg. There was no significant relationship between RNFL thickness measurement and IOP at baseline among all eyes included in the study (β2 = 0.06; P = 0.103). The significant coefficient associated with the interaction term between IOP and TIME (P = 0.001) indicates that higher values of IOP over time were associated with greater loss of the RNFL. For each 1-mmHg higher IOP there was an additional loss of 0.05 μm per year of RNFL.
Table 2.
Results of the Random Coefficients Model Investigating the Relationship between Intraocular Pressure (IOP) and Changes in the Scanning Laser Polarimetry with Enhanced Corneal Compensation Parameter Temporal–Superior–Nasal–Inferior–Temporal (TSNIT) Average Over Time
| Parameter | Coefficient | Estimate | 95% Confidence Interval | P |
|---|---|---|---|---|
| Model A | ||||
| Intercept | β0 | 47.3 | 46.4 to 48.1 | <0.001 |
| TIME | β1 | −0.25 | −0.40 to −0.09 | 0.002 |
| IOP | β2 | 0.06 | −0.01 to 0.13 | 0.103 |
| IOP × TIME | β3 | −0.05 | −0.08 to −0.02 | 0.001 |
| Model B | ||||
| Intercept | β0 | 48.1 | 47.2 to 49.0 | <0.001 |
| TIME | β1 | −0.22 | −0.40 to −0.04 | 0.019 |
| IOP | β2 | 0.02 | −0.06 to 0.09 | 0.648 |
| IOP × TIME | β3 | −0.04 | −0.07 to −0.01 | 0.016 |
| CCT | β4 | 5.12 | 3.16 to 7.08 | <0.001 |
| CCT × TIME | β5 | −0.40 | −0.83 to 0.02 | 0.064 |
| DIAGNOSIS | β6 | −3.53 | −4.74 to −2.31 | <0.001 |
| DIAGNOSIS × TIME | β7 | −0.07 | −0.39 to 0.26 | 0.686 |
CCT = central corneal thickness; DIAGNOSIS = diagnosis at baseline (glaucoma vs suspect).
IOP values were centered on the mean for all eyes (17 mmHg).
CCT values were centered on the mean for all eyes (550 μm).
A and B show models without and with adjustment for corneal thickness and diagnosis at baseline.
We also investigated the effects of IOP on RNFL loss taking into account CCT and diagnosis at baseline (glaucoma vs suspect; Table 2). Higher CCT values were associated with thicker RNFL measurements (P<0.001). Each 100-μm thicker cornea was associated with a 5.12-μm thicker RNFL. However, there was no significant relationship between CCT and rate of RNFL loss over time (P = 0.064). Eyes with a glaucoma diagnosis at baseline had, on average, a 3.53-μm thinner RNFL at baseline (P<0.001) compared with glaucoma suspect eyes. There was no significant relationship between diagnosis at baseline and rate of RNFL loss (P = 0.686) when adjusted for IOP.
Of the 246 glaucoma suspect eyes, 82 (33%) were classified as having ocular hypertension and normal-appearing optic discs on baseline stereophotographs; 164 (67%) were classified as having suspicious appearance of the optic disc at baseline. When adjusted for IOP and CCT, there was no significant difference in the rates of change of the TSNIT average parameter between these 2 groups (−0.29 vs −0.21 μm/year, respectively; P = 0.648). Also, there was no significant difference in the effects of IOP on the rate of RNFL change between these 2 groups (0.05 vs 0.06 μm/year of RNFL loss per 1 mmHg higher IOP, respectively; P = 0.743).
Twenty-nine eyes (8%) showed progression over time on visual fields and/or optic disc stereophotographs. Of the 29 progressing eyes, 13 (45%) progressed only by SAP GPA, 12 (41%) progressed only by optic disc stereophotographs, and 4 (14%) progressed by both methods. Table 3 shows the results of the random coefficients model for investigating the relationship between IOP and RNFL loss, taking into account CCT, diagnosis at baseline, and progression detected by optic disc stereophotos and/or SAP. Eyes that showed progression by photos and/or SAP had significantly thinner RNFL at baseline than eyes that did not show progression (β4 = −3.06; P<0.001). The average rate of RNFL loss in progressing eyes, considering an average IOP of 17 mmHg and average CCT of 550 μm was 0.95 μm per year. Because the model deals with interaction terms, this result was obtained by adding the coefficients β1 and β5. The rate of RNFL loss in progressing eyes (0.95 μm/year) was significantly larger than that of nonprogressing eyes (0.17 μm/year; P = 0.001), adjusting for IOP and CCT. Each 1-mmHg higher IOP was associated with an additional loss of RNFL of 0.13 μm per year. This result was obtained by adding the coefficients β3and β6. Figure 2 shows a scatterplot of the relationship between IOP and slopes of RNFL loss over time for the TSNIT average parameter for eyes that progressed by stereophotographs and/or SAP and eyes that did not.
Table 3.
Results of the Random Coefficients Model Investigating the Relationship between Intraocular Pressure (IOP) and Changes in the Scanning Laser Polarimetry with Enhanced Corneal Compensation Parameter Temporal–Superior–Nasal–Inferior–Temporal (TSNIT) Average Over Time, Taking into Account Progression Based on Optic Disc Stereophotographs and/or Visual Fields
| Parameter | Coefficient | Estimate | 95% Confidence Interval | P |
|---|---|---|---|---|
| Intercept | β0 | 48.3 | 47.4 to 49.1 | <0.001 |
| TIME | β1 | −0.17 | −0.32 to −0.01 | 0.035 |
| IOP | β2 | 0.04 | −0.03 to 0.11 | 0.318 |
| IOP × TIME | β3 | −0.04 | −0.07 to −0.01 | 0.022 |
| PROG | β4 | −3.06 | −4.58 to −1.54 | <0.001 |
| PROG × TIME | β5 | −0.78 | −1.25 to −0.31 | 0.001 |
| PROG × IOP × TIME | β6 | −0.09 | −0.17 to −0.01 | 0.020 |
| CCT | β7 | 4.58 | 2.69 to 6.46 | <0.001 |
| DIAGNOSIS | β8 | −3.25 | −4.36 to −2.15 | <0.001 |
CCT = central corneal thickness; DIAGNOSIS = diagnosis at baseline (glaucoma versus suspect); PROG = progression (1, yes; 0, no) based on optic disc stereophotographs and/or visual fields.
IOP values were centered on the mean for all eyes (17 mmHg).
CCT values were centered on the mean for all eyes (550 μm).
The model adjusts for CCT and diagnosis at baseline.
Figure 2.
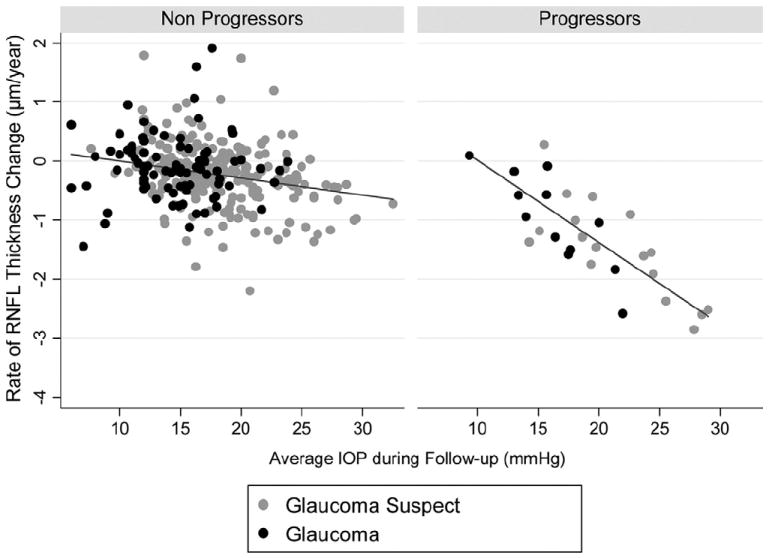
Scatterplot illustrating the relationship between rates of change in the scanning laser polarimetry with enhanced corneal compensation parameter temporal–superior–nasal–inferior–temporal (TSNIT) average and intraocular pressure (IOP). Rates of change are shown for eyes that progressed by visual fields and/or stereophotographs (progressors) as well as for eyes that did not (nonprogressors). RNFL = retinal nerve fiber layer.
Similar models were constructed for the parameters Inferior Average and Superior Average, as well as for the 16 sectors around the optic nerve. For Inferior Average, the rate of change was also significantly higher in progressing eyes compared with nonprogressing eyes (−1.30 vs −0.11μm/year, respectively; P = 0.001). In progressing eyes, each 1-mmHg higher IOP was associated with an additional loss of 0.21 μm per year of Inferior Average RNFL thickness (P<0.001). For Superior Average, the rate of change was also significantly higher in progressing eyes compared with nonprogressing eyes (−1.42 vs −0.55 μm/year, respectively; P = 0.004). In progressing eyes, each 1-mmHg higher IOP was associated with an additional loss of 0.09 μm per year of Superior Average RNFL thickness (P = 0.027).
Figure 3 shows a polar plot illustrating the rates of GDx ECC RNFL measurement change in progressing and nonprogressing eyes according to the sectors around the optic disc. As expected, rates of change were higher on inferior temporal and superior temporal sectors. Figures 4A and B (available online at http://aaojournal.org) show scatterplots of the relationship between IOP and slopes of RNFL change for the inferior temporal (S13) and superior temporal sectors (S4). For comparison, Figure 4C (available online at http://aaojournal.org) shows a scatterplot for the temporal (S16) sector, corresponding to the area of the papillomacular bundle.
Figure 3.
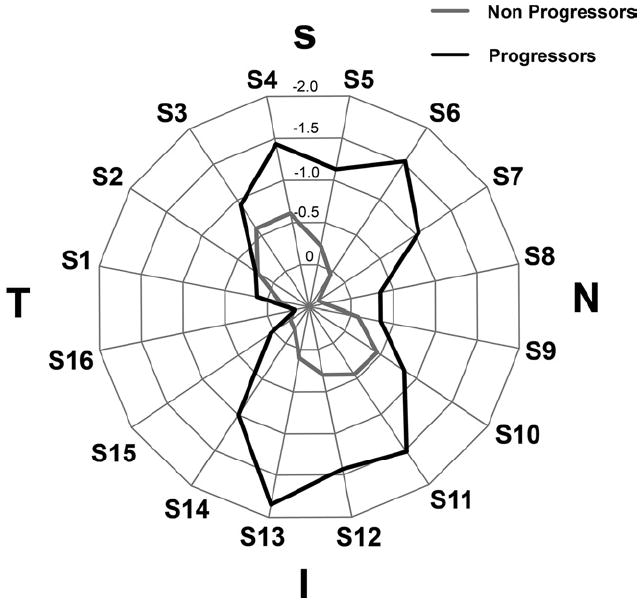
Radar plot illustrating the rates of change in scanning laser polarimetry with enhanced corneal compensation retinal nerve fiber layer measurements according to the sectors around the optic disc. Eyes that showed progression on visual fields and/or optic disc stereophotographs had greater loss of the RNFL in the inferior and superior sectors. I = inferior; N = nasal; S = superior; T = temporal.
Figure 4.
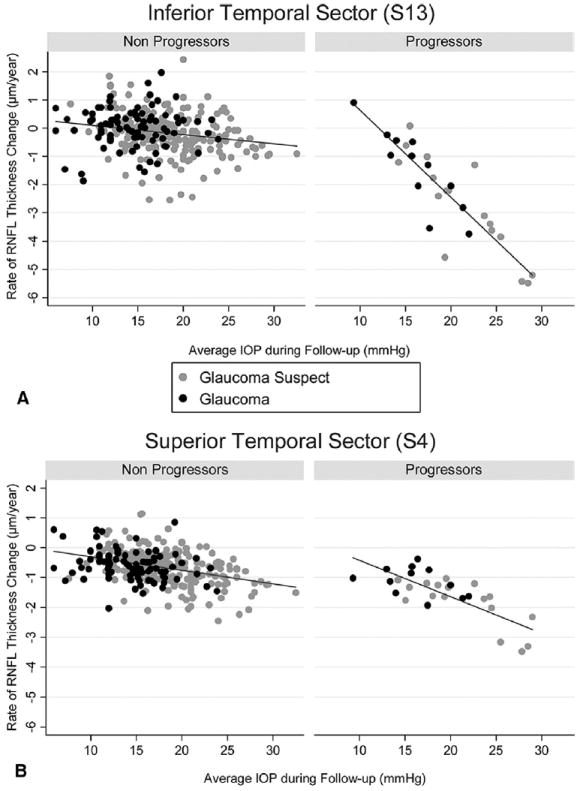
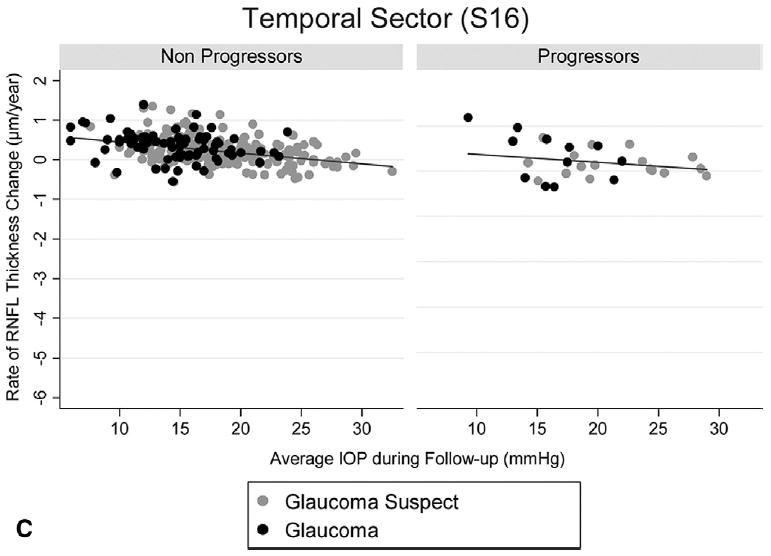
Scatterplots illustrating the relationship between intraocular pressure (IOP) and rates of change in the scanning laser polarimetry with enhanced corneal compensation for the inferior temporal (A, S13), superior temporal (B, S4), and temporal sectors (C, S16). Rates of change are shown for eyes that progressed by visual fields and/or stereophotographs (progressors) as well as for eyes that did not (nonprogressors). RNFL = retinal nerve fiber layer.
Figure 5 (available online at http://aaojournal.org) shows the GDx ECC retardation maps and parameter values during follow-up for an eye suspected of having glaucoma at baseline that showed progression on optic disc stereophotographs during follow-up with an average IOP of 27 mmHg.
Figure 5.
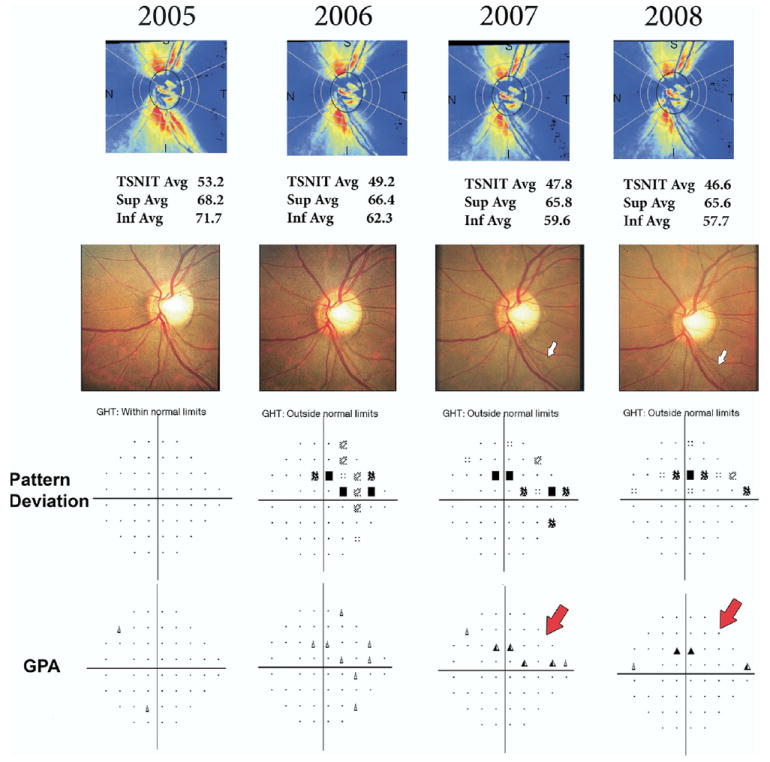
Scanning laser polarimetry with enhanced corneal compensation measurements in an eye that showed progression on optic disc stereophotographs with an average intraocular pressure (IOP) of 27 mmHg during follow-up. The optic disc photographs (middle row) show progressive development and enlargement of an inferior temporal localized retinal nerve fiber layer (RNFL) defect (blank arrows). The standard automated perimetry Guided Progression Analysis (SAP GPA; bottom row) shows corresponding progression on the superior nasal portion of the visual field (red arrows), however, as only 2 points showed repeatable change by this method, it was not sufficient to be flagged as likely progression. The scanning laser polarimetry with enhanced corneal compensation retardation maps (upper row) show progressive loss of the RNFL in the corresponding location. Avg = average; GHT = glaucoma hemifield test; GPA = Guided Progression Analysis; Inf = inferior; Sup = superior; TSNIT = temporal–superior–nasal–inferior–temporal.
Discussion
In this study, higher levels of IOP during follow-up were significantly related to progressive RNFL loss detected by the GDx ECC scanning laser polarimeter. To our knowledge, this is the first study to report such an association using structural evaluation by an imaging instrument. Our findings may have significant implications for the clinical use of GDx ECC in monitoring glaucoma eyes and eyes suspected of having the disease. Furthermore, they may help to elucidate the relationship between IOP and progression in these subjects.
The average rate of RNFL loss for the parameter TSNIT Average in all 344 eyes included in the study was 0.25 μm per year, considering a mean IOP during follow-up of 17 mmHg. However, there was a wide variation in the rate of change among eyes and the IOP levels were found to be a major determinant of the individual rate. Each 1-mmHg higher IOP was associated with a 0.05-μm greater loss of average RNFL over time. Therefore, an eye with an average IOP during follow-up of 30 mmHg would be expected to have a rate of loss in the TSNIT Average parameter of 0.90 μm per year. Considering average baseline levels of 47 μm for the TSNIT Average, this would represent an approximate loss of almost 2% per year. In contrast, an eye with average IOP levels of 12 mmHg during follow-up would have an estimated rate of change in the TSNIT Average parameter close to zero. These findings are in agreement with the results of recent major clinical trials in glaucoma that have evaluated the relationship between IOP and visual field loss.1-6 In the Early Manifest Glaucoma Trial, each 1-mmHg higher mean IOP during follow-up was associated with a 12% increase in the chance of developing progressive visual field loss over time.1 In the Advanced Glaucoma Intervention Study, patients with advanced glaucoma who were kept under control with mean IOP levels of 12.3 mmHg had a mean change in visual field scores close to zero.4
Most clinical trials in glaucoma have used visual fields as the sole end point to determine progression or development of the disease. However, although automated perimetry has been the standard method for detecting progression, it is known that many patients can have progressive structural damage that precedes detectable associated changes in the visual field.2,3 The OHTS and the similarly designed European Glaucoma Prevention Study2 used structural information to determine disease development over time in ocular hypertensive subjects. These 2 studies included stereophotographic assessment of the optic nerve as a structural end point. Considering the 2 studies together, approximately 50% of the eyes showed changes in the optic disc as the first sign of disease development. However, in both studies evaluation of the RNFL was not part of the end point assessment. In fact, color stereophotographs offer suboptimal visualization of the RNFL and identification of changes in this structure may be extremely difficult, especially for detection of diffuse change. By using the GDx ECC scanning laser polarimeter, we were able to objectively identify and quantify progressive RNFL loss and its relationship with IOP. The RNFL measurements obtained by the GDx ECC have been shown to be reproducible16 and able to discriminate glaucomatous from healthy eyes as well as to identify early signs of damage in eyes suspected of having the disease.14 Furthermore, there is evidence to support that RNFL changes may precede detectable changes in the optic disc9 and, therefore, objective assessment of the RNFL may provide a more sensitive method for monitoring development and progression of the disease.
Eyes that showed progression on optic disc stereophotographs and/or SAP had significantly higher rates of GDx ECC RNFL change than eyes that did not show progression in these 2 methods. Considering an average IOP during follow-up of 17 mmHg, the average rate of RNFL loss in progressing eyes was 0.95 μm per year (2% per year) compared with only 0.17 μm per year (0.3% per year) in nonprogressors (P = 0.001). These results support the ability of the GDx ECC to detect progressive RNFL loss in glaucoma and are in agreement with our previous results using another version of this technology.21 Furthermore, these estimates are higher than rates of progression found using topographic information of the optic nerve with confocal scanning laser ophthalmoscopy. A recent study by Poli et al24 reported a rate of change of confocal scanning laser ophthalmoscopy rim area of approximately 1% per year in ocular hypertensive eyes that progressed to glaucoma, which is lower than the average 2% per year rate of RNFL loss for progressors found in our study. This could support the hypothesis that longitudinal assessment of the RNFL is more sensitive than optic disc evaluation for detection of progression. However, these studies had different designs and included different populations; therefore, estimates of rates of change might not be directly comparable. No study has yet been performed comparing these methods for monitoring glaucoma progression in the same population.
In eyes that progressed by optic disc stereophotographs and/or visual fields, IOP also had a significant effect on the rate of RNFL loss. Each 1-mmHg higher IOP was associated with a 0.13 μm per year higher rate of RNFL loss for the parameter TSNIT average. For example, the rate of change in the TSNIT parameter for an eye that showed progression on conventional methods and had an average IOP level of 30 mmHg was 3.16 μm per year or approximately 7% per year. This indicates that an eye that is showing progression on visual fields or optic nerve and maintains IOP levels of 30 mmHg would be expected to reach end-stage disease in approximately 10 years, considering a residual RNFL thickness of 20 μm, which has been reported for end-stage glaucoma eyes.25 This seems to be in agreement with expected rates of progression to blindness in untreated eyes with glaucoma.26 In contrast, rates of change in eyes with low IOP levels were much lower (Fig 2). A few eyes that were classified as progressing by visual fields and/or stereophotographs during follow-up had apparently very low IOPs and low rates of RNFL change as measured by the GDx ECC (Fig 2). This could be related to false-positive classifications of the conventional methods. No gold standard exists for detection of progression with visual fields, and although the GPA has been a widely used method in clinical practice and also supported by one large clinical trial,27 it frequently disagrees with other methods for detecting change.28 Also, assessment of optic nerve progression by stereophotographs is a subjective task and, as such, may have suboptimal reproducibility. The apparent disagreement between GDx ECC and conventional methods in some cases could also be explained by temporal dissociation in the results of the tests. Owing to the imperfect relationship between structure and function in glaucoma, patients are often detected as progressing by 1 method and not another when followed for a relatively short period of time. This has also been the case for evaluation of progression using other imaging technologies in glaucoma.29,30
Eyes that were suspected of having glaucoma at baseline and that sustained higher levels of IOP during follow-up had higher rates of RNFL loss detected by the GDx ECC. This was true even for eyes that did not show detectable progression on optic disc photographs or visual fields. For example, a glaucoma suspect eye with no changes in SAP or photos during follow-up and with average IOP of 30 mmHg had an estimated rate of RNFL loss of 0.85 μm per year, which is well above the expected rate of RNFL change from aging. Using the GDx VCC, Da Pozzo et al31 imaged 384 eyes of 384 healthy subjects and estimated an age-related loss of 0.08 μm per year in the average RNFL thickness. These findings indicate that the GDx ECC is likely detecting progressive RNFL loss in eyes that are not detected as changing by conventional methods. This may have significant implications for the use of this instrument for early glaucoma detection. Because of the wide variability of optic nerve and RNFL measurements among individuals, it is frequently not possible to establish a diagnosis of glaucoma based on a single visit. Our results suggest that RNFL monitoring with the GDx ECC may be able to identify glaucoma suspect patients who develop disease over time. However, not all eyes suspected of having the disease and with high IOPs during follow-up showed high rates of RNFL loss over time. In fact, there was a wide variation in the rate of RNFL change in these eyes (Fig 2). This is probably related to other factors that govern the individual susceptibility of an eye for developing glaucoma damage. Further studies are necessary to ascertain the clinical relevance of progression detected by the GDx ECC and not by standard methods.
Rates of RNFL change detected by the GDx ECC were higher for the inferior and superior sectors around the optic nerve (Fig 3). This is in agreement with the expected pattern of RNFL and neuroretinal rim loss in glaucoma.32 The inferior temporal sector showed the highest rates of change in eyes that progressed by visual fields and/or optic disc photographs and had high IOP levels during follow-up (Fig 4). For example, the rate of RNFL loss in the inferior temporal sector for a progressing eye with an average IOP of 30 mmHg during follow-up was −6.84 μm per year, which corresponds to approximately 13% per year. In contrast, Figure 5 shows rates of changes for the temporal sector, corresponding with the area of the papillomacular bundle. As expected, rates of change in this sector were low and showed only a small relationship with the IOP levels. This is in agreement with the knowledge that the papillomacular bundle is preserved until very late stages in the course of glaucoma.33
We used a statistical model to relate IOP and RNFL changes in glaucoma. This model may provide a framework for investigation of risk factors that could be related to rates of change in glaucoma. It is known that only a proportion of glaucoma patients progress at a significantly fast rate to result in functional damage that can affect quality of vision during their lifetime. Identification of these patients, as well as of the risk factors associated with faster rates of change, may help to improve the allocation of resources, such as therapy and number of follow-up visits. Patients with more risk factors and/or higher rates of change may benefit from more aggressive treatment and closer follow-up. Our results also suggest that the GDx ECC could be used as a tool to monitor structural deterioration in glaucoma patients and as a potential end point for detecting progression in clinical trials of glaucoma.
Our study has limitations. The follow-up time with the GDx ECC was relatively short. However, this technology was introduced in 2004 and longer follow-up times were therefore not possible. Despite this limitation, our results were able to demonstrate a clear relationship between IOP, progression by conventional methods, and rates of RNFL change detected by the GDx ECC, which were in agreement with a previous study with longer follow-up time that used the GDx VCC.21 Rates of change found in the current study were higher than those reported for the GDx VCC, suggesting that the ECC algorithm may provide an additional benefit in longitudinal assessment of the RNFL with SLP. As more longitudinal data accumulate, a refinement of current models should be possible by incorporating additional data from follow-up of these subjects.
One fundamental requirement for any instrument to have acceptable use in clinical practice is to provide reproducible measurements. Good reproducibility is required both for reliable use of the instrument for diagnosis as well as for assessment of progression of disease. Although the design of our study did not allow the evaluation of measurement repeatability with the GDx ECC, a recent report by Mai et al16 found intraclass correlation coefficients >0.90 for most parameters, indicating very good repeatability. In a previous study, we also reported RNFL measurements obtained with the GDx VCC to be highly reproducible in a long-term test–retest situation, supporting the use of this technology for longitudinal assessment of the RNFL.34
In conclusion, higher levels of IOP during follow-up were significantly related to higher rates of progressive RNFL loss detected by the GDx ECC scanning laser polarimeter. Patients who progressed by standard methods such as optic disc photographs or visual fields showed the highest rates of RNFL change. These findings suggest that the GDx ECC may be helpful in monitoring progression and estimating rates of change in patients with glaucoma or suspected of having the disease.
Acknowledgments
Supported in part by the National Eye Institute grants EY11008 (LMZ) and EY08208 (PAS). Participant retention incentive grants in the form of glaucoma medication at no cost: Alcon Laboratories Inc., Allergan, Pfizer Inc., and SANTEN Inc.
Footnotes
Financial Disclosure(s): FAM (Carl Zeiss Meditec: S, L; Heidelberg Engineering: L); LMA: none; LMZ (Carl Zeiss Meditec: S; Heidelberg Engineering: S, L); PAS (Carl Zeiss Meditec: S); RNW (Carl Zeiss Meditec: C, S; Heidelberg Engineering: C, S).
References
- 1.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366–75. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 4.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 6.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 7.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 8.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA, Katz J, Derick RJ, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99:19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros FA, Bowd C, Zangwill LM, et al. Detection of glaucoma using scanning laser polarimetry with enhanced corneal compensation. Invest Ophthalmol Vis Sci. 2007;48:3146–53. doi: 10.1167/iovs.06-1139. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb RN, Shakiba S, Zangwill L. Scanning laser polarimetry to measure the nerve fiber layer of normal and glaucomatous eyes. Am J Ophthalmol. 1995;119:627–36. doi: 10.1016/s0002-9394(14)70221-1. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros FA, Zangwill LM, Bowd C, et al. Comparison of scanning laser polarimetry using variable corneal compensation and retinal nerve fiber layer photography for detection of glaucoma. Arch Ophthalmol. 2004;122:698–704. doi: 10.1001/archopht.122.5.698. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros FA, Zangwill LM, Bowd C, et al. Use of progressive glaucomatous optic disk change as the reference standard for evaluation of diagnostic tests in glaucoma. Am J Ophthalmol. 2005;139:1010–8. doi: 10.1016/j.ajo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros FA, Vizzeri G, Zangwill LM, et al. Comparison of retinal nerve fiber layer and optic disc imaging for diagnosing glaucoma in patients suspected of having the disease. Ophthalmology. 2008;115:1340–6. doi: 10.1016/j.ophtha.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reus NJ, Zhou Q, Lemij HG. Enhanced imaging algorithm for scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2006;47:3870–7. doi: 10.1167/iovs.05-0067. [DOI] [PubMed] [Google Scholar]
- 16.Mai TA, Reus NJ, Lemij HG. Retinal nerve fiber layer measurement repeatability in scanning laser polarimetry with enhanced corneal compensation. J Glaucoma. 2008;17:269–74. doi: 10.1097/IJG.0b013e31815c3a6b. [DOI] [PubMed] [Google Scholar]
- 17.Mai TA, Reus NJ, Lemij HG. Diagnostic accuracy of scanning laser polarimetry with enhanced versus variable corneal compensation. Ophthalmology. 2007;114:1988–93. doi: 10.1016/j.ophtha.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Weinreb RN, Bowd C, Greenfield DS, Zangwill LM. Measurement of the magnitude and axis of corneal polarization with scanning laser polarimetry. Arch Ophthalmol. 2002;120:901–6. doi: 10.1001/archopht.120.7.901. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Weinreb RN. Individualized compensation of anterior segment birefringence during scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2002;43:2221–8. [PubMed] [Google Scholar]
- 20.Bagga H, Greenfield DS, Feuer WJ. Quantitative assessment of atypical birefringence images using scanning laser polarimetry with variable corneal compensation. Am J Ophthalmol. 2005;139:437–46. doi: 10.1016/j.ajo.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50:1675–81. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–80. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Feldman HA. Families of lines: random effects in linear regression analysis. J Appl Physiol. 1988;64:1721–32. doi: 10.1152/jappl.1988.64.4.1721. [DOI] [PubMed] [Google Scholar]
- 24.Poli A, Strouthidis NG, Ho TA, Garway-Heath DF. Analysis of HRT Images: Comparison of Reference Planes. Invest Ophthalmol Vis Sci. 2008;49:3970–5. doi: 10.1167/iovs.08-1764. [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal EZ, Horani A, Sasikumar R, et al. Correlating structure with function in end-stage glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:218–23. doi: 10.3928/15428877-20060501-06. [DOI] [PubMed] [Google Scholar]
- 26.Wilson MR, Kosoko O, Cowan CL, Jr, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am J Ophthalmol. 2002;134:399–405. doi: 10.1016/s0002-9394(02)01585-4. [DOI] [PubMed] [Google Scholar]
- 27.Heijl A, Leske MC, Bengtsson B, Hussein M. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmol Scand. 2003;81:286–93. doi: 10.1034/j.1600-0420.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 28.Vesti E, Johnson CA, Chauhan BC. Comparison of different methods for detecting glaucomatous visual field progression. Invest Ophthalmol Vis Sci. 2003;44:3873–9. doi: 10.1167/iovs.02-1171. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan BC, McCormick TA, Nicolela MT, LeBlanc RP. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol. 2001;119:1492–9. doi: 10.1001/archopht.119.10.1492. [DOI] [PubMed] [Google Scholar]
- 30.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47:2904–10. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]
- 31.Da Pozzo S, Iacono P, Marchesan R, et al. The effect of ageing on retinal nerve fibre layer thickness: an evaluation by scanning laser polarimetry with variable corneal compensation. Acta Ophthalmol Scand. 2006;84:375–9. doi: 10.1111/j.1600-0420.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 32.Jonas JB, Fernandez MC, Sturmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993;100:63–8. doi: 10.1016/s0161-6420(13)31694-7. [DOI] [PubMed] [Google Scholar]
- 33.Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43:293–320. doi: 10.1016/s0039-6257(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 34.Medeiros FA, Doshi R, Zangwill LM, et al. Long-term variability of GDx VCC retinal nerve fiber layer thickness measurements. J Glaucoma. 2007;16:277–81. doi: 10.1097/IJG.0b013e3180391a3c. [DOI] [PubMed] [Google Scholar]


