Abstract
Indolent Non Hodgkin's lymphoma (NHL) comprises a group of incurable, generally slow growing lymphomas highly responsive to initial therapy with a relapsing and progressive course. Rituximab, an anti CD-20 antibody, has had a large impact on treatment of indolent NHL. Its effectiveness as a single agent and in conjunction with known chemotherapy regimens has made it a standard of care in the treatment of NHL. Analysis of data obtained from NHL clinical trials as well as data from the National Cancer Institute indicates that the overall survival of indolent NHL has improved since the discovery of rituximab. Given its effectiveness and tolerability, it is currently being investigated as a maintenance agent with encouraging results. This review summarizes several landmark trials utilizing rituximab as a single agent and in combination with chemotherapy for treatment of NHL. In addition, a review of the studied rituximab maintenance dosing schedules and its impact on NHL will also be presented. Overall, rituximab has changed the landscape for treatment of indolent NHL however additional research is necessary to identify the optimal dosing schedule as well as patients most likely to respond to prolonged rituximab therapy.
Introduction
Indolent Non-Hodgkin's lymphoma (NHL) represents a group of incurable slow growing lymphomas that are highly responsive to initial therapy but relapse with less responsive disease.1-4 The landscape for treatment of indolent NHL has dramatically changed with the introduction of rituximab (Rituxan, Genetech, San Francisco, CA). Its greatest impact has been in follicular lymphoma (FL), which constitutes approximately 70% of indolent lymphomas and up to 25% of all cases of NHL.5,6 Although there are no defined first line therapies for indolent NHL, rituximab has become a standard component in treatment of FL.7 While indolent lymphoma remains an incurable disease, recent data from the Surveillance Epidemiology and End Results (SEER) database and retrospective analysis of clinical trials in indolent NHL suggest an improved overall survival (OS) with the use of rituximab (Figure 1).8-10 It is hoped that overall survival can be further improved with the use of extended rituximab dosing schedules.
Figure 1.
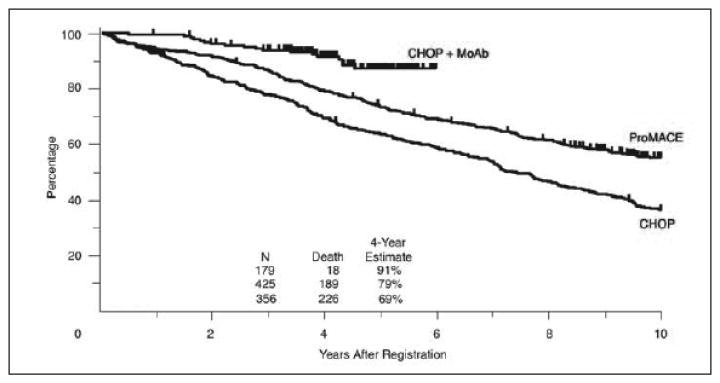
Overall survival according to chemotherapy regimen: CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; MoAb, monoclonal antibody; ProMACE, prednisone, methotrexate, doxorubicin, cyclophosphamide and etoposide. Reprinted with permission.8
Follicular Lymphoma – Rituximab Monotherapy
The initial trials investigating rituximab for treatment of FL was as a single agent. In a pivotal trial conducted by McLaughlin et al.11, 166 patients with heavily treated relapsed low grade lymphoma including 136 with follicular lymphoma were given rituximab 375mg/m2 weekly for four doses. Of the patients enrolled, 48% responded with a median time to progression (TTP) of 13.0 months among responders.11 Toxicity was mild and greatest during the first infusion without development of treatment related cytopenias. Compared to single agent cytotoxic therapy, single agent rituximab was better tolerated and had similar efficacy.11
Rituximab monotherapy has significant clinical activity in previously untreated patients as well. A trial of fifty patients with newly diagnosed stage II/III/IV FL received rituximab 375mg/m2 weekly for four doses. Patients were required to have a low tumor burden defined as an absence of bulky lymphadenopathy, B symptoms, significant splenomegaly and normal serum LDH.12 The observed response rate was 73% four weeks following completion of therapy.12 Polymerase chain reaction (PCR) analysis of BCL-2 rearrangement was performed pre and post therapy as well. Long term follow-up revealed a median PFS of 37 months for patients who became BCL-2 negative following therapy as compared to 12 months for those who remained BCL-2 positive13 suggesting that patients with a molecular response to rituximab have a more indolent course.
Rituximab monotherapy has also been evaluated in combination with other forms of immunotherapy. Using patient-specific B-cell immunoglobulin idiotypes, a therapeutic vaccine can be created which in theory can produce a durable clinical response. This was tested in a randomized clinical trial of 364 patients with follicular lymphoma, the majority of which were treatment naïve. All patients were treated with rituximab 375mg/m2 weekly for four weeks. Those with an objective response were then randomized to receive a vaccine or placebo. Treatment naïve patients who were randomized to receive a vaccine had a TTP of 11.9 months as compared to 17.2 months in the placebo arm (P = 0.258).14 Patients with relapsed disease had a TTP of 6.0 months in the vaccine arm as compared to 11.2 months for the placebo arm (P = 0.004).14 The authors concluded that the difference in TTP among patients with relapsed disease is related to an imbalance in FLIPI risk scores among both treatment arms. While a negative vaccine effect cannot be excluded, when adjusting for FLIPI risk score, there was no significant difference in TTP for those with relapsed disease. Although addition of a patient-specific vaccine did not improve TTP, results of this trial highlight the effectiveness of single agent rituximab in follicular lymphoma and support the results seen in the aforementioned trials.
Follicular Lymphoma – Single Agent Rituximab Maintenance
The concept of maintenance chemotherapy is a recurrent theme in oncology that dates back decades from experience in the curability of acute lymphoblastic leukemia in children. Several studies in indolent lymphoma have attempted to improve OS and PFS in NHL with the use of interferon, chlorambucil and multi-agent chemotherapy with mixed results and a high incidence of adverse effects limiting patient adherence.15-18 To date, rituximab is the first non-chemotherapy drug that is highly effective without treatment associated cytopenias or severe cumulative toxicity.19,20 The advent of a well tolerated, effective drug with a favorable pharmacokinetic profile has led to a resurgence in studying maintenance therapy (Table 1).
Table 1.
Clinical trials of maintenance rituximab
| Number of Patients | |||||
|---|---|---|---|---|---|
| First author, year | Total | Follicular Lymphoma | Induction Regimen | Rituximab Maintenance Schedule | Results |
| Hainsworth, 2002 21 | 62 | 38 | R Qwk × 4 | Weekly × 4 Q6mo | ORR: 76% PFS: 34 mo |
| Ghielmini, 2004 23 | 202 | 202 | R Qwk × 4 | Q2mo × 4 vs. observation | ORR: 75 vs. 77% EFS: 23.2 vs. 11.8 mo DR: 36 vs. 16 mo |
| Van Oers, 2006 43 | 474 | 474 | R-CHOP vs. CHOP × 6 | Q3mo × 24 mo vs. observation | CR: 29 vs. 16%* PFS: 3.7 vs. 1.3 yrs* 5yr OS: 74 vs. 64%*,& |
| Hochster, 2009 45 | 164 | 109 | CVP × 6-8 | Weekly × 4 Q6mo | PFS: 4.3 vs. 1.3 yrs 3 yr OS: 91 vs. 86%& |
ORR, overall response rate; PFS, progression free survival; OS, overall survival; TTF, time to treatment failure; DR, duration of response; EFS, event free survival;
Long term data;
Not significant.
The first trial of rituximab maintenance by Hainsworth et al.21 was a phase II trial to assess response duration with an extended rituximab schedule. Sixty-two patients (38 FL, 24 CLL) with stage II/III/IV disease were enrolled. The authors also accepted patients with stage I/II disease who had relapsed following prior radiation therapy; however, patients with prior chemotherapy were excluded from this study. Enrolled patients received rituximab 375mg/m2 weekly for a total of four weeks. Disease response was assessed two weeks following induction and patients with an objective response or stable disease received rituximab 375mg/m2 weekly for four weeks every 6 months for 24 months.
The addition of a rituximab maintenance schedule led to an improved overall response rate (ORR) such that with continued therapy, 16 of 27 patients (59%) with stable disease achieved an objective response.21 Median PFS following the addition of prolonged rituximab dosing was 34 months. It is important to note that this analysis contains patients with CLL; however ORR and PFS are not significantly different across NHL subtypes.21 Prolonged rituximab therapy was well tolerated with few grade 3 or 4 adverse events.
Although Hainsworth demonstrated an improved ORR and progression free survival (PFS) with an extended rituximab schedule, it is unknown whether maintenance therapy is more effective than re-treatment at the time of progression. This was addressed initially in a randomized phase II study where 90 patients (62 with FL) were given rituximab 375mg/m2 weekly for a total of four doses. Those with an objective response were randomized to maintenance rituximab as in the previously mentioned trial by Hainsworth21 or rituximab re-treatment at time of progression. For patients with FL, PFS with maintenance rituximab was 31 months versus 13 months with rituximab re-treatment (Figure 2).22 However, duration of response was similar between both study arms (31vs. 35 months for maintenance and re-treatment, respectively) (Figure 3).22 Moreover, 3-year survival was not significantly improved in the maintenance arm as compared to the re-treatment arm (72% vs. 68% respectively).22 The recently completed phase III rituximab extended schedule or re-treatment trial (RESORT) study is hoped to definitively address the benefit of maintenance rituximab as compared to re-treatment at the time of progression.
Figure 2.
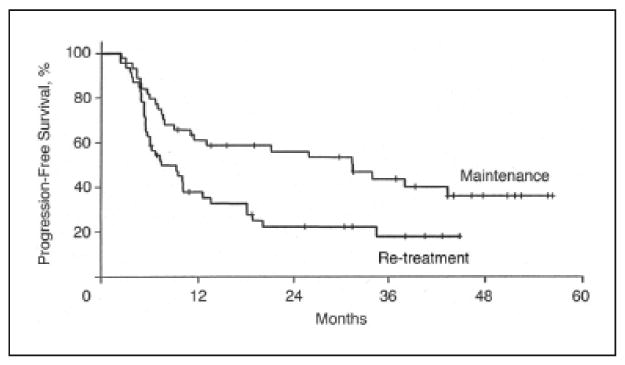
Progression-free survival in those randomized to maintenance rituximab versus retreatment at time of progression. Reprinted with permission.22
Figure 3.
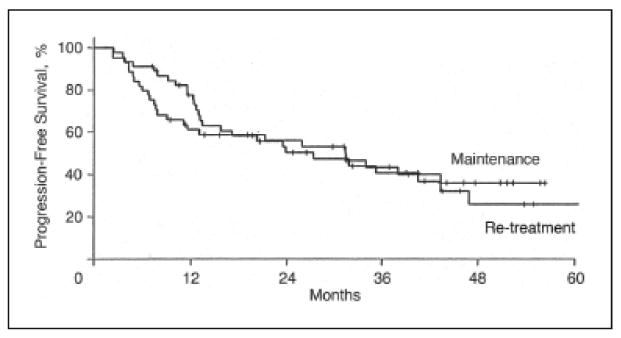
Duration of response to rituximab treatment according to maintenance or re-treatment at time of progression. Reprinted with permission.22
A similar trial of prolonged rituximab therapy by Ghielmini et al.23 was performed using a different treatment schedule based upon pharmacokinetic data. The goal of this schedule was to maintain a mean rituximab drug level greater than 25.4 mcg/ml, a level which in prior studies was observed in responding patients.24 In this study, 202 patients with stage I/II/III FL who have not received prior antibody therapy were enrolled to receive rituximab 375mg/m2 weekly for four weeks. Eight weeks following induction therapy, those with an objective response or stable disease were randomized to receive rituximab 375mg/m2 every two months for four doses or observation.
Following induction therapy, response rate (RR) was 52% with no significant difference between the study arms. 23 However, the response rate of decline following induction therapy was significantly less pronounced in those randomized to rituximab maintenance 12 months following randomization.23 Furthermore, patients who achieved CR following induction therapy remained in CR for a greater period of time if randomized to rituximab maintenance (36 vs. 16 mo).23 Event free survival (EFS) also improved with the addition of a prolonged rituximab dosing schedule. This improvement was greatest in chemotherapy naïve patients (36 vs. 19 mo, P = 0.009) and responders (36 vs. 16 mo, P = 0.004) as compared to those randomized to observation.23
As in their initial report, the Ghielmini extended rituximab dosing schedule did not result in improved event free survival (EFS) in previously treated patients. Subsequent long term follow-up data in abstract form revealed a 5 year EFS of 26% for those randomized to prolonged rituximab dosing as opposed to 10% in the observation arm independent of prior therapy.25 Whereas initial results indicated that chemotherapy naïve patients had an improved EFS as compared to pretreated patients, long term follow-up does not support this.25 It is unknown what characteristics predispose a patient to a prolonged EFS thus highlighting the need for continued research in extended rituximab dosing. This is currently underway in a randomized study of rituximab maintenance for 2 years versus 5 years. Thus far, data presented at the 2009 American Society of Clinical Oncology (ASCO) annual meeting did not reveal any safety concerns associated with rituximab maintenance beyond 2 years.26
Given the number of available treatment options for follicular lymphoma, it is important to identify patients in whom single agent therapy should be considered. In patients with relapsed or refractory follicular lymphoma, single agent rituximab therapy should be considered when other options cannot be tolerated. Unfortunately, the optimal dosing schedule for single agent therapy remains unknown. Clinical studies are underway to ascertain the optimal dosing schedule as the relapsing nature of indolent lymphoma makes rituximab resistance a valid concern. Although rituximab resistance has been demonstrated in in vitro models, rituximab retreatment has been evaluated in clinical trials without any significant loss of treatment effect.19,27
Follicular Lymphoma – Rituximab Combined with Chemotherapy
Following the effectiveness of rituximab as a single agent treatment, rituximab was added to frontline combination chemotherapy in an attempt to improve long term outcome. Encouraging results from a phase II study of rituximab combined with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP)28 prompted five pivotal trials to examine the benefit of adding rituximab to chemotherapy regimens commonly used to treat FL (Table 2). One such trial was a phase III trial that compared R-CHOP to CHOP alone. In this multicenter prospective randomized trial, patients with symptomatic, previously untreated, advanced stage FL, grades I and II were enrolled. Participants were randomized to receive either CHOP or R-CHOP for a total of 6 to 8 cycles. Overall response rates were 96% for R-CHOP versus 90% for CHOP (P =0.011).29 R-CHOP was associated with a significantly improved time to treatment failure (TTF) and a longer duration of response.29
Table 2.
Clinical trials of rituximab combined with chemotherapy versus chemotherapy alone
| Number of Patients | ||||
|---|---|---|---|---|
| First author, year | Total | Follicular Lymphoma | Treatment Arms | Results |
| Forstpointer, 2004 36 | 147 | 65 | R-FCM/FCM | ORR: 94 vs. 70% PFS: NR vs. 21 mo OS: NR for both arms |
| Marcus, 2005 6 | 321 | 321 | R-CVP/CVP | ORR: 81 vs. 57% TTF: 27 vs. 7 mo DR: 34 vs. 14 mo |
| Hiddemann, 2005 29 | 428 | 428 | R-CHOP/CHOP | ORR: 96 vs. 90% |
| Czuczman, 2005 35 | 40 | 31 | RF | ORR: 90% OS: NR TTP: NR |
| Herold, 2007 33 | 360 | 360 | R-MCP/MCP | ORR: 92 vs. 75% PFS: NR vs. 28.8 mo EFS: NR vs. 26 mo 4yr OS: 87 vs. 74% |
R, rituximab; FCM, fludarabine, cyclophosphamide, mitoxantrone; CVP, cyclophosphamide, vincristine, prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; F, fludarabine; MCP, mitoxantrone, chlorambucil, prednisolone; ORR, overall response rate; PFS, progression free survival; OS, overall survival; TTF, time to treatment failure; DR, duration of response; EFS, event free survival; NR: not reached.
Median TTF and response duration were achieved in the CHOP study arm but not in the R-CHOP arm. Therefore, neither TTF nor response duration could be accurately established for the R-CHOP arm in the predetermined follow up period of this study. Treatment related adverse effects were similar among both arms with the exception of increased neutropenia in those receiving R-CHOP. However, this did not lead to an increase in neutropenic infections.29 Infusion related adverse effects were mild and subsided by the second infusion.
Efforts at obtaining accurate time to progression and duration of response following R-CHOP have led to extended follow up periods. One such follow up of 9 years was conducted following completion of R-CHOP for FL as part of a phase II trial of R-CHOP for indolent NHL. Analysis of the 38 patients with advanced stage FL who received six cycles of R-CHOP revealed a median TTP of 82.3 months with a median duration of response of 83.5 months.30
In addition to combining rituximab to CHOP chemotherapy, rituximab was also added to another widely used chemotherapy regimen consisting of cyclophosphamide, vincristine and prednisone (CVP). In this multicenter randomized phase III trial, patients with symptomatic, previously untreated, advanced stage FL grades I to III were randomized to receive R-CVP or CVP (dose of cyclophosphamide only 750mg/m2) for a maximum of 8 cycles. The response rate for R-CVP was 81% as compared to 57% in the CVP arm (P <0.0001) and treatment with R-CVP significantly improved TTF by 20 months (27 mo vs. 7 mo in R-CVP and CVP arms respectively, P < 0.0001).6 Response duration was enhanced with the addition of rituximab to 35 months versus 14 months in the CVP only group.6 The addition of rituximab was well tolerated with a small number of grade 3 or 4 rituximab related reactions.
In vitro studies of commonly used chemotherapy agents revealed synergism with the addition of rituximab to either prednisone or mitoxantrone.31,32 This led to the evaluation of mitoxantrone, chlorambucil and prednisone with rituximab (R-MCP). In a study by Herold et al.33, 358 previously untreated, symptomatic patients with indolent NHL (201 with FL) were randomized to R-MCP or MCP for a maximum of 8 cycles. At the completion of therapy, overall response rate (ORR) for those randomized to R-MCP was 92% as compared to 75% in the MCP arm (P = 0.0009).33 Further analysis revealed that the number of those in CR were twice as great in the R-MCP arm as compared to MCP (P = 0.0004).33 PFS was not reached in the R-MCP arm as compared to 28.8 mo in those randomized to MCP. Although median OS was not reached in either study arm, 4 year survival rates favored R-MCP (87% vs. 74% for R-MCP vs. MCP respectively, P = 0.0096).33 Adverse events were similar among both study arms with the exception of an increase in grade 3 or 4 leukopenia observed in the R-MCP study arm, however this did not lead to an increase in infections.33
As there is no consensus in regards to optimal first line therapy for FL, several other regimens have been utilized to a lesser extent in the treatment of FL.7 One such regimen involves the use of fludarabine and rituximab (FR). Based upon in vitro studies suggesting synergism of this combination34 and separate mechanisms of action, FR combination therapy was studied in indolent NHL. Forty patients, 31 with histologically confirmed FL, received FR for a total of 6 cycles.
Previously treated patients were enrolled and comprised 33% of the study population. Overall response rate (ORR) following FR was 90% with no significant difference in response according to prior treatment.35 Despite a median follow-up period of 44 months, response duration, overall survival and time to progression could not be assessed. Grade 3 or 4 neutropenia was common among patients however reversible with G-CSF support. Typical rituximab associated infusion reactions were observed with initial doses as described in previous trials of rituximab.
Treatment of previously treated FL patients can be challenging as there are cumulative dose limitations and adverse effects related to chemotherapy. For patients previously treated with CHOP or CHOP-like regimens, fludarabine based regimens can be used. The addition of rituximab to a fludarabine based regimen was studied by Forstpointner et al.36 in which 147 previously treated patients (72 with FL) were randomized to receive 4 cycles of fludarabine, cyclophosphamide and mitoxantrone (FCM) with or without rituximab (R-FCM). ORR in FL patients randomized to R-FCM was 94% and consisted of 40% CR, 54% PR.36 Those randomized to FCM experienced an ORR of 70% with 23% CR and 47% PR (P = 0.011).36 R-FCM was superior to FCM irrespective of the number of prior therapies.36 Median PFS was not reached for those randomized to R-FCM as opposed to 21 months in the FCM study arm.36 OS was not reached for both arms during the study observation period; however 2 year estimated median OS was 90% for those randomized to R-FCM and 70% for those in the FCM arm (P = 0.0943).36 Perhaps with further observation one would expect this difference to become significant given the significant difference in PFS.
The addition of rituximab to chemotherapy has enhanced response rates and progression free survival in all of the aforementioned trials. Efforts at long term survival analysis have been attempted and although an improved survival is suggested, it is not clearly definitive. Furthermore, sample sizes are small and long term follow up data does not apply for all chemotherapy regimens used in indolent NHL. Data from a recent meta-analysis of immunochemotherapy evaluating 1943 patients revealed a pooled hazard ratio for death of 0.65 (95% CI = 0.54 – 0.78) in favor of immunochemotherapy.37 When stratified for the 1480 patients with FL, the pooled hazard ratio for mortality was 0.63 (95% CI = 0.51 – 0.79) in favor of immunochemotherapy.37 Based upon this hazard ratio and an assumed 2 year OS of 90%, the number needed to treat with immunochemotherapy to prevent one death in 2 years is 28.37
Rituximab has greatly changed the manner in which indolent NHL is treated. Its application as a single agent has led to an improved PFS with minimal toxicity and has emerged as first line therapy for those unable to tolerate aggressive measures. The addition of rituximab to chemotherapy has had a clear impact on response rate, PFS and response duration with a suggestion towards improved OS irrespective of the chemotherapy regimen to which it is coupled. The improvement in RR, PFS and duration of response in patients treated with immunochemotherapy makes rituximab the standard of care for FL treatment.
Other Indolent Lymphomas
Lymphoplasmacytic lymphoma (LPL) [Waldenstrom macroglobulinemia (WM)] is an uncommon indolent NHL which as a result is underrepresented in the literature. Use of rituximab in this disease is particularly important as it does not promote cytopenias associated with LPL. This was evaluated by the German Low-Grade Lymphoma Study Group (GLSG) in a phase III trial of 70 previously untreated patients with advanced LPL randomized to R-CHOP vs. CHOP. The addition of rituximab to CHOP resulted in an ORR of 94 vs. 67% and a TTF of 63 vs. 22 months.38 Treatment was well tolerated without any difference in side effects among study treatment arms.
The combination of rituximab with fludarabine was also evaluated in a recent phase II trial for treatment of LPL.39 Forty-three patients with FR naïve LPL who had received at most two prior therapies received 8 infusions of rituximab along with 6 cycles of fludarabine. The combination of FR was effective in reducing IgM levels as well as bone marrow involvement with an ORR of 95.3%.39 Response rates were not significantly different for previously treated versus previously untreated patients. However, TTP was significantly greater in treatment naïve patients, 77.6 months, compared to previously treated patients, 51.2 months.39 Neutropenia was common necessitating fludarabine dose reduction. IgM flare, a phenomenon by which IgM levels increase following initial dosing of rituximab, was seen in this study although it was uncommon. Data from similar studies of FR in LPL indicated that concurrent immunochemotherapy rather than sequential dosing of FR decreases the potential for rituximab-associated IgM flare.40
Similarly, the uncommon nature of marginal zone lymphoma (MZL) has limited the number of dedicated studies on the optimal therapeutic approach. Historically, this subtype of indolent NHL has been studied in conjunction with other more prevalent indolent lymphomas. As a result, treatment of MZL is approached in a similar fashion to treatment of FL with similar results for rituximab monotherapy as well as rituximab based therapies.41,42
Maintenance Rituximab Therapy Following Chemotherapy
Prolonged rituximab dosing has also been studied in relapsed FL by Van Oers et al.43 In this study, 465 previously treated patients with advanced stage FL were randomized to R-CHOP versus CHOP for a total of 6 cycles. Those with an objective response were randomized to rituximab 375mg/m2 every three months for a total of 24 months or observation. Data in abstract form from long term follow-up indicates that patients randomized to prolonged rituximab therapy had a PFS of 3.7 years as compared to 1.3 years in the observation arm.44 This improvement in PFS was maintained regardless of whether patients received CHOP or R-CHOP during induction.43,44 Three year OS was also improved by the addition of prolonged rituximab therapy as initially reported (85.1% vs. 77.1%).43 However, data from long term follow-up revealed a trend towards improved OS with a 5-year OS of 74% for prolonged rituximab dosing versus 64% for observation (P = 0.07).44 One possible explanation for a nonsignificant increase in OS despite a significant increase in PFS is that several patients from both study arms received rituximab monotherapy following disease progression.44 It should be noted this rituximab-naïve patient population is different from most patients in the United States, and it is inappropriate to extrapolate these results to patients relapsing after rituximab containing chemotherapy regimens.
The role of maintenance rituximab following initial chemotherapy for treatment of naïve patients is unknown. A recent phase III study by Hochester et al.45 evaluated the impact of maintenance rituximab in the upfront setting following non-rituximab containing chemotherapy. In this study, 109 patients with previously untreated FL were given CVP. Those with an objective response or stable disease were then randomized to rituximab 375mg/m2 weekly for four doses every 6 months for 24 months or observation. Those randomized to maintenance rituximab experienced an improved PFS as compared to the observation arm (4.3 vs. 1.3 yrs).45 Overall survival however was not improved by the addition of maintenance rituximab; however a subset analysis indicated that patients with a high tumor burden had an improved OS with maintenance rituximab.45
Unfortunately, this study did not use rituximab upfront with CVP as is common practice today. At the time this study was conducted, rituximab was not used with chemotherapy in the upfront setting. Since rituximab based chemotherapy is the standard of care for treatment of FL, it is unclear how applicable this study can be given the lack of upfront rituximab. In unusual circumstances where non-rituximab containing regimens have been used, rituximab maintenance should be considered in the relapsed setting if other treatment strategies cannot be employed.
The concept of maintenance rituximab is an appealing treatment option for FL given its efficacy, tolerability and ease of administration. The ability to extend OS has not been proven in long term follow-up although data from the Van Oers study indicates that rituximab naïve patients have an improved PFS with the addition of maintenance rituximab.43 Perhaps additional follow up is necessary in order to demonstrate a survival benefit. However, if a survival benefit cannot be established in the relapsed setting, it is difficult to expect that an effect will be seen in previously untreated patients, where the TTF is much longer.
It is hoped that the Primary Rituximab and Maintenance (PRIMA) study will address the question of whether to incorporate maintenance rituximab following upfront R-chemotherapy in previously untreated FL. The largest FL trial of its kind, it has accrued over 1000 patients with previously untreated FL and randomized them to maintenance rituximab according to the Ghielmini schedule versus observation following R-chemotherapy. Given its large sample size, this trial should be adequately powered to detect a survival difference. The preliminary results are expected soon.
Future Directions
Despite the successful improvement of disease free intervals with the addition of rituximab maintenance, there are several needed areas of further research. Several groups have attempted to improve efficacy of rituximab with immunostimulants to enhance antibody-dependent cellular cytotoxicity or complement mediated cytotoxicity.46 Rituximab has been safely combined with IL-2,47,48 alpha interferon,49 and IL-12.50 We51,52 and others53 have studied rituximab in combination with TLR-9 agonists, which have pleiotropic immunostimulatory effects. In general, these single arm trials have suggested enhanced progression-free survival when rituximab is combined with immunostimulatory agents compared to historical controls of single agent rituximab. However, randomized studies are required to definitively determine the effect of adding immunostimulants to rituximab, and whether these agents would have a role in combination with chemotherapy.
To date, there have been three published trials of rituximab maintenance, each with a different number of rituximab doses and different duration of therapy. However, all three have similar results thus highlighting a lack of knowledge regarding the optimal rituximab dosing schedule. Long term follow up from the initial study conducted by Ghielmini et al. suggests that the effects of rituximab can be long lasting in a certain percentage of patients25; however there is no method by which to identify these patients. Research on host-associated resistance mechanisms to rituximab has identified various polymorphisms of the Fcgamma RIIIA receptor that affect response to rituximab containing regimens in follicular lymphoma, but this has not been validated prospectively.54,55 Given the cost of rituximab maintenance and potential undetermined long-term risks, a greater emphasis on identification of potential responders as well as an optimal dosing schedule and duration are greatly needed.
In this issue of Seminars in Hematology, there is a chapter on novel anti-CD20 monoclonal antibodies. Many of these antibodies have been engineered to have increased cytotoxicity compared with rituximab. Until superior activity is demonstrated in randomized clinical trials, we feel it is unlikely these antibodies will replace rituximab in the routine treatment of indolent lymphoma. Therefore, continued study of this most valuable therapeutic agent is warranted.
Acknowledgments
Dr. Friedberg is a Scholar in Clinical Research of the Leukemia & Lymphoma Society, and is supported in part by the University of Rochester SPORE in Lymphoma (1P50CA130805)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362:516–522. doi: 10.1016/s0140-6736(03)14110-4. [DOI] [PubMed] [Google Scholar]
- 2.Horning SJ. Natural history of and therapy for the indolent non-Hodgkin's lymphomas. Seminars in oncology. 1993;20:75–88. [PubMed] [Google Scholar]
- 3.Johnson PW, Rohatiner AZ, Whelan JS, et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13:140–147. doi: 10.1200/JCO.1995.13.1.140. [DOI] [PubMed] [Google Scholar]
- 4.Montoto S, López-Guillermo A, Ferrer A, et al. Survival after progression in patients with follicular lymphoma: analysis of prognostic factors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2002;13:523–530. doi: 10.1093/annonc/mdf119. [DOI] [PubMed] [Google Scholar]
- 5.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 6.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Fayad L, Cabanillas F, et al. Improvement of Overall and Failure-Free Survival in Stage IV Follicular Lymphoma: 25 Years of Treatment Experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24:1582–1589. doi: 10.1200/JCO.2005.03.3696. [DOI] [PubMed] [Google Scholar]
- 10.Pulte D, Gondos A, Brenner H. Ongoing improvement in outcomes for patients diagnosed as having Non-Hodgkin lymphoma from the 1990s to the early 21st century. Archives of Internal Medicine. 2008;168:469–476. doi: 10.1001/archinternmed.2007.125. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 12.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–106. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Colombat P, Brousse N, Morschhauser F, et al. Single Treatment with Rituximab Monotherapy for Low-Tumor Burden Follicular Lymphoma (FL): Survival Analyses with Extended Follow-Up (F/Up) of 7 Years. ASH Annual Meeting Abstracts. 2006;108:486. [Google Scholar]
- 14.Freedman A, Neelapu SS, Nichols C, et al. Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. Journal of Clinical Oncology. 2009;27:3036–3043. doi: 10.1200/JCO.2008.19.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezdinli EZ, Harrington DP, Kucuk O, Silverstein MW, Anderson J, O'Connell MJ. The effect of intensive intermittent maintenance therapy in advanced low-grade non-Hodgkin's lymphoma. Cancer. 1987;60:156–160. doi: 10.1002/1097-0142(19870715)60:2<156::aid-cncr2820600205>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Hagenbeek A, Carde P, Meerwaldt JH, et al. Maintenance of remission with human recombinant interferon alfa-2a in patients with stages III and IV low-grade malignant non-Hodgkin's lymphoma. European Organization for Research and Treatment of Cancer Lymphoma Cooperative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:41–47. doi: 10.1200/JCO.1998.16.1.41. [DOI] [PubMed] [Google Scholar]
- 17.Rohatiner A, Radford J, Deakin D, et al. A randomized controlled trial to evaluate the role of interferon as initial and maintenance therapy in patients with follicular lymphoma. British journal of cancer. 2001;85:29–35. doi: 10.1054/bjoc.2001.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steward WP, Crowther D, McWilliam LJ, et al. Maintenance chlorambucil after CVP in the management of advanced stage, low-grade histologic type non-Hodgkin's lymphoma. A randomized prospective study with an assessment of prognostic factors. Cancer. 1988;61:441–447. doi: 10.1002/1097-0142(19880201)61:3<441::aid-cncr2820610306>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T, Ohtsu T, Fujii H, et al. Re-treatment of relapsed indolent B-cell lymphoma with rituximab. International journal of hematology. 2001;73:213–221. doi: 10.1007/BF02981940. [DOI] [PubMed] [Google Scholar]
- 21.Hainsworth JD, Litchy S, Burris HA, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:4261–4267. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma--a randomized phase II trial of the Minnie Pearl Cancer Research Network. Journal of Clinical Oncology. 2005;23:1088–1095. doi: 10.1200/JCO.2005.12.191. see comment. [DOI] [PubMed] [Google Scholar]
- 23.Ghielmini M, Schmitz SFH, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 24.Berinstein NL, Grillo-Lopez AJ, White CA, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 25.Ghielmini ME, Hsu Schmitz S, Martinelli G, et al. Long-term follow-up of patients with follicular lymphoma (FL) receiving single agent rituximab at two different schedules in study SAKK 35/98. J Clin Oncol (Meeting Abstracts) 2009;27:8512. doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
- 26.Taverna CJ, Bassi S, Hitz F, et al. First results of long-term rituximab maintenance treatment in follicular lymphoma: Safety analysis of the randomized phase III trial SAKK 35/03. J Clin Oncol (Meeting Abstracts) 2009;27:8534. [Google Scholar]
- 27.Czuczman MS, Olejniczak S, Gowda A, et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clinical Cancer Research. 2008;14:1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 28.Czuczman MS, Grillo-Lopez AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. Journal of Clinical Oncology. 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 29.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 30.Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-Lopez AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. Journal of Clinical Oncology. 2004;22:4711–4716. doi: 10.1200/JCO.2004.04.020. erratum appears in. [DOI] [PubMed] [Google Scholar]; J Clin Oncol. 2005 Jan 1;23(1):248. [Google Scholar]
- 31.Chow KV, Carroll R, Branley P, Nicholls K, Becker G, Hogan C. Anti-CD20 antibody in thrombotic thrombocytopenic purpura refractory to plasma exchange. Internal Medicine Journal. 2007;37:329–332. doi: 10.1111/j.1445-5994.2007.01338.x. [DOI] [PubMed] [Google Scholar]
- 32.Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro: synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100:1765–1773. [PubMed] [Google Scholar]
- 33.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. Journal of Clinical Oncology. 2007;25:1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 34.Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. British Journal of Haematology. 2001;114:800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- 35.Czuczman MS, Koryzna A, Mohr A, et al. Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:694–704. doi: 10.1200/JCO.2005.02.172. [DOI] [PubMed] [Google Scholar]
- 36.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 37.Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2007;99:706–714. doi: 10.1093/jnci/djk152. [DOI] [PubMed] [Google Scholar]
- 38.Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to frontline therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG) Leukemia. 2009;23:153–161. doi: 10.1038/leu.2008.261. [DOI] [PubMed] [Google Scholar]
- 39.Treon SP, Branagan AR, Ioakimidis L, et al. Long-term outcomes to fludarabine and rituximab in Waldenström macroglobulinemia. Blood. 2009;113:3673–3678. doi: 10.1182/blood-2008-09-177329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols GL, Savage DG. Timing of Rituximab/Fludarabine in Waldenstrom's Macroglobulinemia May Avert Hyperviscosity. ASH Annual Meeting Abstracts. 2004;104:4612. [Google Scholar]
- 41.Conconi A, Martinelli G, Thiéblemont C, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003;102:2741–2745. doi: 10.1182/blood-2002-11-3496. [DOI] [PubMed] [Google Scholar]
- 42.Jennifer RB, Jonathan WF, Yang F, et al. A phase 2 study of concurrent fludarabine and rituximab for the treatment of marginal zone lymphomas. British Journal of Haematology. 2009;145:741–748. doi: 10.1111/j.1365-2141.2009.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Oers MHJ, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 44.Van Oers MHJ, Van Glabbeke M, Baila L, et al. Rituximab Maintenance Treatment of Relapsed/Resistant Follicular Non- Hodgkin's Lymphoma: Long-Term Outcome of the EORTC 20981 Phase III Randomized Intergroup Study. ASH Annual Meeting Abstracts. 2008;112:836. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedberg JW, Freedman AS. Antibody and immunomodulatory agents in treatment of indolent non-Hodgkin's lymphoma. Current Treatment Options in Oncology. 2006;7:276–284. doi: 10.1007/s11864-006-0037-2. [DOI] [PubMed] [Google Scholar]
- 47.Friedberg JW, Neuberg D, Gribben JG, et al. Combination immunotherapy with rituximab and interleukin 2 in patients with relapsed or refractory follicular non-Hodgkin's lymphoma. British Journal of Haematology. 2002;117:828–834. doi: 10.1046/j.1365-2141.2002.03535.x. [DOI] [PubMed] [Google Scholar]
- 48.Gluck WL, Hurst D, Yuen A, et al. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-hodgkin's lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clinical Cancer Research. 2004;10:2253–2264. doi: 10.1158/1078-0432.ccr-1087-3. [DOI] [PubMed] [Google Scholar]
- 49.Davis TA, Maloney DG, Grillo-Lopez AJ, et al. Combination immunotherapy of relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma with rituximab and interferon-alpha-2a. Clinical Cancer Research. 2000;6:2644–2652. [PubMed] [Google Scholar]
- 50.Ansell SM, Witzig TE, Kurtin PJ, et al. Phase 1 study of interleukin-12 in combination with rituximab in patients with B-cell non-Hodgkin lymphoma. Blood. 2002;99:67–74. doi: 10.1182/blood.v99.1.67. [DOI] [PubMed] [Google Scholar]
- 51.Friedberg JW, Kim H, McCauley M, et al. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood. 2005;105:489–495. doi: 10.1182/blood-2004-06-2156. [DOI] [PubMed] [Google Scholar]
- 52.Jonathan WF, Jennifer LK, Donna N, et al. Phase II study of a TLR-9 agonist (1018 ISS) with rituximab in patients with relapsed or refractory follicular lymphoma. British Journal of Haematology. 2009;146:282–291. doi: 10.1111/j.1365-2141.2009.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leonard JP, Link BK, Emmanouilides C, et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin's lymphoma. Clinical Cancer Research. 2007;13:6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 54.Friedberg JW. Unique Toxicities and Resistance Mechanisms Associated with Monoclonal Antibody Therapy. Hematology. 2005;2005:329–334. doi: 10.1182/asheducation-2005.1.329. [DOI] [PubMed] [Google Scholar]
- 55.Ghielmini M, Rufibach K, Salles G, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Annals of Oncology. 2005;16:1675–1682. doi: 10.1093/annonc/mdi320. [DOI] [PubMed] [Google Scholar]


