SYNOPSIS
Objectives
Hepatitis C, an important cause of premature mortality, is the most common chronic bloodborne infection in the United States. The severity of disease is strongly affected by a number of other medical conditions and health behaviors. We sought to estimate the association of several exposures with hepatitis C on death certificates.
Methods
We enrolled 63,189 hepatitis C deaths as cases in a case-control study using multiple-cause-of-death data for the U.S. from 1999 to 2004. Three control groups were assembled from all remaining deaths with no mention of hepatitis C, including a random sample of all deaths, digestive disease deaths, and circulatory disease deaths.
Results
Hepatitis B, human immunodeficiency virus (HIV), hemochromatosis, and alcohol use were all strongly associated with hepatitis C, even after controlling for confounding variables. The simultaneous presence of many of these exposures had a synergistic association with hepatitis C being listed as a cause of death. Hepatitis B, HIV, and alcohol use were recorded among 6.4%, 10.5%, and 18.2% of case deaths, respectively.
Conclusions
The strong association of alcohol use, HIV, and hepatitis B with hepatitis C, as well as the frequent occurrence of these conditions, indicates that targeted interventions for mitigating the potential effect of these exposures may present an efficient means of limiting progression of hepatitis C-related liver disease and reducing the population burden of hepatitis C mortality.
Hepatitis C virus infection is the most common chronic bloodborne infection in the United States, with an estimated prevalence of 1.3% in the general U.S. population.1 About 10% to 20% of chronically infected people will develop liver cirrhosis and 1% to 5% will develop hepatocellular carcinoma within 20 to 30 years of infection.2 As a result, chronic hepatitis C infection is now the leading indication for liver transplantation3 and has the potential to result in a substantial amount of premature mortality,4 especially as the number of people chronically infected for more than 20 years increases.5
The clinical course of hepatitis C can vary substantially according to a number of factors, including gender, age at infection, viral genotype, and treatment with ribavirin and interferon.6–8 In addition to these factors, a number of other behaviors and medical conditions have been observed to be associated with progression of chronic liver disease among hepatitis C-infected individuals. People infected with human immunodeficiency virus (HIV) are known to have more rapid progression of hepatitis C-related liver disease than people without HIV infection.9–12 Similarly, alcohol consumption appears to accelerate the development of more severe hepatitis C-related liver disease.6,13,14 Hepatitis C-infected individuals with high iron levels also appear to have more severe liver disease and are less likely to benefit from treatment with interferon.15,16 It has been postulated that treatment of tuberculosis (TB) with hepatotoxic drugs may exacerbate chronic hepatitis C infection, and this theory has been explored in a number of small studies.17–19 Hepatitis B is frequently observed among people infected with hepatitis C, and while the presence of the hepatitis B virus may inhibit replication of hepatitis C virus, it also appears to be associated with more severe liver disease.20–22
As leading causes of death (CODs) have transitioned from acute infections to chronic diseases over the last century in the U.S., the usefulness of examining mortality based on single CODs has been called into question.23 Multiple-cause-of-death (MCOD) data represent a large, population-based data source that has been underutilized for exploring associations among causes of death. Given the growing number of people with longstanding chronic hepatitis C infections in the U.S., identification and confirmation of conditions that worsen the prognosis of hepatitis C infection will be important for effective clinical management of these patients as well as limiting the population burden of hepatitis C disease. To assess the potential impact of such comorbid conditions on hepatitis C mortality, we conducted a case-control analysis using U.S. MCOD data.
METHODS
We obtained MCOD data from the National Center for Health Statistics for all deaths from 1999 to 2004 occurring in the U.S. to evaluate the association between hepatitis C and other CODs. At the time of analysis, 2004 was the most recent data year available. Data were used from 1999 because International Classification of Diseases, 10th Revision (ICD-10) coding of death data began in that year.
The 2003 U.S. Standard Death Certificate, upon which each state's death certificate is based, has two sections for COD information.24 Part I includes information on the underlying COD as well as other conditions involved in the causal chain of events leading to death.25 Part II includes information on other “significant conditions contributing to death but not resulting in the underlying cause given in Part I.” For the purposes of this analysis, we considered a condition mentioned anywhere in Parts I or II of the death certificate to be present for that decedent.
Use of a case-control design for analysis of study populations comprised solely of deaths has been suggested to mitigate many of the limitations associated with proportional mortality studies.26 For this study, case deaths were classified as any death with mention of hepatitis C (ICD-10 codes B17.1 and B18.2). One method suggested for selecting controls in a study using only deaths is to select the control group based on other CODs thought to have no association, positive or negative, with the exposure of interest.26 Given the difficulty in selecting an appropriate control group from deaths, we chose to conduct the analysis using three distinct control groups. The first control group was a random sample of deaths (n=1,000,000) taken from all deaths with no mention of hepatitis C. The second control group consisted of all non-hepatitis C deaths with mention of certain digestive diseases (n=487,947: diseases of esophagus, stomach and duodenum; diseases of appendix; hernia; non-infective enteritis and colitis; other diseases of intestines; and diseases of peritoneum). The third control group consisted of all non-hepatitis C deaths with mention of certain circulatory diseases (n=5,075,790: hypertensive diseases, ischemic heart diseases, pulmonary heart disease, and diseases of pulmonary circulation). Both the second and third control groups were selected based on conditions lacking a strong association with most, if not all, of the exposures related to hepatitis C, and each group was likely subjected to different sets of medical screenings.
We evaluated several exposure conditions in this analysis including TB (ICD-10 codes A15–A19.9), HIV disease (ICD-10 codes B20–B24.9), evidence of alcohol use (ICD-10 codes K70–K70.9 and F10–F10.9), hepatitis B (ICD-10 codes B16–B16.9, B17.0, and B18.0–B18.1), and hemochromatosis (ICD-10 code E83.1). Other variables considered as possible confounders and/or effect-measure modifiers in the analysis included age, race/ethnicity, and gender. We also included HIV disease, alcohol use, and injection drug use in several models as potential confounders or effect-measure modifiers. We estimated the association of each of the exposures with hepatitis C using unconditional logistic regression in SAS® version 9.1.27
We built separate models, each with hepatitis C as the dependent variable, for the five exposures examined. We included confounding variables in models based on biologic plausibility of confounding, reduction in the model deviance, and approximately 10% or greater change in the coefficient for the primary exposure variable. We evaluated the inclusion of possible effect-measure modifiers based upon biologic plausibility and reduction in model deviance as well as the magnitude of modification observed (an interaction coefficient approximately greater than 0.4 or less than –0.4). Given the extremely large sample sizes, p-values, especially those based on Chi-squared statistics, were not informative and are not presented in this article. We assessed model fit based on visual examination of the partition for the Hosmer and Lemeshow goodness-of-fit test. Differences between observed and expected numbers of hepatitis C deaths and controls within categories of the estimated logistic probability were proportionally small for all models. We identified influential observations by examining delta beta values for the primary exposure coefficient, and less than 1% of observations were found to have large values (greater than 1 or less than –1) in each model.
Institutional Review Board approval was not required for this analysis, as human subjects review is only applicable to living people.
RESULTS
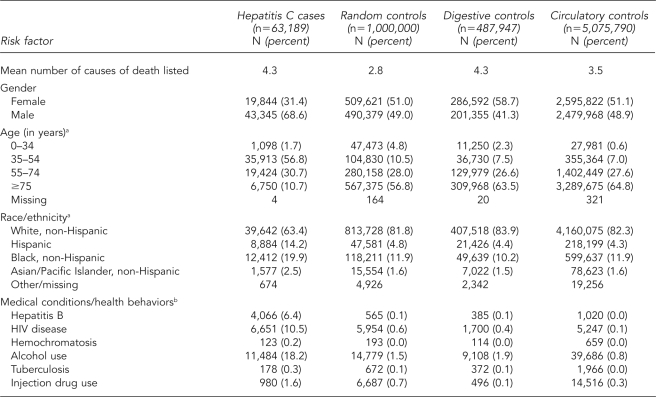
During the study period, we identified 63,189 deaths with mention of hepatitis C, of which 68.6% were male, 56.8% were aged 35 to 54 years, 14.2% were Hispanic, and 19.9% were black, non-Hispanic. About 10.5% of hepatitis C deaths mentioned HIV disease, and 18.2% reported alcohol use. All three control groups were more likely to be female, older, and white compared with the hepatitis C cases; the control groups also mentioned HIV disease, alcohol use, and injection drug use less frequently. The digestive disease and circulatory disease control groups were older than the randomly selected control group, and the digestive disease control group was more likely to be female than the circulatory disease or randomly selected control groups. The mean number of CODs recorded was higher among hepatitis C cases and the digestive disease control group than among the random and circulatory disease control groups (Table 1).
Table 1.
Comparison of characteristics of hepatitis C cases and three control groups, U.S. Multiple-Cause-of-Death Data Study, 1999–2004
aPercentage based on number of available responses (does not include “missing” or “other/missing”)
bCategories of medical conditions are not mutually exclusive.
HIV = human immunodeficiency virus
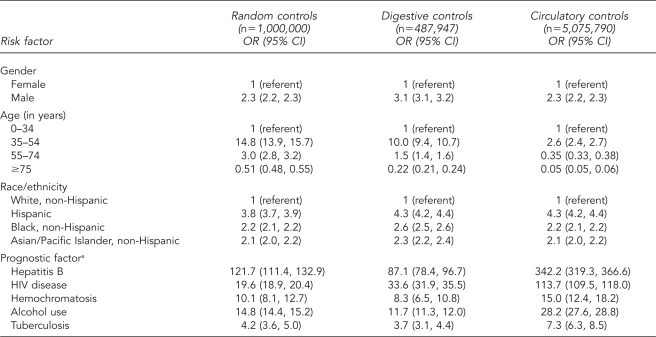
Univariate logistic analysis showed nearly all variables to be positively associated with hepatitis C using each of the three control groups. The odds of hepatitis C being listed on the death certificate were two to three times higher for males than females and were between two and four times higher for decedents who were not white, non-Hispanic. Age was also strongly associated with hepatitis C, with the odds of decedents aged 35–54 years listing hepatitis C being significantly higher than for those aged 0–34 years, especially when using random and digestive disease control groups. Each of the medical conditions examined was a consistently positive predictor of hepatitis C being recorded on the death certificate across the three control groups employed (Table 2).
Table 2.
Univariate logistic regression ORs and 95% CIs for the association between hepatitis C and demographic, confounder, and risk factor variables using three control groups, U.S. Multiple-Cause-of-Death Data Study, 1999–2004
aFor each prognostic factor, the referent group is composed of decedents with no mention of the factor on the death certificate.
OR = odds ratio
CI = confidence interval
HIV = human immunodeficiency virus
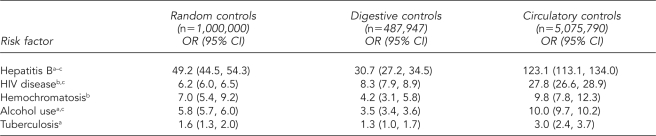
After adjusting for age, race/ethnicity, gender, injection drug use, HIV disease, and alcohol use, hepatitis B was the strongest positive predictor of hepatitis C being listed on the death certificate in multivariate analysis, with odds ratios (ORs) of 49.2 (95% confidence interval [CI] 44.5, 54.3) for the random control group, 30.7 (95% CI 27.2, 34.5) for the digestive disease control group, and 123.1 (95% CI 113.1, 134.0) for the circulatory disease control group. HIV disease, hemochromatosis, and alcohol use also were positively associated with hepatitis C being recorded as a COD, with adjusted ORs for the three control groups ranging from 6.2 to 27.8 for HIV disease, 4.2 to 9.8 for hemochromatosis, and 3.5 to 10.0 for alcohol use. TB exhibited a relatively weak but consistently positive association with hepatitis C (Table 3). Only alcohol use, hepatitis B, and HIV disease were observed in more than 1% of hepatitis C deaths (Table 1).
Table 3.
Multivariate logistic regression ORs and 95% CIs for the association between hepatitis C and selected risk factors using three control groups controlling for gender, age, and race/ethnicity, U.S. Multiple-Cause-of-Death Data Study, 1999–2004
aAlso controlling for HIV
bAlso controlling for alcohol use
cAlso controlling for injection drug use
OR = odds ratio
CI = confidence interval
HIV = human immunodeficiency virus
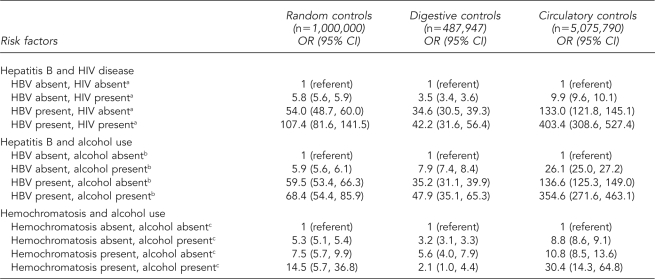
Substantial effect-measure modification was observed infrequently in this analysis. The simultaneous presence of hepatitis B and HIV disease was a stronger predictor of hepatitis C than hepatitis B alone. Similarly, the simultaneous presence of hepatitis B and alcohol use was a stronger predictor of hepatitis C than alcohol use and hepatitis B individually. The statistical interactions observed for hepatitis B and both HIV disease and alcohol use were consistent across the three control groups, although the magnitude of the associations did vary. The association of hemochromatosis with hepatitis C also appeared to vary according to alcohol use status when using random and circulatory disease controls. When using digestive disease controls, -however, the simultaneous presence of hemochromatosis and alcohol use was a weaker predictor (OR=2.1) of hepatitis C than hemochromatosis alone (OR=5.6) (Table 4).
Table 4.
Multivariate logistic regression for the joint effect of two exposures on hepatitis C, U.S. Multiple-Cause-of-Death Data Study, 1999–2004
aControlling for age, race/ethnicity, gender, alcohol use, and injection drug use
bControlling for age, race/ethnicity, gender, HIV, and injection drug use
cControlling for age, race/ethnicity, and gender
OR = odds ratio
CI = confidence interval
HIV = human immunodeficiency virus
HBV = hepatitis B virus
DISCUSSION
These findings indicate that hepatitis B, HIV disease, hemochromatosis, and alcohol use are strongly associated with hepatitis C and may be important in contributing to increased mortality in hepatitis C-infected individuals, consistent with previous research on the influence of these diseases on the clinical course of hepatitis C. Hepatitis B, HIV disease, and alcohol use were common among hepatitis C deaths, indicating that interventions targeting these conditions may be an efficient means for attempting to limit death and severe disease from hepatitis C. This analysis further demonstrates a synergistic influence of multiple exposures on hepatitis C being listed as a COD.
Hepatitis B was the condition most strongly associated with hepatitis C, consistent with previous studies on hepatitis B exacerbating hepatitis C-related liver disease. These studies have shown that the two viruses act synergistically in the development of chronic liver disease.20–22 The strong association between these two conditions is consistent with the synergistic effects described in these other studies. However, given the high likelihood of residual confounding due to poor measurement of injection drug use, as well as confounding due to unmeasured common risk factors for both infections (such as blood transfusions), much of this association is probably driven by shared modes of transmission. Detection bias may also have influenced these results, with hepatitis C deaths more likely to be screened for other viral hepatitis infections than control deaths. Nevertheless, an association of this magnitude is unlikely to be accounted for by bias and uncontrolled confounding alone.
The association of hepatitis B with hepatitis C differed according to alcohol use and HIV disease. Although alcohol use, HIV disease, and hepatitis B have each been identified as potentially exacerbating the progression of hepatitis C disease,6,9–12,20–22 the extent to which these factors interact with one another has not been well studied. The results of this analysis suggest that co-occurrence of hepatitis B and alcohol use or hepatitis B and HIV disease may act synergistically in advancing the progression of hepatitis C disease. This should be interpreted cautiously because of possible detection bias, in which people with HIV disease and hepatitis B or those with hepatitis B and alcohol use are more likely to be screened for hepatitis C than people with only one of those conditions present.
HIV disease was strongly associated with hepatitis C and occurred in more than 10% of hepatitis C deaths, making it an important contributor to the population burden of advanced hepatitis C disease. These results are consistent with the large body of literature showing that HIV infection hastens progression of chronic liver disease among hepatitis C-infected people.9–12,28 Potential mechanisms for this observation include the hepatotoxic properties of highly active antiretroviral therapy (HAART), the tendency of HAART drugs to cause an increase in hepatitis C viral load, and a reduction in hepatitis C antibodies due to HIV-induced impairment of B-cell function.11,29 Despite the relatively well-described role of HIV in hepatitis C-related disease, some of the observed effect is certainly due to residual confounding from shared modes of transmission as well as detection bias in which people with hepatitis C are more likely to be screened for HIV. Nevertheless, given both the strong association of HIV disease with hepatitis C and its common occurrence among hepatitis C deaths, people co-infected with these viruses as well as with HIV alone may warrant targeted intervention to mitigate the effects of chronic hepatitis C or prevent hepatitis C infection from occurring.
Alcohol use was strongly associated with hepatitis C mortality and was reported in nearly one-fifth of all hepatitis C deaths, a proportion that is likely an underestimate.30 Previous research has shown alcohol use to be a strong risk factor for development of severe liver disease among hepatitis C-infected individuals.6,31,32 The results of this analysis are consistent with that body of research, although some of the observed association is likely due to residual confounding from injection drug use. Hepatitis C deaths also may be more likely to have their alcohol use status ascertained and subsequently recorded on the death certificate. Given both the strength of the association between alcohol use and severe hepatitis C disease6,13,33 as well as the high occurrence of alcohol use among hepatitis C deaths, mitigation of this exposure will be critical in reducing the burden of hepatitis C-related liver disease on a population level.
Although relatively strongly associated with hepatitis C, hemochromatosis occurred infrequently among hepatitis C deaths. Prior studies have shown that elevated levels of iron in the liver may promote persistence of chronic viral hepatitis infections, development of more severe liver disease in hepatitis C-infected people, and less successful interferon treatment.15,16,34 Hemochromatosis also may be a risk factor for acquiring hepatitis C, as therapeutic phlebotomy and injected chelation therapy may increase parenteral exposure. However, people with hepatitis C were probably more likely than those in the control groups to be screened for high iron levels, which would have resulted in positive bias of ORs. As hemochromatosis occurred infrequently among hepatitis C deaths, clinical interventions to lower iron levels among individuals with hepatitis C would likely only mitigate a small proportion of hepatitis C-related disease and mortality, but may be useful in individual clinical situations.
The association of hemochromatosis with hepatitis C appeared to be modulated by alcohol use, although results were somewhat inconsistent across control groups, likely due to the extremely low frequency of hemochromatosis among hepatitis C cases. While both high iron levels and alcohol use separately have been implicated in exacerbating hepatitis C disease,6,16 little research has explored the existence of a synergistic effect of these exposures.
The moderate association between hepatitis C and TB may be explained by residual confounding due to HIV disease, but several of the first-line drugs for TB treatment, including isoniazid and rifampin, are known to have hepatotoxic effects.17,18 It is possible that administration of such drugs, especially given the long duration of TB treatment, may hasten the progression of hepatitis C-related liver disease. The association between TB and hepatitis C must be interpreted cautiously, however, as TB was extremely rare among hepatitis C deaths. The low frequency of this exposure would also make any intervention to assess hepatitis C status before initiation of directly observed therapy an inefficient means to reduce the burden of hepatitis C-related liver disease.
Limitations
Our findings must be interpreted with caution for several reasons. Limitations of using death certificate data can include varying quality and type of information collected across states, differences in how physicians complete death certificates, and lack of information on other important risk factors for hepatitis C, such as treatment and duration of infection.
In addition, despite the careful selection of three control groups, selection bias was impossible to avoid entirely. Results were qualitatively consistent across the three control groups, however, with nearly all observed associations being in the same direction and of similar magnitude. Circulatory disease controls tended to result in ORs of greater magnitude (more positive), while digestive disease controls tended to have ORs of lesser magnitude (less positive). This may be due to the fact that circulatory diseases were actually negatively associated with many of the exposure conditions. While only non-liver-related digestive conditions were used to select the digestive disease controls, this group was probably more similar to hepatitis C case deaths than the other two control groups with respect to certain factors. The number of CODs recorded for digestive disease controls was similar to that of the hepatitis C cases, indicating that these two groups may tend to either experience similar rigor in medical screening or truly have more conditions present at the time of death. Unfortunately, the largely consistent results across control groups could be an indicator of appropriate control group selection or a common bias operating across all control groups.26
Another bias, analogous to Berksonian bias,35 may have affected our results. If hepatitis C and the exposure under study both influence the probability of death, then conducting a study using only deaths amounts to stratification on what is termed a “collider variable,” which would induce an association between hepatitis C and the exposure under study.36
It is likely that there was substantial measurement error in classification of both hepatitis C status and the comorbid conditions assessed. A number of published studies have indicated that death certificate data only capture a modest proportion of all hepatitis C deaths.37,38 Furthermore, the hepatitis C case definition employed may have been nonspecific, as deaths were included that listed hepatitis C as either an underlying or contributing COD. Results of this analysis were similar, however, when cases were limited to only those listing hepatitis C as an underlying cause. The degree of misclassification of hepatitis C as a COD may also vary according to exposure status, with some deaths, such as those listing hepatitis B, much more likely to have their hepatitis C status ascertained. Likewise, exposures such as HIV disease, alcohol use, or hemochromatosis may be more likely to be ascertained for hepatitis C deaths than for other deaths. Simultaneous differential misclassification of exposure and disease can lead to bias toward or away from the null.39
Ambiguity over the temporal order of exposure and outcome was also an issue. It is difficult to accurately determine the temporal occurrence of CODs on death certificates, even under circumstances in which a physical copy of the death certificate can be examined. As such, there is no way of knowing whether the comorbid conditions actually preceded the occurrence of hepatitis C-related disease.40 Given the long, latent nature of chronic hepatitis C infection, however, it is likely that the vast majority of exposures examined were, at a minimum, concurrent with hepatitis C infection.
CONCLUSIONS
Hepatitis B, for which an effective vaccine is available, was strongly associated with hepatitis C being listed as a COD, an indicator of its probable importance in exacerbating hepatitis C-related liver disease. HIV disease, hemochromatosis, and alcohol use were also observed to be associated with hepatitis C. The strong associations of hepatitis B, HIV, hemochromatosis, and alcohol use with hepatitis C are consistent with other published studies examining the relationship of these conditions. The simultaneous presence of hepatitis B and HIV, hepatitis B and alcohol use, and hemochromatosis and alcohol use appeared to exhibit synergism in their association with hepatitis C, suggesting a possible role for interactions between comorbid conditions in the progression of hepatitis C disease. Given the frequent occurrence of alcohol use, HIV, and hepatitis B among hepatitis C deaths, interventions to reduce the population burden of hepatitis C-related liver disease and mortality would likely be enhanced by incorporating components targeted toward these conditions.
Footnotes
This project was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases Training Program in HIV/AIDS Epidemiology (T32AI07481). The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the funding agency.
REFERENCES
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 3.Department of Health and Human Services (US) Annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: transplant data 1995–2004. Rockville (MD): HHS, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation (US); 2005. [Google Scholar]
- 4.Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995–2004. Hepatology. 2008;47:1128–35. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–82. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, Bedossa P, Opolon P The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 7.Roffi L, Redaelli A, Colloredo G, Minola E, Donada C, Picciotto A, et al. Outcome of liver disease in a large cohort of histologically proven chronic hepatitis C: influence of HCV genotype. Eur J Gastroenterol Hepatol. 2001;13:501–6. doi: 10.1097/00042737-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 9.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 10.Guitton E, Montastruc JL, Lapeyre-Mestre M, French Network of Pharmacovigilance Centres Influence of HCV or HBV coinfection on adverse drug reactions to antiretroviral drugs in HIV patients. Eur J Clin Pharmacol. 2006;62:243–9. doi: 10.1007/s00228-005-0080-0. [DOI] [PubMed] [Google Scholar]
- 11.Leen CL. Hepatitis C and HIV co-infection. Int J STD AIDS. 2004;15:289–94. doi: 10.1177/095646240401500502. [DOI] [PubMed] [Google Scholar]
- 12.Merriman NA, Porter SB, Brensinger CM, Reddy KR, Chang KM. Racial difference in mortality among U.S. veterans with HCV/HIV coinfection. Am J Gastroenterol. 2006;101:760–7. doi: 10.1111/j.1572-0241.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1150–9. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 14.Singal A, Anand B. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761–72. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- 15.Fontana RJ, Israel J, LeClair P, Banner BF, Tortorelli K, Grace N, et al. Iron reduction before and during interferon therapy of chronic hepatitis C: results of a multicenter, randomized, controlled trial. Hepatology. 2000;31:730–6. doi: 10.1002/hep.510310325. [DOI] [PubMed] [Google Scholar]
- 16.Fujita N, Sugimoto R, Urawa N, Araki J, Mifuji R, Yamamoto M, et al. Hepatic iron accumulation is associated with disease progression and resistance to interferon/ribavirin combination therapy in chronic hepatitis C. J Gastroenterol Hepatol. 2007;22:1886–93. doi: 10.1111/j.1440-1746.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwon YS, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ. Hepatitis C virus infection and hepatotoxicity during antituberculosis chemotherapy. Chest. 2007;131:803–8. doi: 10.1378/chest.06-2042. [DOI] [PubMed] [Google Scholar]
- 18.Sadaphal P, Astemborski J, Graham NM, Sheely L, Bonds M, Madison A, et al. Isoniazid preventive therapy, hepatitis C virus infection, and hepatotoxicity among injection drug users infected with Mycobacterium tuberculosis. Clin Infect Dis. 2001;33:1687–91. doi: 10.1086/323896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Villar A, Sopeña B, Vázquez R, Ulloa F, Fluiters E, Mosteiro M, et al. Isoniazid hepatotoxicity among drug users: the role of hepatitis C. Clin Infect Dis. 2003;36:293–8. doi: 10.1086/345906. [DOI] [PubMed] [Google Scholar]
- 20.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed Ael S, al Karawi MA, Mesa GA. Dual infection with hepatitis C and B viruses: clinical and histological study in Saudi patients. Hepatogastroenterology. 1997;44:1404–6. [PubMed] [Google Scholar]
- 22.Kew M, Yu M, Kedda M, Coppin A, Sarkin A, Hodkinson J. The relative roles of hepatitis B and C viruses in the etiology of hepatocellular carcinoma in southern African blacks. Gastroenterology. 1997;112:184–7. doi: 10.1016/s0016-5085(97)70233-6. [DOI] [PubMed] [Google Scholar]
- 23.Israel R, Rosenberg H, Curtin L. Analytical potential for multiple cause-of-death data. Am J Epidemiol. 1986;124:161–79. doi: 10.1093/oxfordjournals.aje.a114375. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. 2003 revision of the U.S. Standard Death Certificate. [cited 2008 Dec 1]. Available from: URL: http://www.cdc.gov/nchs/data/dvs/DEATH11-03final-ACC.pdf.
- 25.National Center for Health Statistics (US) Instructions for classifying multiple causes of death. Hyattsville (MD): NCHS; 2005. [cited 2008 Dec 1]. Also available from: URL: http://www.cdc.gov/nchs/data/dvs/2a2005a.pdf. [Google Scholar]
- 26.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 27.SAS Institute, Inc. SAS®: Version 9.1. Cary (NC): SAS Institute, Inc.; 2003. [Google Scholar]
- 28.Sterling RK, Sulkowski M. Hepatitis C virus in the setting of HIV or hepatitis B virus coinfection. Semin Liver Dis. 2004;24(Suppl 2):61–8. doi: 10.1055/s-2004-832930. [DOI] [PubMed] [Google Scholar]
- 29.Netski DM, Mosbruger T, Astemborski J, Mehta SH, Thomas DL, Cox AL. CD4+ T cell-dependent reduction in hepatitis C virus-specific humoral immune responses after HIV infection. J Infect Dis. 2007;195:857–63. doi: 10.1086/511826. [DOI] [PubMed] [Google Scholar]
- 30.Hanzlick R. Death certificates, natural death, and alcohol: the problem of underreporting. Am J Forensic Med Pathol. 1988;9:149–50. doi: 10.1097/00000433-198806000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Freeman AJ, Law MG, Kaldor JM, Dore GJ. Predicting progression to cirrhosis in chronic hepatitis C virus infection. J Viral Hepat. 2003;10:285–93. doi: 10.1046/j.1365-2893.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen CM, Yoon YH, Yi HY, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: a life table analysis. Alcohol Clin Exp Res. 2007;31:285–92. doi: 10.1111/j.1530-0277.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 33.Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761–72. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- 34.Shan Y, Lambrecht RW, Bonkovsky HL. Association of hepatitis C virus infection with serum iron status: analysis of data from the third National Health and Nutrition Examination Survey. Clin Infect Dis. 2005;40:834–41. doi: 10.1086/428062. [DOI] [PubMed] [Google Scholar]
- 35.Feinstein AR, Walter SD, Horwitz RI. An analysis of Berkson's bias in case-control studies. J Chronic Dis. 1986;39:495–504. doi: 10.1016/0021-9681(86)90194-3. [DOI] [PubMed] [Google Scholar]
- 36.Greenland S. Quantifying biases in causal models: classical confounding vs. collider-stratification bias. Epidemiology. 2003;14:300–6. [PubMed] [Google Scholar]
- 37.Leyden W, Murphy R, Bell B, Terrault N, Manos M. AASLD abstract 33: a comprehensive assessment of chronic liver disease deaths reveals limitations of statistics based on standard methods. Hepatology. 2004;38(4 Part 2):49A. [Google Scholar]
- 38.Wu C, Chang HG, McNutt LA, Smith PF. Estimating the mortality rate of hepatitis C using multiple data sources. Epidemiol Infect. 2005;133:121–5. doi: 10.1017/s0950268804003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenner H, Savitz DA, Gefeller O. The effects of joint misclassification of exposure and disease on epidemiologic measures of association. J Clin Epidemiol. 1993;46:1195–202. doi: 10.1016/0895-4356(93)90119-l. [DOI] [PubMed] [Google Scholar]
- 40.Redelings MD, Wise M, Sorvillo F. Using multiple cause-of-death data to investigate associations and causality between conditions listed on the death certificate. Am J Epidemiol. 2007;166:104–8. doi: 10.1093/aje/kwm037. [DOI] [PubMed] [Google Scholar]