SYNOPSIS
Objective
A higher incidence of infectious disease has been documented in U.S. regions bordering Mexico compared with non-border areas. We assessed the prevalence of important gastrointestinal infections in Ciudad Juarez, Mexico, and El Paso, Texas, the largest binational community along the U.S.-Mexico border.
Methods
Fecal specimens from a sample of the asymptomatic population representing all ages were tested for Helicobacter pylori (H. pylori), Cryptosporidium spp., Giardia spp., and other intestinal parasitic pathogens using flotation, immunoassays, and/or polymerase chain reaction. We also measured indicators of microbiological contamination of drinking water, hands of food preparers, and kitchen surfaces.
Results
Overall, of the 386 participants, H. pylori was present in 38.2%, Taenia spp. in 3.3%, Giardia spp. in 2.7%, Cryptosporidium spp. in 1.9%, Entamoeba dispar in 1.3%, and Ascaris lumbricoides and Necator americanus in 0.3% of the study subjects; Cyclospora spp. and Entamoeba histolytica were not found. H. pylori infection was associated with handwashing (prevalence ratio [PR] = 1.3, 95% confidence interval [CI] 1.0, 1.8). Taenia spp. was found more often on the U.S. side (PR=8.6, 95% CI 2.3, 30.8). We did not find an association between these infections and the occurrence of total coliforms or fecal coliforms on kitchen surfaces. In addition, Escherichia coli was not found in any drinking water sample.
Conclusion
The study results indicated that H. pylori and Taenia spp. infections may be highly prevalent along the U.S.-Mexico border. Additional research is necessary to adequately characterize the prevalence, as well as determine whether interventions that reduce these infections are warranted.
Gastrointestinal (GI) infectious diseases are prevalent in economically developing countries including Mexico, and, thus, these infections are thought to overflow into regions of the United States that border Mexico. For example, in the 1990s, an excess of certain reportable infectious diseases—saliently hepatitis A, shigellosis, and botulism—was reported in U.S. counties bordering Mexico, suggesting that improvements in the water and sanitation infrastructure were needed.1 Previous studies in communities of Ciudad Juarez, Chihuahua, Mexico, lacking municipal sanitation or piped water to their homes found Giardia in 82% and Cryptosporidium in 70% of samples taken from the biosolid waste in composting toilets.2 One such border area is El Paso, Texas, and Ciudad Juarez. These cities are separated by the Rio Grande River and form a single metropolitan area with a combined population of approximately two million. Large numbers of this multicultural population actively move back and forth across the border. In 2007, 10 million cars and 12.2 million pedestrians crossed the border through the bridges of the El Paso/Ciudad Juarez area.
Helicobacter pylori (H. pylori) infection causes gastritis, peptic ulcer, atrophic gastritis, iron deficiency anemia, and gastric malignancy.3 This mostly asymptomatic infection is more common among minority groups including Hispanics in the U.S., who, in turn, also experience a twofold increased incidence of non-cardia adenocarcinoma of the stomach.4
Our study assessed the prevalence of selected enteric parasitic pathogens and H. pylori in this binational population, and addressed the role of domestic sanitation, including microbiologically safe water and foods, sanitary disposal, and hygienic behavior. We previously reported findings from this research focusing exclusively on the prevalence of Taenia spp.;5 this article includes all of the pathogens addressed in the study.
METHODS
Study design and population
We conducted a cross-sectional study based on data collected from a sampling survey. The study included a sample of individuals of all ages, both male and female, to represent the target of the noninstitutionalized population of Ciudad Juarez and El Paso. We selected the sample by means of a two-stage cluster self-weighted design. In the first stage, a systematic random sample of census blocks was selected with probability proportional to size (i.e., 20 Areas Geográficas Estadísticas Básicas [AGEBs] in Ciudad Juarez and 20 census tracts in El Paso), using the most complete sampling frame of geo-referenced information by census tracts (AGEBs for Ciudad Juarez and from the U.S. Census Bureau for El Paso). One block was selected within each of the 40 primary sampling units using simple random sampling from all blocks mapped on available maps. In selected blocks, all households were listed, and a sample of households was subsequently selected using systematic random sampling. All the data and specimens were collected from July through September 2004. The sampling design ensured a representative sample across both cities and representing all socioeconomic strata.
Institutional Review Board approval and consent
We obtained approval from the Institutional Review Board at the University of Texas Health Science Center. Within selected households, all eligible individuals were invited to participate in the study. Field personnel visited the selected blocks one or two days in advance of the actual visit and left a leaflet to introduce the study. In a subsequent visit, signed informed consent and parental consent/child assent were obtained.
Interviews
All interviews were conducted in English or Spanish depending on the preference of the interviewee. Caretakers of children younger than 18 years of age—or the meal preparer(s) if no child lived in the household—were interviewed using a structured questionnaire. The questionnaire addressed history of recent illnesses, dwelling characteristics, socioeconomic factors such as income and education, hygienic practices, and travel frequency to the other side of the U.S.-Mexico border. Specifically, we asked questions about handwashing in scale form, such as, “How often would you say you wash your hands with soap after using the toilet?”, and read aloud the answer options, such as “always, most times, sometimes, seldom, or never.”
Kitchen surfaces, hand, and drinking water contamination
We took environmental samples from 135 households: 52 in El Paso and 83 in Ciudad Juarez, as described elsewhere.4 Briefly, different surfaces throughout the kitchen as well as the hands of the person who was the head of the household were sampled, and total and fecal coliforms were enumerated. We also enumerated total coliforms and Escherichia coli (E. coli) in drinking water samples.
Infection status
A sample of at least 5 grams of recently eliminated morning stool was requested from each household member. Study staff picked up containers of stool samples collected by participants and transported them to the study lab at 4°C. Fecal specimens were aliquoted, and fresh specimens were examined for helminths and frozen for subsequent parasitologic and immunodiagnostic testing.
Methods used to assess the prevalence of helminths (specifically Taenia spp.) have been previously described.3 Briefly, we used microscopy after centrifugal fecal flotation (Sheather's sugar solution) to isolate helminth eggs and larvae. We measured Taenia infection using a copro-antigens enzyme-linked immunosorbent assay test previously developed and validated.5 We assessed H. pylori infection status using a native catalase antigen test described elsewhere.6 Testing for Cryptosporidium spp. oocysts and Giardia spp. cysts was done with a direct immunofluorescence assay (Merifluor kit, Meridian Diagnostics, Inc., Cincinnati, Ohio). Concentrated samples were used to look for unsporulated Cyclospora spp. oocysts using the ultraviolet epifluorescent illumination method. Frozen specimens were analyzed for Entamoeba histolytica (E. histolytica) and Entamoeba dispar (E. dispar) using a polymerase chain reaction test described previously.7
Data analysis
The data collected were double-entered using a front-end application developed in Epi Info™ and subsequently merged and analyzed using SAS® version 9.1 and SUDAAN® software.8–10 The infection status for each pathogen was the outcome variable, and the prevalence figures were estimated taking into account the sampling weights and the cluster design in SUDAAN. We explored the relation between infection with these pathogens and measures of microbial contamination of kitchen surfaces, hands, and water; self-reported frequency of handwashing; age and gender; place of birth; educational attainment of the head of household; and the number of people per bedroom. We used comparison of means and linear regression techniques to assess the relationship between indicators of contamination in households and the prevalence of infections. We also assessed the relationship using two-by-two tables and stratified analyses followed by negative binomial logistic regression analyses.
RESULTS
A total of 280 households were visited; in five instances (1.8%), individuals in a household refused to participate, and in 76 households (27.1%), no one was home after three visits. Of the 199 consenting households, 104 were in El Paso and 95 were in Ciudad Juarez. A total of 746 enumerated individuals participated, with 386 (51.7%) of them providing fecal samples; the final response rate was 36.7% (Table 1). Those from whom fecal samples were collected were more likely to be older (p<0.05) and residents of Mexico, and Mexican respondents were 50.0% more likely to provide fecal samples than their U.S. counterparts (prevalence ratio [PR] = 1.5, 95% confidence interval [CI] 1.3, 1.6; p<0.001). The age-gender distribution of the study populations resembled that of El Paso and Ciudad Juarez, as portrayed by the U.S. and Mexican Census data.11,12 The mean number of people per household was greater in Ciudad Juarez (5.0) than in El Paso (2.6), which was only slightly different from the mean number of household members according to the Census data (i.e., 4.6 and 3.2, respectively). However, the study population had a lower income and education level than the overall population in both cities.11,12
Table 1.
Number of households and subjects selected and participating in a study of gastrointestinal infections in El Paso, Texas, and Ciudad Juarez, Mexico, 2004
Prevalence of pathogens
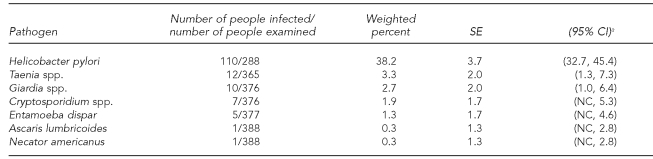
As shown in Table 2, H. pylori was the most common pathogen identified (38.2%) followed by Taenia spp. (3.3%), Giardia spp. (2.7%), Cryptosporidium spp. (1.9%), E. dispar (1.3%), and Ascaris lumbricoides (A. lumbricoides) and Necator americanus (N. americanus) (both 0.3%). We found no Cyclospora spp. or E. histolytica in the stool samples examined. Only six individuals reported diarrhea in the previous week, all of them from a single family in Ciudad Juarez; however, they did not have diarrhea at the time of the visit. The number of specimens examined for each pathogen varied, as aliquots from specimens provided by some participants could not be prepared; this particularly affected the specimens sent for H. pylori testing. In 10 specimens, co-infection was observed, nine of them involving H. pylori. Cryptosporidium spp. concomitant with Giardia spp. were found in one other person harboring two pathogens.
Table 2.
Prevalence of pathogens examined in a study of gastrointestinal infections in El Paso, Texas, and Ciudad Juarez, Mexico, 2004
aVariance estimates obtained using complex survey estimators available in SUDAAN® (Research Triangle Institute. SUDAAN®: Version 9.0. Research Triangle Park [NC]: Research Triangle Institute; 2008.)
SE = standard error
CI = confidence interval
NC = not calculable
Risk factors
We found enough informative events (i.e., ≥10) for H. pylori, Taenia spp., and Giardia spp. to be compared by side of the border (Table 2). We found no difference in the prevalence of H. pylori and Giardia spp. infection between the two sides of the U.S.-Mexico border. All samples testing positive for Cryptosporidium spp., E. dispar, A. lumbricoides, and N. americanus were collected among residents of Ciudad Juarez, but there were too few observations to draw any conclusion. On the other hand, the prevalence of Taenia spp. infection was higher on the El Paso side of the border: 9.7% (or 9/93) (95% CI 3.7, 15.1) compared with 1.1% (or 3/280) (95% CI 0.6, 3.8) in Ciudad Juarez, a difference that reached statistical significance (p<0.05). According to the PR of taeniasis observed in the two cities, individuals living in El Paso appeared to be 8.6-fold (95% CI 2.3, 30.8) more likely to be positive for taeniasis than those living in neighboring Juarez.
We did not find differences in the levels of domestic contamination on kitchen surfaces, flora of the hands of meal preparers, or drinking water between El Paso and Ciudad Juarez.4 There was more crowding (i.e., two or more people per room) in the households in the study in Ciudad Juarez (70%) compared with El Paso (45%), but hygienic practices, access to piped running water, sewage, and a working refrigerator were present in 100% of the households included in this random sample on both sides of the border.
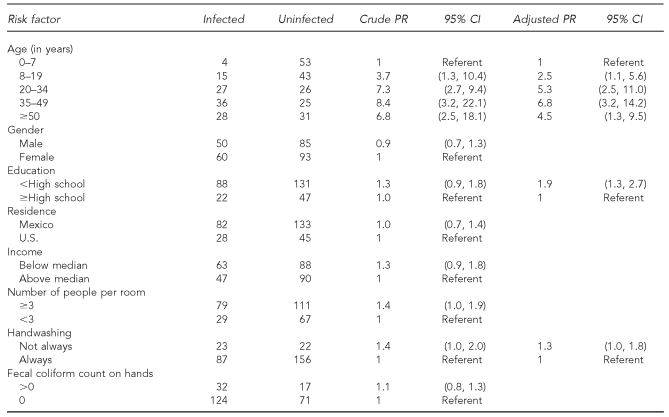
As shown in Table 3, the prevalence of H. pylori infection increased significantly with age. H. pylori prevalence was inversely related to education level but was not associated with the prevalence of fecal contamination of the hands of food preparers. H. pylori prevalence increased with crowding (defined as three or more people per bedroom), with a household income level lower than the median, and with not always washing hands after using the toilet. However, once age and socioeconomic status (as measured by educational attainment) were entered in stratified or multivariate analysis, these effects disappeared. In addition, people living in households where the informant reportedly did not wash his or her hands all the time with soap after using the toilet had an increased prevalence of H. pylori infection. Furthermore, using unweighted analyses, we did not find significant differences between the presence of total and fecal coliforms on the different surfaces and the presence of H. pylori in the household either at the individual or household level.
Table 3.
Risk factors for Helicobacter pylori infection in El Paso, Texas, and Ciudad Juarez, Mexico, 2004
PR = prevalance ratio
CI = confidence interval
DISCUSSION
To our knowledge, this is the first study describing the prevalence of infections in a representative sample of the largest urban metropolitan area of the U.S.-Mexico border. We found that H. pylori infection is highly prevalent in this population, at levels comparable with those reported among the Mexico-born U.S. population.13 However, the prevalence of Taenia spp. among U.S. immigrants from Mexico underscores the -appropriateness of a call for action on the emerging nature of neurocysticercosis in the U.S.14 Moreover, our study further characterizes that Mexican Americans (and particularly immigrants from Mexico, who largely populate the U.S.-Mexico border) tend to be a high-risk group for infection with H. pylori and Taenia spp.
Our previous study2 evaluating the use of urine-diverting latrines in Ciudad Juarez had found Giardia and Cryptosporidium in 82.0% and 70.0% of the samples, respectively, while the present study found them at 2.7% and 1.9%, respectively. However, in the earlier study2 the samples of biosolid waste were obtained from the urine-diverting toilets rather than from identifiable individuals in a representative population-based sample.
Our findings on the prevalence of intestinal parasites are very different from those reported in children in U.S. areas along the border with Mexico.15–18 The differences could be real or could reflect differences in the populations studied (facility-based vs. population-based) and in the sampling methodologies and organisms ascertained. It can be argued that the sustained development of the infrastructure in water supply and sanitation associated with the economic boom of the maquiladora industry in Ciudad Juarez could be responsible for the low prevalence of infections. In addition, the region is in the middle of the large Chihuahuan Desert, and the dry, sandy soil provides an adverse environment for the survival of geo-helminths.
The findings on the prevalence of Giardia spp. (4%) and Cryptosporidium spp. (1% to 3%) are consistent with the reported frequency of infection with these parasites in the U.S. On the other hand, we found that 38% of the study population was infected with H. pylori, which was the most prevalent infection of the GI tract in this population. The status of H. pylori infection does not mean that the individual has precancer lesions; however, it seems from volunteer studies that most infected people develop a so-called H. pylori gastritis with intense polymorphonuclear cell infiltration and interleukin-8 induction in gastric mucosa even in the absence of infections with cagA gene strains.19 Given that infections with most of the pathogens studied—such as Cryptosporidium and Giardia—typically last no more than three months while the duration of H. pylori infections tends to be lifelong, we cannot draw conclusions as to the incidence of infection with each pathogen.
When comparing infection prevalence on either side of the border, our study findings suggested that the prevalence of Taenia spp. was significantly higher on the U.S. side of this study population, probably due to the use of mass deworming campaigns among schoolchildren in Mexico that have proved successful.19 This intervention, as well as the dry and urban setting that ensures safe drinking water and sanitation to almost 100% of the population, could also explain the relatively low prevalence of helminths.
Limitations
Some of the limitations of our study are worth discussing, including the relatively low response of 51% submission of fecal samples. However, it should be noted that we obtained the consent and interview from the household head, whereas other members of the household may have been less inclined to participate. Surveys using only interviews on average obtain response rates of 70% to 85%;20 one study of migrant farm workers obtained a 50% response rate for fecal specimens.21
Second, the study lacked the statistical power to detect increased PRs >3.5 for Cryptosporidium spp., Giardia spp., E. dispar, A. lumbricoides, N. americanus, and Cyclospora spp. We failed to find an association between the prevalence of H. pylori infection and the occurrence of domestic fecal contamination, which could be due to limited microbiological sampling. It is also possible that participants disinfected their kitchens in anticipation of the visits.
We did not collect specific data on individuals who live/work in both cities (i.e., commute or have family members on the other side of the border). Border crossing in 2004 between these two cities was extensive, according to our data:3 52% of residents in Ciudad Juarez and and 48% of residents in El Paso crossed the border at least once a month. One-third of residents of Ciudad Juarez reported never crossing to El Paso, and almost one-fifth of residents of El Paso said they never visited Ciudad Juarez. Our study did not find any association between these patterns and the prevalence of any of the infections examined (data not shown).
Handwashing was associated with the prevalence of fecal coliforms on the hands of meal preparers in the study population,4 giving support to the importance of within-household H. pylori transmission via the fecal-oral route. Other cross-sectional studies have documented such evidence of self-reported hygienic behaviors and prevalence of H. pylori infection,22 which is consistent with transmission—at least most times—through the oral-fecal route. The protective effect of handwashing on the prevalence of H. pylori infection in this population is noteworthy, as a large binational health education campaign has been in place, the “Handwashing Viva Las Manos” campaign, which received more than $3.2 million from the Paso del Norte Health Foundation. Our data suggest that these types of programs need continuing support, as almost one-sixth of the population reported that they do not always wash hands after using the toilet.
CONCLUSION
In this population-based binational probabilistic survey, we found that H. pylori and Taenia spp. were the most prevalent infections of the GI tract in El Paso and Ciudad Juarez. Of great concern was our finding of a large prevalence of Taenia spp., as it could represent evidence of autochthonous transmission of Taenia solium and increased risk of neurocysticercosis. We did not find an association between the occurrence of fecal contamination of kitchen surfaces, drinking water, and hands of food preparers; however, the prevalence of H. pylori infection was related to self-reported frequency of handwashing.
Acknowledgments
The authors acknowledge the financial support of the Paso del Norte Health Foundation's Center for Border Health Research Grant (CBHR FMS Project 004288). The work was supported in part by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Gulf Coast Digestive Diseases Center. Dr. Variyam's laboratory is supported by institutional funds (Haggerton Chair in Gastroenterology and Dean's Research funds) and by the CH Foundation. The authors thank the following individuals for their assistance in data collection: Dolores Perez, Maria Concepcion Lopez, Mario Escobedo, Fernando Quiroz, and Martha Macias.
REFERENCES
- 1.Doyle TJ, Bryan RT. Infectious disease morbidity in the US region bordering Mexico, 1990–1998. J Infect Dis. 2000;182:1503–10. doi: 10.1086/315876. [DOI] [PubMed] [Google Scholar]
- 2.Redlinger T, Corella-Barud V, Graham J, Galindo A, Avitia R, Cardenas V. Hyperendemic Cryptosporidium and Giardia in households lacking municipal sewer and water on the United States-Mexico border. Am J Trop Med Hyg. 2002;66:794–8. doi: 10.4269/ajtmh.2002.66.794. [DOI] [PubMed] [Google Scholar]
- 3.Barton Behravesh C, Mayberry LF, Bristol JR, Cardenas VM, Mena KD, Martinez-Ocaña J, et al. Population-based survey of taeniasis along the United States-Mexico border. Ann Trop Med Parasitol. 2008;102:325–33. doi: 10.1179/136485908X300788. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco L, Mena KD, Mota LC, Ortiz M, Bristol JR, Mayberry L, et al. Prevalence of potential domestic fecal contamination along the U.S.-Mexico border, 2004. Letters Appl Microbiol. 2008;46:682–7. doi: 10.1111/j.1472-765X.2008.02378.x. [DOI] [PubMed] [Google Scholar]
- 5.Flisser A, Gyorkos TW. Contribution of immunodiagnostic tests to epidemiological/intervention studies of cysticercosis/taeniosis in Mexico. Parasite Immunol. 2007;29:637–49. doi: 10.1111/j.1365-3024.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas VM, Dominguez DC, Puentes FA, Aragaki CC, Goodman KJ, Graham DY, et al. Evaluation of a novel stool native catalase antigen test for Helicobacter pylori infection in asymptomatic North American children. J Pediatr Gastroenterol Nutr. 2008;46:399–402. doi: 10.1097/MPG.0b013e318148b688. [DOI] [PubMed] [Google Scholar]
- 7.Troll H, Marti H, Weiss N. Simple differential detection of Entamoeba histolytica and Entamoeba dispar in fresh stool specimens by sodium acetate-acetic acid-formalin concentration and PCR. J Clin Microbiol. 1997;35:1701–5. doi: 10.1128/jcm.35.7.1701-1705.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (US) Epi Info™: Version 3.5.1. Atlanta: CDC; 2004. [Google Scholar]
- 9.SAS Institute, Inc. SAS®: Version 9.1. Cary (NC): SAS Institute, Inc.; 2002. [Google Scholar]
- 10.Research Triangle Institute. SUDAAN®: Version 9.0. Research Triangle Park (NC): Research Triangle Institute; 2008. [Google Scholar]
- 11.Census Bureau (US) 2005–2007 American Community Survey. Table S1901, income in the past 12 months, and Table S1501, educational attainment. American FactFinder: El Paso County, Texas. [cited 2009 Sep 4]. Available from: URL: http://factfinder.census.gov.
- 12.National Institute of Statistics, Geography and Informatics, Mexico Department of the Treasury. II Count of population and housing, Mexico 2005. Table on educational attainment for persons 5+ years of age in households. Interactive Data Query System. Municipality of Juarez, Chihuahua, Mexico. [cited 2009 Sep 4]. Available from: URL: http://www.inegi.org.mx.
- 13.Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol. 2006;163:127–34. doi: 10.1093/aje/kwj018. [DOI] [PubMed] [Google Scholar]
- 14.Schantz PM, Wilkins PP, Tsang VCW. Immigrants, imaging, and immunoblots: the emergence of neurocysticercosis as a significant public health problem. In: Scheld WM, Craig WA, Hughes JM, editors. Emerging infections. 2nd ed. Washington: ASM Press; 1998. pp. 213–42. [Google Scholar]
- 15.Escobedo LG, Homedes N, von Alt K, Escobedo MA. Intestinal parasites in children from three West Texas border communities. J Sch Health. 2004;74:411–3. doi: 10.1111/j.1746-1561.2004.tb06609.x. [DOI] [PubMed] [Google Scholar]
- 16.Huh S, Ahn C, Chai JY. Intestinal parasitic infections in the residents of an emigration camp in Tijuana, Mexico. Korean J Parasitol. 1995;33:65–7. doi: 10.3347/kjp.1995.33.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Leach CT, Koo FC, Kuhls TL, Hilsenbeck SG, Jenson HB. Prevalence of Cryptosporidium parvum infection in children along the Texas-Mexico border and associated risk factors. Am J Trop Med Hyg. 2000;62:656–61. doi: 10.4269/ajtmh.2000.62.656. [DOI] [PubMed] [Google Scholar]
- 18.Flisser A, Valdespino JL, García-García L, Guzman C, Aguirre MT, Manon ML, et al. Using national health weeks to deliver deworming to children: lessons from Mexico. J Epidemiol Community Health. 2008;62:314–7. doi: 10.1136/jech.2007.066423. [DOI] [PubMed] [Google Scholar]
- 19.Graham DY, Opekun AR, Osato MS, El-Zimaity HM, Lee CK, Yamaoka Y, et al. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 2004;53:1235–43. doi: 10.1136/gut.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aday LA. Designing and conducting health surveys. 2nd ed. San Francisco: Jossey-Bass; 1998. [Google Scholar]
- 21.Ciesielski SD, Seed JR, Ortiz JC, Metts J. Intestinal parasites among North Carolina migrant farmworkers. Am J Public Health. 1992;82:1258–62. doi: 10.2105/ajph.82.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown LM, Thomas TL, Ma JL, Chang YS, You WC, Liu WD. Helicobacter pylori infection in rural China: demographic, lifestyle and environmental factors. Int J Epidemiol. 2002;31:638–45. doi: 10.1093/ije/31.3.638. [DOI] [PubMed] [Google Scholar]