Tobacco use is the second major cause of death in the world and the fourth most common risk factor for disease worldwide. A recent estimation attributes more than five million deaths a year to tobacco consumption, a figure expected to rise to more than eight million deaths a year by 2030 if current smoking patterns continue.1 In addition, smoking affects not only the health of smokers, but also the health of those around them who are exposed to environmental tobacco smoke (ETS) such as children, relatives at home, and coworkers in the workplace. ETS involves inhaling carcinogens and other toxic components, and scientific evidence has unequivocally established that exposure to ETS causes death, disease, and disability in children and adults who do not smoke. Several recent reports have synthesized this evidence and reached clear and firm conclusions with regard to the adverse consequences of exposure to ETS.2–4
The U.S. Surgeon General review from 2006 suggested that ETS exposure is causally associated with a wide range of developmental and respiratory effects in children: low birthweight, sudden infant death syndrome (SIDS), lower respiratory tract infections such as bronchitis and pneumonia, middle-ear infections, symptoms of upper respiratory tract irritation, small reductions in lung function, asthma onset, and additional episodes and increased severity of symptoms in children with asthma. Recent studies have also shown a possible correlation between the use of cigarettes and alcohol during pregnancy and risk for attention deficit hyperactivity disorder in the offspring.5,6 On the other hand, for adults, outcomes with the strongest evidence of the effect of ETS exposure include lung cancer, ischemic heart disease, and asthma onset. In addition, some condition-specific impacts of ETS include chronic respiratory symptoms such as wheezing, coughing, phlegm, and dyspnoea among children and adults; serous otitis media among children; and decreased pulmonary function in children.7,8
Tobacco use is one of the chief preventable causes of death and health consequences for nonsmokers in the world. Smoking bans restrict tobacco smoking in workplaces and indoor public spaces.9,10 The adoption and implementation of these public policies are gaining momentum, with an increasing number of countries adopting complete smoking bans in public places.11,12 However, assuming the law is observed in public areas, unregulated indoor environments such as private residences or vehicles are still significant remaining indoor sites of ETS exposure, especially for children. A larger portion of children's ETS exposure occurs in such indoor environments, where many parents and other household members still expose their children to ETS.
Children are more vulnerable to the physiological effects of ETS and more sensitive to the adverse health effects of ETS than adults: physical development is ongoing with sensitivity in several organs, the immune system is less protective, and a child's breathing rate is higher than an adult's. Children have limited or no control over their indoor environments.13 They often sit near or on parents, family members, or caregivers, closer to the source of the pollutant than other passive smokers. Because the home is a predominant location for smoking after the implementation of smoking bans in public places, children of smokers are exposed to ETS, especially in the first years of life, while eating, playing, and even sleeping. This exposure at home may be added to exposure at school and in vehicles.14
The World Health Organization's (WHO's) Implementing Environment and Health Information System (ENHIS) program set up a comprehensive information and knowledge system to support relevant policies in Europe (www.enhis.org). This WHO program developed a set of indicators to protect children's health from environmental risk factors. These indicators cover most of the priority topic areas specified in the Children's Environment and Health Action Plan for Europe (CEHAPE) as adopted in the Fourth Ministerial Conference on Health and Environment in 2004 and will be used to monitor the implementation of CEHAPE.15 In addition, health impact assessment (HIA) methods were developed in the frame of this program and applied to selected indicators, and the assessment results were integrated into the indicator fact sheets.16
In this article, we present the HIA findings on children exposed to tobacco smoke using one specific indicator defined by the ENHIS program for this purpose. We have estimated the ETS impact for SIDS (infants from one month to one year of age) and asthma episodes (children younger than 14 years of age) on children exposed at home in 27 European countries.
MATERIALS AND METHODS
In brief, we first carried out a feasibility study to test the available information for ETS HIA in international databases. Second, we followed the methods for generating information from existing data sources provided by ENHIS-2.17 Finally, we applied the HIA approach to assess the potential health impacts of ETS on the European children population.18
Data sources
Exposure data and exposure scenarios.
The exposure measure was adult smoking prevalence from population-based datasets. According to reports from WHO19 and others,20 the use of surveys of smoking prevalence in the adult population as a proxy of children's ETS exposure is valid under the assumption that smoking prevalence (percent of the adult population that smokes tobacco products regularly) among all adults aged 20 to 50 years is similar to that among parents with children. Smoking prevalence was available online by country, gender, and time period (1994–1998, 1999–2001, or 2002–2005) in the WHO Tobacco Control Database.21 In addition, the Global Youth Tobacco Survey (GYTS),22 developed by WHO and the U.S. Centers for Disease Control and Prevention (CDC), inquired about children's exposure to ETS in their home or in other places during the last seven days and about current smoking habits of the parents. GYTS represents the largest school-based survey on ETS exposure of children (aged 13 to 15 years) in 130 countries.
We considered different scenarios based on ETS exposure variations:
-
Upper ETS exposure estimate. The data provided by the GYTS survey were used as the upper ETS exposure estimate. For countries that have not implemented or completed the GYTS, children's ETS exposure was estimated through the adult smoking prevalence provided by the WHO Tobacco Control Database.21 If we assume that families consist of a father and a mother living together and that parents have the same smoking prevalence and the same number of children as the general population, the percentage of children with at least one smoking parent could be estimated as follows:20
Any parental smoking = male smoking prevalence + female smoking prevalence − (male smoking prevalence * female smoking prevalence)
-
Lower ETS exposure estimate. We used the most recent, highest smoking prevalence rate by gender for each country (often the male, except in Sweden) as an alternative lower estimate.21
We assumed that smoking prevalence among parents should be in between these figures—the upper and the lower estimate.
Health data.
We based the selection of health outcomes (SIDS cases and asthma episodes) on two criteria: (1) strength of scientific evidence of association with ETS and (2) suitable exposure-response function (ERF).
The number of SIDS cases (International Classification of Diseases, 10th Revision [ICD-10] code R95)23 for each country in 1998, 2001, and 2005 was retrieved from Eurostat databases,24 according to the proposed ETS exposure scenarios. Conversely, the lack of routine data on asthma symptoms prevalence in international databases did not allow us to estimate the number of attributable cases due to ETS exposure. Therefore, we calculated the population-attributable fraction (PAF).
ERFs.
The most suitable ERFs expressed by odds ratios (ORs) between the population exposed to a factor and that not exposed for the quantification of health effects due to ETS exposure were selected according to a recent epidemiologic evidence review by WHO.25 For SIDS, the pooled adjusted OR of 1.94 (95% confidence interval [CI] 1.55, 2.43)26 for maternal smoking was considered the most appropriate ERF for our HIA. This study included relevant studies with adjustment for confounding factors that could influence the SIDS prevalence, and the OR estimate was proposed as the basis for quantification of disease burden in relation to any parental smoking. For asthma prevalence, the most recent meta-analysis4 provided an overall pooled OR of 1.23 (95% CI 1.14, 1.33) for children younger than 14 years of age, related to any parental smoking.
Population data.
Children population data in each country were retrieved from Eurostat databases.27 According to the proposed scenarios, we selected data for children younger than one year of age in 1998, 2001, and 2005 to estimate the impact on SIDS. The participating countries were Austria, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Switzerland, The Former Yugoslav Republic of Macedonia (TFYR Macedonia), and United Kingdom.
HIA methodology
Using ORs and different ETS exposure scenarios, we calculated country-specific estimates of the PAF, which may be interpreted as the proportion of health outcomes in the population attributable to ETS exposure. We consider the general equation to calculate PAF, as described by Rockhill et al.:28
where Pe is the proportion of the children population exposed to the ETS, and OR is the odds ratio of mortality or morbidity associated with ETS. We assumed Pe to be similar to the proposed scenarios of ETS exposure.
We then multiplied these PAFs by the rate of disease and child population size in each country to estimate the annual excess cases attributable to ETS:
where N is the total number of people in the population, and Ip is the rate of SIDS in the infant population.
For SIDS cases, we estimated the attributable number of deaths due to ETS exposure. For asthma, we calculated country-specific estimates of PAF, as data on diagnosed asthma or asthma symptoms have not been an object of reporting in the inter-country comparable form.
Trend analysis
We examined the trends of the highest national smoking prevalence (lower ETS exposure scenario) seen in 10 selected countries from 1998 to 2005 as a possible progressive reduction in ETS exposure scenario to assess the potential impact on SIDS. We selected the Czech Republic, Finland, France, Germany, Hungary, Netherlands, Norway, Spain, Sweden, and United Kingdom because SIDS cases27 (children younger than one year of age)24 and smoking prevalence data (for males, except in Sweden) were available for 1998, 2001, and 2005.21
RESULTS
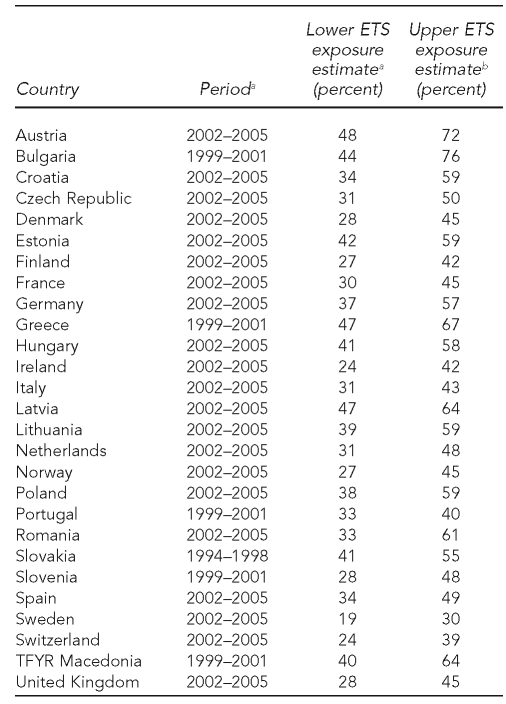
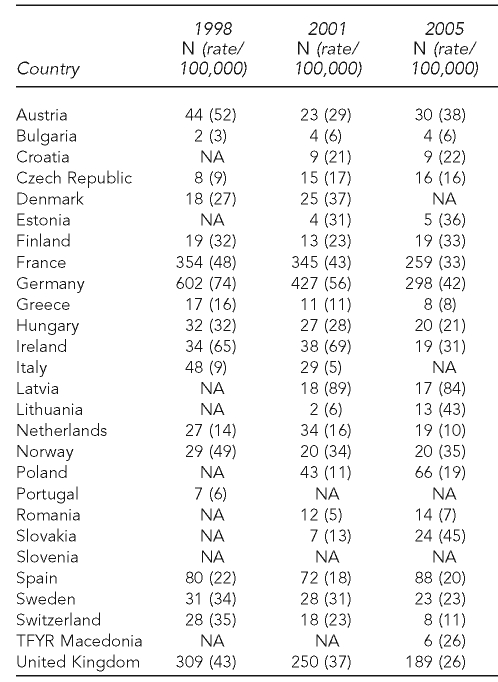
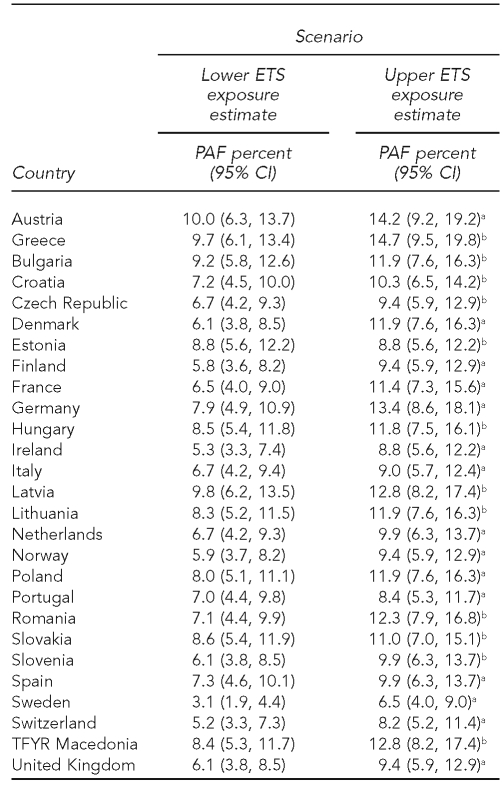
Table 1 presents the data reported or estimated for ETS exposure in the participating countries. Twenty-one countries reported data for 2002 through 2005, five countries reported data for 1999 through 2001, and Slovakia reported data for 1994 through 1998. There were remarkable differences for lower and upper ETS exposure estimates across countries. The percentage of children exposed to ETS ranged from 48% in Austria to 19% in Sweden for the lower scenario. Considering the upper scenario, Bulgaria, Austria, Greece, Latvia, and Romania showed the highest smoking prevalence (more than 60%), and Sweden and Switzerland the lowest (30% and 39%, respectively). Table 2 includes data on the absolute number of SIDS cases and crude death rate by country in 1998, 2001, and 2005.
Table 1.
ETS exposure scenarios for health impact assessment estimates in European countries
aSource: World Health Organization, Regional Office for Europe. Tobacco control database [cited 2010 Jan 19]. Available from: URL: http://data.euro.who.int/tobacco (Note: smoking prevalence for males, except in Sweden)
bSource: Centers for Disease Control and Prevention (US). Smoking and tobacco use: global tobacco control: global tobacco surveillance system: Global Youth Tobacco Survey (GYTS) [cited 2010 Jan 20]. Available from: URL: http://www.cdc.gov/tobacco/global/gyts/index.htm (Note: Smoking prevalence was modeled in Austria, Denmark, Finland, France, Germany, Ireland, Italy, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and United Kingdom.)
ETS = environmental tobacco smoke
TFYR = The Former Yugoslav Republic
Table 2.
Absolute number of sudden infant death syndrome (ICD-10 R95) cases and crude death rate per 100,000 children younger than one year of agea
aSource: European Commission. Eurostat [cited 2010 Jan 20]. Available from: URL: http://epp.eurostat.ec.europa.eu/portal/page/portal/eurostat/home
ICD-10 = International Classification of Diseases, 10th Revision
NA = not available
TFYR = The Former Yugoslav Republic
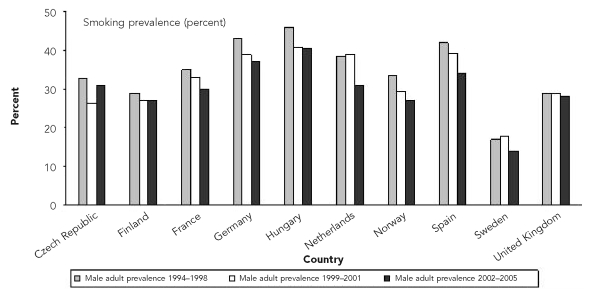
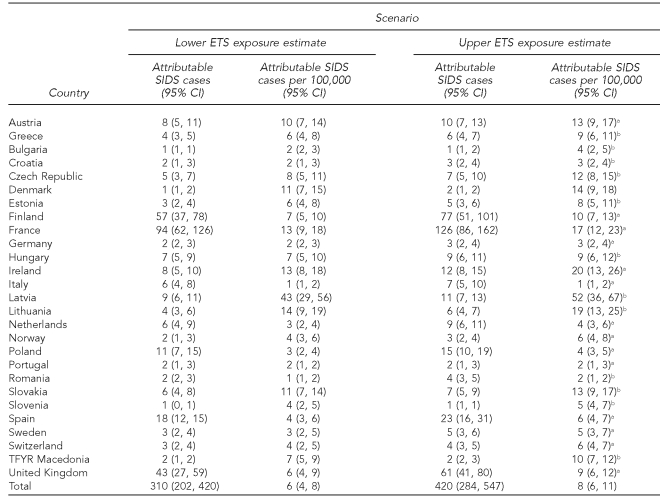
The trends in reported highest smoking prevalence rates in selected European countries are shown in Figure 1. There was an overall improvement of 5% from the period 1994–1998 (35%) to 2002–2005 (30%) in all countries, except the Czech Republic. For SIDS cases, the total population younger than one year of age was around five million in the participating European countries. Depending on the ETS exposure scenario, we estimated that between 24% (n=310) and 32% (n=420) of SIDS cases could be attributable to exposure to ETS (Table 3). The highest impact of ETS exposure was observed in Latvia, Ireland, and Lithuania.
Figure 1.
Trends in the smoking prevalence rates in the male adult population (except in Sweden) for selected European countriesa
aSource: World Health Organization, Regional Office for Europe. Tobacco control database [cited 2010 Jan 19]. Available from: URL: http://data.euro.who.int/tobacco/Default.aspx?TabID=2444
Table 3.
Health impact assessment of ETS exposure for SIDS cases in European countries
aSmoking prevalence modeled by using the formula: any parental smoking = male smoking prevalence + female smoking prevalence − (male smoking prevalence * female smoking prevalence)
bSource: Centers for Disease Control and Prevention (US). Smoking and tobacco use: global tobacco control: global tobacco surveillance system: Global Youth Tobacco Survey (GYTS) [cited 2010 Jan 20]. Available from: URL: http://www.cdc.gov/tobacco/global/gyts/index.htm
ETS = environmental tobacco smoke
SIDS = sudden infant death syndrome
CI = confidence interval
TFYR = The Former Yugoslav Republic
In relation to the impact of ETS exposure on asthma episodes in children younger than 14 years of age, in the lower exposure scenario, the PAFs (Table 4) were between 3% and 10%. The highest estimated proportion of asthma episodes attributable to ETS exposure was observed in Austria and Greece. The mean proportion in the evaluated countries was 7% to 11% of attributable asthma episodes, depending on the ETS exposure scenario.
Table 4.
Health impact assessment of ETS exposure for asthma in children younger than 14 years of age in European countries
aSmoking prevalence modeled by using the formula: any parental smoking = male smoking prevalence + female smoking prevalence − (male smoking prevalence * female smoking prevalence)
bSmoking prevalence from Global Youth Tobacco Survey database. Source: Centers for Disease Control and Prevention (US). Smoking and tobacco use: global tobacco control: global tobacco surveillance system: Global Youth Tobacco Survey (GYTS) [cited 2010 Jan 20]. Available from: URL: http://www.cdc.gov/tobacco/global/gyts/index.htm
ETS = environmental tobacco smoke
PAF = population-attributable fraction
CI = confidence interval
TFYR = The Former Yugoslav Republic
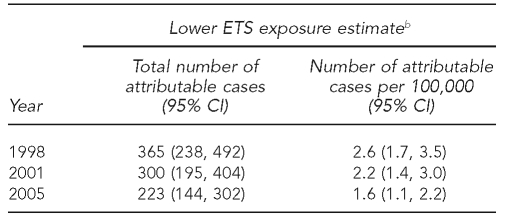
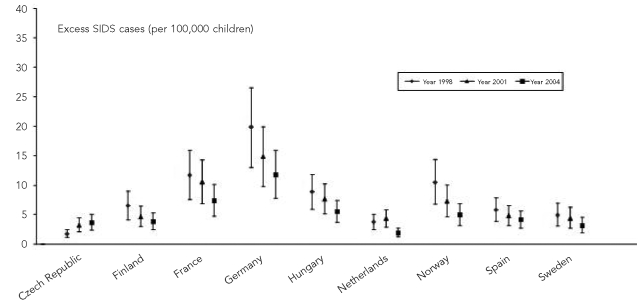
The decreasing trends in the highest national smoking prevalence seen in 10 selected countries from 1998 to 2005 may have contributed to a 38% decrease in the number of attributable SIDS cases, from 2.6 cases per 100,000 children younger than one year of age in 1998 to 1.6 cases per 100,000 children younger than one year of age in 2005 (Table 5). All participating countries, except Czech Republic, presented a decreasing trend in the number of attributable SIDS cases (Figure 2).
Table 5.
Trends in the number of excess SIDS cases attributable to lower ETS exposure estimate in selected European countries by yeara
aSelected countries: Czech Republic, Finland, France, Germany, Hungary, Netherlands, Norway, Spain, Sweden, and United Kingdom
bSource: World Health Organization, Regional Office for Europe. Tobacco control database [cited 2010 Jan 19]. Available from: URL: http://data.euro.who.int/tobacco (Note: smoking prevalence for males, except in Sweden)
SIDS = sudden infant death syndrome
ETS = environmental tobacco smoke
CI = confidence interval
Figure 2.
Trends in the number of excess SIDS cases attributable to lower ETS exposure estimate by country and year
SIDS = sudden infant death syndrome
ETS = environmental tobacco smoke
DISCUSSION
ETS is a major indoor air pollutant and a significant risk factor for children including risk of fatal (SIDS) and nonfatal (asthma episodes) adverse health effects. In Europe, infants' exposure to ETS may be responsible for 24% to 32% of SIDS cases and may increase the number of asthma episodes by a mean proportion of 7% to 11% in children younger than 14 years of age. These findings are consistent with findings from other international studies.4,29 The estimates of population impact presented in this article are given in ranges to reflect the uncertainty of ETS exposure assessment. Confidence in these estimates is based greatly on the assumption of a causal association and the strong validity of parental smoking as a surrogate of relevant ETS exposure in infants and children.
The estimates derived in this article are based on a number of assumptions and uncertainties, which could bias the estimated number of cases for each health outcome. Three key components of the HIA analyses must be considered: exposure assessment, health outcomes, and ERFs.
Exposure assessment
Children's involuntary exposure to ETS is frequently measured in several ways: air sampling in enclosed spaces, use of biomarkers, and application of survey instruments. Several studies found a strong association between air nicotine concentrations, urinary cotinine levels, and questionnaire reported smoking,30–32 which validates the use of residential ETS exposure among children to assess the impact of ETS.30 The percentage of children exposed to ETS at home was 71.5% in Europe;33 thus, the data provided by GYTS were included in the upper ETS exposure estimate in this study.
On the other hand, smoking prevalence in the European Region based on country-reported data was estimated at approximately 28.6% (40.0% among males and 18.2% among females) in 2005.34 In this HIA, the general survey on smoking prevalence among adults provided by the WHO Tobacco Control Database was considered an acceptable proxy for the lower estimates of children ETS exposure. Member states agreed to report data, using identical sampling procedures and methodology, as a part of their commitment to Tobacco-free Europe and the Framework Convention on Tobacco Control.
Limitations.
We recognize the limitations of our results associated with the evaluation of the real exposure, and it could lead to over- or underestimations of our findings. A potential error may arise from using the nationwide smoking prevalence mean to represent exposures of children at risk. Exposure varies considerably according to socioeconomic status,35–37 and the composition of tobacco smoke inhaled involuntarily is variable quantitatively and depends on the smoking patterns of the smokers who are producing the smoke, as well as the composition and design of the cigarettes or other smoking devices.3 Moreover, there are variations in societal attitudes toward ETS, which may influence indoor smoking behavior.
Finally, our model assumed that parents have the same smoking prevalence as the average adult population and that the number of children is equally distributed between smokers and nonsmokers. This assumption may be a limitation of our study, although, as we stated previously, empirical studies show general concordance when using different methods to measure ETS exposure. Our findings support the validity of these surveys in capturing variations in child population exposures to ETS and strengthen the reliability of HIA that depend on ETS exposures.
Health outcomes
The selection of health outcomes (SIDS and asthma episodes) was based on the strength of scientific evidence of association with ETS and the availability of suitable ERFs. SIDS mortality is a well-defined health outcome in HIA. Even though the actual cause (or causes) of SIDS remains a mystery, it is generally accepted that SIDS may be a reflection of multiple interacting risk factors,38 including prenatal or postnatal exposure to tobacco smoke.26 Nevertheless, the temporal relationship between changes in ETS exposure and resulting changes in health outcomes can be further explained by other public health measures or factors. Since early 1990, the incidence of SIDS has dropped sharply because of public health campaigns (e.g., the “Back to Sleep” campaign initiated in several European countries at the beginning of the 1990s39–41 decrying the dangers of the prone sleep position).42 The change in infants' sleeping positions can partially explain the continuing decline in the number of SIDS cases in European countries since 1998.39,41,43,44 Some authors have argued that the attributed risk associating maternal smoking and SIDS has increased following these campaigns, due to a decreased overall rate of SIDS resulting from those campaigns.45 In addition, it should be noted that SIDS remains the highest cause of infant death beyond the neonatal period in Europe, and there are still several potentially modifiable risk factors (e.g., maternal smoking).
On the other hand, data on morbidity (asthma episodes) are usually less comprehensive and the availability needs to be improved in national and international databases. The results of the International Study of Asthma and Allergies in Childhood (ISAAC) phases I (1992–1998) and III (1999–2004) showed an increase in prevalence, namely of asthma symptoms, in most European countries,46 and epidemiologic evidence shows that asthmatic children are at a greater risk of having their symptoms worsen upon exposure to ETS in their homes.47,48 However, at present, it is not possible to estimate the absolute number of asthma attacks related to ETS exposure because the available data on asthma prevalence49 are not updated, only cover selected populations, and show large inter-regional variability. Therefore, the number of attacks occurring in children with asthma is difficult to evaluate in HIA analysis.
This study assessed only two adverse health outcomes, but the quantity of the potential health damage due to ETS exposure is much greater. We must highlight that many health outcomes related to ETS exposure are not quantified because there is insufficient data in international databases (e.g., low birthweight or otitis media) or because inclusion of certain health effects in the analysis could lead to double-counting (e.g., wheezing and coughing are symptoms of asthma, but not a disease per se).
ERFs
The ERF was a central element in the impact analysis. In our study, the quantification of health effects for 95% CIs represented the lower and upper confidence bounds from the ORs, and it did not take into account other possible uncertainties in the HIA estimation.
Anderson and Cook in 1997 estimated that maternal smoking doubles the risk of SIDS.26 The evidence is sufficient to infer a causal relationship between parental smoking and ever having asthma among children of school age. The causal interpretation is further strengthened by the trend for the OR to increase with the number of parents who smoke (i.e., none, one, or both). Therefore, evidence exists of an exposure-response relationship with the number of parents who smoke, and maternal smoking probably has a greater impact on the exposure of children to ETS.4 This HIA applied a conservative approach using the pooled random effect associated with smoking by either parent, slightly lower than those for smoking by both parents.
CONCLUSION
ETS is linked with adverse health effects among children in Europe. A decrease in smoking prevalence could provide substantial health gains in children. Because the effects of ETS are dose-response and there is no known safe level of exposure to ETS, WHO encourages member states to follow these recommendations to protect workers and the public from exposure to ETS:50
Provide 100% smoke-free environments.
Ensure universal protection by law.
Provide proper implementation and adequate enforcement of the law.
Use public education to reduce ETS exposure indoors.
Therefore, our key message to decision-makers is to follow the WHO recommendations to develop and implement enforceable smoke-free policies to avoid the ETS impact on children's health.
Footnotes
The World Health Organization's (WHO's) European Environment and Health Information System (ENHIS) program was funded by the European Commission Directorate General for Health and Consumer Affairs (DG SANCO), Grant SPC 2004124. The authors acknowledge the contribution of ENHIS-2 project partners (available at www.enhis.org). (Source: WHO. Children's health and the environment in Europe: a baseline assessment, 2007 [cited 2010 Jan 18]. Available from: URL: http://www.euro.who.int/InformationSources/Publications/Catalogue/20071007_1).
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.California Environmental Protection Agency. Proposed identification of environmental tobacco smoke as a toxic air contaminant. California EPA: Sacramento (CA); 2005. [Google Scholar]
- 3.World Health Organization; International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, volume 83: tobacco smoke and involuntary smoking. Lyon (France): WHO, IARC; 2004. [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services (US), Office of the Surgeon General. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta: DHHS, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health (US); 2006. [PubMed] [Google Scholar]
- 5.Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96:1269–74. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 6.Brondum J. Environmental exposures and ADHD. Environ Health Perspect. 2007;115:A398. doi: 10.1289/ehp.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaakkola MS, Jaakkola JJ. Impact of smoke-free workplace legislation on exposures and health: possibilities for prevention. Eur Respir J. 2006;28:397–408. doi: 10.1183/09031936.06.00001306. [DOI] [PubMed] [Google Scholar]
- 8.Vineis P, Hoek G, Krzyzanowski M, Vigna-Taglianti F, Veglia F, Airoldi L, et al. Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health. 2007;6:7. doi: 10.1186/1476-069X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO framework convention on tobacco control (FCTC) Geneva: WHO; 2003. [cited 2010 Jan 20]. Also available from: URL: http://www.who.int/fctc/en/index.html. [Google Scholar]
- 10.World Health Organization, Regional Office for Europe. European Environment and Health Information System (ENHIS): policies to reduce the exposure of children to environmental tobacco smoke [ENHIS-2 fact sheet no. 3.7] 2007. May 9, [cited 2010 Jan 20]. Available from: URL: http://www.enhis.org/object_document/o4727n27382.html.
- 11.Radke PW, Schunkert H. Public smoking ban: Europe on the move. Eur Heart J. 2006;27:2385–6. doi: 10.1093/eurheartj/ehl266. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt CW. A change in the air: smoking bans gain momentum worldwide. Environ Health Perspect. 2007;115:A412–5. doi: 10.1289/ehp.115-a412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck Louis G, Damstra T, Díaz-Barriga F, Faustman E, Hass U, Kavlock R, et al. Environmental health criteria 237: principles for evaluating health risks in children associated with exposure to chemicals. Geneva: World Health Organization; 2007. [Google Scholar]
- 14.Samet J, Yang G. Passive smoking, women, and children. In: Samet JM, Yoon S-Y, editors. Women and the tobacco epidemic: challenges for the 21st century. Geneva: World Health Organization; 2001. pp. p. 17–45. [Google Scholar]
- 15.Pond K, Kim R, Carroquino MJ, Pirard P, Gore F, Cucu A, et al. Workgroup report: developing environmental health indicators for European children: World Health Organization Working Group. Environ Health Perspect. 2007;115:1376–82. doi: 10.1289/ehp.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization, Regional Office for Europe. European Environment and Health Information System (ENHIS): exposure of children to environmental tobacco smoke [ENHIS-2 fact sheet no. 3.4] 2007. May 9, [cited 2007 Sep 25]. Available from: URL: http://www.enhis.org/object_document/o4744n27382.html.
- 17.Galan A, Puklová V, Muszynska M, Zurlyté I. Hands-on guidance for information retrieval of 30 “core” CEHAPE-RPGs indicators from international databases: ENHIS-2 project, Grant Agreement SPC 2004124. 2007. [cited 2010 Jan 20]. Available from: URL: http://enhiscms.rivm.nl/object_binary/o2801_ENHIS_Hands_on_2007_v11_uneditedVerison.pdf.
- 18.World Health Organization. Evaluation and use of epidemiological evidence for environmental health risk assessment: WHO guideline document. Environ Health Perspect. 2000;108:997–1002. doi: 10.1289/ehp.00108997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Tobacco free initiative. International consultation on environmental tobacco smoke (ETS) and child health. Geneva: WHO; 1999. [Google Scholar]
- 20.Patja K, Hakala S, Prattala R, Ojala K, Boldo E, Oberg M. Adult smoking as a proxy for environmental tobacco smoke exposure among children—comparing the impact of the level of information in Estonia, Finland, and Latvia. Prev Med. 2008;49:240–4. doi: 10.1016/j.ypmed.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization, Regional Office for Europe. Tobacco control database. [cited 2010 Jan 19]. Available from: URL: http://data.euro.who.int/tobacco.
- 22.Centers for Disease Control and Prevention (US) Smoking and tobacco use: global tobacco control: global tobacco surveillance system: Global Youth Tobacco Survey (GYTS) [cited 2010 Jan 20]. Available from: URL: http://www.cdc.gov/tobacco/global/gyts/index.htm.
- 23.World Health Organization. International statistical classification of diseases and related health problems, 10th revision. Geneva: WHO; 2007. [Google Scholar]
- 24.European Commission. Eurostat (public health—causes of death/population—demography) [cited 2010 Jan 20]. Available from: URL: http://epp.eurostat.ec.europa.eu/portal/page/portal/eurostat/home.
- 25.Öberg M, Jaakkola MS, Woodward A. Second-hand smoke: assessing the environmental burden of disease at national and local levels. Geneva: World Health Organization; 2010. [Google Scholar]
- 26.Anderson HR, Cook DG. Passive smoking and sudden infant death syndrome: review of the epidemiological evidence. Thorax. 1997;52:1003–9. doi: 10.1136/thx.52.11.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Commission. Eurostat. [cited 2010 Jan 19]. Available from: URL: http://epp.eurostat.ec.europa.eu/portal/page/portal/eurostat/home.
- 28.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rushton L, Courage C, Green E. Estimation of the impact on children's health of environmental tobacco smoke in England and Wales. J R Soc Promot Health. 2003;123:175–80. doi: 10.1177/146642400312300315. [DOI] [PubMed] [Google Scholar]
- 30.Gehring U, Leaderer BP, Heinrich J, Oldenwening M, Giovannangelo ME, Nordling E, et al. Comparison of parental reports of smoking and residential air nicotine concentrations in children. Occup Environ Med. 2006;63:766–72. doi: 10.1136/oem.2006.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovell MF, Zakarian JM, Wahlgren DR, Matt GE, Emmons KM. Reported measures of environmental tobacco smoke exposure: trials and tribulations. Tob Control. 2000;9(Suppl 3):III22–8. doi: 10.1136/tc.9.suppl_3.iii22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jatlow P, McKee S, O'Malley SS. Correction of urine cotinine concentrations for creatinine excretion: is it useful? Clin Chem. 2003;49:1932–4. doi: 10.1373/clinchem.2003.023374. [DOI] [PubMed] [Google Scholar]
- 33.Exposure to secondhand smoke among students aged 13–15 years—worldwide, 2000–2007. MMWR Morb Mortal Wkly Rep. 2007;56(20):497–500. [PubMed] [Google Scholar]
- 34.World Health Organization, Regional Office for Europe. The European tobacco control report 2007. [cited 2010 Jan 20]. Available from: URL: http://www.euro.who.int/Document/E89842.pdf.
- 35.Fernandez E, Garcia M, Schiaffino A, Borras JM, Nebot M, Segura A. Smoking initiation and cessation by gender and educational level in Catalonia, Spain. Prev Med. 2001;32:218–23. doi: 10.1006/pmed.2000.0794. [DOI] [PubMed] [Google Scholar]
- 36.Mannino DM, Siegel M, Husten C, Rose D, Etzel R. Environmental tobacco smoke exposure and health effects in children: results from the 1991 National Health Interview Survey. Tob Control. 1996;5:13–8. doi: 10.1136/tc.5.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rainio SU, Rimpela AH. Home smoking bans in Finland and the association with child smoking. Eur J Public Health. 2008;18:306–11. doi: 10.1093/eurpub/ckm098. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell EA. SIDS: facts and controversies. Med J Aust. 2000;173:175–6. doi: 10.5694/j.1326-5377.2000.tb125594.x. [DOI] [PubMed] [Google Scholar]
- 39.Blair PS, Sidebotham P, Berry PJ, Evans M, Fleming PJ. Major epidemiological changes in sudden infant death syndrome: a 20-year population-based study in the UK. Lancet. 2006;367:314–9. doi: 10.1016/S0140-6736(06)67968-3. [DOI] [PubMed] [Google Scholar]
- 40.Guala A, Cozzi M, Guarino R, Campra D, Pastore G. Back to sleep: a longitudinal report from an infant population based study in the Local Health Service 11, Piedmont, Italy. Minerva Pediatr. 2005;57:52. [PubMed] [Google Scholar]
- 41.Vennemann M, Fischer D, Jorch G, Bajanowski T. Prevention of sudden infant death syndrome (SIDS) due to an active health monitoring system 20 years prior to the public “Back to Sleep” campaigns. Arch Dis Child. 2006;91:324–6. doi: 10.1136/adc.2005.082172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert R, Salanti G, Harden M, See S. Infant sleeping position and the sudden infant death syndrome: systematic review of observational studies and historical review of recommendations from 1940 to 2002. Int J Epidemiol. 2005;34:874–87. doi: 10.1093/ije/dyi088. [DOI] [PubMed] [Google Scholar]
- 43.Hollebecque V, Briand E, Bouvier-Colle MH. [Information campaign on child care practices: measure of the effects on sleep position and sudden infant death syndrome] Rev Epidemiol Sante Publique. 1998;46:115–23. [PubMed] [Google Scholar]
- 44.Willinger M, Hoffman HJ, Hartford RB. Infant sleep position and risk for sudden infant death syndrome: report of meeting held January 13 and 14, 1994, National Institutes of Health, Bethesda, MD. Pediatrics. 1994;93:814–9. [PubMed] [Google Scholar]
- 45.Anderson ME, Johnson DC, Batal HA. Sudden infant death syndrome and prenatal maternal smoking: rising attributed risk in the Back to Sleep era. BMC Med. 2005;3:4. doi: 10.1186/1741-7015-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 47.Jindal SK, Gupta D. The relationship between tobacco smoke & bronchial asthma. Indian J Med Res. 2004;120:443–53. [PubMed] [Google Scholar]
- 48.Leem JH, Kim JH, Lee KH, Hong Y, Lee KH, Kang D, et al. Asthma attack associated with oxidative stress by exposure to ETS and PAH. J Asthma. 2005;42:463–7. doi: 10.1080/02770900500200733. [DOI] [PubMed] [Google Scholar]
- 49.The International Study of Asthma and Allergies in Childhood (ISAAC) ISAAC: phase one. [cited 2010 Jan 19]. Available from: URL: http://isaac.auckland.ac.nz/phases/phaseone/phaseone.html.
- 50.World Health Organization. Protection from exposure to second-hand tobacco smoke: policy recommendations. Geneva: WHO; 2007. [cited 2010 Jan 19]. Also available from: URL: http://whqlibdoc.who.int/publications/2007/9789241563413_eng.pdf. [Google Scholar]