Abstract
Context
Aspirin reduces risk of colorectal neoplasia in randomized trials and inhibits tumor growth and metastases in animal models. However, the influence of aspirin on survival after diagnosis of colorectal cancer is unknown.
Objective
To examine the association between aspirin use after colorectal cancer diagnosis on colorectal cancer–specific and overall survival.
Design, Setting, and Participants
Prospective cohort study of 1279 men and women diagnosed with stage I, II, or III colorectal cancer. Participants were enrolled in 2 nationwide health professional cohorts in 1980 and 1986 prior to diagnosis and followed up through June 1, 2008.
Main Outcome Measure
Colorectal cancer–specific and overall mortality.
Results
After a median follow-up of 11.8 years, there were 193 total deaths (35%) and 81 colorectal cancer–specific deaths (15%) among 549 participants who regularly used aspirin after colorectal cancer diagnosis, compared with 287 total deaths (39%) and 141 colorectal cancer–specific deaths (19%) among 730 participants who did not use aspirin. Compared with nonusers, participants who regularly used aspirin after diagnosis experienced a multivariate hazard ratio (HR) for colorectal cancer–specific mortality of 0.71 (95% confidence interval [CI], 0.53-0.95) and for overall mortality of 0.79 (95% CI, 0.65-0.97). Among 719 participants who did not use aspirin before diagnosis, aspirin use initiated after diagnosis was associated with a multivariate HR for colorectal cancer–specific mortality of 0.53 (95% CI, 0.33-0.86). Among 459 participants with colorectal cancers that were accessible for immunohistochemical assessment, the effect of aspirin differed significantly according to cyclooxygenase 2 (COX-2) expression (P for interaction = .04). Regular aspirin use after diagnosis was associated with a lower risk of colorectal cancer–specific mortality among participants in whom primary tumors overexpressed COX-2 (multivariate HR, 0.39; 95% CI, 0.20-0.76), whereas aspirin use was not associated with lower risk among those with primary tumors with weak or absent expression (multivariate HR, 1.22; 95% CI, 0.36-4.18).
Conclusion
Regular aspirin use after the diagnosis of colorectal cancer is associated with lower risk of colorectal cancer–specific and overall mortality, especially among individuals with tumors that overexpress COX-2.
Numerous Prospective, OB-servational studies demonstrate that regular aspirin use is associated with a lower risk of colorectal adenoma or cancer.1 In addition, randomized, placebo-controlled trials have shown that use of aspirin,2-5 as well as celecoxib and rofecoxib,6-8 significantly reduces the risk of adenoma among high-risk patients with a prior history of colorectal neoplasia. Aspirin is likely, at least in part, to prevent colorectal neoplasia through inhibition of cyclooxygenase 2 (COX-2), the rate-limiting step for the conversion of arachidonic acid to prostaglandins and related eicosanoids.9,10 COX-2 promotes inflammation and cell proliferation,11 and is overexpressed in the majority of human colorectal cancers.12,13 Overexpression of COX-2 in tumor tissue has been associated with a poorer prognosis among colorectal cancer patients in some13-16 but not all studies.17,18
Nevertheless, it remains uncertain if aspirin use can influence the prognosis for patients with established colorectal cancer. In animal models, aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) with activity against COX-2 isoenzyme have been shown to inhibit tumor growth and metastases, as well as prolong survival.19-25 In a study of patients with stage III colon cancer enrolled in an adjuvant chemotherapy trial, aspirin use was associated with a lower risk of disease recurrence and death.26 However, this study only assessed aspirin use after diagnosis.
We therefore studied the effect of aspirin use among patients with nonmetastatic (stage I, II, and III) colorectal cancer who were participating in 2 large prospective cohort studies (the Nurses' Health Study [NHS] and the Health Professionals Follow-up Study [HPFS]) that were initiated prior to cancer diagnosis. Within these cohorts, we previously have demonstrated that regular aspirin use was associated with a reduction in the subsequent risk of developing an initial primary colorectal cancer,27,28 particularly tumors with COX-2 overexpression.9 Because these participants have provided biennially updated data on aspirin use, we had a unique opportunity to extend these findings by examining the influence of prediagnosis and postdiagnosis aspirin use on the survival of patients with established colorectal cancer. In addition, we assessed whether the effect of aspirin differed according to levels of tumoral expression of COX-2. Specificity in the association between aspirin and colorectal cancer survival to particular tumor markers would further enhance the case for causality, provide important insight into the anti-cancer mechanism of aspirin, and suggest the potential for use of these markers to tailor cancer therapy.
METHODS
Study Population
The NHS was established in 1976 when 121 701 US women who were registered nurses aged 30 to 55 years completed a mailed questionnaire. The HPFS was established in 1986 as a parallel cohort of 51 529 US men who were dentists, optometrists, osteopathic physicians, podiatrists, pharmacists, and veterinarians aged 40 to 75 years at entry. In each cohort, with a follow-up rate of 92%, we mailed biennial questionnaires to update information and identify new cases of cancer. In 1980, the NHS questionnaire was expanded to include a validated assessment of diet and aspirin use29,30; a similar instrument was administered in the 1986 HPFS questionnaire.31,32 In both cohorts, participants self-reported their race and ethnicity.
On each biennial follow-up questionnaire, participants were asked whether they had received a diagnosis of colorectal cancer during the prior 2 years. When a participant reported a diagnosis of colorectal cancer, we asked for permission to obtain hospital medical records and pathology reports. Study physicians, blinded to exposure data, reviewed all medical records related to colorectal cancer and classified disease stage according to the sixth version of the American Joint Committee on Cancer.33 In the NHS, beginning in 1993, we also sent participants who reported a diagnosis of colorectal cancer a supplementary questionnaire that inquired if patients had received chemotherapy. For this analysis, we included the 1279 participants (840 women from NHS and 439 men from HPFS) with pathologically confirmed stage I, II, or III colorectal adenocarcinoma who were diagnosed through 2002 and provided data on aspirin use before and after their diagnosis of the disease. The 154 patients who did not provide data on aspirin use after diagnosis, compared with those included in the analysis, were more likely to have stage III disease (56% vs 32%; P<.0001), but were otherwise similar according to other baseline characteristics (mean age at diagnosis, 65.8 vs 65.0 years; nonwhite, 4% vs 3%; former or current smoker, 62% vs 58%; mean body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]), 26.6 vs 26.2; and aspirin users before diagnosis, 47% vs 44%; P>.21 for all comparisons). We excluded participants if they reported any cancer (other than nonmelanoma skin) previous to colorectal cancer diagnosis. Similar to prior analyses in this cohort, we did not include patients with stage IV colorectal cancer since the vast majority would die of their disease within 1 year of diagnosis.34 We were not funded to collect information on chemotherapy received after diagnosis until 1993, when we began to administer a supplementary treatment questionnaire among participants in the NHS.
The institutional review boards at the Brigham and Women's Hospital and the Harvard School of Public Health approved this study; completion and return of the questionnaire was considered to imply informed consent.
Assessment of COX-2 Expression
We have previously described our procurement of tumor specimens and methodology for COX-2 immunostaining in detail.9,35 Beginning in 1997 in the HPFS and 2001 in the NHS, we began retrieving, from treating hospital pathology departments, representative pathological specimens from the primary tumor for participants in whom we confirmed development of colorectal cancer. We successfully obtained specimens for 76% of cases over 16 years of follow-up in HPFS and 58% of cases over 22 years of follow-up in NHS. We did not obtain tissue specimens for recurrent cancers. To determine COX-2 tumor expression, we limited our analysis to those participants for whom we were able to obtain sufficient amounts of tumor tissue from unstained paraffin blocks as well as adjacent mucosa for comparison (n=459; 207 from NHS and 252 from HPFS). Baseline characteristics among participants with colorectal cancer whom we did and did not analyze for COX-2 expression were largely similar (mean age at diagnosis, 66.3 vs 64.4 years; non-white, 3% vs 4%; stage I, 32% vs 34%; stage II, 36% vs 34%; stage III, 32% vs 32%; rectal site, 24% vs 23%; former or current smoker, 61% vs 57%; mean BMI, 26.5 vs 26.1; aspirin users before diagnosis, 42% vs 45%; and aspirin use after diagnosis, 42% vs 43%; P>.21 for all comparisons).9
We incubated deparaffinized tissue sections in a citrate buffer by microwave for 15 minutes and cooled for 40 minutes (BioGenex, San Ramon, California). Tissue sections were then incubated with 3% H2O2 for 20 minutes, then avidin block for 15 minutes (Vector Laboratories, Burlingame, California), and then biotin block for 15 minutes (Vector Laboratories). Primary anti-COX-2 antibody (Cayman Chemical, Ann Arbor, Michigan) diluted 1:300 in phosphate-buffered saline was applied overnight at 4°C. We then applied secondary anti–mouse antibody for 20 minutes followed by avidin-biotin complex conjugate (Vector Laboratories). Sections were visualized by diaminobenzidine and methyl-green counter-stain. For each assay run, we included a positive control (cancer with COX-2 overexpression) and a negative control (normal colonic tissue). We also treated a positive control specimen with phosphate-buffered saline without anti–COX-2 antibody. As previously described,9,35 a pathologist (blinded to any other participant data) interpreted tumor COX-2 expression using a standardized grading system (absent, weak, moderate, or strong) comparing the immunohistochemical reactions of tumor cells with adjacent normal colonic epithelium and inflammatory cells, which served as internal built-in controls (Figure 1). A random sample of 108 cancers was reread by a second pathologist and the concordance between readers was 0.92 (κ = 0.62; P < .001). If immunostaining intensity was moderate or strong, tumors were classified as cancers with COX-2 overexpression (COX-2–positive). If immunostaining intensity was weak or absent, tumors were classified as cancers with negative COX-2 overexpression (COX-2–negative). These specific categories were defined a priori based on our prior analysis and additional categories were not examined.9
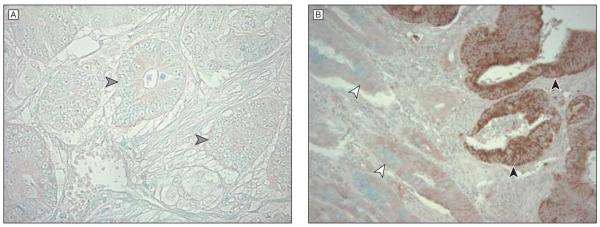
Figure 1. COX-2 Immunohistochemistry in Colorectal Cancer.
Primary anti–cyclooxygenase 2 (COX-2) antibody diluted 1:300 in phosphate-buffered saline was applied overnight at 4°C. We then applied secondary anti–mouse antibody for 20 minutes followed by avidin-biotin complex conjugate. Sections were visualized by diaminobenzidine and methyl-green counterstain. A, Representative section from a COX-2–negative tumor (original magnification ×400). The gray arrowheads indicate colorectal cancer cells without COX-2 overexpression. B, Representative section from a COX-2–positive tumor (original magnification ×400). Black arrowheads indicate colorectal cancer cells with strong COX-2 expression (dark brown color) compared with normal colonic epithelium indicated by white arrowheads.
Ascertainment of Death
We included deaths that occurred after the completion of the baseline aspirin use questionnaire (1980 in the NHS and 1986 in the HPFS) and before June 1, 2008. We identified deaths through the National Death Index and next of kin. Mortality follow-up was more than 98% complete.36,37 For all deaths, we sought information to determine the cause (including death certificates) and when appropriate, requested permission from next of kin to review medical records.
Assessment of Medication Use
We have previously detailed our assessment of aspirin use in both the NHS and HPFS cohorts.9,27,30,38,39 In 1980 we asked NHS participants if they regularly used aspirin in most weeks, the number of pills or capsules taken each week, and the number of years of use. We updated this information biennially (except in 1986) with specific questions on the number of aspirin tablets used per week (in categories). In the 1986 HPFS questionnaire and every 2 years thereafter, we inquired if participants regularly used aspirin 2 or more times per week. Beginning in 1992, we also asked the average number of tablets used per week (in categories). In both cohorts, we specifically inquired about standard-dose (325 mg) aspirin tablets. However, to reflect secular trends in consumption of low-dose ([baby], 81 mg) aspirin, questionnaires after 1992 asked participants to convert intake of 4 baby aspirin to 1 adult standard-dose tablet when responding. We did not collect consistent data over follow-up on nonaspirin NSAIDs in both cohorts. In addition, there was insufficient follow-up after the introduction of COX-2–selective inhibitors in the United States in 1999 and only 17 participants used these agents. Thus, we did not examine these drugs in the present study.
Reasons for aspirin use were not assessed for the entire cohort, but supplementary validation questionnaires were sent in 1990 to a sample of 200 women (91% response) and in 1993 to a sample of 211 men (88% response) who reported aspirin use on the main questionnaire. The major reasons for use among women taking 1 to 6 aspirin and 7 or more aspirin per week were headache (32% and 18%, respectively); arthritis and other musculoskeletal pain (30% and 50%); a combination of headache and musculoskeletal pain (16% and 15%); cardiovascular disease prevention (9% and 8%); and other reasons (13% and 9%).30 Among men, the major reasons for use were cardiovascular disease (25.4%); to decrease risk of cardiovascular disease (58.4%); headaches (25.4%); joint or musculoskeletal pain (33.0%); and other reasons (7.0%).32
As previously described, we asked NHS participants in 2004 and HPFS participants in 2006 to report any major episodes of gastrointestinal bleeding that required either hospitalization or a blood transfusion. Among the women, the incidence of events per 1000 person-years was 0.77 among those who reported no aspirin use; 1.07 for 0.5 to 1.5 standard aspirin tablets per week; 1.07 for 2 to 5 aspirin per week; 1.40 for 6 to 14 aspirin per week; and 1.57 for more than 14 aspirin per week. Among the men, the incidence of events per 1000 person-years was 0.92 among those who reported no aspirin use; 1.02 for 0.5 to 1.5 standard aspirin tablets per week; 1.65 for 2 to 5 aspirin per week; and 1.84 for 6 or more aspirin per week.27
Power Analysis
Our a priori hypothesis was that aspirin use after diagnosis is associated with a lower risk of colorectal cancer–related death among participants with nonmetastatic colorectal cancer. Based on the sample size of 1279 participants diagnosed with stage I, II, and III colorectal cancer between 1980 and 2002 in the NHS cohort and 1986 and 2002 in the HPFS cohort, the number of colorectal cancer–related deaths, and the prevalence of aspirin use after diagnosis, we had 80% power to detect a hazard ratio (HR) of 0.70 comparing aspirin users with nonusers.40
Statistical Analysis
In both the NHS and HPFS cohorts, we have administered similar biennial questionnaires, ascertained cancers and deaths with comparable methodologies, collected pathological specimens through a common biospecimen repository, and concurrently assayed for COX-2 with a uniform protocol. Thus, as in our prior analysis,9 we pooled data from both cohorts and tested for heterogeneity using the Cochran 𝒬 statistic. We observed no heterogeneity between the cohorts regarding the association of aspirin use after diagnosis and colorectal cancer–specific survival (P=.93, Cochran 𝒬 test).41
Participants eligible for analysis accrued follow-up time beginning on the month of their diagnosis of colorectal cancer and ending on the month of death from colorectal cancer, death from any cause, or June 1, 2008, whichever came first. We categorized participants according to aspirin data provided prior to the date of diagnosis of colorectal cancer and after the date of diagnosis. We used Kaplan-Meier curves and the log-rank test to compare colorectal cancer–specific and overall mortality according to aspirin use.
We used Cox proportional hazards modeling to control for multiple risk factors that have been shown to influence colorectal cancer survival to compute 95% confidence intervals (CIs). We have used this model-building approach in previous analyses of these cohorts.9,27,28 The validity of the proportional hazards assumption was assessed by using time-dependent covariates; the Wald test showed no evidence of departure from this assumption. We assessed overfitting and collinearity of the model by using collinearity diagnostics; variance inflation factors showed no significant collinearity between each of our included covariates.
We conducted preplanned stratified analyses according to COX-2 status of the primary tumors as well as subgroups defined by our prior study of physical activity and survival in this cohort. Consistent with this prior analysis, we also observed, comparing extreme tertile categories, a significant association between colorectal cancer–specific mortality and postdiagnosis physical activity (multivariate HR, 0.50; 95% CI, 0.36-0.71), but not BMI at diagnosis (multivariate HR, 0.99; 95% CI, 0.71-1.39).34 We assessed statistical interaction of subgroups by including cross-product terms in our models and assessing their significance using the Wald test. As in our prior study, we also performed an analysis excluding deaths that occurred within 12 months of completing the postdiagnosis aspirin assessment.
In a secondary analysis, to further evaluate for potential bias related to differences among participants who chose to use aspirin after diagnosis compared with those who did not, we computed a propensity score by using logistic regression assigning postdiagnosis aspirin use as the dependent variable and the risk factors listed in Table 1 as independent variables. We categorized the propensity scores into quintiles and adjusted for these quintile categories in Cox proportional hazards models.42,43
Table 1.
Baseline Characteristics of the Study Cohort by Aspirin Usea
| No. (%) |
||||
|---|---|---|---|---|
| Prediagnosis |
Postdiagnosis |
|||
| Characteristic | Nonuser (n = 719) |
Aspirin User (n = 560) |
Nonuser (n = 730) |
Aspirin User (n = 549) |
| Age, mean (SD), y | 65.2 (8.3) | 64.7 (9.1) | 65.0 (8.4) | 65.0 (9.1) |
|
| ||||
| Women | 459 (64) | 381 (68) | 495 (68) | 345 (63) |
|
| ||||
| Race | ||||
| White | 691 (96) | 545 (97) | 705 (97) | 531 (97) |
|
| ||||
| Nonwhite | 28 (4) | 15 (3) | 25 (3) | 18 (3) |
|
| ||||
| Body mass index, mean (SD)b | 26.1 (4.5) | 26.2 (4.6) | 25.9 (4.6) | 26.5 (4.6) |
|
| ||||
| Smoking status | ||||
| Past smoker | 341 (47) | 265 (47) | 320 (44) | 286 (52) |
|
| ||||
| Current smoker | 88 (12) | 68 (12) | 91 (12) | 65 (12) |
|
| ||||
| Physical activity, mean (SD)c | 22.8 (36.9) | 18.6 (21.3) | 22.4 (36.2) | 18.8 (21.7) |
|
| ||||
| Postmenopausald | 421 (92) | 338 (89) | 454 (92) | 305 (88) |
|
| ||||
| Past use of hormonesd | 126 (30) | 88 (26) | 125 (28) | 89 (29) |
|
| ||||
| Current use of hormonesd | 91 (22) | 86 (25) | 106 (23) | 71 (23) |
|
| ||||
| Colorectal cancer in parent or sibling | 117 (16) | 103 (18) | 111 (15) | 109 (20) |
|
| ||||
| Stage of disease | ||||
| I | 228 (32) | 193 (35) | 218 (30) | 203 (37) |
|
| ||||
| II | 260 (36) | 186 (33) | 265 (36) | 181 (33) |
|
| ||||
| III | 231 (32) | 181 (32) | 247 (34) | 165 (30) |
|
| ||||
| Site of disease | ||||
| Colon | 546 (76) | 430 (77) | 551 (75) | 425 (77) |
|
| ||||
| Rectum | 173 (24) | 130 (23) | 179 (25) | 124 (23) |
|
| ||||
| Grade of differentiation | ||||
| Well | 105 (15) | 84 (15) | 109 (15) | 80 (14) |
|
| ||||
| Moderate | 449 (62) | 351 (63) | 454 (62) | 346 (63) |
|
| ||||
| Poor/undifferentiated | 95 (13) | 64 (11) | 101 (14) | 58 (11) |
|
| ||||
| Unknown | 70 (10) | 61 (11) | 66 (9) | 65 (12) |
|
| ||||
| Year of colorectal cancer diagnosis | ||||
| Before 1990 | 172 (24) | 175 (31) | 187 (26) | 160 (29) |
|
| ||||
| 1990-1995 | 228 (32) | 146 (26) | 232 (31) | 142 (26) |
|
| ||||
| After 1995 | 319 (44) | 239 (43) | 311 (43) | 247 (45) |
Baseline characteristics are from time of diagnosis of colorectal cancer. Prediagnosis aspirin use was defined as regular use of aspirin during most weeks on the biennial questionnaire prior to diagnosis of colorectal cancer. Postdiag-nosis aspirin use was defined as regular use of aspirin during most weeks on the biennial questionnaire after diagnosis of colorectal cancer.
Body mass index is calculated as weight in kilograms divided by the height in meters squared.
Physical activity was assessed as metabolic equivalent of tasks per week.
Percentage of postmenopausal participants is among women only. Hormone use was defined as postmenopausal estrogen or estrogen-progesterone preparations. The percentage of past and current use was calculated among postmenopausal women only.
The linear trend test across categories was calculated by using the median value of each category as a continuous variable, consistent with prior studies.9,27,34 We used SAS software version 9.1.3 (SAS Institute Inc, Cary, North Carolina). All P values were 2-sided and a level of significance of less than .05 was considered statistically significant.
RESULTS
Among the 1279 eligible participants with stage I, II, or III colorectal cancer, we documented 480 total deaths among which 222 deaths were due to colorectal cancer. For participants who were alive through the end of follow-up, the median time of follow-up from date of diagnosis was 11.8 years (interquartile range, 7.6-16.2). There were 536 participants who did not regularly use aspirin before and after diagnosis (42%), 366 regularly used aspirin before and after diagnosis (29%), 194 regularly used aspirin before diagnosis but then discontinued use after diagnosis (15%), and 183 did not regularly use aspirin before diagnosis but initiated use after diagnosis (14%). Baseline characteristics of the participants are shown in Table 1. Compared with nonusers, regular users of aspirin before diagnosis appeared to be less physically active. After diagnosis, regular users of aspirin after also appeared to be less physically active, were more likely to have previously smoked cigarettes, and were less likely to be diagnosed with stage III disease compared with nonusers.
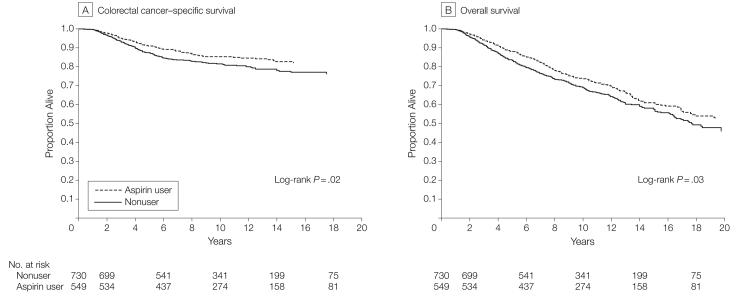
There were 193 total deaths (35%) and 81 colorectal cancer–specific deaths (15%) among 549 participants who regularly used aspirin after colorectal cancer diagnosis, compared with 287 (39%) total and 141 (19%) colorectal cancer–specific deaths among 730 participants who did not use aspirin. For the entire cohort, the overall 5-year survival was 88% for those participants who used aspirin compared with 83% for those who did not. The corresponding 10-year survival rates were 74% and 69%. Regular use of aspirin after diagnosis was associated with a significant reduction in risk of colorectal cancer–specific mortality (log-rank P=.02; Figure 2A) and a reduction in overall mortality (log-rank P=.03; Figure 2B). The relationship remained largely unchanged even after adjusting for use of aspirin before diagnosis as well as other predictors of cancer recurrence (Table 2). Compared with nonusers, the multivariate HR associated with regular aspirin use after diagnosis was 0.71 (95% CI, 0.53-0.95) for colorectal cancer–specific mortality and 0.79 (95% CI, 0.65-0.97) for overall mortality. Because the prognosis among stage I participants is generally favorable, we also examined the influence of aspirin use among those diagnosed with stage II or III disease and observed similar results (multivariate HR, 0.72; 95% CI, 0.53-0.99 for colorectal cancer–specific mortality and multivariate HR, 0.82; 95% CI, 0.66-1.04 for overall mortality).
Figure 2. Survival According to Aspirin Use After Diagnosis.
Table 2.
Risk of Colorectal Cancer-Specific Mortality and Overall Mortality According to Use of Aspirin After Diagnosisa
| Colorectal Cancer-Specific Mortality |
Overall Mortality |
|||
|---|---|---|---|---|
| Aspirin Nonuser | Aspirin User | Aspirin Nonuser | Aspirin User | |
| All participants (n = 1279) | ||||
| No. of events/No. at risk | 141/730 | 81/549 | 287/730 | 193/549 |
|
| ||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.73 (0.55-0.96) | 1 [Reference] | 0.81 (0.67-0.97) |
|
| ||||
| Age and prediagnosis aspirin-adjusted HR (95% CI) | 1 [Reference] | 0.67 (0.50-0.90) | 1 [Reference] | 0.81 (0.66-0.98) |
|
| ||||
| Multivariate-adjusted HR (95% CI)b | 1 [Reference] | 0.71 (0.53-0.95) | 1 [Reference] | 0.79 (0.65-0.97) |
|
| ||||
| Aspirin nonusers prediagnosis (n = 719) | ||||
| No. of events/No. at risk | 100/536 | 21/183 | 213/536 | 64/183 |
|
| ||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.57 (0.35-0.91) | 1 [Reference] | 0.76 (0.58-1.01) |
|
| ||||
| Multivariate-adjusted HR (95% CI)c | 1 [Reference] | 0.53 (0.33-0.86) | 1 [Reference] | 0.68 (0.51-0.92) |
|
| ||||
| Aspirin users prediagnosis (n = 560) | ||||
| No. of events/No. at risk | 41/194 | 60/366 | 74/194 | 129/366 |
|
| ||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.76 (0.51-1.13) | 1 [Reference] | 0.86 (0.65-1.15) |
|
| ||||
| Multivariate-adjusted HR (95% CI)c | 1 [Reference] | 0.89 (0.59-1.35) | 1 [Reference] | 0.95 (0.71-1.28) |
|
| ||||
| COX-2–positive primary cancer (n = 314)d | ||||
| No. of events/No. at risk | 38/182 | 13/132 | 74/182 | 43/132 |
|
| ||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.44 (0.24-0.83) | 1 [Reference] | 0.69 (0.47-1.00) |
|
| ||||
| Age and prediagnosis aspirin-adjusted HR (95% CI) | 1 [Reference] | 0.39 (0.20-0.74) | 1 [Reference] | 0.64 (0.43-0.94) |
|
| ||||
| Multivariate-adjusted HR (95% CI)e | 1 [Reference] | 0.39 (0.20-0.76) | 1 [Reference] | 0.62 (0.42-0.93) |
|
| ||||
| COX-2–negative primary cancer (n = 145)f | ||||
| No. of events/No. at risk | 7/84 | 7/61 | 28/84 | 22/61 |
|
| ||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 1.45 (0.50-4.16) | 1 [Reference] | 1.02 (0.58-1.80) |
|
| ||||
| Age and prediagnosis aspirin-adjusted HR (95% CI) | 1 [Reference] | 1.26 (0.41-3.85) | 1 [Reference] | 1.11 (0.62-2.02) |
|
| ||||
| Multivariate-adjusted HR (95% CI)e | 1 [Reference] | 1.22 (0.36-4.18) | 1 [Reference] | 1.05 (0.55-2.02) |
Abbreviations: CI, confidence interval; COX-2, cyclooxygenase 2; HR, hazard ratio.
Prediagnosis aspirin use was defined as regular use of aspirin during most weeks on the biennial questionnaires prior to diagnosis of colorectal cancer. Postdiagnosis aspirin use was defined as regular use of aspirin during most weeks on the biennial questionnaires after diagnosis of colorectal cancer.
Multivariate HRs were adjusted for age at diagnosis (years), sex, date of diagnosis of cancer (years), stage of cancer (I, II, III), site of primary cancer (colon or rectum), histologi-cal grade of cancer (well, moderate, poor/unknown), time from diagnosis to first measurement of postdiagnosis aspirin use (months), smoking (never vs current/past), metabolic equivalent tasks per week after diagnosis, body mass index (BMI), colorectal cancer in parent or sibling (yes or no), and use of aspirin prediagnosis.
Multivariate HRs were adjusted for age at diagnosis (years), sex, date of diagnosis of cancer (years), stage of cancer (I, II, III), site of primary cancer (colon vs rectum), histologi-cal grade of cancer (well, moderate, poor/unknown), time from diagnosis to first measurement of postdiagnosis aspirin use (months), smoking (never vs current/past), metabolic equivalent tasks per week after diagnosis, BMI, and colorectal cancer in parent or sibling (yes or no).
Primary cancers with immunohistochemical COX-2 staining of moderate-to-strong intensity are classified as COX-2–positive primary cancers.
Multivariate hazard ratios are adjusted for age at diagnosis (years), sex, date of diagnosis of cancer (years), stage of cancer (I, II, III), site of primary cancer (colon or rectum), histological grade of cancer (well, moderate, poor/unknown), time from diagnosis to first measurement of postdiagnosis aspirin use (months), smoking (never vs current/past), metabolic equivalent tasks per week after diagnosis, BMI, colorectal cancer in parent or sibling (yes or no), and use of aspirin prediagnosis. For the analyses of COX-2–negative tumors, the multivariate models omitted site and grade due to an insufficient number of events in some categories.
Primary cancers with no immunohistochemical COX-2 staining or with staining of weak intensity are classified as COX-2–negative primary cancers.
In contrast to aspirin use after diagnosis, aspirin use prior to cancer diagnosis did not appear to be associated with either colorectal cancer–specific mortality (multivariate HR, 1.05; 95% CI, 0.80-1.37) or overall mortality (multivariate HR, 0.93; 95% CI, 0.77-1.11). This relationship was consistent after including those participants with colorectal cancer who did not return a postdiagnosis aspirin questionnaire (multivariate HR, 1.00; 95% CI, 0.81-1.25). A formal test for interaction between aspirin use before diagnosis vs aspirin use after diagnosis on colorectal cancer–specific mortality was not statistically significant (P=.09). However, simultaneous inclusion of both prediagnosis and postdiagnosis aspirin use in models confirmed that post-diagnosis aspirin use remained independently associated with colorectal cancer–specific mortality (P=.008) and overall mortality (P=.03), while pre-diagnosis aspirin use was not significantly associated with either colorectal cancer–specific mortality (P=.14) or overall mortality (P=.98).
To better characterize this relationship, we examined postdiagnosis aspirin use according to use of aspirin prior to cancer diagnosis (Table 2). Among the 719 participants who did not use aspirin before diagnosis, initiation of use postdiagnosis was associated with a multivariate HR for colorectal cancer–specific mortality of 0.53 (95% CI, 0.33-0.86) and overall mortality of 0.68 (95% CI, 0.51-0.92). In contrast, among participants who were using aspirin before diagnosis, continuation of aspirin use postdiagnosis was not associated with a significant reduction in colorectal cancer–specific survival (multivariate HR, 0.89; 95% CI, 0.59-1.35) or overall survival (multivariate HR, 0.95; 95% CI, 0.71-1.28).
We have previously shown that aspirin use is associated with a lower risk of subsequently developing colorectal COX-2–positive cancers but not colorectal COX-2–negative cancers.9 Thus, we examined whether the effect of postdiagnosis aspirin use on survival differed according to COX-2 expression status within the primary tumors. Among 459 participants for whom we had sufficient tumor tissue with adjacent normal mucosa to assay for COX-2, the benefit of aspirin use after diagnosis appeared to be confined to those with COX-2–positive primary tumors (Table 2). Among participants with COX-2–positive tumors, regular aspirin use after diagnosis was associated with a lower risk of colorectal cancer–specific (multivariate HR, 0.39; 95% CI, 0.20-0.76) and overall (HR, 0.62; 95% CI, 0.42-0.93) mortality, whereas postdiagnosis aspirin use was not associated with lower risk of either colorectal cancer–specific or overall mortality for those with COX-2–negative tumors. A test for heterogeneity of the effect of regular aspirin use after diagnosis on survival for COX-2–positive tumors vs COX-2–negative tumors was statistically significant (P for interaction = .04).
We considered the possibility that the superior benefit of postdiagnosis aspirin use among COX-2–positive cancers reflected the observation that COX-2–positive cancers are more likely to develop among individuals who abstain from aspirin use prior to cancer diagnosis. In a post hoc analysis, we therefore examined the joint effect of prediagnosis and postdiagnosis aspirin use according to tumoral COX-2 expression. Although participants who reported aspirin use following diagnosis but not before diagnosis (postdiagnosis use only) experienced a significant reduction in colorectal cancer–specific mortality from COX-2–positive cancers (HR, 0.22; 95% CI, 0.07-0.74), COX-2–positive participants who reported aspirin use both before and after diagnosis also experienced a non-significant reduction in colorectal cancer–specific mortality (HR, 0.56; 95% CI, 0.23-1.33).
Among participants who did not use aspirin before diagnosis, the association between postdiagnosis aspirin use and survival was modestly dose-responsive (Table 3). Compared with individuals who used any aspirin, the multivariate-adjusted HR for colorectal cancer–specific mortality was 0.57 (95% CI, 0.32-0.99) for those who used 0.5 to 5 standard aspirin tablets and 0.49 (95% CI, 0.18-1.35) for individuals who used 6 or more tablets per week (P for trend = .04).
Table 3.
Risk of Colorectal Cancer-Specific Mortality and Overall Mortality According to Dose of Aspirin Used After Colorectal Cancer Diagnosisa
| No. of Aspirin Tablets per Week |
||||
|---|---|---|---|---|
| 0 | 0.5-5 | ≥6 |
P for Trend |
|
| Colorectal cancer-specific mortality | ||||
| No. of events/No. at risk | 86/460 | 15/127 | 4/39 | |
|
| ||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.57 (0.33-0.99) | 0.46 (0.17-1.26) | .03 |
|
| ||||
| Multivariate-adjusted HR (95% CI)b | 1 [Reference] | 0.57 (0.32-0.99) | 0.49 (0.18-1.35) | .04 |
|
| ||||
| Overall mortality | ||||
| No. of events/No. at risk | 172/460 | 40/127 | 13/39 | |
|
| ||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.71 (0.50-1.00) | 0.68 (0.38-1.19) | .05 |
|
| ||||
| Multivariate-adjusted HR (95% CI)b | 1 [Reference] | 0.69 (0.48-0.99) | 0.61 (0.34-1.09) | .03 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NHS, Nurses' Health Study; HPFS, Health Professionals Follow-up Study.
Aspirin dose is classified according to the number of standard 325-mg tablets taken per week after diagnosis of co-lorectal cancer. Analysis was conducted among NHS and HPFS participants who did not regularly use aspirin prior to diagnosis. Among this cohort, cases diagnosed between 1980 and 2002 in the NHS and between 1992 and 2002 in the HPFS provided data on the number of standard 325-mg aspirin tablets used per week for this analysis.
Multivariate HRs were adjusted for age at diagnosis (years), sex, date of diagnosis of cancer (years), stage of cancer (I, II, III), site of primary cancer (colon or rectum), histological grade of cancer (well, moderate, poor/unknown), time from diagnosis to first measurement of postdiagnosis aspirin use (months), smoking (never vs current/past), metabolic equivalent tasks per week after diagnosis, body mass index (calculated as weight in kilograms divided by height in meters squared), and colorectal cancer in parent or sibling (yes or no).
Since the decision to use or avoid aspirin could be influenced by occult cancer recurrence or impending death, we performed a secondary analysis in which we excluded participants who died within 12 months of completing the assessment of postdiagnosis aspirin use. Our results were essentially unchanged. Compared with nonusers, participants who regularly used any aspirin after diagnosis had a multivariate-adjusted HR for colorectal cancer–specific mortality of 0.71 (95% CI, 0.53-0.95) and a multivariate HR for overall mortality of 0.80 (95% CI, 0.65-0.97). Moreover, when we examined survival from the date of return of the questionnaire regarding postdiagnosis aspirin use rather than the date of diagnosis of colorectal cancer, we also observed similar results (multivariate HR, 0.71; 95% 0.53-0.95 for colorectal cancer–specific mortality and multivariate HR, 0.79; 95% CI, 0.65-0.97 for overall mortality).
Because aspirin use was not randomly assigned in this population, we also conducted a secondary analysis using a propensity score in which we computed each participant's probability to use aspirin after diagnosis of colorectal cancer without regard to outcome. Adjusting for quintile categories of this propensity score did not alter our findings (propensity-adjusted HR, 0.70; 95% CI, 0.52-0.94 for colorectal cancer–specific mortality and propensity-adjusted HR, 0.82; 95% CI, 0.67-0.99 for overall mortality).
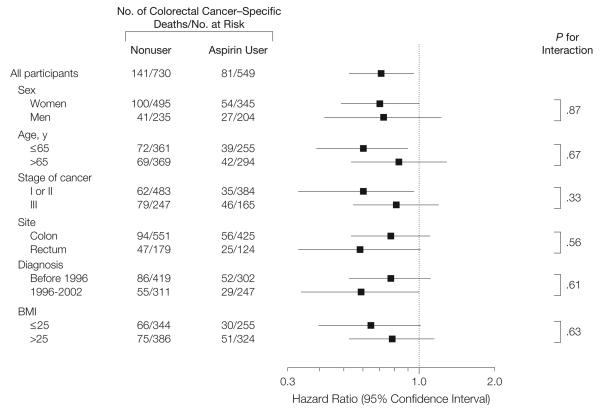
We also evaluated potential differences in the influence of aspirin according to strata of clinical characteristics. There were no significant differences in the influence of aspirin in strata defined by sex (cohort), age, cancer stage, site of primary tumor, year of diagnosis, or BMI (Figure 3). We also confirmed that the inverse association between postdiagnosis aspirin use and colorectal cancer–specific mortality was also observed among the 241 participants for whom we collected data on treatment. Although statistical power was limited, the association between postdiagnosis aspirin use and mortality did not appear to be materially changed, even after accounting for receipt of chemotherapy (multivariate HR, 0.40; 95% CI, 0.15-1.10 for colorectal cancer–specific mortality and multivariate HR, 0.53; 95% CI, 0.26-1.07 for overall mortality). Moreover, postdiagnosis aspirin use was not associated with likelihood of receiving adjuvant chemotherapy across stages of colorectal cancer. Among participants with stage I colorectal cancer, 11% of aspirin users received chemotherapy compared with 12% of nonusers (P for difference = .82). Similarly, among participants with stage II or III colorectal cancer, 73% of aspirin users received chemotherapy compared with 77% of nonusers (P for difference = .53).
Figure 3. Multivariate-Adjusted Stratified Analyses of Colorectal Cancer–Specific Survival According to Aspirin Use After Diagnosis.
Multivariate hazard ratios are adjusted for age at diagnosis (years), sex, date of cancer diagnosis (years), stage of cancer (I, II, or III), site of primary cancer (colon or rectum), histological grade of cancer (well, moderate, or poor/unknown), time from diagnosis to first measurement of postdiagnosis aspirin use (months), smoking (never vs current/past), metabolic equivalent tasks per week after diagnosis, body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]), and colorectal cancer in a parent or sibling (yes or no). For each stratified analysis, the stratification variable was omitted from the model.
COMMENT
In summary, we observed that use of aspirin after a diagnosis of nonmeta-static colorectal cancer was associated with a decreased risk of colorectal cancer–specific mortality. This inverse association appeared to be strongest among participants whose primary tumors overexpressed COX-2. This relationship appeared to be independent of the intake of aspirin prior to diagnosis and was modestly related to increasing aspirin dose.
Our results are consistent with findings based on a detailed survey of medication use and lifestyle administered to 846 patients with stage III colon cancer enrolled in a postoperative adjuvant chemotherapy trial comparing bolus fluorouracil and leucovorin to bolus irinotecan and bolus fluorouracil and leucovorin.26 In that study, consistent aspirin use was associated with an HR for cancer recurrence, death, or both combined of 0.48 (95% CI, 0.24-0.99), comparable with our findings. Moreover, regular use of the COX-2–selective inhibitors celecoxib or rofecoxib was similarly associated with a lower risk of cancer recurrence and mortality. However, aspirin use was only assessed after initial diagnosis of cancer. Thus, it was unclear if the observed benefit might actually reflect a more favorable behavior of tumors initiated within an environment of aspirin use before diagnosis rather than an effect of aspirin on established cancer. Our data demonstrate that aspirin in-take after, but not before the development of stage I, II, or III cancer is associated with improved survival. This suggests that aspirin may have a specific effect on the prevention or progression of micrometastases among individuals with established disease. Notably, among participants who used aspirin before diagnosis, continuation of aspirin use after diagnosis did not appear to influence survival. This suggests the possibility that tumors that initially developed despite exposure to aspirin may be less susceptible to any potential effect of aspirin on tumor progression.
Several mechanisms have been hypothesized to underlie the influence of aspirin on colorectal neoplasia, including inhibition of nuclear factor-κB,44 induction of apoptosis by activation of p38 kinase,45 and catabolism of polyamines.46 However, we had previously shown that regular aspirin use was associated with a significant reduction in the subsequent risk of developing a primary COX-2–positive colorectal cancer but not a COX-2–negative tumor, suggesting that aspirin works principally by inhibiting COX-2 or its downstream effectors.9,10 In the current study, we found that aspirin use after diagnosis was associated with lower colorectal cancer–specific mortality among individuals with COX-2–positive tumors but not COX-2–negative tumors. This supports the hypothesis that COX-2–positive tumors may be relatively sensitive to the anticancer effect of aspirin, whereas COX-2–negative tumors may be relatively aspirin-resistant. Moreover, it potentially explains the observation that the benefit of postdiagnosis aspirin use on patient survival was not apparent among patients who used aspirin prior to cancer diagnosis.
Our findings are also supported by other studies in humans. A placebo-controlled study of 135 malnourished patients with various solid tumors reported that patients randomized to indomethacin had a prolonged survival that was statistically significant.47 A randomized trial of standard-dose aspirin in patients with prior Dukes stage A or B1 colon or rectal cancer who had undergone curative resection of their primary tumor demonstrated a 35% reduction in risk of colorectal adenoma after a median 30 months of treatment.3 In addition, intratumoral expression of COX-2 has been independently associated with tumor differentiation,17 angiogenesis,48,49 recurrence,50 and metastasis.51 Moreover, COX-2 expression has been correlated with worsened patient survival in some13-16,48 but not all studies.17,18,52,53
Our results are also supported by substantial experimental data. In colon cancer cell lines and mice, aspirin and other NSAIDs interrupt tumor growth,20,21,23-25,54 inhibit angiogenesis,22,25,55,56 abrogate invasiveness,24,57,58 and retard metastasis.19,22-25 Aspirin and NSAIDs also enhance responsiveness to chemotherapy,23,25,59,60 increase expression of mismatch repair proteins,61 and improve overall survival.23,25 Moreover, aspirin and NSAIDs inhibit the production of prostaglandins that promote cancer cell growth and the production of angiogenic factors.10,62,63
Our study has several important strengths. First, we used prospectively collected data on aspirin use. Thus, we were able to minimize potential bias related to differential recall of aspirin use according to disease activity, and any errors in recall would have tended to attenuate rather than exaggerate true associations. Second, we obtained data on aspirin use both before and after cancer diagnosis. This permitted us to disentangle the effect of aspirin use after diagnosis from aspirin use before diagnosis. We considered the possibility that regular aspirin users who develop colorectal cancer simply acquire tumors that are biologically less aggressive. However, the benefit of regular aspirin use was largely restricted to patients who initiated aspirin use following cancer diagnosis; after adjusting for postdiagnosis aspirin use, regular aspirin use before cancer diagnosis was not associated with any reduction in colorectal cancer or overall mortality. Finally, since all participants were health professionals, the accuracy of self-reported aspirin use is likely to be high and more likely to reflect actual consumption of these largely over-the-counter medications.
Several limitations of this study warrant comment. First, our study was observational and aspirin use was self-selected. Thus, despite the strong biological plausibility of our results, it is possible that our findings could be related to the reason for which participants used aspirin. However, most participants reported using aspirin primarily for analgesia.27 During much of the study period, data regarding an association between aspirin and colorectal neoplasia were also not widely available, suggesting that it is unlikely participants took aspirin for the purpose of cancer prevention. Moreover, an analysis adjusting for an individual's propensity to use aspirin also did not materially change our results. We also cannot completely exclude the possibility that aspirin use may be reflective of other occult predictors for improved prognosis. However, we did not observe any significant association between aspirin use and other predictors of cancer outcome and our findings remained unchanged after adjusting for other potential risk factors for colorectal cancer mortality. Finally, the differential effect of aspirin according to COX-2 expression is consistent with a causal mechanism. To minimize any bias by occult cancer recurrence, we also performed a secondary analysis in which we excluded deaths within 12 months of the aspirin assessment and continued to observe a significant influence of regular aspirin use on patient outcome.
Beyond causes of mortality, data on cancer recurrences were not available in this cohort. Nonetheless, since median survival for recurrent (meta-static) colorectal cancer was approximately 10 to 12 months during much of the time period of this study,64 colorectal cancer–specific mortality should be a reasonable surrogate for cancer-specific outcome. In this cohort, we also had limited data on chemotherapy. However, it is unlikely that differential receipt of chemotherapy could explain the observed findings. First, the association of aspirin use and survival was similar among participants with stage I or II disease (for which surgery alone would represent a standard of care) and among those with stage III cancer (for which adjuvant chemotherapy would represent a routine approach). Second, since our cohort consisted of health professionals, considerable heterogeneity in use of adjuvant chemotherapy would be unlikely. Third, in this cohort, aspirin use was not associated with the likelihood of receiving adjuvant chemotherapy. Among participants with stage I colorectal cancer, 11% of aspirin users received chemotherapy compared with 12% of nonusers (P for difference = .82). Similarly, among participants with stage II or III colorectal cancer, 73% of asprin users received chemotherapy compared with 77% of nonusers (P for difference = .53). Fourth, when we repeated our analyses adjusting for receipt of chemotherapy among participants for whom data on chemotherapy use was available, the association between aspirin use and survival was not materially altered.
Importantly, our results are consistent with findings in a separate cohort nested in a National Cancer Institute–sponsored adjuvant chemotherapy trial that similarly found that regular aspirin use was associated with a significant reduction in cancer recurrence and mortality. In that latter cohort, data on disease recurrence as well as mortality were consistently collected and chemotherapy treatment was uniformly administered by protocol.26
We were unable to obtain tumor tissue on all cases of confirmed colorectal cancer over follow-up. However, it is unlikely that COX-2 expression or mortality would be differential according to retrieval success. Moreover, an assessment of risk factors and the effect of aspirin on colorectal cancer risk did not appreciably differ among those participants for whom we were unable to obtain tumor tissue.9
Finally, although we had sufficient statistical power for our primary analysis of postdiagnosis aspirin use and risk of colorectal cancer–specific and overall mortality, we had comparatively limited sample sizes in some of our subgroups, such as the cohort of participants with treatment data or available archived tumor tissue. Thus, although the results of our secondary analyses within these subgroups support our primary findings, the precise risk estimates should be cautiously interpreted in light of the relatively wide associated CIs.
Our findings show that use of aspirin after diagnosis of nonmetastatic colorectal cancer is associated with improved survival from the disease, particularly among individuals with primary tumors that overexpress COX-2. These results suggest that aspirin may influence the biology of established colorectal tumors in addition to preventing their occurrence. Our data also highlight the potential for using COX-2 or related markers to tailor aspirin use among patients with newly diagnosed colorectal cancer. Nonetheless, because our data are observational, routine use of aspirin or related agents as cancer therapy cannot be recommended, especially in light of concerns over their related toxicities, such as gastrointestinal bleeding.1,65 Further studies among patients with colorectal cancer, including placebo-controlled trials of aspirin or related agents as adjuncts to other routine therapies, are required.
Acknowledgments
Funding/Support: This work was supported by grants CA87969, CA55075, CA118553, CA127003, and CA137178 from the National Cancer Institute (NCI), National Institutes of Health (NIH). Dr Chan is a recipient of the Damon Runyon Cancer Research Foundation Clinical Investigator Award and a career development award from the NCI (CA107412). Dr Ogino is a recipient of a career development award from the NCI (CA122826).
Role of the Sponsor: The NCI, the NIH, and the Damon Runyon Cancer Research Foundation had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures: None reported.
Previous Presentations: An abstract of this data was presented on June 1, 2009, at the clinical plenary session of the American Gastroenterological Association/ Digestive Disease Week, Chicago, Illinois.
REFERENCES
- 1.Dubé C, Rostom A, Lewin G, et al. US Preventive Services Task Force. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the US Preventive Services Task Force. Ann Intern Med. 2007;146(5):365–375. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 2.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 3.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 4.Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125(2):328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 5.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR, ukCAP Trial Group Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Bertagnolli MM, Eagle CJ, Zauber AG, et al. APC Study Investigators. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 7.Arber N, Eagle CJ, Spicak J, et al. PreSAP Trial Investigators. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 8.Baron JA, Sandler RS, Bresalier RS, et al. APPROVe Trial Investigators. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 9.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz SD. Aspirin and colon cancer-targeting prevention? N Engl J Med. 2007;356(21):2195–2198. doi: 10.1056/NEJMe078044. [DOI] [PubMed] [Google Scholar]
- 11.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23(12):2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 12.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 13.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10(24):8465–8471. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan KM, Sheahan K, O'Donoghue DP, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282(13):1254–1257. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Schneider S, Yochim JM, et al. Intratumoral COX-2 gene expression is a predictive factor for colorectal cancer response to fluoropyrimidine-based chemotherapy. Clin Cancer Res. 2005;11(9):3363–3368. doi: 10.1158/1078-0432.CCR-04-1650. [DOI] [PubMed] [Google Scholar]
- 16.Ogino S, Kirkner GJ, Nosho K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. 2008;14(24):8221–8227. doi: 10.1158/1078-0432.CCR-08-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002;97(4):1037–1041. doi: 10.1111/j.1572-0241.2002.05625.x. [DOI] [PubMed] [Google Scholar]
- 18.Fux R, Schwab M, Thon KP, Gleiter CH, Fritz P. Cyclooxygenase-2 expression in human colorectal cancer is unrelated to overall patient survival. Clin Cancer Res. 2005;11(13):4754–4760. doi: 10.1158/1078-0432.CCR-04-2586. [DOI] [PubMed] [Google Scholar]
- 19.Tomozawa S, Nagawa H, Tsuno N, et al. Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522. Br J Cancer. 1999;81(8):1274–1279. doi: 10.1038/sj.bjc.6694262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60(21):6045–6051. [PubMed] [Google Scholar]
- 21.Williams CS, Sheng H, Brockman JA, et al. A cyclooxygenase-2 inhibitor (SC-58125) blocks growth of established human colon cancer xenografts. Neoplasia. 2001;3(5):428–436. doi: 10.1038/sj.neo.7900177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi T, Watanabe M, Hasegawa H, et al. The potential for a selective cyclooxygenase-2 inhibitor in the prevention of liver metastasis in human colorectal cancer. Anticancer Res. 2003;23(1A):245–249. [PubMed] [Google Scholar]
- 23.Yao M, Kargman S, Lam EC, et al. Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res. 2003;63(3):586–592. [PubMed] [Google Scholar]
- 24.Yao M, Lam EC, Kelly CR, Zhou W, Wolfe MM. Cyclooxygenase-2 selective inhibition with NS-398 suppresses proliferation and invasiveness and delays liver metastasis in colorectal cancer. Br J Cancer. 2004;90(3):712–719. doi: 10.1038/sj.bjc.6601489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao M, Zhou W, Sangha S, et al. Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res. 2005;11(4):1618–1628. doi: 10.1158/1078-0432.CCR-04-1696. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs C, Meyerhardt JA, Heseltine DL, et al. Influence of regular aspirin use on survival for patients with stage III colon cancer: findings from intergroup trial CALGB 89803 [abstract] J Clin Oncol. 2005;23(suppl 16):3530. [Google Scholar]
- 27.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134(1):21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Stampfer MJ, Colditz GA, et al. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA. 1991;266(4):521–527. [PubMed] [Google Scholar]
- 31.Rimm EB, Giovannucci E, Stampfer M, Colditz G, Litin L, Willett W. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121(4):241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Greene FL, Fleming ID, Fritz A, Balch CM. AJCC Cancer Staging Handbook. 6th ed. Springer-Verlag; New York, NY: 2002. Page DL. [Google Scholar]
- 34.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 35.Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19(1):59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 36.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 37.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci E, Egan KM, Hunter DJ, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333(10):609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 39.Chan AT, Giovannucci EL, Schernhammer ES, et al. A prospective study of aspirin use and the risk of colorectal adenoma. Ann Intern Med. 2004;140(3):157–166. doi: 10.7326/0003-4819-140-3-200402030-00006. [DOI] [PubMed] [Google Scholar]
- 40.Rosner B. Fundamentals of Biostatistics. 5th ed. Duxbury Thomson Learning; Pacific Grove, CA: 2000. [Google Scholar]
- 41.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 42.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. John Wiley & Sons, Inc; Hoboken, NJ: 2000. [Google Scholar]
- 43.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: a propensity analysis. JAMA. 2001;286(10):1187–1194. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]
- 44.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 45.Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci U S A. 1997;94(7):2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez ME, O'Brien TG, Fultz KE, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 2003;100(13):7859–7864. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundholm K, Gelin J, Hyltander A, et al. Anti-inflammatory treatment may prolong survival in under-nourished patients with metastatic solid tumors. Cancer Res. 1994;54(21):5602–5606. [PubMed] [Google Scholar]
- 48.Masunaga R, Kohno H, Dhar DK, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6(10):4064–4068. [PubMed] [Google Scholar]
- 49.Cianchi F, Cortesini C, Bechi P, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121(6):1339–1347. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 50.Tomozawa S, Tsuno NH, Sunami E, et al. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer. 2000;83(3):324–328. doi: 10.1054/bjoc.2000.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petersen S, Haroske G, Hellmich G, Ludwig K, Petersen C, Eicheler W. COX-2 expression in rectal carcinoma: immunohistochemical pattern and clinical outcome. Anticancer Res. 2002;22(2B):1225–1230. [PubMed] [Google Scholar]
- 52.Joo YE, Kim HS, Min SW, et al. Expression of cyclooxygenase-2 protein in colorectal carcinomas. Int J Gastrointest Cancer. 2002;31(1-3):147–154. doi: 10.1385/IJGC:31:1-3:147. [DOI] [PubMed] [Google Scholar]
- 53.Yamac D, Celenkoglu G, Coskun U, et al. Prognostic importance of COX-2 expression in patients with colorectal cancer. Pathol Res Pract. 2005;201(7):497–502. doi: 10.1016/j.prp.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Sheng H, Shao J, Kirkland SC, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99(9):2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93(5):705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 56.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105(11):1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94(7):3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2–selective inhibitor, etodolac. Int J Cancer. 2001;91(6):894–899. doi: 10.1002/1097-0215(200102)9999:9999<894::aid-ijc1146>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 59.Trifan OC, Durham WF, Salazar VS, et al. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 2002;62(20):5778–5784. [PubMed] [Google Scholar]
- 60.Lin J, Hsiao PW, Chiu TH, Chao JI. Combination of cyclooxygenase-2 inhibitors and oxaliplatin increases the growth inhibition and death in human colon cancer cells. Biochem Pharmacol. 2005;70(5):658–667. doi: 10.1016/j.bcp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 61.Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9(1):383–390. [PubMed] [Google Scholar]
- 62.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276(21):18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 63.Buchanan FG, Chang W, Sheng H, Shao J, Morrow JD, DuBois RN. Up-regulation of the enzymes involved in prostacyclin synthesis via Ras induces vascular endothelial growth factor. Gastroenterology. 2004;127(5):1391–1400. doi: 10.1053/j.gastro.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 64.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 65.Arber N. Cyclooxygenase-2 inhibitors in colorectal cancer prevention: point. Cancer Epidemiol Bio-markers Prev. 2008;17(8):1852–1857. doi: 10.1158/1055-9965.EPI-08-0167. [DOI] [PubMed] [Google Scholar]