Abstract
Objective
The aims of the study were to determine the immune responses to candidate viral triggers of multiple sclerosis (MS) in patients with clinically isolated syndromes (CIS), and to evaluate their potential value in predicting conversion to MS.
Methods
Immune responses to Epstein-Barr virus (EBV), human herpesvirus 6, cytomegalovirus (HCMV), and measles were determined in a cohort of 147 CIS patients with a mean follow-up of 7 years and compared with 50 demographically matched controls.
Results
Compared to controls, CIS patients showed increased humoral (p<0.0001) and cellular (p=0.007) immune responses to the EBV-encoded nuclear antigen-1 (EBNA1), but not to other EBV-derived proteins. IgG responses to other virus antigens and frequencies of T cells specific for HCMV and influenza virus gene products were unchanged in CIS patients. EBNA1 was the only viral antigen towards which immune responses correlated with number of T2 lesions (p=0.006) and number of Barkhof criteria (p=0.001) at baseline, and with number of T2 lesions (p=0.012 both at 1 and 5 years), presence of new T2 lesions (p=0.003 and p=0.028 at 1 and 5 years), and EDSS (p=0.015 and p=0.010 at 1 and 5 years) during follow-up. In a univariate Cox regression model, increased EBNA1-specific IgG responses predicted conversion to MS based on McDonald criteria [hazard ratio (95% confidence interval), 2.2 (1.2–4.3); p=0.003].
Interpretation
Our results indicate that elevated immune responses towards EBNA1 are selectively increased in CIS patients and suggest that EBNA1-specific IgG titers could be used as a prognostic marker for disease conversion and disability progression.
Introduction
Environmental insults, such as viral infections, are thought to be important determinants of the risk to develop multiple sclerosis (MS).1,2 Genetic epidemiological studies provided evidence that such factors are likely ubiquitous and operative on a population-level and that they require a permissive genetic trait to trigger the disease since studies in twins, siblings and adopted relatives of MS patients found no indication for non-genetic transmissibility of MS.3,4
The human γ-herpesvirus Epstein-Barr virus (EBV) has long been considered as one biologically plausible trigger factor in MS, because it infects nearly the whole human population, establishes a lifelong dormant persistence with continuous virus production due to reactivation, and modulates the human immune system. In its immune-modifying function, EBV rescues infected B cells via latent antigen expression and assists their differentiation into memory B cells, the long-lived reservoir of EBV persistence. In addition, the virus continuously stimulates strong T-cell responses via chronic antigen presence, and this immune control is crucial for preventing EBV-associated malignancies. Evidence implicating EBV in MS development includes the similarity in the epidemiology of MS and infectious mononucleosis,5 the two to three folds increased risk of developing MS among individuals with history of infectious mononucleosis compared with subjects who acquired EBV without symptoms,6 the almost universal seropositivity for EBV in adults and children with MS,7,8 and the steep and monotonic increase in MS risk with increasing titers of antibodies to EBV in apparently healthy adults.9,10
In the present study, we determined immune responses to candidate viral triggers of MS in patients presenting with clinically isolated syndromes (CIS) compared to demographically matched healthy controls. CIS patients had a mean follow-up of 7 years and were evaluated by serial MRI and clinical examinations.
Methods
Patients
Of a total cohort of 596 patients with CIS recruited at the Centre d’Esclerosi Multiple de Catalunya (CEM-Cat) between 1995 and 2005, 147 patients in whom serum samples were available were included in the study. Demographic, clinical, and MRI characteristics of these 147 patients were similar to those CIS patients that were not part of the study (n=449), except for mean time of follow-up which was significantly longer in the study subgroup (5.3 vs. 7.0 years; p-value = 3.4 × 10−9). Patients presenting for the first time with monophasic neurologic symptoms of the type seen in MS were recruited at the CEM-Cat. Inclusion criteria were: (1) a CIS suggestive of central nervous system demyelination involving the optic nerve, brainstem, spinal cord, or other topography, not attributable to other diseases; (2) age < 50 years; (3) onset of symptoms within three months of both clinical and MRI examinations. The study was approved by the Ethics Committee of Vall d’Hebron University Hospital.
Clinical, cerebrospinal fluid, and MRI assessments have been previously described elsewhere.11 Briefly, during the initial assessment patients were invited to undergo a lumbar puncture as part of our diagnostic workout. All patients were asked about any previous history of neurological disturbances. Any neurological symptom suggestive of MS lasting more than 24 hours, even not confirmed by a previous clinician, was considered as an exclusion criterion. Patients were seen every three to six months and instructed to report any new or worsening of pre-existing symptoms. Brain MRI scans were performed at baseline and after 1 and 5 years of follow-up on a 1.0-T or 1.5-T magnet with a standard head coil. MRI included the following sequences: transverse proton-density and T2-weighted conventional spin echo, and in some patients contrast-enhanced T1-weighted spin-echo. The number of Barkhof criteria,12,13 number of T2 lesions, and number of new T2 lesions were scored. IgG oligoclonal bands were determined by agarose isoelectric focusing combined with immunoblotting and immunoperoxidase staining. Disability was evaluated according to the Expanded Disability Status Scale (EDSS) score in each visit and only EDSS performed during stability periods were considered. Patients were considered stable when symptoms were stable for at least 30 days. A diagnosis of clinically definite MS (CDMS) was made when new symptoms occurred after an interval of at least one month and only when other diagnoses had been excluded. CDMS was diagnosed when there was a second attack with a new neurological abnormality that was confirmed by examination.14 Evidence of dissemination in space was provided by the presence of (i) 3 out of 4 MRI Barkhof parameters or (ii) at least two T2 lesions plus oligoclonal bands. Dissemination in time was fulfilled when at least one new T2 lesion had appeared in the follow-up MRI.15 The McDonald criteria were met when 1/ patients fulfilled the MRI definitions for dissemination in time and space, or 2/ patients had a second clinical attack. Time of follow-up was calculated as the difference between the date of the last visit and the date of the event. Baseline demographic, clinical, and radiological characteristics of the CIS patients are summarized in Table 1. At follow-up, 64% of patients who converted to CDMS and 53% of those who met McDonald criteria received treatment with immunomodulatory agents.
Table 1.
Demographic and baseline clinical characteristics of CIS patients and controls
| Characteristics | CIS | HD |
|---|---|---|
| n | 147 | 50 |
| Age (years) | 29.5 (7.3) | 29.9 (6.0) |
| Female/male (% women) | 110/37 (75.5) | 38/12 (76.0) |
| Follow-up (years)a | 7.0 (2.8–12.5) | - |
| Oligoclonal bands (% positive)* | 93 (65.5) | - |
| Clinical presentation [n (%)] | - | |
| Optic neuritis | 66 (44.9) | - |
| Spinal | 34 (23.1) | - |
| Brainstem | 33 (22.4) | - |
| Others | 14 (9.5) | - |
| Number of Barkhof criteria [n (%)] | ||
| 0 | 54 (37.0) | - |
| 1–2 | 39 (26.7) | - |
| 3–4 | 53 (36.3) | - |
Data are expressed as mean (standard deviation) unless otherwise stated.
Data are expressed as mean (range).
Oligoclonal bands were not available in 5 patients. CIS: patients with clinically isolated syndromes. HD: healthy donors
A healthy control group of 50 individuals recruited from the same geographical area (Barcelona, Spain) was also included in the study (Table 1). A frequency matching was performed to select controls, using sex and age as matching criteria. Thus, controls had the same distribution for the two main variables considered: sex (75.5% and 76.0% of women for CIS patients and controls, respectively) and age in two categories using 34 years as a cut-off point (74.8% vs. 74.0% for age under 34 years and 25.2% vs. 26.0% for age over 34 years in CIS patients and controls, respectively).
ELISA
Peripheral blood was collected from untreated CIS patients at baseline and controls, and serum was prepared after centrifugation of the clotted blood and stored frozen at −80°C until used. The median time (interquartile range) between sample extraction and CIS onset was 1.8 months (0.9–3.2 months). Virus antigen-specific IgG responses were assessed using commercially available ELISA kits according to the manufacturers’ recommendations. The following kits were used: EBNA1 (p72 encoded by BKRF1) IgG and EBV-early antigen (EA) (p54/p138) IgG (Biotest, Dreieich, Germany), VCA (p23/p28) IgG (Diamedix Corporation, Miami, USA), human cytomegalovirus (HCMV) and measles virus (viral lysates) IgG (BioMerieux, Marcy-l’Etoile, France), human herpesvirus 6 (HHV-6) (viral lysates) IgG (Panbio, Brisbane, Australia). IgG activity towards EBV-encoded VCA defined persistent EBV infection/EBV carrier status. All individuals tested for EBV-specific T cell responses as well as for EBV viral loads had detectable levels of VCA-specific IgG antibodies.
ELISPOT for IFN-γ release
Ninety-six-well plates (Millititer; Millipore, Bedford, Massachusetts, USA) were coated overnight at 4°C with 10 mg/ml of the primary anti-IFN-γ mAb (Mabtech, Stockholm, Sweden). The antibody-coated plates were washed four times with phosphate buffered saline (PBS) and blocked with RPMI containing 5% PHS for 1 hour at 37°C. 1.25 × 105 peripheral blood mononuclear cells (PBMC) in 100 μl culture medium containing 1% human AB serum were added per well and stimulated with pooled peptides (5 micromol per peptide). Cells were stimulated with (i) an overlapping peptide library covering the C-terminal domain of EBNA1 (aa 400–641), towards which most of the T cell responses of healthy virus carriers are directed,16 as well as (ii) with pooled immunodominant MHC class I-restricted T cell epitopes derived from three latent (EBNA3A, EBNA3B, EBNA3C) and three lytic (BZLF1, BRLF1, BMLF1) antigens.17 The MHC class I-restricted epitopes were selected based on their previous identification as immunodominant CD8+ T cell epitopes,16 whereas EBNA1 was chosen as a dominant EBV-encoded CD4+ T cell antigen.16,18 MHC class I-restricted HCMV phosphoprotein 65 (pp65)-derived epitopes were used as control antigens.19,20,21 In addition to peptides stimulation, PBMC were exposed to influenza A virus (A/Aichi/68; H3N2) infection.21,22 PBMC were incubated for 24 hours at 37°C in 5% CO2. Wells were washed four times with PBS containing 0.05% Tween-20 (Sigma Chemicals Co., St Louis, Missouri, USA) followed by a 2 hours incubation with 50 μl of the secondary antibody (1 mg/ml, Biotin conjugated anti- IFN-γ mAb; Mabtech). Plates were washed four times in PBS with 0.1% Tween-20. Avidin-bound biotinylated horseradish peroxidase H (Vectastain Elite kit; Vector laboratories, Inc., Burlingame, California, USA) was added to the wells for 1 hour at room temperature. The plates were washed four times in PBS with 0.1% Tween-20 followed by a 5 minutes incubation in stable diaminobenzene (Research Genetics, Huntsville, Alabama, USA) to develop the reaction; tap water was added to stop the reaction. The spots were counted with an ELISPOT reader (AID Autoimmun Diagnostika GmbH, Strassberg, Germany). Only spots with a fuzzy border and a brown colour were counted. A response was considered positive, if there was at least twice the number of spots compared to unstimulated wells. Frequencies of T cells were calculated by subtracting the mean spot number of peptide-stimulated cells from non-stimulated wells.22
Peptide preparation
Peptides were synthesized by the Proteomics Resource Center, Rockefeller University. All peptides were created using a Protein Technologies SYMPHONY multiple-peptide synthesizer (Rainin Instruments, Tucson, AZ) on Wang resin (P-alkoxy-benzyl alcohol resin; BACHEM Bioscience, King of Prussia, PA, and Midwest Bio-Tech, Fishers, IN) using N-Fmoc (9-fluorenylmethyloxycarbonyl) nitrogen terminal–protected amino acids (aa’s; Anaspec, San Jose, CA). Couplings were conducted using HBTU (2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) and HOBT (1-hydroxybenzotriazole) in NMP (N-methylpyrrolidinone) as the primary solvent. Simultaneousresin cleavage and side-chain deprotection were achieved bytreatment with concentrated, sequencing-grade trifluoroacetic acid with triisopropylsilane, water, and also ethanedithiol (if indicated by Cys or Met in sequence) added as ion scavengers in a ratio of 95:2:2:1 (all chemical reagents purchased from Fisher, Hampton, NH; Fluka, Steinheim, Switzerland; and Anaspec). Peptides were then released in 8 M acetic acid, filtered from resin, rotary evaporated, and redissolved in high-performance liquid chromatography (HPLC)–grade water for lyophilization. All crude lyophilized products were subsequently analyzed by reverse-phase HPLC (Waters Chromatography, Milford, MA) using a Merck Chromolith Performance C18 column (West Point, PA). Individual peptide integrity was determined and verified by matrix-assisted laser desorption ionization-mass spectrometry using a Perkin-Elmer/Applied Biosystems Voyager (PE/ABI, Foster City, CA) spectrometer system. Overlapping peptides of 12- to 22-aa length (average of 15-aa length) with 11-aa overlap were designed for the EBNA1400–641 sequence of the B95-8 EBV strain using the peptide-generator tool of the HIV sequence database at the Los Alamos National Laboratory (Los Alamos National Security. PeptGen: HIV Molecular Immunology Database. http://www.hiv.lanl.gov/content/hiv-db/PEPTGEN/PeptGenSubmitForm.html Accessed October 29, 2003).17 For CD8+ T-cell epitopes from EBV and HCMV, we synthesized nonamer peptides that are part of the CEF control peptide pool of the NIH AIDS Research & Reference Reagent Program.17,20 Influenza A virus (A/Aichi/68; H3N2) infection served as additional control.22
Viral loads
EBV DNA was quantified from PBMC by quantitative real time PCR using a TaqMan PCR kit and a Model 7500 Sequence Detector (Applied Biosystems, Foster City, CA)19 in 29 CIS patients and 34 controls from whom cryopreserved cells were available. Demographic and baseline clinical characteristics were comparable between patients and controls with and without viral loads assessed, except for mean time of follow-up which was significantly longer in CIS patients with viral loads determined (8.1 vs. 6.7 years; p-value = 0.013). In these individuals, DNA was extracted from PBMC using the QIAamp DNA Blood Mini Kit (Qiagen) following the manufacturer’s protocol. A region from the Bam HIW fragment of EBV was amplified using primers 5′-GGACCACTGCCCCTGGTAAA-3′ and 5′-TTTGTGTGGACTCCTGGGG-3′ and detected with fluorogenic probe 5′-FAM-TCCTGCAGCTATTTCTGGTCGCATCA-TAMRA-3′. The human bcl-2 gene was amplified using primers 5′-CCTGCCCTCCTTCCGC-3′ and 5′-TGCATTTCAGGAAGACCCTGA-3′ and detected with fluorogenic probe 5′-FAM-CTTTCTCATGGCTGTCC-TAMRA-3′. The EBV BamHI W fragment copy number per cell was calculated using the formula N=2xW/B, where N is the EBV BamHI W copy number/cell, W is the EBV BamHI W copy number, and B is the bcl-2 copy number. All samples were tested in at least duplicate and the mean results were determined.17,19
Statistical analysis
Statistical analysis was performed by using the SPSS 15.0 package (SPSS Inc, Chicago, IL) for MS-Windows. A Mann-Whitney’s test was used to test for significant differences in cellular and humoral immune responses between CIS patients and controls. Correlations between viral IgG titers and MRI and clinical variables in CIS patients were assessed by the Spearman rank correlation coefficient. In 4 out of 147 patients (2.7%) the second clinical relapse occurred shortly after the CIS event, and serum samples were collected after the second attack (in three patients blood was collected 2 months after the second relapse, and in one patient 3 days after the second relapse). Insomuch as viral titers were similar between both groups of patients, all patients were included in the analysis. Univariate and multivariate Cox proportional hazard regression models including the baseline Barkhof criteria, oligoclonal bands, age, and gender as covariates were used to evaluate the association between viral IgG titers and time to conversion to MS defined by the Poser or McDonald criteria. An adjustment for use of disease modifying therapies after the CIS event was not possible, owing to the fact that treatment initiation was not a baseline variable, but rather a consequence of the subsequent disease evolution.
Results
Selective increase of EBNA1-specific immune responses in patients with CIS
To evaluate immune responses to candidate viral triggers of MS in patients with CIS versus healthy virus carriers, we first determined levels of IgG specific for different EBV-encoded antigens: the EBV-encoded immunogenic C-terminal domain of EBNA1 (p72), pooled viral capsid antigens (EBV-VCA) and pooled early antigens (EBV-EA). EBV-specific antibody responses were compared to IgG reactivities against lysates of other ubiquitous viruses such as HCMV, HHV-6, and measles virus.
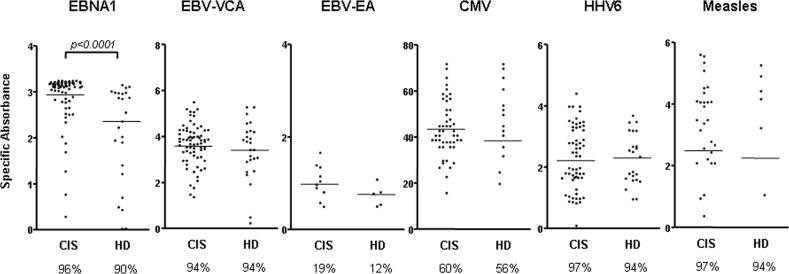
As shown in Figure 1, the percentage of individuals showing a positive response towards EBV antigens was similar between CIS patients (n = 147) and healthy EBV carriers (n = 50) (96% vs. 90% for EBNA1; 94% vs. 94% for VCA; 19% vs. 12% for EA). However, patients with CIS at the time of disease onset showed a selective and highly significant increase of IgG responses towards EBNA1 compared to healthy EBV carriers [mean values (standard error of the mean, SEM): 2.94 (0.04) vs. 2.36 (0.13) respectively; p < 0.0001, which corresponds to 1.25-fold higher IgG activity to EBNA1 in CIS patients compared to controls]. Antibody responses to other EBV-encoded antigens as well as to proteins derived from other viruses were similar in patients and controls (Fig 1).
Figure 1. Selective increase of EBNA1-specific IgG response in patients with CIS.
Antibody reactivity was determined in 147 untreated CIS patients and 50 demographically matched controls recruited from the same geographical area (Barcelona, Spain), as described in Methods. IgG responses towards EBNA1, EBV-encoded viral VCA and pooled EA as well towards lysates of other viruses previously associated with MS (HHV6, measles, HCMV) were quantified using commercially available ELISA kits. The percentage of individuals showing a positive response towards each antigen preparation. i.e. seroprevalence, is given below the diagrams. The non-parametric Mann Whitney U test was used to compare frequencies between CIS patients and healthy donors (HD).
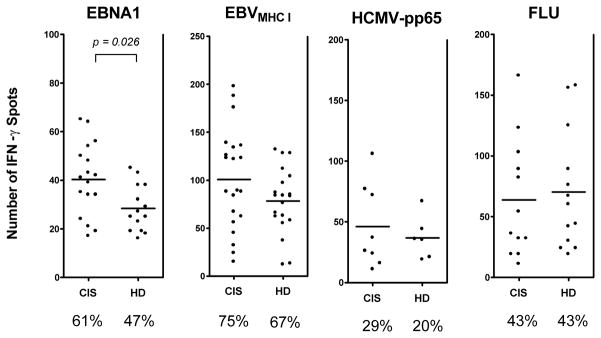
To investigate cellular immune responses to ubiquitous viruses in patients with CIS, we determined frequencies of viral antigen-specific T cells by IFN-γ ELISPOT assays. PBMC from 28 EBV-infected patients with CIS and 30 healthy EBV carriers were stimulated with an overlapping peptide library of EBNA1 (aa 400–641), pooled immunodominant MHC class I-restricted EBV- and HCMV-derived epitopes as well as infected with influenza A virus. Compared to healthy EBV carriers, patients with CIS showed a moderate increased frequency of EBNA1-specifc IFN-γ producing T cells [mean (SEM): 48.0 (6.0) vs. 26.2 (2.2); p= 0.026, which corresponds to 1.83-fold higher frequency of EBNA1-specific IFN-γ producing T cells in CIS patients compared to controls]. The frequency of T cells specific for EBV-derived immunodominant CD8+ T cell epitopes was elevated in some patients but the overall difference was not statistically significant compared with controls (Fig 2). Moreover, frequencies of HCMV-pp65 and influenza A virus targeting T cells were unchanged in CIS patients. These data suggest a selective increase of cellular and humoral immune responses specific for EBNA1 in patients with CIS.
Figure 2. Increased frequency of EBNA1-specific IFN-γ producing T cells in patients with CIS.
T cell frequencies were quantified in 28 untreated EBV-infected patients with CIS and 30 healthy EBV carriers using an ELISPOT assay. PBMC were stimulated with an overlapping peptide library covering the C-terminal domain of EBNA1 (aa 400–641), pooled immunodominant MHC class I restricted EBV-derived epitopes and MHC class I restricted immunodominant CMV-encoded epitopes derived form the phosphoprotein 65 (pp65). In addition, PBMC were infected with human influenza virus (FLU). Frequencies are displayed as number of spots per 250,000 PBMC. The percentage of individuals showing a positive IFN-γ response towards each set of viral antigens is given below the diagrams. The non-parametric Mann Whitney U test was used to compare frequencies between CIS patients and controls (HD).
Higher cellular EBV copy numbers in patients with CIS
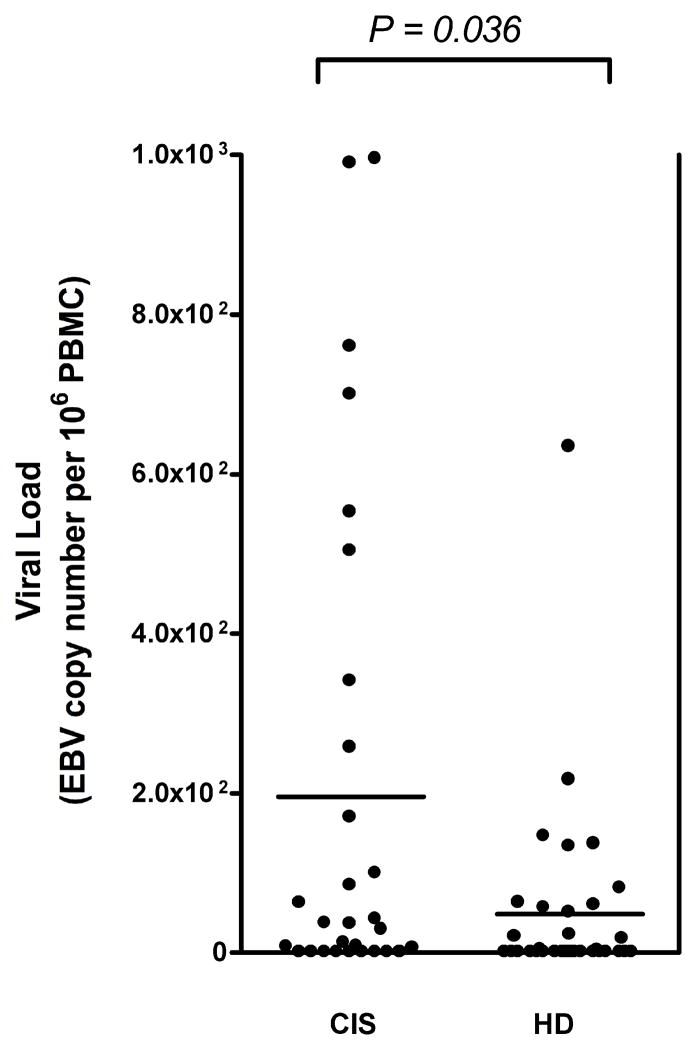
To determine whether the increased immune response to EBNA1 observed in CIS patients at baseline correlate with higher viral loads, we quantified levels of cell-bound viral genomes in circulating blood cells.17,19 EBV DNA was detectable in 20 out of 29 (69%) CIS patients and in 16 out of 34 (47%) controls from whom PBMC were accessible for viral load quantification. Surprisingly, CIS patients showed approximately fourfold increased levels of cell-associated EBV DNA copies compared to healthy EBV carriers [mean EBV copy number per 106 PBMC (SEM) in CIS vs. controls: 196 (58) vs. 48 (20); p= 0.036] (Fig 3) suggesting that EBV infection is dysregulated early in the development of MS.
Figure 3. Elevated viral load in patients with CIS compared to demographically matched controls.
Viral titers were determined in 29 untreated EBV-infected patients with CIS and 34 healthy EBV carriers by quantitative real time PCR in relation to bcl-2 expression. All samples were tested in duplicates. Bars represent mean viral titers [mean (SEM) in CIS vs. controls (HD): 196 (58) vs. 48 (20)].
Humoral immune responses to EBV are associated with brain MRI abnormalities in CIS patients at baseline
We next investigated whether immune responses to candidate viral triggers of MS correlate with brain MRI-derived metrics in patients with CIS at the time of disease onset. Baseline data on number of T2 lesions and of Barkhof criteria which have shown high specificity for predicting early conversion to MS11,12 were available for all patients (n = 147). IgG responses to EBV-encoded VCA and EBNA1 correlated with the number of T2 lesions and the number of Barkhof criteria (Table 2). Antibody responses to antigens expressed by HCMV, HHV-6, measles virus and to EBV-encoded EA did not significantly correlate with brain MRI-derived metrics. Data on number of gadolinium-enhancing lesions was available for a small subgroup of CIS patients (n=32). Within this subgroup, we did not detect statistically significant correlations between virus-specific IgG responses and the number of contrast enhancing lesions. In addition, frequencies of virus-specific T cells (subgroup of 28 patients) and EBV copy numbers in circulating blood cells (subgroup of 30 patients) did not significantly correlate with the aforementioned MRI measures (data not shown). Altogether, these data indicate that IgG responses to EBV-encoded VCA and to EBNA1 are associated with brain MRI measures of dissemination in space in treatment-naive patients with CIS.
Table 2.
Correlations between viral IgG titers and clinical and brain MRI parameters
| Baseline |
1 year1 |
5 years2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Serologies | BC | NT2L | NT2L | NNT2L | EDSS | NT2L | NNT2L | EDSS |
| Measles | −0.02 (0.796) | −0.02 (0.799) | 0.05 (0.595) | 0.01 (0.876) | 0.08 (0.372) | −0.01 (0.926) | −0.01 (0.961) | 0.02 (0.859) |
| HCMV | −0.06 (0.455) | 0.03 (0.728) | 0.02 (0.801) | 0.01 (0.950) | 0.07 (0.403) | 0.01 (0.946) | −0.03 (0.767) | 0.12 (0.197) |
| HHV-6 | −0.06 (0.473) | −0.05 (0.559) | 0.05 (0.633) | −0.01 (0.969) | −0.17 (0.044) | 0.13 (0.219) | 0.14 (0.182) | −0.01 (0.962) |
| EBV-VCA | 0.22 (0.009) | 0.27 (0.002) | 0.21 (0.020) | 0.12 (0.161) | 0.15 (0.085) | 0.18 (0.077) | 0.08 (0.438) | 0.14 (0.152) |
| -EBNA1 | 0.30 (0.001) | 0.24 (0.006) | 0.24 (0.012) | 0.22 (0.012) | 0.21 (0.015) | 0.31 (0.003) | 0.23 (0.028) | 0.25 (0.010) |
| -EA | −0.01 (0.979) | −0.01 (0.957) | −0.15 (0.501) | −0.15 (0.478) | 0.08 (0.696) | 0.16 (0.547) | 0.04 (0.888) | −0.19 (0.400) |
Data are expressed as Spearman correlation coefficient (p value).
Number of patients with 1 year follow-up: 147.
Number of patients with 5 year follow-up: 119. HCMV: cytomegalovirus. HHV-6: human herpesvirus 6. EBV: Epstein-Barr virus. VCA: viral capsid antigen. EBNA1: EBV nuclear antigen 1. EA: early antigen. BC: Barkhof criteria. NT2L: number of T2 lesions. NNT2L: number of new T2 lesions. EDSS: Expanded Disability Status Scale.
Increased EBNA1-specific immune response is associated with disability progression and conversion to MS
Taking advantage of the long follow-up of the CIS cohort (7 years of mean follow-up), we investigated whether antiviral immune responses were associated MRI abnormalities and disability after 1 and 5 years of follow-up. Almost 90% of CIS patients with abnormal baseline MRI develop CDMS during a follow-up period of 14 years23 and even patients with normal baseline MRI have a 20% risk of developing CDMS at 20 years.24 We therefore included all patients in our correlation analysis instead of focusing on those who converted to MS within the follow-up period. Similar to our findings at baseline, IgG responses to both EBV-encoded VCA and EBNA1 significantly correlated with the number of T2 lesions at 1 year (Table 2). In contrast, EBNA1 was the only viral antigen towards which immune responses correlated with the presence of new T2 lesions at 1 year, as well as number of T2 lesions and presence of new T2 lesions at 5 years (Table 2). Furthermore, only EBNA1-specific antibody responses correlated with neurological disability, i.e. EDSS, at 1 and 5 years after disease onset (Table 2). During the follow-up period, 67 patients (45.6%) converted to CDMS according to the Poser criteria, in which conversion is based solely on clinical findings, and 87 patients (59.2%) converted to MS according to McDonald criteria,15,25 in which conversion is based on clinical and/or MRI findings. Of note, in a univariate Cox regression model, increased EBNA1-specific antibody responses were associated with an increased risk for conversion to CDMS [1.7 (0.9–3.2); p=0.074] and a significantly higher risk of conversion to MS [2.2 (1.2–4.3); p=0.003] as defined by McDonald criteria (Table 3). However, significance was lost in a multivariate model that includes strong predictors of conversion to MS such as the Barkhof criteria and oligoclonal bands, most likely reflecting the influence that these covariates have in the predictive model. These data indicate that, even though they are not an independent risk factor, EBNA1-specific immune responses increase the risk of conversion to MS as defined by MRI and clinical criteria, and correlate with disability progression at 1 and 5 years.
Table 3.
EBNA1 IgG titers and risk of conversion to MS defined by Poser and McDonald criteria
| Criteria | Univariate | Multivariate*9 | |||||
|---|---|---|---|---|---|---|---|
| N (%) | HR | 95% CI | P value | HR | 5% CI | P value | |
| Poser | |||||||
| EBNA1 | 67 (45.6) | 1.7 | 0.9 – 3.2 | 0.074 | 1.2 | 0.6 – 2.1 | 0.619 |
| McDonald | |||||||
| EBNA1 | 87 (59.2) | 2.2 | 1.2 – 4.3 | 0.003 | 1.6 | 0.9 – 3.0 | 0.128 |
For Poser criteria, Cox regression model was adjusted by baseline Barkhof criteria, oligoclonal bands, sex, and gender. For McDonald criteria, inasmuch as the number of Barkhof criteria is included in the McDonald criteria for conversion to MS, the multivariate analysis was adjusted by oligoclonal bands, sex, and gender but not baseline Barkhof criteria. HR: hazard ratio. 95% CI: 95% confidence intervals.
Discussion
Exposures to ubiquitous environmental factors during the first two decades of life such as childhood infections with common viruses are considered important risk factors in the development of MS. We found that, compared to matched healthy EBV carriers, patients with CIS show increased cellular and humoral immune responses to EBNA1, the most consistently recognized EBV-derived CD4+ T cell antigen,16,18 but not to other EBV-encoded proteins such as VCA and EA. Moreover, IgG responses to HHV-6, HCMV, and measles virus derived antigens as well as frequencies of T cells specific for HCMV and influenza virus gene products were unchanged in patients with CIS. EBNA1 was the only viral antigen towards which immune responses correlated with brain MRI abnormalities at baseline and during follow-up, and with disability progression over a 5 year period. Moreover, in a univariate regression model increased IgG responses to EBNA1 predicted conversion to MS. These data indicate that EBNA1 is a potential target antigen of the pathogenic immune response in patients with CIS and suggest that EBNA1-specific IgG titers could be used as a prognostic marker for disability progression and conversion to MS.
The selective increase of EBNA1-specific T cell and antibody responses in CIS patients without a concomitant increase of immune responses to other EBV-encoded antigens and to proteins derived from other ubiquitous viruses is consistent with previous studies performed in patients with MS7,9,10,19,22 and in healthy individuals who will develop MS. EBNA1 is the only EBV-encoded protein consistently expressed in proliferating EBV-infected memory B cells and the most consistently recognized EBV-derived CD4+ T-cell antigen in healthy virus carriers.18,26 EBNA1 maintains EBV infection by distributing viral DNA into progeny cells through cross-linking of the episome to mitotic chromosomes during cell division.27 Structural proteins such as viral nucleocapsid antigens (VCA) are expressed during acute infection and, in healthy virus carriers, following occasional reactivations into lytic cycle, triggered by B cell receptor antigen stimulation or receipt of another plasma cell differentiation signal.16 Antibody responses to VCA peak during acute infection, whereas EBNA1 reach their highest titer during convalescence from infectious mononucleosis. Notably, epidemiological studies reported that the increase in EBNA1-specific IgG titers precede the onset of MS in various populations.7–9 Investigating longitudinal serum samples form the Nurses’ Health Study, Ascherio et al.7 found that IgG specific for EBV nuclear antigens were significantly elevated in plasma collected before the onset of MS and did not change significantly after MS onset. Sundstrom et al.9 reported that individuals who will develop MS show increased IgG responses to EBNA1 but not to EBV-VCA, this being most pronounced in the 5-year period preceding MS onset. In line with these results, Levin et al.10, studying longitudinal samples from US military personnel, found that EBNA1-specific IgG titers were elevated 5 or more years before the onset of MS and noted that this increase occurred between the late teens and the mid to late twenties, independently from the age of MS onset. Three recently published longitudinal studies investigated whether humoral immune responses to EBV correlate with clinical and MRI-based markers for MS disease activity and progression over time. Zivadinov and colleagues28 reported that initial levels of EBV VCA-specific IgG were associated with accelerated loss of whole brain parenchyma (BPF) measured over a period of three years. Unfortunately, initial IgG titers specific for other EBV antigens such as EBNA1 or towards other viruses were not determined. In another study,29 the same group reported that expression of certain MHC class I alleles are significantly associated with MRI-based markers of MS disease activity and progression. In the aforementioned study, VCA-IgG titers were similar in MS patients and controls and not significantly associated with any clinical or neuroimaging disease parameter. Again, immune responses to EBV antigens other than VCA were not determined. In line with our results, Farrell at al.30 reported that elevated humoral immune responses to EBNA1 are associated with the development of gadolinium-enhancing lesions and predictive for T2 lesion volume change and EDSS progression in patients with CIS and relapsing-remitting MS followed over a period of 5 years. We previously reported that untreated patients with relapsing-remitting MS show a selective increase of CD4+ T cell responses to EBNA1, but not to other EBV-encoded proteins or to influenza and HCMV-derived antigens,19,22 and EBNA1-specific IgG responses are predominantly increased in children and adults with MS.19,31 Here, we extended our previous findings by demonstrating that patients with CIS have a selective increase of EBNA1-specific cellular and humoral immune responses, and report that higher EBNA1-specific IgG levels are predictive of conversion to MS.
In contrast to patients with relapsing remitting MS,19 patients with CIS showed moderately increased cell-bound EBV copy numbers. Notably, viral loads in CIS patients were quantified using the same protocol as in the aforementioned study. EBV manipulates the human B cell compartment to achieve persistence in memory B cells and is strongly regulated by and responsive to the biology of its main host cell. Antigen stimulation and/or receipt of another plasma cell differentiation signal drives occasional reactivations into the viral lytic cycle.18,32 It has previously been proposed that B cell dysfunction in autoimmune diseases associated with prominent autoantibody production alters regulatory mechanisms of EBV persistence since patients with systemic lupus erythematosus show up to 40-fold increased viral loads33 as well as aberrant expression of viral latent and lytic genes in the blood and abnormally high frequencies of circulating EBV-infected cells associated with disease flares.34 We found that patients with rheumatoid arthritis show 7-fold higher levels of cell-associated viral genomes in circulating blood cells associated with increased frequencies of EBV-specific and IFN-γ producing CD8+ T cells17 and suggested that immune dysfunctions associated with B cell-driven autoimmune diseases, e.g. less stringent activation/differentiation requirements, drive enhanced EBV replication in B cells, thereby stimulating increased lytic and latent EBV antigen-specific T cell responses.17 Our results in CIS patients are compatible with an early dysregulation of host-EBV interactions at the level of EBV-infected B cells. In line with this assumption, Wagner et al.35 reported that the presence of detectable EBV particles in plasma samples is indicative for an increased in risk of MS. Although MS is considered a primarily T cell-mediated autoimmune disease, the finding that clonally expanded populations of immunoglobulin variable gene-mutated B cells are present in MS lesion tissue36,37 as well as the therapeutic efficacy of CD20-targeting immunotherapies38,39 indicate that B cells critically contribute to the development of MS. Jilek et al.40 recently reported that CIS patients but not patients with CDMS show increased frequencies of T cells specific for MHC class I-restricted EBV antigens, including those expressed during B cell transformation and lytic replication. Although some patients in our study showed higher frequencies of IFN-γ producing T cell specific for EBV-encoded immunodominant CD8+ T cell epitopes if compared to healthy EBV carriers, the overall difference was not statistically significant and EBNA1 was the only differentially recognized EBV antigen. Likewise, EBNA1-specific IgG titers appear to be the strongest predictor of MS risk in healthy individuals.10 However, slightly elevated levels of IgG specific for lytic EBV antigens such as VCA and EA where also observed in the above mentioned Nurses’ Health Study7 and the US Army personnel study.10 Although we did not detect significantly elevated immune responses to EBV antigens expressed during B cell transformation and productive viral replication in our CIS cohort, we cannot exclude that such specificities predominate at earlier time points during the evolution of MS. Such a scenario would be reminiscent of infectious mononucleosis, during which most antibody responses follow the availability of their respective antigens and peak during acute infection, whereas antibody responses against EBNA1 reach their highest titer at later time points during convalescence from infection.41 We therefore suggest that subtle changes in regulatory mechanisms of EBV persistence early in the development of MS, maybe triggered by autoreactive immune responses, lead to increased viral replication and antigen recognition. These alterations in EBV specific immune control might not unlike infectious mononucleosis expand EBNA1 specific T and B cell responses that remain elevated due to cross-reactivity with autoantigens and/or restimulation by central nervous system infiltrating EBV infected B cells.42–45
Even so the mechanisms behind elevated EBNA1 specific immune responses in CIS and MS patients are far from understood and our findings do not imply a pathogenic role of EBNA1-specifc immune responses in MS, the observation that EBNA1 IgG responses observed in CIS patients at baseline are associated with conversion to MS and correlate with disability over a 5 year period strongly suggests that, if replicated in other CIS cohorts, EBNA1-IgG titers might be used as prognostic marker for conversion to MS and disability progression. An individual biomarker is likely to reflect only one of many ongoing pathogenic processes in a complex autoimmune disease such as MS, and it is thus likely to be most useful when integrated with others.46 Further investigations will be necessary to clarify whether increased EBNA1-specific immune responses are causally related to MS or a consequence of MS-associated immune dysfunction.
Acknowledgments
The authors thank the “Red Española de Esclerosis Múltiple (REEM)” sponsored by the “Fondo de Investigación Sanitaria” (FIS), Ministry of Science and Innovation, Spain, and the “Ajuts per donar Suport als Grups de Recerca de Catalunya (SGR 2005-1081)”, sponsored by the “Agència de Gestió d’Ajuts Universitaris i de Recerca” (AGAUR), Generalitat de Catalunya, Spain. The authors are supported by the Burroughs Wellcome Fund, the Starr Foundation, the National Cancer Institute (R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious Diseases (RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health (Grand Challenges in Global Health), and an Institutional Clinical and Translational Science Award (to the Rockefeller University Hospital) to CM; by a Dana Foundation and Irvington Institute’s Human Immunology Fellowship and an Institutional Clinical and Translational Science Pilot and Collaborative Project Grant (to the Rockefeller University Hospital) to JDL.
References
- 1.Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ascherio A, Munger KL. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 2.Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ascherio A, Munger KL. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 3.Ebers GC, Sadovnick AD, Risch NJ. A genetic basis for familial aggregation in multiple sclerosis. Canadian Collaborative Study Group. Nature. 1995;377:150–151.4. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- 4.Sadovnick AD, Ebers GC, Dyment DA, Risch NJ. Evidence for genetic basis of multiple sclerosis. The Canadian Collaborative Study Group. Lancet. 1996;347:1728–1730. doi: 10.1016/s0140-6736(96)90807-7. [DOI] [PubMed] [Google Scholar]
- 5.Warner HB, Carp RI. Multiple sclerosis and Epstein-Barr virus. Lancet. 1981;2:1290. doi: 10.1016/s0140-6736(81)91527-0. [DOI] [PubMed] [Google Scholar]
- 6.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59:499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- 7.Ascherio A, Munger KL, Lennette ET, et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA. 2001;286:3083–3088. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 8.Alotaibi S, Kennedy J, Tellier R, et al. Epstein-Barr virus in pediatric multiple sclerosis. JAMA. 2004;291:1875–1879. doi: 10.1001/jama.291.15.1875. [DOI] [PubMed] [Google Scholar]
- 9.Sundstrom P, Juto P, Wadell G, et al. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology. 2004;62:2277–2282. doi: 10.1212/01.wnl.0000130496.51156.d7. [DOI] [PubMed] [Google Scholar]
- 10.Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of epstein-barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293:2496–2500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 11.Tintoré M, Rovíra A, Río J, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology. 2006;67:968–972. doi: 10.1212/01.wnl.0000237354.10144.ec. [DOI] [PubMed] [Google Scholar]
- 12.Tintore M, Rovira A, Rio J, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology. 2008;70:1079–1083. doi: 10.1212/01.wnl.0000280576.73609.c6. [DOI] [PubMed] [Google Scholar]
- 13.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120:2059–2069. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 14.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 15.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 16.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from epstein-barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 17.Lunemann JD, Frey O, Eidner T, et al. Increased frequency of EBV-specific effector memory CD8+ T cells correlates with higher viral load in rheumatoid arthritis. J Immunol. 2008;181:991–1000. doi: 10.4049/jimmunol.181.2.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munz C, Bickham KL, Subklewe M, et al. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunemann JD, Edwards N, Muraro PA, et al. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain. 2006;129:1493–1506. doi: 10.1093/brain/awl067. [DOI] [PubMed] [Google Scholar]
- 20.Currier JR, Kuta EG, Turk E, et al. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods. 260:157–172. doi: 10.1016/s0022-1759(01)00535-x. [DOI] [PubMed] [Google Scholar]
- 21.Heller KN, Upshaw J, Seyoum B, et al. Distinct memory CD4+ T-cell subsets mediate immune recognition of Epstein Barr virus nuclear antigen 1 in healthy virus carriers. Blood. 2007;109:1138–1146. doi: 10.1182/blood-2006-05-023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunemann JD, Jelcic I, Roberts S, et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-gamma and IL-2. J Exp Med. 2008;205:1763–1773. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 24.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131(Pt 3):808–817. doi: 10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- 25.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci U S A. 2004;101:239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munz C. Epstein-barr virus nuclear antigen 1: from immunologically invisible to a promising T cell target. J Exp Med. 2004;199:1301–1304. doi: 10.1084/jem.20040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zivadinov R, Zorzon M, Weinstock-Guttman B, et al. Epstein-Barr virus is associated with grey matter atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:620–625. doi: 10.1136/jnnp.2008.154906. [DOI] [PubMed] [Google Scholar]
- 29.Zivadinov R, Weinstock-Guttman B, Zorzon M, et al. Gene-environment interactions between HLA B7/A2, EBV antibodies are associated with MRI injury in multiple sclerosis. J Neuroimmunol. 2009;209:123–130. doi: 10.1016/j.jneuroim.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Farrell RA, Antony D, Wall GR, et al. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology. 2009;73:32–38. doi: 10.1212/WNL.0b013e3181aa29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunemann JD, Huppke P, Roberts S, et al. Broadened and elevated humoral immune response to EBNA1 in pediatric multiple sclerosis. Neurology. 2008;71:1033–1035. doi: 10.1212/01.wnl.0000326576.91097.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 33.Kang I, Quan T, Nolasco H, et al. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J Immunol. 2004;172:1287–1294. doi: 10.4049/jimmunol.172.2.1287. [DOI] [PubMed] [Google Scholar]
- 34.Gross AJ, Hochberg D, Rand WM, Thorley-Lawson DA. EBV and systemic lupus erythematosus: a new perspective. J Immunol. 2005;174:6599–6607. doi: 10.4049/jimmunol.174.11.6599. [DOI] [PubMed] [Google Scholar]
- 35.Wagner HJ, Munger KL, Ascherio A. Plasma viral load of Epstein-Barr virus and risk of multiple sclerosis. Eur J Neurol. 2004;11:833–834. doi: 10.1111/j.1468-1331.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 36.Colombo M, Dono M, Gazzola P, et al. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000;164:2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- 37.Colombo M, Dono M, Gazzola P, et al. Maintenance of B lymphocyte-related clones in the cerebrospinal fluid of multiple sclerosis patients. Eur J Immunol. 2003;33:3433–3438. doi: 10.1002/eji.200324144. [DOI] [PubMed] [Google Scholar]
- 38.Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 39.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 40.Jilek S, Schluep M, Meylan P, et al. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. 2008;131:1712–1721. doi: 10.1093/brain/awn108. [DOI] [PubMed] [Google Scholar]
- 41.Henle W, Henle G, Andersson J, et al. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc Natl Acad Sci USA. 1987;84:570–574. doi: 10.1073/pnas.84.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannoni G, Cutter GR, Lunemann J, et al. Infectious causes of multiple sclerosis. Lancet Neurol. 2006;5:887–894. doi: 10.1016/S1474-4422(06)70577-4. [DOI] [PubMed] [Google Scholar]
- 43.Lünemann JD, Kamradt T, Martin R, Münz C. Epstein Barr Virus: Environmental Trigger of Multiple Sclerosis? J Virol. 2007;81:6777–6784. doi: 10.1128/JVI.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serafini B, Rosicarelli B, Franciotta D, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franciotta D, Salvetti M, Lolli F, et al. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 46.Bielekova B, Martin R. Development of biomarkers in multiple sclerosis. Brain. 2004;127(Pt 7):1463–1478. doi: 10.1093/brain/awh176. [DOI] [PubMed] [Google Scholar]