Abstract
Apoptosis or programmed cell death has been demonstrated to play a role in the development of lung injury following hemorrhagic shock. A major pathway modulating the apoptotic response is the phosphatidylinositol 3-kinase/serine/threonine kinase (PI3K/Akt) pathway. Ciglitazone, a peroxisome proliferator-activated receptor-y (PPARy) ligand has previously been shown to attenuate lung inflammation following hemorrhagic shock. In vivo similar ligands have demonstrated anti-apoptotic effects with a reduction in organ injury in models of acute illness. In this study we examined the effect of ciglitazone on apoptosis and PI3K/Akt signaling in the lung following severe hemorrhage and resuscitation. Hemorrhagic shock was induced in male Wistar rats by withdrawing blood from the femoral artery to a mean arterial pressure of 50 mmHg. Animals were kept in shock for 3h at which time they were rapidly resuscitated by returning their shed blood. At the time of resuscitation and every hour thereafter, groups of animals received ciglitazone (10mg/kg) or DMSO intraperitoneally. Vehicle-treated rats had increased lung apoptosis following hemorrhage and resuscitation by Tunel staining. This was associated with increased activity of caspase-3. Ciglitazone treatment reduced lung apoptosis with a significant reduction in caspase-3 activity. This was associated with increased phosphorylation of the pro-survival kinase Akt. Thus, our data suggest that ciglitazone, a PPARy ligand, promotes cell survival in the lung following hemorrhagic shock.
Keywords: Hemorrhagic shock, lung injury, ciglitazone, peroxisome proliferator-activated receptor-y, apoptosis, phospho-Akt
Introduction
Apoptosis or programmed cell death represents an alternative pathway for cell death in the setting of critical illness [1]. It is characterized by the activation of intracellular signaling pathways which ultimately lead to DNA cleavage through activation of enzymes known as caspases. Caspases are cysteine proteases which when activated commit a cell to die. In mammals caspase-3 is the major effector caspase and is the final executioner of the apoptotic response [2]. Such a mechanism of cell death may represent an important phenomenon in the pathogenesis of lung injury [3]. On the contrary, a major signaling pathway that regulates cell growth, differentiation and survival is the phosphatidylinositol 3-kinase/serine/threonine kinase (PI3K/Akt) pathway [4]. Modulation of this pathway in animal models of ischemia-reperfusion and sepsis is associated with amelioration of organ injury [5].
In the setting of hemorrhagic shock, lung dysfunction is common [6]. Moreover lung injury may perpetuate the development of multiple organ failure [6, 7]. Neutrophils play a major role in the pathogenesis of lung injury following severe hemorrhage; however apoptosis may also contribute to this process [8]. Hemorrhage has been shown to induce apoptosis in the alveolar epithelial cells, vascular endothelial cells, neutrophils and macrophages in the lung which may contribute to organ dysfunction [9, 10]. Hence, modulating apoptosis may limit cell death and thereby ameliorate lung injury.
We have previously demonstrated that ciglitazone, a PPARy ligand belonging to the thiazolidinedione group, ameliorates the inflammatory response in the lung following severe hemorrhage [11]. Additionally, PPAFty has been shown to regulate cellular differentiation, proliferation and apoptosis [12, 13]. More recently in a rodent model of myocardial ischemia-reperfusion PPARy activation was associated with increased activity of the pro-survival serine-threonine kinase Akt [14]. However, the effects of PPAFty activation on apoptosis and the PI3K/Akt pathway during severe hemorrhage are not known.
Therefore, the purpose of this study was to investigate whether ciglitazone, a PPAFty ligand, would modulate the apoptotic response and PI3K/Akt signaling in a rodent model of hemorrhagic shock. Our data demonstrated that administration of ciglitazone following severe hemorrhage at the time of resuscitation was associated with a reduction in lung apoptosis and upregulation of the serine-threonine kinase Akt in the lung. Hence, our data suggest that ciglitazone treatment may be beneficial in promoting cell survival in the lung during hemorrhage.
Materials and methods
Rodent model of hemorrhagic shock
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996) and commenced with the approval of the institutional Animal Care and Use Committee. Male Wistar rats (200-300 g, Charles River Laboratories, Wilmington MA) were anesthetized with pentobarbital (80 mg/kg) intraperitoneally (IP). The right femoral artery and left common carotid artery were cannulated (PE-50 tubing) and used for drawing blood and measuring mean arterial blood pressure (MAP), respectively. The trachea was cannulated and the animals were placed on a rodent ventilator (Harvard Apparatus, Holliston, MA) with similar settings, tidal volume 2 ml, rate 60 breaths/min and FiO2 of 0.4. Hemorrhagic shock was induced as previously described [15]. The animals were kept at a MAP of 50 mmHg for 3h by withdrawing or re-injecting blood through the femoral artery. At 3h rats were rapidly resuscitated over 10 min with their own shed blood supplemented with Ringer Lactate solution to a final volume equal to total shed blood and monitored for another 3h. Heart rates (HR) and MAP were measured using a pressure transducer and digitized using a Maclab A/D converter. This data was analyzed using Chart 5 software (AD Instruments Colorado Springs, CO) at 30 min intervals during the entire experiment.
Rats were assigned to 3 groups- sham, vehicle, and ciglitazone. Rats in the sham group underwent the surgical procedure, but were not bled. Rats in the vehicle and ciglitazone group received dimethyl sulphoxide (0.1 ml/kg DMSO IP) or ciglitazone (10 mg/kg IP) respectively at resuscitation and hourly thereafter. Rats were sacrificed at 3h following resuscitation. Lungs and plasma samples were collected from each rat and stored at -70 °C.
Determination of apoptosis
Cell death by apoptosis in lungs was evaluated by measurement of oligonucleosomal DNA fragments by a histochemical terminal deoxynucleotidyl transferase (TdT) TUNEL-like staining (TdT-FragEL kit; Oncogene Research Products, Cambridge, MA). In brief, after deparaffinization, paraffin-embedded sections were permeabilized with protease K (2 mg/ml) in 10 mM Tris, pH 8, at room temperature for 20 min. Endogenous peroxidase was quenched with 3% H2O2 in methanol for 5 min. Sections were incubated with a reaction buffer composed by biotin-dCTP and unlabeled dCTP and TdT enzyme in a humidified chamber at 37°C. In this assay, TdT binds to exposed 3'-OH ends of DNA fragments and catalyzes the addition of biotin-labeled and unlabeled deoxynucleotides. Biotinylated nucleotides were then detected using a streptavidin-horseradish peroxidase conjugate and diaminobenzidine [16]. Apoptotic cells were counted under 40× magnification utilizing an imaging software (Image-Pro Plus, Media Cybernetics, Bethesda MD) by independent observers.
Subcellular fractionation and nuclear protein extraction
Lung samples were homogenized with a Polytron homogenizer (Brinkmann Instruments, West Orange, NY) in a buffer containing 0.32 M sucrose, 10 mM Tris-HCI (pH 7.4), 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM 2-ME, 20 μM leupeptin, 0.15 μM pepstatin A, 0.2 mM PMSF, 50 mM NaF, 1 mM sodium orthovanadate, and 0.4 nM microcystin. The homogenates were centrifuged (1,000 × g, 10 min), and the supernatant (cytosol plus membrane extract) was collected. The pellets were solubilized in Triton buffer (1% Triton X-100, 150 mM NaCI, 10 mM Tris-HCI (pH 7.4), 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 20 μM leupeptin A, and 0.2 mM PMSF). The lysates were centrifuged (15,000 × g, 30 min, 4°C), and the supernatant (nuclear extract) was collected.
Measurement of activity of caspase-3
Activity of caspase-3 was measured by the cleavage of the fluorogenic tetrapeptide-amino-4-methylcoumarine conjugates DEVD-amino-fluoro-coumarine (AFC) as described (25). Cytosol extracts and substrate (50 μM) were combined in the caspase reaction buffer (100 mM HEPES, 10% sucrose, 5 mM dithiothreitol, 0.1% CHAPS, pH 7.25). AFC liberation was monitored for more than 30 sec with a Perkin-Elmer fluorimeter using 400-nm excitation and 505-nm emission wavelength. Fluorescence units were converted to picomoles of AFC using a calibration curve generated with free AFC. Data are given as DEVD-fluoro-methyl-ketone inhibitable AFC generation.
Western blot analysis for phospho-Akt
Nuclear content of phosphorylated form of Akt (phospho-Akt) was determined by immunoblot analyses. Nuclear extracts were boiled in equal volumes of loading buffer (125 mM Tris-HCI (pH 6.8), 4% SDS, 20% glycerol, and 10% 2-mercaptoethanol), and 40 μg of protein was loaded per lane on a 10% Tris-glycine gradient gel. Proteins were separated electrophoretically and transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with 5% nonfat dried milk in Tris-buffered saline for 1 h and then were incubated with primary antibody against phospho-Akt for 1 h. The membranes were washed in Tris-buffered saline with 0.05% Tween 20 and incubated with secondary peroxidase-conjugated antibody. Immunoreaction was visualized by chemiluminescence and on a photographic film. Densitometric analysis of blots was performed using ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Materials
The primary antibodies directed against phospho-Akt were obtained form Santa Cruz Biotechnology (Santa Cruz, CA). Ciglitazone was obtained from Biomol (Plymouth Meeting, PA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Data analysis
Data was analyzed using SigmaStat for Windows Version 3.10 (SysStat Software, San Jose, CA). MAP values are expressed as mean ± SD; the remainder of the values in the figures and text are expressed as mean ± SEM of n observations, where n represents the number of animals in each group. Specifically, MAP results were analyzed using a general linear model for repeated measures. For the remainder of the data analysis a one way ANOVA or a Kruskal Wallis one way ANOVA on ranks was utilized. A value of p < 0.05 was considered significant.
Results
Ciglitazone treatment improved MAP following shock and resuscitation
Ciglitazone and vehicle treated animals had similar MAP at baseline (124.3 ± 17.3 vs. 123.8 ± 10.4 mmHg respectively). During the shock phase there was no difference in MAP (53.3 ± 0.7 vs. 51 ± 0.9 mmHg). At 3h following resuscitation ciglitazone treated animals had higher MAP (86.9 ± 9.4 mmHg) when compared to animals that were vehicle-treated (67.9 ± 13.6 mmHg; p<0.05). These results are similar to our previous data wherein ciglitazone treated animals had a significantly higher MAP following resuscitation [11].
Effect of ciglitazone on lung apoptosis
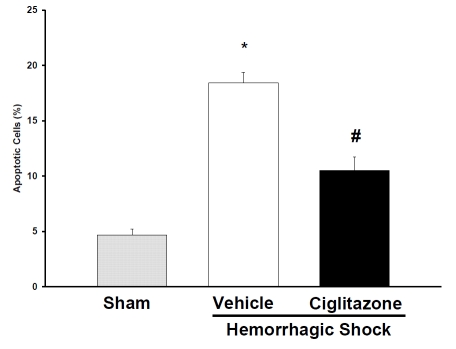
To determine the effect of ciglitazone on lung apoptosis, lung tissue was examined by Tunel staining at the end of the experiment. Alveolar epithelial cells seemed to be the most commonly observed cell type undergoing apoptosis (Figure 1). Vehicle-treated animals had 18.4 ± 0.96 % apoptotic cells per lung field. This apoptotic count was significantly higher than sham animals (4.7 ± 0.53 %; p<0.05). In contrast, ciglitazone treatment reduced the amount of apoptotic cells (10.5 ± 1.2 % per lung field; p<0.05 in comparison to the vehicle-treated group) (Figure 2).
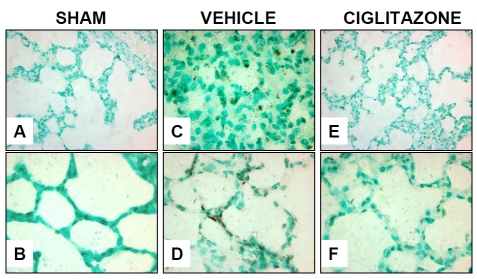
Figure 1.
Effect of in vivo treatment with vehicle (DMSO) or ciglitazone on lung apoptosis 3h after resuscitation. (A) Representative photomicrograph of Tunel staining from a sham rat, rare dark staining nuclei visualized (magnification 400×). (B) Representative photomicrograph of Tunel staining from a sham rat, no dark staining nuclei seen (magnification 1000×). (C) Representative photomicrograph of Tunel staining from a vehicle-treated rat showing increase in dark staining nuclei indicating apoptosis (magnification 400×). (D) Representative photomicrograph of Tunel staining from a vehicle-treated rat showing dark staining nuclei of alveolar epithelial cell (magnification 1000×). (E) Representative photomicrograph of Tunel staining from a ciglitazone-treated rat showing occasional dark staining nuclei (magnification 400×). (F) Representative photomicrograph of Tunel staining from a ciglitazone-treated rat showing reduction in dark staining nuclei compared to vehicle-treated rat (magnification 1000×).
Figure 2.
Effect of in vivo treatment with vehicle (DMSO) or ciglitazone on percentage of Tunel positive apoptotic cells in the lung per high power field (counted at 40× magnification). Each data point represents the mean ± SEM of 3 to 5 rats. *p < 0.05 vs. sham rats; # p < 0.05 vs. vehicle`-treated rats.
Effect of ciglitazone on Akt signaling
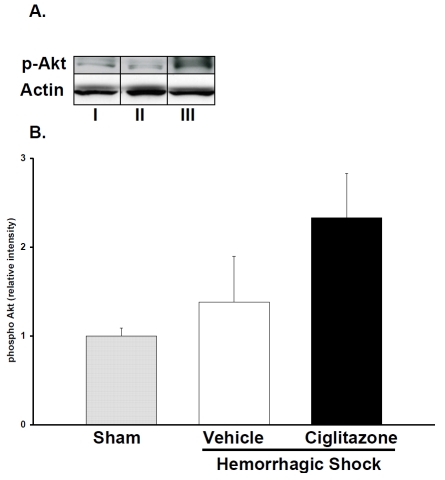
To elucidate a possible signaling mechanism for the reduction in apoptosis in the lung of ciglitazone-treated rats, we measured the activation of pro-survival kinase Akt. Akt is a multifaceted kinase that inhibits apoptosis thereby promoting cell survival. We examined the phosphorylation of Akt by western blot. Ciglitazone treatment increased nuclear phospho-Akt content when compared to both vehicle and sham rats following resuscitation (Figure 3). This would suggest that that ciglitazone may be exerting an anti-apoptotic effect by activating Akt in the lung.
Figure 3.
Effect of in vivo treatment with vehicle (DMSO) or ciglitazone on phosphorylated form of Akt in lung nuclear extracts. (A), Representative western blot of phosphorylated form of Akt. I. Sham II. vehicle treatment. III. ciglitazone treatment. (B), image analysis of nuclear content of phosphorylated form of Akt as determined by densitometry. The fold increase was calculated vs. the respective sham value (time zero), which was set at 1.0. Each data point represents the mean ± SEM of 3 to 5 rats.
Effect of ciglitazone on caspase-3 activity
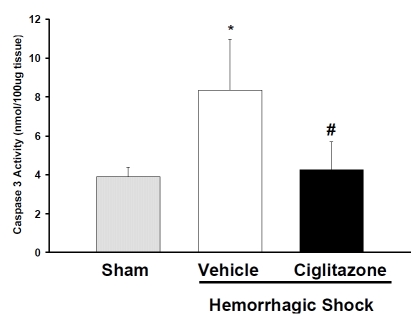
Since activation of caspase-3 represents the final step in the apoptotic pathway. We investigated the effect of ciglitazone on the activity of caspase-3 in the lung. Following shock and resuscitation vehicle-treated rats had significantly higher caspase-3 activity compared to ciglitazone-treated rats (Figure 4).
Figure 4.
Effect of in vivo treatment with vehicle (DMSO) or ciglitazone on activity of caspase-3 in lung cytosol at 3h following hemorrhage and resuscitation. Each data point represents the mean ± SEM of 3 to 5 rats. *p < 0.05 vs. sham rats; # p < 0.05 vs. vehicle-treated rat.
Discussion
Lung injury is a common consequence of severe hemorrhage. We have previously demonstrated that ciglitazone administration following severe hemorrhage ameliorated the inflammatory response with a reduction in lung neutrophil infiltration and NF-kB activation [11]. Our current study further expands these findings and demonstrates that administration of ciglitazone also reduces apoptosis in the lung; reduction of apoptosis is associated with a reduction in caspase-3 activity and increased expression of the pro-survival kinase Akt.
When compared to vehicle, ciglitazone-treated rats had a significantly higher blood pressure at the end of the experiment. This finding is in keeping with our previous study [11]. Additionally, prior studies in models of polymicrobial sepsis and myocardial ischemia/ reperfusion have demonstrated improved blood pressure following PPAFty ligand administration [17, 18]. In our current study this effect on blood pressure is likely a reflection of ciglitazone's anti-inflammatory properties and further confirms the protective effect of this drug in shock.
Lung injury in the setting of severe hemorrhage represents an indirect form of tissue damage as a result of development of a dysregulated systemic inflammatory state. In this state the alveolar-capillary barrier is compromised leading to interstitial and alveolar edema, neutrophil infiltration and alveolar macrophage activation. Dysfunction and eventually cell death of both endothelial and alveolar epithelial cells has also been described [19]. More specifically, hemorrhage can induce apoptosis of these cells [9, 20-23]. Apoptosis or programmed cell death is a form of regulated cell death which may contribute significantly to the occurrence of lung injury [3, 8]. Apoptosis is characterized by activation of intracellular signaling pathways which lead to activation of intracellular serine rich proteases, caspases, which lead to DNA cleavage and cell death. Activation of these pathways may be due to intrinsic signals from within a cell due to release of mitochondrial products such as cytochrome C or extrinsic signals that activate death receptors in the cell membrane [3]. Both pathways lead to activation of Caspase-3. This enzyme represents the final executioner of the apoptotic response its activation commits a cell to die.
In our study we observed that vehicle treated animals had a significant increase in apoptotic cells in the lung as evidenced by Tunel staining. The occurrence of apoptosis was associated with increased activity of caspase-3. Ciglitazone treatment at the time of fluid resuscitation and hourly thereafter reduced lung cell apoptosis and caspase-3 activity significantly when compared to the vehicle-treated group. Tunel staining in our study suggests that this anti-apoptotic effect mainly involves the alveolar epithelial cell. Although we cannot rule out the involvement of other cell types, recent work done by Barlos et al. has demonstrated that both lung endothelial and epithelial cell death is seen following hemorrhage [21]. In their study, the authors observed that epithelial cell death was a caspase-3 mediated event, whereas endothelial cell death was caspase independent [21]. Therefore, similarly in our study, we can suggest that a reduction in caspase-3 activity may well reflect a reduction in apoptosis in alveolar epithelial cells.
To further define a possible signaling mechanism by which ciglitazone may modulate the apoptotic response we evaluated the PI3K/Akt pathway. Akt is a serine threonine kinase that regulates cell survival by multiple mechanisms. Specifically, it can sequester the pro-apoptotic proteins (BAD) and increase anti-apoptotic proteins (Bcl-xl), it can inhibit caspase-9 and inhibit transcription factors of the forkhead family thereby reducing transcription of Fas ligand [24, 25]. Activation of the PI3K/Akt pathway has been demonstrated to have a protective effect following an acute inflammatory insult [5, 14]. In our study the anti-apoptotic effects of ciglitazone were associated with an increased expression of the pro-survival kinase Akt. In a similar manner, other PPAFty ligands have been shown to have an anti-apoptotic effect by increasing Akt expression in PI3K dependent manner [26, 27]. For example, Zingarelli et al. demonstrated a cardioprotective effect with increased Akt expression following a PPARy ligand administration in a rodent model of myocardial ischemia reperfusion [14]. Recently, Wu et al. demonstrated a cytoprotective effect with rosiglitazone treatment in murine neuroblastoma cells exposed to oxygen and glucose deprivation via a PI3K/Akt pathway [26]. Furthermore, PPARy ligands may promote cell survival by other mechanisms [28-30]. For example, Liu et al. in a rabbit model of myocardial ischemia reperfusion demonstrated that rosiglitazone afforded cardioprotection by reducing myeloperoxidase (MPO) expression and reducing caspase-3 activity [30]. In their model administration of a MPO inhibitor reduced post-ischemic caspase-3 activation in the myocardium. Hence, it appears that rosiglitazone could indirectly affect apoptosis by downregulating the inflammatory response by inhibiting leukocyte MPO activity. Our previous work showed a reduction in lung MPO following ciglitazone administration [11]. Hence, the anti-apoptotic effect of ciglitazone may partly be due to a reduction in MPO activity in the lung.
Another major pathway that may contribute to apoptosis is the death receptor pathway. This pathway includes the Fas/FasL system. Activation of this pathway is important in both human and animal models of lung injury [8, 31-33]. Of note, it appears that inhibition of this system reduces apoptosis and preserves lung function. In this regard, it has been demonstrated that RNA siliencing of Fas attenuated lung injury and apoptosis in a murine model of hemorrhage and sepsis [23, 34]. Although our investigation did not explore the effect of ciglitazone on the Fas/FasL pathway, it is also possible that ciglitazone by down regulating the inflammatory response following hemorrhage and resuscitation may indirectly affect the Fas pathway.
Our study has several limitations. Our choice of the PPARy ligand, ciglitazone is based on our previous work, whether other ligands would have similar effects in our model is not known [11]. Other than the PI3K/Akt pathway we did not explore other pathways that may have contributed to the anti-apoptotic effects. Therefore, it is unclear from our work if ciglitazone's anti-apoptotic effects in our study are a consequence of modulation of the intrinsic and/or extrinsic apoptotic pathways.
In conclusion, ciglitazone administration following severe hemorrhage and resuscitation exerted a cytoprotective effect in the lung with increased activity of the pro-survival kinase Akt. Of clinical relevance, ciglitazone exerted anti-apoptotic effects when given at the time of resuscitation. Thus our data suggest that PPARy activation may be an attractive therapeutic option to combat lung injury following severe hemorrhage. Moreover, since thiazolidinediones are currently FDA approved for human use, drugs belonging to this group may have therapeutic potential for treating lung injury in trauma/shock conditions.
References
- 1.Yasuhara S, Asai A, Sahani ND, Martyn JA. Mitochondria, endoplasmic reticulum, and alternative pathways of cell death in critical illness. Crit Care Med. 2007;35:S488–495. doi: 10.1097/01.CCM.0000278045.91575.30. [DOI] [PubMed] [Google Scholar]
- 2.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 3.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31:S184–188. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- 4.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 5.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 6.Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma. 2003;55:608–616. doi: 10.1097/01.TA.0000092378.10660.D1. [DOI] [PubMed] [Google Scholar]
- 7.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery. 2005;138:749–757. doi: 10.1016/j.surg.2005.07.020. discussion 757-748. [DOI] [PubMed] [Google Scholar]
- 8.Perl M, Lomas-Neira J, Chung CS, Ayala A. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury-a unifying hypothesis? What we have learned from small interfering RNAs. Mol Med. 2008;14:465–475. doi: 10.2119/2008-00011.Perl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jernigan TW, Croce MA, Fabian TC. Apoptosis and necrosis in the development of acute lung injury after hemorrhagic shock. Am Surg. 2004;70:1094–1098. [PubMed] [Google Scholar]
- 10.Guan J, Jin DD, Jin LJ, Lu Q. Apoptosis in organs of rats in early stage after polytrauma combined with shock. J Trauma. 2002;52:104–111. doi: 10.1097/00005373-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. CritCare Med. 2008;36:2849–2857. doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou FS, Wang PS, Kulp S, Pinzone JJ. Effects of thiazolidinediones on differentiation, proliferation, and apoptosis. Mol Cancer Res. 2007;5:523–530. doi: 10.1158/1541-7786.MCR-06-0278. [DOI] [PubMed] [Google Scholar]
- 13.Fajas L, Egler V, Reiter R, Miard S, Lefebvre AM, Auwerx J. PPARgamma controls cell proliferation and apoptosis in an RB-dependent manner. Oncogene. 2003;22:4186–4193. doi: 10.1038/sj.onc.1206530. [DOI] [PubMed] [Google Scholar]
- 14.Zingarelli B, Hake PW, Mangeshkar P, O'Connor M, Burroughs TJ, Piraino G, Denenberg A, Wong HR. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-Delta 12,14-prostaglandin J2 and ciglitazone, in reperfusion injury: role of nuclear factor-kappaB, heat shock factor 1, and Akt. Shock. 2007;28:554–563. doi: 10.1097/shk.0b013e31804f56b9. [DOI] [PubMed] [Google Scholar]
- 15.Zingarelli B, Ischiropoulos H, Salzman AL, Szabo C. Amelioration by mercaptoethylguanidine of the vascular and energetic failure in haemorrhagic shock in the anesthetised rat. Eur J Pharmacol. 1997;338:55–65. doi: 10.1016/s0014-2999(97)01325-3. [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zingarelli B, Sheehan M, Hake PW, O'Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003;171:6827–6837. doi: 10.4049/jimmunol.171.12.6827. [DOI] [PubMed] [Google Scholar]
- 18.Zhu P, Lu L, Xu Y, Schwartz GG. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation. 2000;101:1165–1171. doi: 10.1161/01.cir.101.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 20.Alam HB, Austin B, Koustova E, Rhee P. Resuscitation-induced pulmonary apoptosis and intracellular adhesion molecule-1 expression in rats are attenuated by the use of Ketone Ringer's solution. J Am Coll Surg. 2001;193:255–263. doi: 10.1016/s1072-7515(01)01004-3. [DOI] [PubMed] [Google Scholar]
- 21.Barlos D, Deitch EA, Watkins AC, Caputo FJ, Lu Q, Abungu B, Colorado I, Xu DZ, Feinman R. Trauma hemorrhagic shock-induced pulmonary epithelial and endothelial cell injury utilizes different programmed cell death signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2008 doi: 10.1152/ajplung.00491.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayuste EC, Chen H, Koustova E, Rhee P, Ahuja N, Chen Z, Valeri CR, Spaniolas K, Mehrani T, Alam HB. Hepatic and pulmonary apoptosis after hemorrhagic shock in swine can be reduced through modifications of conventional Ringer's solution. J Trauma. 2006;60:52–63. doi: 10.1097/01.ta.0000200156.05397.0b. [DOI] [PubMed] [Google Scholar]
- 23.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khwaja A. Akt is more than just a Bad kinase. Nature. 1999;401:33–34. doi: 10.1038/43354. [DOI] [PubMed] [Google Scholar]
- 25.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JS, Lin TN, Wu KK. Rosiglitazone and PPAR-gamma overexpression protect mitochondrial membrane potential and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol. 2009 doi: 10.1002/jcp.21730. [DOI] [PubMed] [Google Scholar]
- 27.Lin CY, Gurlo T, Haataja L, Hsueh WA, Butler PC. Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3'-kinase-dependent pathway. J Clin Endocrinol Metab. 2005;90:6678–6686. doi: 10.1210/jc.2005-0079. [DOI] [PubMed] [Google Scholar]
- 28.Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- 29.Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN. Ligand-Activated Peroxisome Proliferator-Activated Receptor-{gamma} Protects Against Ischemic Cerebral Infarction and Neuronal Apoptosis by 14-3-3{varepsilon} Upregulation. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu HR, Tao L, Gao E, Qu Y, Lau WB, Lopez BL, Christopher TA, Koch W, Yue TL, Ma XL. Rosiglitazone inhibits hypercholesterolaemia-induced myeloperoxidase upregulation-a novel mechanism for the cardioprotective effects of PPAR agonists. Cardiovasc Res. 2009;81:344–352. doi: 10.1093/cvr/cvn308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 32.Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, Kiener PA, Martin TR. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol Lung Cell Mol Physiol. 2001;281:L328–335. doi: 10.1152/ajplung.2001.281.2.L328. [DOI] [PubMed] [Google Scholar]
- 33.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perl M, Chung CS, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, Ayala A. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]