Abstract
Aims
We sought to examine the relationship between circulating interleukin-6 (IL-6) level and regional left-ventricular (LV) function among apparently healthy individuals free of cardiovascular disease.
Methods and results
Using magnetic resonance myocardial tagging, we determined peak systolic circumferential strain (Ecc) as a measure of regional systolic function in 894 asymptomatic participants in the Multi-Ethnic Study of Atherosclerosis. Ecc was analysed by harmonic phase imaging separately in the LV anterior wall, septum, lateral wall, and inferior wall. Global Ecc was calculated as the average of Ecc in all myocardial segments. We performed multivariable linear regression to evaluate the independent associations between log IL-6 and Ecc, after adjusting for demographic features, cardiovascular risk factors, and markers of subclinical atherosclerosis. The inverse relationships between IL-6 and absolute Ecc were similar in both genders. In multivariable analysis, higher IL-6 level was independently associated with reduced systolic function (less negative Ecc) in the septum [regression coefficient = 1.03 per unit higher log IL-6, 95% confidence interval (CI) 0.26–1.79, P = 0.008] and inferior wall (regression coefficient = 1.65, 95% CI 0.74–2.56, P < 0.001), but not in the anterior wall (P = 0.27) or lateral wall (P = 0.52). Overall, there was an independent inverse association between IL-6 and global Ecc (regression coefficient = 0.94, 95% CI 0.37–1.51, P = 0.001). Compared with C-reactive protein, higher IL-6 level demonstrates a stronger independent association with reduced regional systolic function.
Conclusion
In asymptomatic men and women without documented cardiovascular disease, there is a strong, independent, inverse relationship between IL-6 and regional LV systolic function. These findings suggest that IL-6 may underlie the pathogenetic link between inflammation, LV dysfunction and incipient heart failure. The observed variable relationships between IL-6 and systolic function across different LV regions warrant further investigations.
Keywords: Heart failure, Myocardial contraction, Interleukin-6, Magnetic resonance imaging
See page 768 for the editorial comment on this article (doi:10.1093/eurheartj/ehq014)
Introduction
The pivotal role of inflammation in cardiovascular disease has been increasingly recognized.1 Heart failure (HF) is a state of immune activation and systemic inflammation characterized by increased circulating levels of various cytokines, including interleukin-6 (IL-6), a pleiotropic cytokine with diverse humoral and cellular immunomodulatory effects.2,3 Experimental data support the existence of a complex relationship between IL-6 and chronic HF.4–8
Accumulating clinical evidence also suggests that IL-6 may promote the development and progression of left-ventricular (LV) dysfunction leading to HF.9–11 Because coronary artery disease is a major cause of HF, myocardial ischaemia or infarction from atherosclerotic plaque rupture may be the causal link between inflammation and HF.10
Inflammation may also exert a more direct and detrimental effect on myocardial function.2,3 Although several epidemiological studies have demonstrated an association between inflammation and incident HF,9,11–13 the precise pathogenetic mechanisms remain to be elucidated. Cardiac magnetic resonance imaging (MRI) is considered a reference standard for assessment of LV structure and function,14 and may provide valuable insights into the intriguing link between inflammation and HF. Furthermore, magnetic resonance tagging detects subtle changes in regional myocardial function, which may afford unique pathophysiologic insights.14,15
Accordingly, we evaluated the association between circulating IL-6 level and the presence and extent of regional and global LV myocardial dysfunction as measured by MRI tagging, among asymptomatic participants in the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesized that IL-6 level is inversely correlated with regional and global LV function independent of other established cardiovascular risk factors, among individuals without known cardiovascular disease.
Methods
MESA is a prospective, community-based cohort study designed to investigate the pathogenetic mechanisms of subclinical cardiovascular disease development and progression in asymptomatic individuals. The design and methodology have been previously published,16 and are briefly described herein. Between July 2000 and September 2002, MESA recruited 6814 men and women of four ethnic groups (non-Hispanic white, African American, Hispanic, and Chinese), aged 45–84 years, from six US participating field centres (Wake Forest University, Winston Salem; Columbia University, New York; Johns Hopkins University, Baltimore; University of Minnesota, Minneapolis; Northwestern University, Chicago; and University of California, Los Angeles). Eligible individuals had no known cardiovascular disease at enrolment. At study entry, all participants underwent a comprehensive baseline evaluation that included a medical history, physical examination, and laboratory investigations. Of the entire MESA cohort, this present study examined a subset of 894 participants from all six field centres for an ancillary study of tagged MRI who had analysable tagging data and IL-6 measurement (290 participants were excluded due to incomplete data). The institutional review board at each centre approved the study protocol, and all participants provided written informed consent.
Cardiac magnetic resonance imaging
Details of the cardiac MRI protocol have been described.17 All cardiac MRI examinations were performed using 1.5T whole-body scanners [Signa (LX and Cvi) General Electric Medical Systems, Waukesha, WI, and Siemens Medical Solutions (Vision and Sonata, Erlangen, Germany)] with phase-arrayed coils and electrocardiographic gating. All images were obtained during expiratory breath-hold. Cine images were acquired using segmented k-space fast spoiled gradient-echo (SPGR or FLASH). Short-axis, four-chamber, and two-chamber cine images were prescribed according to a standardized protocol, with temporal resolution maintained at 50 ms or less.
After completion of the cine imaging, tagged images of three short-axis slices (covering the LV base to apex) were obtained. Parallel stripe tags were prescribed in two orthogonal orientations using a fast gradient echo sequence with spatial modulation of magnetization (SPAMM). Typical imaging parameters were: field of view 40 cm; slice thickness 8–10 mm; tag spacing 7 mm; repetition time 6 ms; echo time 3.0 ms; 4–9 phase encoding views per segment to yield a temporal resolution of 20–41 ms.
Image analysis
Left-ventricular structural parameters (mass, end-diastolic, and end-systolic volumes) and ejection fraction were calculated using a standard commercially available software (MASS 4.2, MEDIS, Leiden, The Netherlands).
Regional myocardial systolic function was analysed by harmonic phase imaging. Harmonic phase imaging is a validated technique for rapid determination of myocardial strain.18 Regional peak systolic circumferential strain (Ecc) was analysed in the mid-wall layer of each of the four LV walls (septal, anterior, inferior, and lateral) at the basal, mid-cavity, and apical levels of the LV (Figure 1). A previous study has demonstrated good inter-observer and intra-observer reproducibility for measurement of peak Ecc.19 We computed average systolic strain for each of the four LV walls (at the basal, mid, and apical levels), and overall for all 12 segments in the LV (global Ecc).
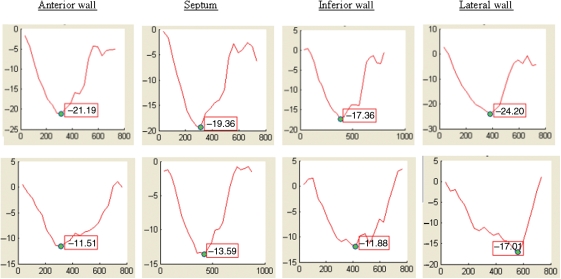
Figure 1.
Myocardial circumferential shortening strain curves (% strain on y-axis; time in milliseconds on x-axis) showing normal Ecc (top panel) and reduced Ecc (bottom panel) in the anterior wall, septum, inferior wall, and lateral wall.
Determination of cardiovascular risk factors
Hip and waist circumferences were measured at the baseline examination. Participants completed a questionnaire including family history, medication use, and smoking status, which was classified as currently smoking, formerly smoked, or never smoked. Blood pressure was measured three times in the seated position, and we used the average of the second and third measurements for this study. We defined hypertension as diastolic blood pressure ≥90 mmHg, systolic blood pressure ≥140 mmHg, or receiving treatment for hypertension. Heart rate was monitored and recorded during the cardiac MRI examination. Plasma glucose, triglycerides, total cholesterol, and HDL-cholesterol levels were determined after a 12 h fast. We defined diabetes as fasting plasma glucose ≥126 mg/dL and/or use of anti-hyperglycaemic treatment.
Measurements of inflammatory biomarkers and subclinical atherosclerosis
Before the exam, participants were instructed to fast for 12 h, and to refrain from smoking or strenuous exercise. Blood samples were stored at −80C until analysed. Interleukin-6 was measured using an ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay, R&D Systems, Minneapolis, MN; inter-assay coefficient of variation 6.3%), and C-reactive protein by a high-sensitivity assay (N High-Sensitivity C-reactive protein, Dade Behring, Deerfield, IL; inter-assay coefficient of variation 2.1–5.7%).
Study participants underwent high-resolution B mode ultrasonography of the carotid arteries. A blinded core laboratory measured the maximal intima–media thickness of near and far walls of the internal carotid arteries, and calculated the mean of the left and right sides. Coronary artery calcium score was determined with either a cardiac-gated electron-beam computed tomography scanner or a multi-detector computed tomography system. Participants had two scans, and we used the average phantom-adjusted Agatston score (derived from plaque densities and areas) in all analyses.
Statistical analysis
Continuous variables are reported as median and interquartile ranges (IQR), and categorical variables as frequencies or percentages; Kendall tau-b and χ2 for trend were used to test for trends across ordinal groups (IL-6 quartiles), respectively.
We log-transformed IL-6 and C-reactive protein levels for analysis, because of their skewed distributions. We constructed multiple linear regression models to examine the independent relationships between IL-6 and (i) LV structural parameters (LV end-diastolic and LV end-systolic volumes), (ii) global LV function (LV ejection fraction), and (iii) each of the regional myocardial functional parameters (systolic Ecc of the LV anterior, septal, lateral, and inferior walls), and global Ecc (12-segment-averaged Ecc). By convention, Ecc is negative to represent circumferential shortening. Therefore, a less negative Ecc (lower absolute value) reflects reduced systolic function (e.g. Ecc = −12% indicates lower systolic function than Ecc = −16%).
To determine the independent associations between IL-6 levels and LV structural and functional parameters, we adjusted for other covariates in a hierarchical order in the following multivariable linear regression models: Model I included demographics variables (age, gender, ethnicity); Model II included traditional atherosclerotic risk factors (waist-to-hip circumference ratio, smoking, total cholesterol, HDL cholesterol, systolic and diastolic blood pressure, and diabetes status), serum creatinine, and use of anti-hypertensive and lipid-modifying medications, in addition to the all predictor variables in Model I; and Model III included markers of subclinical atherosclerosis (carotid intima–media thickness and coronary artery calcification) and LV mass index, in addition to all the predictor variables in Model II. We checked the linearity assumption, and examined the variance inflation factors to detect any collinearity in the models.
Because of the known association between inflammatory biomarkers IL-6 and C-reactive protein, we also entered C-reactive protein into a separate multivariable model to further evaluate the independent relationship between IL-6 and Ecc. Since higher C-reactive protein level is associated with lower regional myocardial systolic function in men but not in women,20 we also tested for interaction effects between IL-6 and gender on regional and global Ecc. To confirm the robustness of our results, we conducted sensitivity analyses by substituting body mass index for waist–hip ratio, estimated glomerular filtration rate for serum creatinine, calcium score of corresponding coronary artery territory for the mean total calcium score, and by including microalbuminuria (as a dichotomous variable) in the multivariable models. Finally, we repeated the analyses by assigning the myocardial segments to the three coronary artery territories according to the 17-segment model. We performed statistical analysis using SPSS (version 15.0, SPSS, Inc., Chicago, IL), and considered two-sided P-values <0.05 to be significant.
Results
In this study cohort of 894 participants, the median age was 67 (IQR: 59–74) and 44.6% were women. Compared with the remaining MESA cohort, the present study included older subjects and a higher proportion of men, with a higher prevalence of hypertension, higher systolic blood pressure, and higher Agatston coronary calcium score. The prevalence of diabetes and levels of lipid profile, C-reactive protein, and carotid intima–media thickness were similar between the two groups.
Overall, the median IL-6 level was 1.30 pg/mL (IQR: 0.87–1.98); women had higher IL-6 levels compared with men (median=1.47 vs. 1.23, respectively, P < 0.001). Diabetes and hypertension were also associated with higher IL-6 levels (both P ≤ 0.001). Table 1 shows the baseline demographic characteristics of the study participants, according to quartiles of IL-6 levels. Subjects with higher IL-6 levels were older, more likely to be female, and had a higher prevalence of hypertension, diabetes, and family history of myocardial infarction. In addition, they had higher systolic blood pressure and waist–hip circumference ratio, lower HDL cholesterol, and higher fasting glucose, C-reactive protein, carotid intima–media thickness, and Agatston coronary calcium score (all P for trend < 0.001).
Table 1.
Baseline characteristics of the study participants according to plasma interleukin-6 quartiles
| Plasma interleukin-6 level |
P-value for trend | ||||
|---|---|---|---|---|---|
| First quartile (n = 224) | Second quartile (n =223) | Third quartile (n =224) | Fourth quartile (n =223) | ||
| Age, yearsa | 65 (56,70) | 66 (58,72) | 67 (61,75) | 69 (62,75) | <0.001 |
| Men, % | 67.0 | 53.8 | 54.0 | 46.6 | <0.001 |
| Ethnicity, % | |||||
| Caucasian | 37.0 | 33.2 | 36.2 | 30.5 | 0.016b |
| Asian | 12.5 | 8.1 | 4.0 | 6.7 | |
| African American | 26.8 | 26.5 | 29.0 | 26.5 | |
| Hispanic | 22.8 | 32.3 | 30.8 | 36.3 | |
| Smoking status, % | |||||
| Never | 57.0 | 48.0 | 51.6 | 49.1 | 0.069b |
| Former | 35.9 | 39.5 | 33.0 | 41.0 | |
| Current | 7.2 | 12.6 | 15.4 | 9.9 | |
| Family history of myocardial infarction, % | 36.0 | 46.4 | 48.3 | 48.3 | 0.01 |
| Diabetes mellitus, % | 7.1 | 12.1 | 17.4 | 16.1 | 0.001 |
| Hypertension, % | 35.3 | 45.3 | 52.7 | 58.7 | <0.001 |
| Anti-hypertensive medication, % | 33.5 | 35.4 | 43.8 | 50.7 | <0.001 |
| Lipid-modifying medication, % | 19.2 | 17.5 | 20.5 | 20.6 | 0.54 |
| Waist–hip circumference ratioa | 0.92 (0.88,0.96) | 0.95 (0.89,0.99) | 0.95 (0.90,0.99) | 0.96 (0.91,1.00) | <0.001 |
| Heart rate, b.p.m.a | 60 (55,67) | 62 (57,68) | 62 (55,69) | 64 (57,70) | 0.006 |
| Systolic blood pressure, mmHga | 121 (112,134) | 127 (113,139) | 128 (116,141) | 129 (117,149) | <0.001 |
| Diastolic blood pressure, mmHga | 73 (66,79) | 72 (65,79) | 72 (65,79) | 71 (64,78) | 0.26 |
| Fasting glucose, mg/dLa | 89 (84,98) | 92 (85,101) | 93 (87,101) | 95 (87,103) | <0.001 |
| Total cholesterol, mg/dLa | 192 (169,213) | 200 (178,213) | 191 (172,215) | 189 (166,212) | 0.18 |
| LDL-cholesterol, mg/dLa | 114 (96,130) | 121 (99,142) | 118 (97,131) | 115 (95,135) | 0.77 |
| HDL-cholesterol, mg/dLa | 52 (42,62) | 50 (41,61) | 47 (39,59) | 46 (39,57) | <0.001 |
| Triglycerides, mg/dLa | 95 (68,152) | 115 (77,166) | 115 (78,162) | 117 (82,163) | 0.04 |
| Total cholesterol: HDL ratioa | 3.64 (2.97,4.50) | 4.07 (3.19,4.93) | 4.00 (3.24,4.79) | 4.09 (3.33,4.94) | 0.001 |
| Creatinine, mg/dLa | 1.0 (0.8,1.1) | 0.9 (0.8,1,1) | 0.9 (0.8,1.1) | 0.9 (0.8,1.1) | 0.56 |
| C-reactive protein, mg/La | 0.86 (0.49,1.67) | 1.69 (0.76,3.09) | 2.47 (1.19,4.70) | 3.58 (1.78,7.55) | <0.001 |
| Carotid intima–media thickness, mma | 0.75 (0.60,1.02) | 0.84 (0.66,1.29) | 0.98 (0.70,1.27) | 1.00 (0.69,1.60) | <0.001 |
| Agatston coronary artery calcium scorea | 0 (0,71) | 9 (0,216) | 26 (0,227) | 28 (0,213) | <0.001 |
aMedian (25th, 75th percentiles).
bP-value for Pearson χ2 test (instead of P-value for trend).
Table 2 summarizes the regional and global systolic strain (Ecc) measurements by IL-6 quartiles. Higher IL-6 levels were associated with reduced systolic function in the septum and inferior wall. The relationships were weaker and not significant in the anterior wall and lateral wall. Overall, global Ecc showed a significant positive correlation with IL-6 level (P < 0.001).
Table 2.
Peak regional and global systolic Ecc by interleukin-6 quartiles
| Plasma interleukin-6 level |
P-value for trend | ||||
|---|---|---|---|---|---|
| First quartile (n =224) | Second quartile (n =223) | Third quartile (n =224) | Fourth quartile (n =223) | ||
| Anterior wall Ecc | −17.83 ± 0.23 | −17.71 ± 0.22 | −17.39 ± 0.26 | −17.39 ± 0.21 | 0.089 |
| Septum Ecc | −16.16 ± 0.19 | −15.56 ± 0.21 | −15.70 ± 0.21 | −15.20 ± 0.21 | 0.005 |
| Inferior wall Ecc | −14.30 ± 0.23 | −13.66 ± 0.23 | −13.34 ± 0.25 | −13.26 ± 0.25 | 0.001 |
| Lateral wall Ecc | −20.11 ± 0.21 | −19.65 ± 0.19 | −19.47 ± 0.20 | −19.77 ± 0.19 | 0.089 |
| Global Ecc | −17.15 ± 0.14 | −16.70 ± 0.15 | −16.55 ± 0.16 | −16.44 ± 0.15 | <0.001 |
Data shown as mean ± standard error of mean.
Table 3 presents the results of multivariable analysis. The independent relationships between IL-6 and Ecc did not differ by gender (P for interaction >0.25 for all regional and global Ecc) or race. The positive associations between IL-6 level and Ecc in the septum and inferior wall were maintained after controlling for age, gender, and ethnicity (Model I). Inclusion of cardiovascular risk factors and medication use into model II did not substantially attenuate these associations. Finally, the results remained essentially unchanged after further adjustment for markers of atherosclerosis, namely, carotid intima–media thickness and Agatston coronary calcium score, and LV mass index (Model III). In contrast, there were no significant relationships between IL-6 and Ecc in the anterior wall and lateral wall. The model R and adjusted R2 were 0.36 and 0.11, respectively, for global Ecc.
Table 3.
Relationship between regional and global systolic Ecc and log-interleukin-6 levels after adjustment for demographic characteristics (model I); demographic characteristics and risk factors (model II); demographic characteristics, risk factors, and markers of atherosclerosis
| Model I |
Model II |
Model III |
||||
|---|---|---|---|---|---|---|
| Regression coefficient (95% CI)a | P-value | Regression coefficient (95% CI)a | P-value | Regression coefficient (95% CI)a | P-value | |
| Anterior wall Ecc | 0.52 (−0.34–1.38) | 0.24 | 0.47 (−0.41–1.35) | 0.29 | 0.51 (−0.39–1.41) | 0.27 |
| Septum Ecc | 1.10 (0.34–1.85) | 0.005 | 0.86 (0.09–1.63) | 0.029 | 1.03 (0.26–1.79) | 0.008 |
| Inferior wall Ecc | 1.43 (0.55–2.30) | 0.001 | 1.39 (0.49–2.29) | 0.003 | 1.65 (0.74–2.56) | <0.001 |
| Lateral wall Ecc | 0.16 (−0.59–0.91) | 0.67 | 0.18 (−0.60–0.95) | 0.65 | 0.26 (−0.53–1.05) | 0.52 |
| Global Ecc | 0.90 (0.34–1.45) | 0.002 | 0.81 (0.25–1.38) | 0.005 | 0.94 (0.37–1.51) | 0.001 |
Model I included demographics variables (age, gender, ethnicity); Model II included traditional atherosclerotic risk factors (smoking, cholesterol profile, blood pressure and diabetes status), serum creatinine, and use of anti-hypertensive and lipid-modifying medications, plus all the predictor variables in Model I; and Model III included markers of subclinical atherosclerosis (carotid intima–media thickness and coronary artery calcification) and LV mass index, plus all the predictor variables in Model II.
aRegression coefficients represent the adjusted differences in Ecc per 1 unit higher log IL-6 (pg/mL). Because Ecc is negative by convention, positive regression coefficients indicate worse systolic function (i.e. lower absolute value of Ecc).
All three models demonstrated a strong independent positive association between global Ecc and IL-6 level (Table 3). We obtained similar results in the sensitivity analyses using body mass index, estimated glomerular filtration rate, microalbuminuria, coronary artery calcium score for the corresponding territory, or LV mass index in the multivariable models. The independent associations between IL-6 and Ecc were observed in the left anterior descending (P = 0.013) and right coronary artery (P = 0.005) territories, but not in left circumflex artery territory (P = 0.24).
There was a moderately strong, positive correlation between plasma C-reactive protein and IL-6 levels (Kendall τ-b correlation coefficient = 0.34, P < 0.001). When log- C-reactive protein was also entered to model III, IL-6 retained an independent association with global Ecc (regression coefficient = 0.75 per 1-unit log IL-6, 95% CI 0.12–1.38, P = 0.019), and was a stronger predictor than C-reactive protein (regression coefficient = 0.31 per 1-unit log C-reactive protein, 95% CI −0.06–0.69, P = 0.10).
Interleukin-6 was not significantly associated with LV end-diastolic volume, LV end-systolic volume, and LVEF after adjusting for variables in Models I, II, and III (data not shown).
Discussion
There are three key findings in this study of asymptomatic individuals without known cardiovascular disease. First, higher plasma IL-6 levels were independently associated with reduced regional systolic function after controlling for demographic characteristics, cardiovascular risk factors, and markers of subclinical atherosclerosis. Second, compared with another related inflammatory marker C-reactive protein, IL-6 showed a stronger, independent, inverse relationship with regional systolic function in both men and women. Third, there was considerable variability in associations of IL-6 and Ecc by LV region, with significant correlations only in the LV septum and inferior wall.
Experimental and animal data have implicated a causative role of IL-6 in cardiomyocyte hypertrophy and apoptosis,7,21 adverse LV remodelling,4 and progression to HF.5 IL-6 exerts a negative inotropic effect on cardiac myocytes by augmentation of nitric oxide and peroxynitrite formation,22–24 and by inhibition of sarcoplasmic reticulum Ca-ATPase (SERCA2).25 Transgenic mice overexpressing both IL-6 and IL-6 receptors exhibit pathological cardiac hypertrophy.4 Subcutaneous administration of recombinant human IL-6 in rats leads to pulmonary oedema, dose-dependent ventricular dilatation, reduced end-systolic pressure, and myocardial contractility.5
Clinical studies have corroborated these experimental findings. For example, serum with markedly elevated IL-6 levels from patients with septic shock can depress myocyte contractile function in vitro.6,8 Circulating IL-6 level and myocardial IL-6 expression are increased in donor human hearts, more profoundly in those unsuitable for transplant because of myocardial dysfunction.26 Compared with healthy controls, IL-6 levels are elevated in both HF patients and asymptomatic patients with LV dysfunction.27,28 In patients with chronic HF, IL-6 level correlates with the severity of HF,29 and confers incremental prognostic information beyond LVEF and norepinephrine level.30 Similar observations have been made in patients with advanced HF and acute decompensated HF.31–34 Finally, administration of proven medical therapies for HF is associated with a decline in circulating IL-6 level.30,35
Several epidemiological studies also support the notion that IL-6 is a risk marker of incident HF.9–12 In a nested case–control study of apparently healthy men in the Physicians’ Health Study, Ridker et al.10 demonstrated that elevated IL-6 levels were associated with an increased risk of future myocardial infarction. As a leading cause of HF, coronary artery disease may be a crucial intermediate step in the causal pathway linking IL-6 to overt HF. In the Framingham Heart Study of 732 elderly subjects free of prior myocardial infarction and HF, Vasan et al.9 found that IL-6 was a powerful predictor of HF over a mean follow-up of 5.2 years. Furthermore, stepwise regression analysis identified IL-6 as a better HF predictor than C-reactive protein, although the study utilized a low-sensitivity C-reactive protein assay.9 Similarly, in the larger MESA and Health, Aging and Body Composition studies, baseline IL-6 levels independently predicted new onset HF.11,12 Yet, elucidation of the precise pathophysiological role of IL-6 in the development and progression of HF was beyond the scope of these seminal epidemiological studies.
To the best of our knowledge, the present study is the first to investigate the relationship between IL-6 level and regional LV function in apparently healthy humans. Subjects with elevated IL-6 levels had a higher prevalence of traditional cardiovascular risk factors that are associated with subtle alterations in LV structure and function.36 However, adjustment for these confounding risk factors had a negligible impact on the associations between IL-6 and regional systolic function, lending credence to the concept that IL-6 may have a direct detrimental effect on LV function. Our results furnish novel and unique pathophysiological insights that IL-6 may be directly involved early in the pathogenesis, which complement the clinical observations regarding IL-6 in epidemiological studies of healthy subjects and studies of selected HF patients.9,10,28,30 Moreover, our study underscores the discrepancies between regional LV functional parameters and conventional measures of LV function (such as LV ejection fraction)—the latter may be insensitive to the earliest deterioration in myocardial function.17
The regional differences we observed in association of IL-6 with LV function are intriguing. Previous MESA studies also documented regional differences in the association between risk factors, myocardial perfusion, and Ecc.37–39 For example, carotid intima–media thickness is only weakly correlated with Ecc in the anterior wall.38 Rosen et al.20 reported that the relationship between C-reactive protein and Ecc was weaker in the right coronary artery territory, compared with the left anterior descending artery and left circumflex artery territories. These results were unchanged after adjusting for coronary calcium scores in the corresponding regions, suggesting that such differences may not be due to different atherosclerotic burden. Accordingly, in the present study, we examined the myocardial segments without assigning them to coronary artery territories. Of note, further adjustment of carotid intima–media thickness and coronary artery calcium score (for the corresponding coronary territories, or the mean) did not influence the associations (Model II vs. Model III) between IL-6 and LV septum Ecc, inferior Ecc and global Ecc. It is not clear whether these observations reflect regional differences in myocardial susceptibility to inflammatory insults and deleterious neurohormonal effects. Signal-to-noise ratio may be lower in the lateral wall, thereby compromising the accuracy of Ecc measurement, but this would not explain the non-significant association between IL-6 and Ecc in the anterior wall. Although inadequate statistical power remains possible, the strong independent relationships in the LV septum and inferior wall which were not attenuated after adjustment for multiple confounders, in contrast to the much weaker relationships in the anterior and lateral walls, suggest that these may not merely be chance findings, and potentially complex pathophysiological mechanisms should be explored.
Although both IL-6 and C-reactive protein are considered inflammatory markers, they may have distinct and possibly synergistic roles in the pathogenesis of coronary artery disease and HF.1 Whether C-reactive protein directly promotes atherogenesis has been a subject of controversy.40–42 C-reactive protein is an acute phase reactant synthesized predominantly in the liver in response to stimulation by IL-6 and other cytokines. While we observed a moderate positive correlation between C-reactive protein and IL-6, the latter turned out to be a stronger independent predictor of regional systolic function. Thus, together with prior experimental data,2,3 our findings raise the interesting hypothesis that IL-6 or other inflammatory mediators may underlie the critical pathogenetic link between inflammation and myocardial dysfunction.
Several study limitations are noteworthy. First, due to the potentially selective enrolment of MESA participants in this MRI tagging substudy, our study cohort was not a true population-based sample. Although MRI tagging has inferior temporal resolution compared with echocardiography and other technical limitations, it is widely accepted as a valid and useful tool to assess myocardial mechanical function.14,15 In the absence of global LV dysfunction or clinically evident HF, the long-term prognostic significance and therapeutic implications of regional LV systolic dysfunction remain to be determined. Nonetheless, the present study affords new pathophysiological insights. Our cross-sectional analyses precluded evaluation of the temporal profiles of IL-6 and Ecc and any reliable causal inferences. Serial MRI and longitudinal clinical follow-up in MESA will likely shed light on these important issues. The intra-individual variability in IL-6 measurement might have weakened the observed relationship with regional systolic function.43 Finally, the modest model R2 indicates that IL-6 and other traditional cardiovascular risk factors account for only a small proportion of variance in Ecc. However, this is biologically plausible, and the model R2 may be considerably underestimated by the inclusion of only healthy individuals in MESA (i.e. a relatively narrow range of IL-6 and Ecc in our sample). The strengths of this study are the relatively large sample of subjects from four ethnic groups, standardized and rigorous methods of data collection, and the use of a validated software in a blinded core laboratory with extensive experience in MRI tagging.18,19
In conclusion, there is an independent inverse relationship between IL-6 and regional LV systolic function measured by MRI tagging. This association is strong and consistent in both men and women, even after controlling for multiple confounders including C-reactive protein. The differences in association of IL-6 and systolic function in different LV regions deserve further study.
Funding
This study was supported by the National Heart, Lung, and Blood Institute grant (RO1-HL66075-01) and the Multi-Ethnic Study of Atherosclerosis study contracts (NO1-HC-95162, NO1-HC-95168, and NO1-HC-95169). A.T.Y. is supported by the Canadian Institutes of Health Research and a New Investigator Award from the Heart and Stroke Foundation of Canada.
Conflict of interest: none declared.
Acknowledgements
We thank all the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- 1.Libby P, Ridker PM. Inflammation and atherothrombosis. From population biology and bench research to clinical practice. J Am Coll Cardiol. 2006;48:A33–A36. [Google Scholar]
- 2.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 3.El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J Card Fail. 2008;14:61–74. doi: 10.1016/j.cardfail.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA. 1995;92:4862–4866. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 6.Joulin O, Petillot P, Labalette M, Lancel S, Neviere R. Cytokine profile of human septic shock serum inducing cardiomyocyte contractile dysfunction. Physiol Res. 2007;56:291–297. doi: 10.33549/physiolres.930946. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Wang HY, Bassel-Duby R, Maass DL, Johnston WE, Horton JW, Tao W. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am J Physiol Heart Circ Physiol. 2007;292:H2408–H2416. doi: 10.1152/ajpheart.01150.2006. [DOI] [PubMed] [Google Scholar]
- 8.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O'Gara P, Nadel S, Relman DA, Harding SE, Levin M. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–209. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 11.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 13.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 14.Lima JA, Desai MY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol. 2004;44:1164–1171. doi: 10.1016/j.jacc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Gotte MJ, Germans T, Russel IK, Zwanenburg JJ, Marcus JT, van Rossum AC, van Veldhuisen DJ. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006;48:2002–2011. doi: 10.1016/j.jacc.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Nasir K, Tsai M, Rosen BD, Fernandes V, Bluemke DA, Folsom AR, Lima JA. Elevated homocysteine is associated with reduced regional left ventricular function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2007;115:180–187. doi: 10.1161/CIRCULATIONAHA.106.633750. [DOI] [PubMed] [Google Scholar]
- 18.Garot J, Bluemke DA, Osman NF, Rochitte CE, McVeigh ER, Zerhouni EA, Prince JL, Lima JA. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101:981–988. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 19.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 20.Rosen BD, Cushman M, Nasir K, Bluemke DA, Edvardsen T, Fernandes V, Lai S, Tracy RP, Lima JA. Relationship between C-reactive protein levels and regional left ventricular function in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2007;49:594–600. doi: 10.1016/j.jacc.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Wollert KC, Chien KR. Cardiotrophin-1 and the role of gp130-dependent signaling pathways in cardiac growth and development. J Mol Med. 1997;75:492–501. doi: 10.1007/s001090050134. [DOI] [PubMed] [Google Scholar]
- 22.Yu XW, Liu MY, Kennedy RH, Liu SJ. Both cGMP and peroxynitrite mediate chronic interleukin-6-induced negative inotropy in adult rat ventricular myocytes. J Physiol. 2005;566:341–353. doi: 10.1113/jphysiol.2005.087478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinugawa K, Takahashi T, Kohmoto O, Yao A, Aoyagi T, Momomura S, Hirata Y, Serizawa T. Nitric oxide-mediated effects of interleukin-6 on [Ca2+]i and cell contraction in cultured chick ventricular myocytes. Circ Res. 1994;75:285–295. doi: 10.1161/01.res.75.2.285. [DOI] [PubMed] [Google Scholar]
- 24.Sugishita K, Kinugawa K, Shimizu T, Harada K, Matsui H, Takahashi T, Serizawa T, Kohmoto O. Cellular basis for the acute inhibitory effects of IL-6 and TNF- alpha on excitation-contraction coupling. J Mol Cell Cardiol. 1999;31:1457–1467. doi: 10.1006/jmcc.1999.0989. [DOI] [PubMed] [Google Scholar]
- 25.Villegas S, Villarreal FJ, Dillmann WH. Leukemia Inhibitory Factor and Interleukin-6 downregulate sarcoplasmic reticulum Ca2+ ATPase (SERCA2) in cardiac myocytes. Basic Res Cardiol. 2000;95:47–54. doi: 10.1007/s003950050007. [DOI] [PubMed] [Google Scholar]
- 26.Birks EJ, Burton PB, Owen V, Mullen AJ, Hunt D, Banner NR, Barton PJ, Yacoub MH. Elevated tumor necrosis factor-alpha and interleukin-6 in myocardium and serum of malfunctioning donor hearts. Circulation. 2000;102:III352–358. doi: 10.1161/01.cir.102.suppl_3.iii-352. [DOI] [PubMed] [Google Scholar]
- 27.Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77:723–727. doi: 10.1016/s0002-9149(97)89206-5. [DOI] [PubMed] [Google Scholar]
- 28.Raymond RJ, Dehmer GJ, Theoharides TC, Deliargyris EN. Elevated interleukin-6 levels in patients with asymptomatic left ventricular systolic dysfunction. Am Heart J. 2001;141:435–438. doi: 10.1067/mhj.2001.113078. [DOI] [PubMed] [Google Scholar]
- 29.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 30.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6spillover in the peripheral circulation increases with the severity of heartfailure the high plasma level of interleukin-6is an important prognostic predictor in patients with congestive heartfailure. J Am Coll Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari R. Interleukin-6: a neurohumoral predictor of prognosis in patients with heart failure:light shadow. Eur Heart J. 2002;23:9–10. doi: 10.1053/euhj.2001.3060. [DOI] [PubMed] [Google Scholar]
- 32.Kell R, Haunstetter A, Dengler TJ, Zugck C, Kubler W, Haass M. Do cytokines enable risk stratification to be improved in NYHA functional class III patients? Comparison with other potential predictors of prognosis. Eur Heart J. 2002;23:70–78. doi: 10.1053/euhj.2001.2780. [DOI] [PubMed] [Google Scholar]
- 33.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen KH, Lassus J, Harjola VP, Siirila-Waris K, Melin J, Punnonen KR, Nieminen MS, Laakso M, Peuhkurinen KJ. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10:396–403. doi: 10.1016/j.ejheart.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–1593. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- 36.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, III, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–991. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, Bluemke DA, Nasir K, O'Leary DH, Lima JA. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:2420–2428. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 39.Rosen BD, Lima JA, Nasir K, Edvardsen T, Folsom AR, Lai S, Bluemke DA, Jerosch-Herold M. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the multi-ethnic study of atherosclerosis. Circulation. 2006;114:289–297. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
- 40.Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP, Cushman M, Bis JC, Zeng D, Lin D, Kuller LH, Nickerson DA, Psaty BM, Tracy RP, Reiner AP. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA. 2006;296:2703–2711. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- 41.Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis–chicken or egg? N Engl J Med. 2008;359:1953–1955. doi: 10.1056/NEJMe0807235. [DOI] [PubMed] [Google Scholar]
- 42.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 43.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]