Abstract
Aims
Very few data exist on the occurrence of acute kidney injury (AKI) associated with transcatheter aortic valve implantation (TAVI). The objectives of the present study were (i) to determine the incidence, predictive factors, and prognostic value of AKI following TAVI, and (ii) to compare the occurrence of AKI in TAVI vs. surgical aortic valve replacement (SAVR) in patients with pre-procedural chronic kidney disease (CKD).
Methods and results
A total of 213 patients (mean age 82 ± 8 years) undergoing TAVI for the treatment of severe aortic stenosis were included in the study. Acute kidney injury was defined as a reduction of >25% in estimated glomerular filtration rate (eGFR) within 48 h following the procedure or the need for haemodialysis during index hospitalization. Those patients with pre-procedural CKD (eGFR <60 mL/min/1.73 m2, n = 119) were compared with 104 contemporary patients with CKD who underwent isolated SAVR. The incidence of AKI following TAVI was 11.7%, with 1.4% of the patients requiring haemodialysis. Predictive factors of AKI were hypertension (OR: 4.66; 95% CI: 1.04–20.87), chronic obstructive pulmonary disease (OR: 2.64, 95% CI: 1.10–6.36), and peri-operative blood transfusion (OR: 3.47, 95% CI: 1.30–9.29). Twenty-one patients (9.8%) died during index hospitalization, and the logistic EuroSCORE (OR: 1.03 for each increase of 1%; 95% CI: 1.01–1.06) and occurrence of AKI (OR: 4.14, 95% CI: 1.42–12.13) were identified as independent predictors of postoperative mortality. Patients with CKD who underwent TAVI were older, had a higher logistic EuroSCORE and lower pre-procedural eGFR values compared with those who underwent SAVR (P < 0.0001 for all). The incidence of AKI was lower (P = 0.001; P = 0.014 after propensity score adjustment) in CKD patients who underwent TAVI (9.2%, need for haemodialysis: 2.5%) compared with those who underwent SAVR (25.9%, need for haemodialysis: 8.7%).
Conclusion
Acute kidney injury occurred in 11.7% of the patients following TAVI and was associated with a greater than four-fold increase in the risk of postoperative mortality. Hypertension, chronic obstructive pulmonary disease, and blood transfusion were predictive factors of AKI. In those patients with pre-procedural CKD, TAVI was associated with a significant reduction of AKI compared with SAVR.
Keywords: Aortic stenosis, Transcatheter aortic valve implantation, Acute renal failure, Surgical aortic valve replacement
Introduction
Acute kidney injury (AKI) is one of the most serious complications following cardiac surgery, with an incidence varying between 1 and 30% depending on the definition of AKI and baseline characteristics of the study population.1 Several studies have shown that the occurrence of AKI after cardiac surgery is an independent predictor of in-hospital, mid- and long-term mortality, with a two- to three-fold increase in the risk of death in those patients presenting AKI following the intervention.1–4 Surgical aortic valve replacement (SAVR) is the gold standard for the treatment of symptomatic severe aortic stenosis (AS), and transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment for those patients considered at very high or prohibitive surgical risk.5–8 Patients undergoing TAVI nowadays are thus commonly very old and have a high prevalence of chronic kidney disease (CKD). In fact, trying to avoid potential deterioration of renal function in patients with CKD has become an important argument for choosing TAVI rather than SAVR in those cases. However, very few data exist on the occurrence and prognosis of AKI following TAVI,9 and no studies have as yet determined whether a TAVI strategy is associated with a lower incidence of AKI compared with SAVR. TAVI procedures involve the administration of contrast media, the systematic occurrence of short periods of extreme hypotension (rapid pacing, balloon valvuloplasty, and valve deployment), and the manipulation of large catheters in the aorta of patients with a high prevalence of diffuse atherosclerosis with the risk of cholesterol embolization, all of them are potential risk factors for AKI. Therefore, the objectives of this study were (i) to determine the incidence, predictive factors, and prognostic value of AKI following TAVI, and (ii) to compare the occurrence of AKI in TAVI vs. SAVR in the subset of patients with pre-procedural CKD.
Methods
Patients
A total of 243 patients diagnosed with symptomatic severe AS underwent TAVI at the St Paul's Hospital (n = 188, four operators), Vancouver, British Columbia, Canada, and the Quebec Heart and Lung Institute (n = 55, four operators), Quebec city, Quebec, Canada, between January 2005 and February 2009. Patients on chronic haemodialysis (n = 7), those participating in the PARTNER (Placement of AoRTic traNscathetER valve) trial (n = 15), and those who died within the 24 h precluding creatinine measurements following TAVI (n = 8) were excluded from the study, leading to a final study population of 213 patients. TAVI was approved for compassionate clinical use by the Canadian Department of Health and Welfare (Ottawa, Canada) in patients with symptomatic severe AS considered either non-operable or very high risk surgical candidates, and all patients provided signed informed consent for the procedures. All clinical, echocardiographic, procedural, and post-procedural data were prospectively gathered. A total of 119 patients (56%) undergoing TAVI had pre-procedural CKD [defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2]10 and were compared with 104 consecutive patients (nine operators) with severe AS and CKD who underwent isolated SAVR at the Quebec Heart and Lung Institute from January 2005 to February 2009. This surgical cohort was obtained from the Quebec Heart and Lung Institute surgical database and all data were prospectively collected.
Transcatheter aortic valve implantation and surgical aortic valve replacement procedures
TAVI procedures have been described in detail in previous reports.5–8 The Cribier–Edwards or Edwards-SAPIEN valve (Edwards Lifesciences Inc., Irvine, CA, USA) was used in all cases either by transfemoral (n = 111, 52%) or transapical (n = 102, 48%) approach. Patients with pre-procedural CKD received intravenous hydration before and after the procedure, and the use of a prophylactic treatment (N-acetylcysteine, intravenous bicarbonate) was left at the discretion of the physician performing the procedure. The amount of contrast, number of rapid pacing runs, the occurrence of any complication leading to severe maintained hypotension, and/or the need for haemodynamic support (aortic counterpulsation balloon and extracorporeal circulation) were recorded. A successful procedure was defined as the implantation of a functioning prosthetic valve within the aortic annulus at the end of the procedure without in-laboratory mortality. SAVR procedures were performed through mid-sternotomy, using standard surgical techniques and under extracorporeal circulation.
Serum creatinine measurements and acute kidney injury definition
All patients in both TAVI and SAVR groups had a systematic determination of serum creatinine and eGFR calculation based on the simplified modification of diet in renal disease (MDRD) formula11 before (<7 days) and at 48 h following the procedure. The occurrence of AKI was defined as a decrease of >25% in eGFR at 48 h following the procedure (RIFLE criteria),12 or the need of haemodialysis during index hospitalization. The degree of AKI was further classified as (i) >25% decrease in eGFR, (ii) 50–75% decrease in eGFR, and (iii) >75% decrease in eGFR.
Statistical analysis
Qualitative variables were expressed as percentages and quantitative variables as mean (standard deviation) or median (interquartile range). The normality distribution for continuous data was examined with the Shapiro–Wilk test. Comparison of numerical variables was performed using the two-sided Student's t-test or Wilcoxon rank-sum test, and the chi-square or Fischer's exact tests were used to compare qualitative variables. A stepwise logistic regression analysis including all variables with P-value < 0.2 in the univariate analysis was used to determine the predictive factors of both AKI and hospital mortality. The following variables were included in the model for the prediction of AKI: age, hypertension, chronic obstructive pulmonary disease, logistic EuroSCORE, procedural approach (transfemoral vs. transapical), procedural time, contrast media volume, any complication leading to the need of haemodynamic support, red blood cell (RBC) transfusion, and post-procedural myocardial infarction. The variables included in the model for the prediction of in-hospital death were: male gender, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, pre-procedural serum creatinine, logistic EuroSCORE, left ventricular ejection fraction, pre-procedural mean aortic gradient, pulmonary artery pressure, procedural approach, contrast media volume, RBC transfusion, number of RBC units, post-procedural stroke, post-procedural pneumonia or sepsis, serum creatinine and eGFR at 48 h post-procedure, and AKI. A continuous propensity score analysis was performed to adjust for the intergroup (TAVI vs. SAVR in patients with CKD) differences in baseline clinical characteristics caused by the selection bias inherent to the nonrandomized nature of the study. A propensity score representing the likelihood of having TAVI as opposed to SAVR was calculated for each patient by using a logistic regression analysis that identified variables independently associated with the type of procedure. Variables exhibiting a P-value < 0.2 in the univariate analysis were included in the logistic regression analysis. The variables used for the propensity score were: age, logistic EuroSCORE, congestive heart failure, coronary artery disease, peripheral vascular disease, and baseline eGFR. In addition, the incidence of post-procedural AKI was further evaluated after patient matching based on pre-procedural eGFR values between TAVI and SAVR groups. Differences were considered statistically significant at P-values < 0.05. The data were analysed using SAS statistical software version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
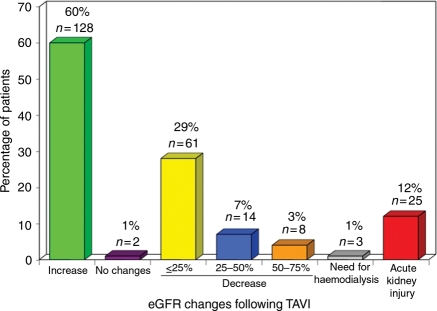
Baseline clinical and echocardiographic characteristics of the study population and the main TAVI peri-procedural characteristics are shown in Table 1. Acute kidney injury occurred in 25 patients (11.7%) and 3 patients (1.4%) required dialysis during index hospitalization. The changes in eGFR at 48 h following TAVI are shown in Table 1 and Figure 1.
Table 1.
Baseline, peri-procedural characteristics, and post-procedural renal function of patients undergoing transcatheter aortic valve implantation
| Variable | n = 213 |
|---|---|
| Clinical characteristics | |
| Age (years) | 82 ± 8 |
| Male sex | 99 (47) |
| Diabetes | 48 (23) |
| Hypertension | 150 (70) |
| Congestive heart failure | 115 (54) |
| New York Heart Association class | |
| I–II | 22 (10) |
| III–IV | 191 (90) |
| Coronary artery disease | 143 (67) |
| Cerebrovascular disease | 61 (29) |
| Peripheral vascular disease | 84 (39) |
| Chronic obstructive pulmonary disease | 69 (32) |
| Serum creatinine (mg/dL) | 1.24 ± 0.5 |
| eGFR (mL/min/1.73 m2), median (interquartile range) | 57 (43–77) |
| eGFR <60 (mL/min/1.73 m2) | 119 (56) |
| Haemoglobin (g/dL) | 12 ± 1.6 |
| Logistic EuroSCORE | 29.3 ± 17.5 |
| Echocardiographic data | |
| Left ventricular ejection fraction | 57 ± 15 |
| Left ventricular ejection fraction <40% | 25 (12) |
| Mean gradient (mmHg) | 44 ± 17 |
| Aortic valve area (cm2) | 0.63 ± 0.16 |
| Pulmonary artery systolic pressure (mmHg) | 50 ± 12 |
| Moderate to severe mitral regurgitation | 72 (35) |
| Peri-procedural variables | |
| Approach | |
| Transapical | 102 (48) |
| Transfemoral | 111 (52) |
| Time of procedure ‘skin to skin’ (min), median (interquartile range) | 85 (50–477) |
| Contrast amount (cm3) | 97 ± 57 |
| Rapid pacing runs | 5.4 ± 2.1 |
| Life-threatening arrhythmias | 14 (7) |
| Any complication leading to severe maintained hypotension | 10 (5) |
| Any complication leading to the need of haemodynamic support | 7 (3) |
| Successful procedure | 205 (96) |
| Lowest haemoglobin (g/dL) | 9.8 ± 1.5 |
| Blood transfusion | 104 (49) |
| Number of units | 3.6 ± 4.3 |
| N-Acetylcysteine/bicarbonate pre-treatment | 28 (13) |
| Post-procedural complications | |
| Myocardial infarction | 7 (3) |
| Stroke | 7 (3) |
| Pneumonia/sepsis | 21 (10) |
| Death | 21 (10) |
| Hospitalization length (days), median (interquartile range) | 6 (3–113) |
| Post-procedural renal impact | |
| Serum creatinine at 48 h (mg/dL) | 1.23 ± 0.6 |
| eGFR at 48 h (mL/min/1.73 m2), median (interquartile range) | 60 (42–83) |
| eGFR changes at 48 h | |
| No change in eGFR | 2 (1) |
| Decrease in eGFR | |
| ≤ 25% | 61 (29) |
| > 25% | 22 (10) |
| Increase in eGFR | 128 (60) |
| % of increase in eGFR | 26 ± 19 |
| Need for haemodialysis | 3 (1) |
| Acute kidney injury | 25 (12) |
Values are expressed as n (%) or mean ± SD unless otherwise noted.
eGFR, estimated glomerular filtration rate.
Figure 1.
Changes in estimated glomerular filtration rate (eGFR) at 48 h following transcatheter aortic valve implantation (TAVI).
Predictive factors of acute kidney injury
Baseline and procedural characteristics of the study population grouped according to the occurrence of AKI are shown in Table 2. Patients who presented AKI had more frequently a history of hypertension (92 vs. 68%, P = 0.010) and chronic obstructive pulmonary disease (52 vs. 30%, P = 0.038), tended to have a TAVI procedure performed by transapical approach (64 vs. 46%, P = 0.093) and required more frequently blood transfusions during the peri-procedural period (76 vs. 45%, P = 0.005). The independent predictors of AKI following the procedure are shown in Table 3.
Table 2.
Baseline and peri-procedural characteristics of patients undergoing transcatheter aortic valve implantation, according to the occurrence of post-procedural acute kidney injury
| Variable | Acute kidney injury |
||
|---|---|---|---|
| Yes (n = 25) | No (n = 188) | P-value | |
| Clinical characteristics | |||
| Age (years) | 84 ± 7 | 82 ± 8 | 0.129 |
| Male sex | 13 (52) | 86 (46) | 0.671 |
| Diabetes | 7 (28) | 41 (22) | 0.456 |
| Hypertension | 23 (92) | 127 (68) | 0.010 |
| Congestive heart failure | 15 (60) | 100 (53) | 0.670 |
| New York Heart Association class | |||
| I–II | 4 (16) | 18 (10) | |
| III–IV | 21 (84) | 170 (90) | 0.302* |
| Coronary artery disease | 14 (56) | 129 (69) | 0.352 |
| Cerebrovascular disease | 6 (24) | 55 (29) | 0.647 |
| Peripheral vascular disease | 12 (48) | 72 (38) | 0.388 |
| Chronic obstructive pulmonary disease | 13 (52) | 56 (30) | 0.038 |
| Serum creatinine (mg/dL) | 1.17 ± 0.5 | 1.25 ± 0.54 | 0.442 |
| eGFR (mL/min/1.73 m2), median (interquartile range) | 70 (49–80) | 57 (43–76) | 0.252 |
| eGFR <60 (mL/min/1.73 m2) | 11 (44) | 108 (57) | 0.284 |
| Haemoglobin (g/dL) | 12 ± 1.7 | 12 ± 1.5 | 0.547 |
| Logistic EuroSCORE | 34.3 ± 21.8 | 28.7 ± 16.8 | 0.131 |
| Echocardiographic data | |||
| Left ventricular ejection fraction | 54 ± 14 | 57 ± 15 | 0.468 |
| Left ventricular ejection fraction <40% | 3 (13) | 22 (12) | 1.000 |
| Mean gradient (mmHg) | 41 ± 14 | 44 ± 17 | 0.319 |
| Aortic valve area (cm2) | 0.62 ± 0.11 | 0.63 ± 0.17 | 0.884 |
| Pulmonary artery systolic pressure (mmHg) | 54 ± 17 | 50 ± 12 | 0.231 |
| Moderate to severe mitral regurgitation | 10 (44) | 62 (34) | 0.360 |
| Peri-procedural variables | |||
| Approach | |||
| Transapical | 16 (64) | 86 (46) | 0.093** |
| Transfemoral | 9 (36) | 102 (54) | |
| Time of procedure (min), median (interquartile range) | 104 (70–160) | 85 (74–101) | 0.153 |
| Contrast amount (cm3) | 79 ± 55 | 99 ± 57 | 0.190 |
| Rapid pacing runs | 5.9 ± 3.3 | 5.3 ± 1.9 | 0.293 |
| Life-threatening arrhythmias | 3 (12) | 11 (6) | 0.217 |
| Any complication leading to severe maintained hypotension | 2 (8) | 8 (4) | 0.332 |
| Any complication leading to the need of haemodynamic support | 2 (8) | 5 (3) | 0.192 |
| Successful procedure | 24 (96) | 181 (96) | 1.000 |
| Lowest haemoglobin (g/dL) | 9.9 ± 1.5 | 9.8 ± 1.5 | 0.892 |
| Blood transfusion | 19 (76) | 85 (45) | 0.005 |
| Number of red cell blood units | 4.8 ± 7.4 | 3.3 ± 3.2 | 0.379 |
| N-Acetylcysteine/bicarbonate pre-treatment | 1 (4) | 27 (14) | 0.480 |
| Post-procedural complications | |||
| Myocardial infarction | 2 (8) | 5 (3) | 0.192 |
| Stroke | 1 (4) | 6 (3) | 0.588 |
| Pneumonia/sepsis | 2 (8) | 19 (10) | 1.000 |
| Death | 7 (28) | 14 (7) | 0.005 |
| Hospitalization length (days) median (interquartile range) | 9 (5–30) | 6 (4–10) | 0.017 |
| Post-procedural renal impact | |||
| Serum creatinine at 48 h (mg/dL) | 1.86 ± 0.66 | 1.44 ± 0.51 | <0.0001 |
| eGFR at 48 h (mL/min/1.73 m2), median (interquartile range) | 36 (23–45) | 64 (47–87) | <0.0001 |
| Need for haemodialysis | 3 (12) | 0 | 0.001 |
Values are expressed as n (%) or mean ± SD unless otherwise noted.
eGFR, estimated glomerular filtration rate.
*P-value corresponds to both sets of variables, I–II and III–IV.
**P-value corresponds to both sets of variables, Transapical and Transfemoral.
Table 3.
Independent predictors of acute kidney injury following transcatheter aortic valve implantation
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Hypertension | 4.66 (1.04–20.87) | 0.044 |
| Chronic obstructive pulmonary disease | 2.64 (1.10–6.36) | 0.030 |
| Red blood cell transfusion | 3.47 (1.30–9.29) | 0.013 |
Acute kidney injury and hospital mortality
Hospital mortality occurred in 21 patients (9.8%). In those patients presenting AKI, the mortality rate was 28% compared with 7.4% in those patients with no AKI (P = 0.005; P < 0.0001 after including in the analysis the eight patients who died within the 24 h following the procedure and for which post-procedural creatinine value could not be assessed). Table 4 shows the clinical and peri-procedural characteristics of the patients who died in the postoperative period compared with those who survived. Patients who died had more frequently a history of chronic obstructive pulmonary disease (52 vs. 30%, P = 0.049), had a higher logistic EuroSCORE (40.5 ± 21.3 vs. 28.1 ± 16.7%, P = 0.001) and exhibited higher levels of pulmonary artery systolic pressure (56 ± 14 vs. 50 ± 12 mmHg, P = 0.033). The independent predictive factors of hospital mortality are shown in Table 5.
Table 4.
Baseline and peri-procedural characteristics, and post-procedural renal impact of patients undergoing transcatheter aortic valve implantation, according to the occurrence of hospital mortality
| Variable | In-hospital death |
||
|---|---|---|---|
| Yes (n = 21) | No (n = 192) | P-value | |
| Clinical characteristics | |||
| Age (years) | 82 ± 10 | 82 ± 8 | 0.873 |
| Male, n (%) | 14 (67) | 85 (44) | 0.065 |
| Diabetes | 3 (14) | 45 (23) | 0.421 |
| Hypertension | 15 (71) | 135 (70) | 1.000 |
| Congestive heart failure | 11 (52) | 104 (54) | 1.000 |
| New York Heart Association class | |||
| I–II | 2 (9) | 20 (10) | 1.000* |
| III–IV | 19 (91) | 172 (90) | |
| Coronary artery disease | 13 (62) | 130 (68) | 0.633 |
| Cerebrovascular disease | 9 (42) | 52 (27) | 0.135 |
| Peripheral vascular disease | 12 (57) | 72 (38) | 0.100 |
| Chronic obstructive pulmonary disease | 11 (52) | 58 (30) | 0.049 |
| Serum creatinine (mg/dL) | 1.41 ± 0.67 | 1.23 ± 0.52 | 0.136 |
| eGFR (mL/min/1.72 m2), median (interquartile range) | 54 (36–74) | 57 (43–77) | 0.693 |
| eGFR <60 (mL/min/1.72 m2) | 12 (57) | 107 (56) | 1.000 |
| Haemoglobin (g/dL) | 11.7 ± 1.4 | 12.0 ± 1.6 | 0.320 |
| Logistic EuroSCORE | 40.5 ± 21.3 | 28.1 ± 16.7 | 0.001 |
| Echocardiographic data | |||
| Left ventricular ejection fraction | 52 ± 15 | 60 ± 15 | 0.193 |
| Left ventricular ejection fraction <40% | 3 (16) | 22 (12) | 0.708 |
| Mean gradient (mmHg) | 37 ± 13 | 45 ± 17 | 0.044 |
| Aortic valve area (cm2) | 0.65 ± 0.15 | 0.62 ± 0.16 | 0.497 |
| Pulmonary artery systolic pressure (mmHg) | 56 ± 14 | 50 ± 12 | 0.034 |
| Moderate to severe mitral regurgitation | 8 (42) | 64 (34) | 0.461 |
| Peri-procedural variables | |||
| Approach | |||
| Transapical | 14 (67) | 88 (46) | 0.106 |
| Transfemoral | 7 (33) | 104 (54) | |
| Time of procedure (min) median (interquartile range) | 90 (69–110) | 85 (74–105) | 0.756 |
| Contrast amount (cm3) | 74 ± 59 | 99 ± 57 | 0.148 |
| Rapid pacing runs | 5.5 ± 2.0 | 5.4 ± 2.2 | 0.876 |
| Life-threatening arrhythmias | 2 (10) | 12 (6) | 0.634 |
| Any complication leading to severe maintained hypotension | 2 (10) | 8 (4) | 0.257 |
| Any complication leading to need haemodynamic support | 1 (5) | 6 (3) | 0.522 |
| Successful procedure | 21 (100) | 184 (96) | 1.000 |
| Lowest haemoglobin (g/dL) | 9.6 ± 1.7 | 9.9 ± 1.5 | 0.528 |
| Blood transfusion | 14 (67) | 90 (47) | 0.108 |
| Number of red blood cell units | 6.6 ± 8.1 | 3.1 ± 3.1 | 0.137 |
| N-Acetylcysteine/bicarbonate pre-treatment | 4 (19) | 24 (13) | 0.443 |
| Post-procedural complications | |||
| Myocardial infarction | 1 (5) | 6 (3) | 0.521 |
| Stroke | 2 (10) | 5 (3) | 0.144 |
| Pneumonia/sepsis | 5 (24) | 16 (8) | 0.041 |
| Post-procedural renal impact | |||
| Serum creatinine at 48 h (mg/dL) | 1.54 ± 0.71 | 1.19 ± 0.55 | 0.008 |
| eGFR at 48 h (mL/min/1.73 m2), median (interquartile range) | 45 (30–71) | 60 (43–83) | 0.080 |
| eGFR changes at 48 h | |||
| No change in eGFR | 0 | 2 (1) | 0.055** |
| Decrease in eGFR | |||
| ≤ 25% | 4 (19) | 57 (30) | |
| > 25% | 6 (29) | 16 (8) | |
| Increase in eGFR | 11 (52) | 117 (61) | |
| % of increase in eGFR | 22 ± 10 | 27 ± 20 | 0.199 |
| Need for haemodialysis | 1 (5) | 2 (1) | 0.269 |
| Acute Kidney Injury | 7 (33) | 18 (9) | 0.005 |
Values are expressed as n (%) or mean ± SD unless otherwise noted.
eGFR, estimated glomerular filtration rate.
*P-value corresponds to both sets of variables, I–II and III–IV.
**P-value corresponds to all sets of variables, No change in eGFR, Decrease in eGFR, ≤25%, >25%, and Increase in eGFR.
Table 5.
Independent predictors of hospital mortality following transcatheter aortic valve implantation
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Acute kidney injury | 4.14 (1.42–12.13) | 0.010 |
| Logistic EuroSCORE | 1.03a (1.01–1.06) | 0.009 |
aFor each increase of 1%.
Acute kidney injury in transcatheter aortic valve implantation vs. surgical aortic valve replacement procedures
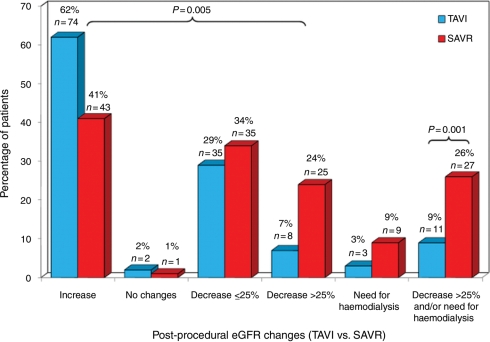
The clinical and procedural characteristics of patients with pre-procedural CKD grouped according to the type of procedure (TAVI vs. SAVR) are shown in Table 6. Compared with patients with CKD who underwent SAVR, patients with CKD who underwent TAVI were older (83 ± 7 vs. 74 ± 8 years, P < 0.0001), and presented more co-morbidities leading to a higher risk clinical profile (logistic EuroSCORE: 31.2 ± 18.1 vs. 21.8 ± 14.6%, P < 0.0001). Also, baseline creatinine and eGFR values were higher and lower, respectively, in TAVI patients compared with SAVR patients (P < 0.0001 for both). Postoperative AKI occurred less frequently in the TAVI group compared with the SAVR group (9.2 vs. 25.9%, OR: 0.29, 95% CI: 0.14–0.62, P = 0.001; OR: 0.33, 95% CI: 0.13–0.79, P = 0.014 after propensity score adjustment), with 2.5 and 8.7% of the patients requiring dialysis during index hospitalization in the TAVI and SAVR groups, respectively (P = 0.070). The differences remained significant (TAVI: 9.4% vs. SAVR: 28.1%; OR: 0.26, 95% CI: 0.10–0.72, P = 0.011) after patient matching on the basis of pre-procedural eGFR values (64 patients per group, mean eGFR: 49 ± 8 mL/min). The changes in eGFR following the procedure and grouped according to the type of intervention (TAVI vs. SAVR) are shown in Figure 2.
Table 6.
Baseline characteristics and post-procedural renal impact in patients with chronic kidney disease, according to the type (transcatheter vs. surgical) of treatment
| Variable | TAVI (n = 119) | SAVR (n = 104) | P-value |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 83 ± 7 | 74 ± 8 | <0.0001 |
| Female gender | 70 (59) | 54 (52) | 0.345 |
| Diabetes | 32 (27) | 42 (40) | 0.046 |
| Hypertension | 91 (77) | 80 (77) | 1.000 |
| Congestive heart failure | 66 (55) | 16 (15) | <0.0001 |
| Coronary artery disease | 83 (70) | 19 (18) | <0.0001 |
| Cerebrovascular disease | 37 (31) | 12 (12) | <0.0001 |
| Peripheral vascular disease | 48 (40) | 12 (12) | <0.0001 |
| Chronic obstructive pulmonary disease | 30 (25) | 23 (22) | 0.218 |
| Serum creatinine (mg/dL) | 1.54 ± 0.54 | 1.35 ± 0.28 | <0.0001 |
| eGFR (mL/min/1.73 m2), median (interquartile range) | 46 (35–52) | 50 (43–55) | 0.0002 |
| eGFR <30 (mL/min/1.73 m2) | 20 (17) | 1 (1) | <0.0001 |
| Haemoglobin (g/dL) | 11.8 ± 1.5 | 12.4 ± 1.6 | 0.002 |
| Logistic EuroSCORE | 31.2 ± 18.1 | 21.8 ± 14.6 | <0.0001 |
| Echocardiographic data | |||
| Left ventricular ejection fraction | 56 ± 15 | 55 ± 16 | 0.616 |
| Left ventricular ejection fraction <40% | 16 (14) | 15 (15) | 0.848 |
| Mean gradient (mmHg) | 42 ± 16 | 48 ± 18 | 0.010 |
| Aortic valve area (cm2) | 0.64 ± 0.15 | 0.65 ± 0.15 | 0.620 |
| Pulmonary artery systolic pressure (mmHg) | 51 ± 13 | 44 ± 17 | 0.023 |
| Moderate to severe mitral regurgitation | 46 (39) | 6 (9) | <0.0001 |
| Post-procedural renal impact | |||
| Serum creatinine at 48 h (mg/dL) | 1.48 ± 0.59 | 1.52 ± 0.58 | 0.678 |
| eGFR at 48 h (mL/min/1.73 m2), median (interquartile range) | 47 (30–60) | 47 (34–38) | 0.899 |
| eGFR changes at 48 h | |||
| No changes in eGFR | 2 (2) | 1 (1) | 0.005* |
| Decrease in eGFR | |||
| ≤25% | 35 (29) | 35 (34) | |
| >25% | 8 (7) | 25 (24) | |
| Increase in eGFR | 74 (62) | 43 (41) | |
| % of increase in eGFR | 28 ± 21 | 22 ± 26 | 0.174 |
| Need for haemodialysis | 3 (3) | 9 (9) | 0.071 |
| Acute kidney injury | 11 (9) | 27 (26) | 0.001 |
Values are expressed as n (%) or mean ± SD unless otherwise noted.
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement; eGFR, estimated glomerular filtration rate.
*P-value corresponds to all sets of variables, No change in eGFR, Decrease in eGFR, ≤25%, >25%, and Increase in eGFR.
Figure 2.
Changes in estimated glomerular filtration rate (eGFR) at 48 h following transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) in patients with pre-procedural chronic kidney disease.
Discussion
Acute kidney injury occurred in 11.7% of the patients following TAVI, with 1.4% of them requiring dialysis. A history of hypertension, chronic obstructive pulmonary disease, and peri-operative blood transfusion were predictive factors of AKI. Patients who presented AKI had a hospital mortality of 28%, and the occurrence of AKI was an independent predictor of mortality, with greater than four-fold increase in the risk of postoperative death among those patients presenting AKI after TAVI. In patients with severe symptomatic AS and pre-procedural CKD, the incidence of AKI was lower in those who underwent TAVI compared with those who underwent SAVR (9.2 vs. 25.9%), despite the fact that TAVI patients exhibited worse clinical risk profile and poorer pre-procedural kidney function.
Aregger et al.9 evaluated the occurrence of AKI in a series of 54 patients who had undergone TAVI. Similarly to our results, most patients (56%) increased eGFR values after TAVI, but the incidence of AKI was 28% with up to 7.4% of the patients requiring dialysis during index hospitalization. Compared with our study, creatinine levels were determined daily during the hospitalization period, baseline eGFR values were lower (mean: 55 ± 26 mL/min/1.73 m2) and the amount of contrast media used during the procedure was higher (mean >200 cc). All these factors might have contributed to the higher rate of AKI following TAVI in that study. The presence of hypertension has been associated with impaired kidney autoregulation which increases the risk of AKI despite maintaining mean arterial pressure within the normal range.13 Hsu et al.14 reported that hypertension increased the risk of nosocomial AKI in patients with CKD. Also, hypertension was shown to be an independent predictor of AKI following cardiothoracic surgery and percutaneous coronary intervention.15,16 Chronic obstructive pulmonary disease has been shown to be an independent predictor of AKI in patients undergoing cardiac surgery,1,17 and the present study also demonstrated that this comorbidity increased the risk of AKI following TAVI by greater than two-fold. Anand et al.18 reported that patients with chronic obstructive pulmonary disease may experience a significant reduction in renal blood flow and glomerular filtration. Also, chronic obstructive pulmonary disease patients are more prone to the occurrence of episodes of severe hypoxemia–hypercapnia during the peri-operative period and this might indeed have contributed to further deterioration of renal function.19 The need for RBC transfusion is a very well recognized predictor of AKI following cardiac surgery.4,20 Consistent with our results, Aregger et al.9 recently showed that the number of blood transfusions was also associated with an increased risk of AKI following TAVI. Preserved RBCs undergo progressive functional and structural changes leading to a reduction in RBC function and viability, and accumulate proinflammatory molecules, free iron, and haemoglobin, and all these changes might favour renal dysfunction, particularly in older patients with impairment of kidney autoregulation.20,21 The present study showed that up to half of the patients undergoing TAVI had received RBC transfusion and that this was associated with a greater than three-fold increase in the risk of AKI. This result suggests that efforts should be made to avoid unnecessary blood transfusions in patients undergoing TAVI.
Interestingly, the amount of contrast media was not associated with AKI following TAVI. A contrast volume >100 cc has been associated with contrast-induced nephropathy following percutaneous coronary intervention.22–24 The fact that the mean contrast volume used in the present study was <100 cc might partially explain the lack of correlation. Nonetheless, continued efforts to minimize the amount of contrast media in these procedures (contrast dilution, contrast hand injections, and echocardiography guiding for valve positioning25) would be important to further reduce the risk of AKI following TAVI. Finally, we found no correlation between the number of rapid pacing runs and the occurrence of AKI, and this suggests that the short periods of severe hypotension induced by rapid pacing do not play an important role in the deterioration of renal function following TAVI. However, the fact that the rapid pacing technique was used in all patients limits our ability to completely rule out a potential role for such short periods of hypotension in AKI following TAVI.
Acute kidney injury and hospital mortality
Several studies have shown AKI to be a powerful predictor of death at short-, mid-, and long-term follow-up after cardiothoracic surgery and percutaneous coronary intervention.1–4,24 The present study is the first to demonstrate that AKI is associated with a higher (four-fold) postoperative mortality following TAVI and that this association was independent of baseline risk profile characteristics and peri-procedural complications. The pathophysiology explaining the prognostic role of AKI in the early postoperative period of TAVI remains unclear. Although AKI could have been only a marker of multisystem failure, our results showed that the high mortality rate in patients with AKI cannot be fully attributed to comorbidities or peri-procedural complications, which suggests that AKI contributes directly to early postoperative mortality. These results highlight the clinical relevance of assessing kidney function within the 48 h following TAVI in order to identify those patients who complicate with AKI and thereby require close follow-up during the post-procedural period.
Surgical aortic valve replacement vs. transcatheter aortic valve implantation in patients with pre-procedure chronic kidney disease
Patients with preoperative CKD undergoing cardiac surgery are at high risk of AKI and dialysis following the operation.1 Gummert et al.26 showed that up to 16% of the patients with CKD undergoing SAVR required haemodialysis during the postoperative period. The results of the present study showed that in patients with previous renal dysfunction, the occurrence of AKI was lower with TAVI (9.2%) compared with SAVR (25.9%). Also, TAVI was associated with a non-significant reduction in the need for haemodialysis following the intervention compared with SAVR. Interestingly, eGFR improved in most CKD patients undergoing TAVI compared with less than half of the patients undergoing SAVR. The deleterious effects of cardiopulmonary bypass on renal function are well known4,27 and its avoidance would probably explain the better response in kidney function obtained with TAVI in the high-risk subset of CKD patients with severe AS. Future prospective randomized studies such as the Placement of AoRTic TraNscathetER Valve (PARTNER) trial should confirm these results and determine whether the sole presence of CKD should be used as a criterion to select TAVI rather than SAVR in patients with symptomatic severe AS.
Study limitations
The results of this study were obtained from a database with prospectively gathered data. However, this was a post hoc non-pre-specified analysis and we cannot rule out the possibility that other potential confounding variables not included in the model might have affected the results. Also, the results were based on a single determination of eGFR at 48 h following the procedure. We cannot exclude the possibility that further measurements of creatinine levels during the hospitalization period might have revealed a higher incidence of AKI. Due to the non-randomized nature of the study, differences in the occurrence of AKI between TAVI and SAVR should be seen as hypothesis generating and will need to be confirmed by prospective randomized studies. Finally, these results may not apply to lower volume centres, especially at the beginning of the learning curve.
Conclusions
Acute kidney injury occurred in only 11.7% of the patients undergoing TAVI despite advanced age (mean >80 years) and the very high-risk clinical profile of the study population. Nonetheless, AKI was a very powerful predictor of death during the postoperative period independently of baseline comorbidities and peri-procedural complications, and this highlights the importance of both preventing and identifying such a complication early after TAVI. Patients with hypertension and chronic obstructive pulmonary disease and those receiving RBC transfusions were at higher risk, suggesting that a more careful monitoring of patients with these risk factors and a more restrictive strategy regarding blood transfusion during TAVI might be clinically relevant. Finally, in the high-risk group of patients with advanced age, symptomatic severe AS, and pre-procedural chronic kidney disease, TAVI was associated with greater than two-fold lower incidence of postoperative AKI compared with SAVR. Future randomized studies are needed to confirm these results and determine whether their prognostic relevance should lead to the favouring of TAVI in this particular subset of patients.
Funding
Funding to pay the Open Access publication charges for this article was provided by the Fondation de l'Institut Universitaire de Cardiologie et Pneumologie de Québec.
Conflict of interest: J.G.W., É.D., and J.R.-C. are consultants for Edwards Lifesciences Inc. P.P. has received honoraria for presentations and research grants from Edwards Lifesciences Inc. The remaining authors report no conflict of interest.
Acknowledgements
We would like to thank Serge Simard, MSc, for the statistical analysis, and Jacinthe Aubé, RN, Stéphanie Dionne, and Diana Ayán, for their exceptional work on data collection.
References
- 1.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 3.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 4.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 5.Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Nercolini D, Tapiero S, Litzler PY, Bessou JP, Babaliaros V. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47:1214–1223. doi: 10.1016/j.jacc.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850. doi: 10.1161/CIRCULATIONAHA.105.582882. [DOI] [PubMed] [Google Scholar]
- 7.Walther T, Simon P, Dewey T, Wimmer-Greinecker G, Falk V, Kasimir MT, Doss M, Borger MA, Schuler G, Glogar D, Fehske W, Wolner E, Mohr FW, Mack M. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation. 2007;116:I240–I245. doi: 10.1161/CIRCULATIONAHA.106.677237. [DOI] [PubMed] [Google Scholar]
- 8.Rodés-Cabau J, Dumont E, De Larochellière R, Doyle D, Lemieux J, Bergeron S, Clavel MA, Villeneuve J, Raby K, Bertrand OF, Pibarot P. Feasibility and initial results of percutaneous aortic valve implantation including selection of the transfemoral or transapical approach in patients with severe aortic stenosis. Am J Cardiol. 2008;102:1240–1246. doi: 10.1016/j.amjcard.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 9.Aregger F, Wenaweser P, Hellige GJ, Kadner A, Carrel T, Windecker S, Frey FJ. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24:2175–2179. doi: 10.1093/ndt/gfp036. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Green T, Kusek JW, Beck GL MDRD Study Group. A simplified equation to predict glomerular filtration rate from serum creatinine (abstract) J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 12.Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33:409–413. doi: 10.1007/s00134-006-0478-x. [DOI] [PubMed] [Google Scholar]
- 13.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conen D, Buerkle G, Perruchoud AP, Buettner HJ, Mueller C. Hypertension is an independent risk factor for contrast nephropathy after percutaneous coronary intervention. Int J Cardiol. 2006;110:237–241. doi: 10.1016/j.ijcard.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Metz LI, LeBeau ME, Zlabek JA, Mathiason MA. Acute renal failure in patients undergoing cardiothoracic surgery in a community hospital. WMJ. 2009;108:109–114. [PubMed] [Google Scholar]
- 17.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, Daley J. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 18.Anand IS, Chandrashekhar Y, Ferrari R, Sarma R, Guleria R, Jindal SK, Wahi PL, Poole-Wilson PA, Harris P. Pathogenesis of congestive state in chronic obstructive pulmonary disease. Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Circulation. 1992;86:12–21. doi: 10.1161/01.cir.86.1.12. [DOI] [PubMed] [Google Scholar]
- 19.Wouters EF. Management of severe COPD. Lancet. 2004;364:883–895. doi: 10.1016/S0140-6736(04)16984-5. [DOI] [PubMed] [Google Scholar]
- 20.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 21.Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Radic Biol Med. 2002;32:568–576. doi: 10.1016/s0891-5849(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 22.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 23.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 24.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR., Jr Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 25.Dumont E, Lemieux J, Doyle D, Rodés-Cabau J. Feasibility of transapical aortic valve implantation fully guided by transesophageal echocardiography. J Thorac Cardiovasc Surg. 2009;138:1022–1024. doi: 10.1016/j.jtcvs.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Gummert JF, Bucerius J, Walther T, Doll N, Falk V, Schmitt DV, Mohr FW. Requirement for renal replacement therapy in patients undergoing cardiac surgery. Thorac Cardiovasc Surg. 2004;52:70–76. doi: 10.1055/s-2004-817806. [DOI] [PubMed] [Google Scholar]
- 27.Hix JK, Thakar CV, Katz EM, Yared JP, Sabik J, Paganini EP. Effect of off-pump coronary artery bypass graft surgery on postoperative acute kidney injury and mortality. Crit Care Med. 2006;34:2979–2983. doi: 10.1097/01.CCM.0000248905.67352.BA. [DOI] [PubMed] [Google Scholar]