Abstract
The IgE of sera from patients with a history of allergy to oranges (Citrus sinensis) bind a number of proteins in orange extract, including Cit s 1, a germin-like protein. In the present study, we have analysed its immunological cross-reactivity and its molecular nature. Sera from many of the patients examined recognise a range of glycoproteins and neoglycoconjugates containing β1,2-xylose and core α1,3-fucose on their N-glycans. These reagents also inhibited the interaction of Cit s 1 with patients’ sera, thus underlining the critical role of glycosylation in the recognition of this protein by patients’ IgE and extending previous data showing that deglycosylated Cit s 1 does not possess IgE epitopes. In parallel, we examined the peptide sequence and glycan structure of Cit s 1 using mass spectrometric techniques. Indeed, we achieved complete sequence coverage of the mature protein as compared to the translation of an expressed sequence tag cDNA clone and demonstrated that the single N-glycosylation site of this protein carries oligosaccharides with xylose and fucose residues. Due to the presumed requirement for multivalency for in vivo allergenicity, our molecular data showing that Cit s 1 is monovalent as regards glycosylation and that the single N-glycan is the target of the IgE response to this protein, therefore, explain the immunological cross-reactive properties of Cit s 1 as well as its equivocal nature as a clinically-relevant allergen.
INTRODUCTION
Cross-reactive IgE recognising carbohydrate epitopes is a major complication in the correct laboratory diagnosis of food and pollen allergy; the phenomenon of carbohydrate cross-reactive determinants was first described twenty-five years ago (Aalberse, et al. 1981). Many studies have shown that plant and insect glycoproteins bind the IgE of the sera of allergic patients, but the biological and clinical significance is in some cases unclear. A number of studies do indeed show that glycoconjugates, particularly in multivalent forms, elicit histamine release (Batanero, et al. 1999, Bublin, et al. 2003, Westphal, et al. 2003, Wicklein, et al. 2004); however, monovalent glycoconjugates tend not to display such activity (Wicklein, et al. 2004). The highly conserved nature of core α1,3-fucosylation of N-glycans, though, is the basis for the cross-reaction of many plant and invertebrate glycoproteins to antisera raised against individual plant glycoproteins such as horseradish peroxidase (Fabini, et al. 2001, Paschinger, et al. 2004, Wilson 2002, Wilson, et al. 1998). The widespread occurrence of this glycostructural determinant is reflected in the presence of relevant core α1,3-fucosyltransferases in plants (Leiter, et al. 1999), nematodes (Paschinger, et al. 2004) and insects (Fabini, et al. 2001). On the other hand, the presence of enzymes transferring β1,2-xylose to N-glycans is primarily a feature of plants (Strasser, et al. 2000), snails (Mulder, et al. 1995) and trematodes (Faveeuw, et al. 2003), but this feature also contributes to anti-glycan cross-reactivity. The immune response to such carbohydrate epitopes varies between species, being apparently higher in rats and rabbits than in some mouse strains (Bardor, et al. 2003, Jin, et al. 2006). However, both core α1,3-fucose and xylose induce Th2 responses and antibody production in Schistosoma-infected mice (Faveeuw, et al. 2003). It is also interesting that a large proportion of non-allergic human blood donors were found, in one study, to have IgM and IgG1 recognising core α1,3-fucose or β1,2-linked xylose (Bardor, et al. 2003), whereas core α1,3-fucose is an important epitope for IgE from nematode-infected sheep (van Die, et al. 1999).
Recently, the presence of carbohydrate epitopes, recognised by IgE, on the orange protein Cit s 1 has been described (Ahrazem, et al. 2006); this protein constitutes one of three recently identified and characterised orange allergens. Whereas Cit s 2 corresponds to orange profilin and represents a major allergen, according to its in vitro and in vivo reactivity in patients with allergy to this fruit (Lopez-Torrejon, et al. 2005), Cit s 3 belongs to the lipid transfer protein (LTP) panallergen family and behaves as a minor allergen in the patients studied (~35% prevalence) (Ahrazem, et al. 2005). Cit s 1, on the other hand, was first detected in a study of six patients as being a protein of 24 kDa recognised by patients’ IgE (Ibañez, et al. 2004), which in a larger study was shown to display high in vitro reactivity with its glycans constituting the major IgE epitopes (Ahrazem, et al. 2006). Cit s 1 was also identified in an independent study as being a major orange allergen (Crespo, et al. 2006). Interestingly, the presence of Cit s 1-reactive IgE did not generally appear to correlate with the ability to elicit positive skin prick tests; only one-eighth of patients showed a significant response in this test with purified Cit s 1 (Ahrazem, et al. 2006). Initial N-terminal sequencing data indicated that Cit s 1 may be a germin-like protein (Ahrazem, et al. 2006, Crespo, et al. 2006); however, the full sequence was not determined and the nature of its N-glycosylation had not been examined. In this study, we present not only the full molecular characterisation of Cit s 1, using mass spectrometry, in terms of its peptide and glycan sequence, but also examine the nature of the anti-carbohydrate cross-reactivity in more detail.
RESULTS
Immunological analysis
Previous data indicated that the carbohydrate of Cit s 1 is a major epitope for the IgE of orange-allergic patients, because deglycosylation of this protein with trifluoromethanesulphonic acid abolished the binding of IgE from pooled and individual sera (Ahrazem, et al. 2006). In order to study this in more detail, orange peel extract, purified Cit s 1, remodelled forms of human transferrin carrying xylose or fucose (i.e., MMX, MMF and relevant MM controls carrying solely Man3GlcNAc2) and BSA neoglycoconjugates carrying bromelain glycopeptides with and without fucose (MUXF and MUX) on a xylosylated Man2GlcNAc2 structure were subject to Western blotting both with anti-horseradish peroxidase, as a model anti-carbohydrate antiserum, and with patients’ IgE. The specificity of anti-horseradish peroxidase has been examined in a number of studies and both xylose and core α1,3-fucose are known epitopes (Bencúrová, et al. 2004, Kurosaka, et al. 1991, Wilson, et al. 1998). Therefore, the MMX, MMF, MUXF and MUX neoglycoforms were employed as positive controls for the interaction with anti-horseradish peroxidase, whereas their use with patients’ IgE was intended to gain a first hint as to the contribution of xylose and fucose to the binding of IgE from orange-allergic patients. Coomassie staining (Figure 1A) was performed to check that the protein content of each sample was approximately equal.
Figure 1. SDS-PAGE and Western blotting of various glycoproteins.
Coomassie staining (A) or Western blotting with either anti-horseradish peroxidase followed by anti-rabbit IgG (B) or a pooled patients’ serum followed by anti-human IgE (C) were performed with the following protein samples: 1, orange peel extract; 2, purified Cit s 1; 3, MMX-transferrin; 4, MM-transferrin (no UDP-Xyl control); 5, MMF-transferrin; 6, MM-transferrin (no GDP-Fuc control); 7, bovine serum albumin; 8, horseradish peroxidase; 9, BSA-MUX; 10, BSA-MUXF. Definitions of glycoforms are given in the abbreviations and also in Figure 2.
The results indicate that anti-horseradish peroxidase reacts strongly with various orange glycoproteins and Cit s 1 (Figure 1B). As expected from previous data, transferrin carrying xylose or fucose (after remodelling with recombinant forms of either rice xylosyltransferase or nematode fucosyltransferase) reacted with anti-horseradish peroxidase (Bencúrová, et al. 2004, Fabini, et al. 2001), whereas the corresponding MM controls did not. On the other hand, all transferrin isoforms reacted with anti-human transferrin (data not shown). The two BSA neoglycoconjugates and horseradish peroxidase itself also reacted with this antibody as expected (Wilson, et al. 1998). With the patients’ serum pool, used in combination with a conjugated anti-human IgE for detection, a similar pattern of reactivity was observed (Figure 1C), indicating binding of IgE to both xylosylated and fucosylated glycans.
Specific IgE determination by direct and inhibition ELISA
Previous ELISA data indicated that horseradish peroxidase could inhibit the binding of patients’ sera to Cit s 1, whereas deglycosylated forms of Cit s 1 and horseradish peroxidase did not diminish this interaction (Ahrazem, et al. 2006); this suggests that the patients’ IgE displays glycan-dependent reactivity towards both Cit s 1 and horseradish peroxidase and no significant interaction with the Cit s 1 polypeptide. To examine the role of plant-like glycans further, the transferrin neoglycoforms and the BSA neoglyconjugates were used in various ELISA tests. In direct ELISA (Figure 2), the serum pool showed a strong reaction towards both the MUX and MUXF forms of BSA; three of four individual sera also showed significant reactivity towards both these neoglycoconjugates, which is compatible for a role at least for xylose, if not also for fucose, in IgE binding. As regards the transferrin neoglycoforms, a more complex picture emerged: the pool and the same three sera showed a reaction towards MMX; only one serum showed a significant reaction towards MMF.
Figure 2. Direct ELISA of glycoproteins with patients’ sera.
ELISA plates were coated with either purified Cit s 1, BSA-MUX, BSA-MUXF, MMX and MMF forms of transferrin (and corresponding controls) or BSA prior to probing with either (A) the pool of patients’ sera, two sera with no reaction to Cit s 1 and buffer or (B) the four individual patients’ sera displaying binding to Cit s 1. The degree of binding is expressed as the absorbance obtained at 490 nm after addition of the chromogenic substrate. (C) Explanation of the oligosaccharide abbreviations using the symbolic nomenclature of the Consortium for Functional Glycomics (http://www.functionalglycomics.org) with GlcNAc as a black square, mannose as a grey circle, fucose as a grey triangle and xylose as a white star; MM indicates the presence of two terminal α-mannoses, MU an unsubstituted 3-hydroxyl on the β-mannose, X a xylose and F a fucose.
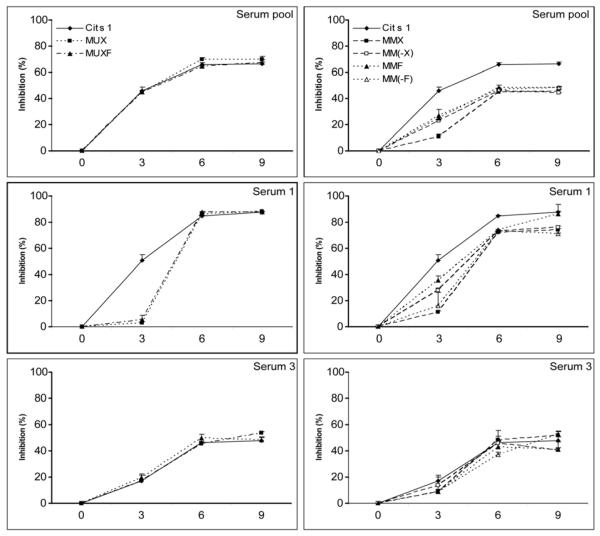
The binding characteristics of Cit s 1 with two of the sera, as well as the serum pool, were investigated further by inhibition ELISA (Figure 3). BSA-MUXF and BSAMUX were effective in inhibiting the binding of Cit s 1 to the sera and the pool; with serum 3 and the pool, these neoglycoconjugates were as effective as Cit s 1 at all concentrations used (left panel). With the transferrin glycoforms (right panel), the situation was somewhat more complex and it was obvious that, under the conditions used, all glycoforms possessed inhibitory activity regardless of the presence of the xylose or fucose residues. This appears contradictory to the other data (blot and direct ELISA), but may be explained by considering that inhibition with trimannosyloligosaccharides may be different from actual specific binding to xylose and fucose. Furthermore, the maximum level of inhibition when employing the native allergen is also attained with the highest neoglycoform concentration; this demonstrates that these reagents are approximately as effective as the allergen itself in inhibiting IgE binding.
Figure 3. Determination of inhibition of binding of IgE to Cit s 1.
Various concentrations (0 - 9 μg/ml) of either Cit s 1, BSA neoglycoconjugates (left panels) or transferrin neoglycoforms (right panels) were incubated with either the patients’ serum pool, serum 1 or serum 3 prior to binding to Cit s 1-coated ELISA plates and subsequent IgE detection. Sera 1 and 3 in the present study correspond to the sera 6 and 10 described by Ahrazem, et al. (2006); in the case of sera 3 in the present study, immunoblotting showed abolition of IgE binding upon deglycosylation of Cit s 1.
Mass spectrometric analysis
The data accumulated to date indicate that Cit s 1 may have a plant-type N-glycan, which is an IgE epitope, but actual glycoanalytical data was missing. Furthermore, the exact nature of the peptide was also unknown. Based solely on N-terminal sequencing, yielding the sequence TDPGHLQDVXVAINDPKXGVFVNRK (Ahrazem, et al. 2006, Crespo, et al. 2006), amino acid homology with a germin-like protein from pepper (Capsicum annuum, Genbank entry AY391748) was found; the pepper protein was previously found as a major allergen when using sera from patients with mugwort-birch-celery-spice syndrome (Leitner, et al. 1998). In order to molecularly define Cit s 1 more fully in terms of both glycan structure and peptide sequence, a mass spectrometric-based analysis was performed.
After the tryptic digestion of the protein, the peptides were initially analysed by LC-ESI-MS. After this run, two peptides were selected and MS-MS (collision induced dissociation, CID) experiments were carried out in order to determine the sequence for an ensuing database search. For this purpose, [M+H]+ ions of m/z 747.5 and 835.5 were fragmented. The analysis was performed in a so-called survey scan. This means that the analyser was scanning in a defined mass range and that, when an intense signal appeared, the mass analyser opens an additional channel and fragments this ion. In the case of 747.5 the analyser sought out not the tryptic fragment T5 itself, but instead selected an ion which was produced in the mass analyser by a loss of an N-terminal alanine; the sequence FQLDPK was determined (Figure 4A). For the second ion, the peptide T6 with the sequence DGVFVNGK was found (Figure 4B).
Figure 4. MSMS fragment spectra of two selected peptides of Cit s 1.
A, fragment spectrum of the [M+H]+ ion 747.39 with the assignment of the peaks to the sequence FQLDPK (the form of peptide T5 lacking one alanine residue). B, fragment spectrum of the [M+H)]+ ion 835.5 with the assignment of the peaks to the sequence DGVFVNGK (peptide T6).
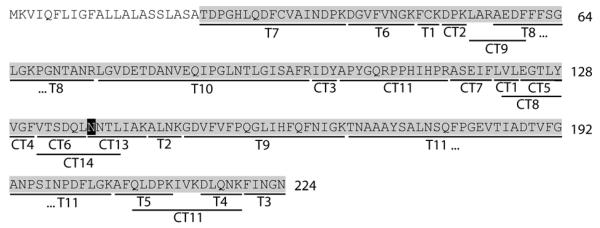
With these sequences and the results of the N-terminal sequencing, a tBLASTn search (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) was performed using the EST database. In this search, sections of a cDNA clone from the peel of Citrus sinensis (Genbank CK937230) were found. Both sequences matched exactly to this cDNA; therefore, the full reconstructed open reading frame was translated in silico (http://us.expasy.org/tools/dna.html) (Figure 5). Analysis of the putative full-length protein (224 residues) revealed that the previously-determined N-terminal sequence of Cit s 1 is probably the result of signal sequence cleavage. Without further posttranslational modification, the protein displays a molecular weight of 21943.79 (average mass); however, one potential N-glycosylation site appears in the sequence. The theoretical tryptic digest of the protein was then used to aid identification of the other peptides detected by LC-ESI-MS; the theoretical mass of each was compared to that observed (Table 1).
Figure 5. Translation of the putative Cit s 1 open reading frame.
The grey highlighted sequence could be confirmed by the MS analysis; the preceding sequence is assumed to be a cleaved signal sequence. The black highlighted asparagine corresponds to the single N-glycosylation site. Peptides T1-T11 are, in order to size, the observed tryptic peptides and peptides CT1-CT12 are those observed after digestion with both chymotrypsin and trypsin. CT13 and CT14 are glycopeptides observed after treatment of tryptic peptides with chymotrypsin.
Table 1. Detected tryptic fragments of the translated Cit s 1 protein.
Listed are those peptides predicted by the in silico digest of the expressed sequence tag open reading frame which could be detected by MS following the tryptic digest. The locations of peptides T1 - T10 within the Cit s 1 sequence are shown in Figure 5.
| Peptide | Sequence of tryptic fragment | [M+H]+ calc. | Predominant ion |
m/z theor. | m/z found | Ret. time [min] |
|---|---|---|---|---|---|---|
| T1 | FCK | 397.1904 | [M+H]+ | 397.1904 | 397.2409 | 28.96 |
| T2 | ALNK | 445.2769 | [M+H]+ | 445.2769 | 445.2050 | 25.50 |
| T3 | FINGN | 564.2776 | [M+H]+ | 564.2776 | 564.2913 | 21.84 |
| T4 | DLQNK | 617.3253 | [M+H]+ | 617.3253 | 617.3528 | 18.44 |
| T5 | AFQLDPK | 818.4406 | [M+H]+ | 818.4406 | 818.4667 | 28.00 |
| T6 | DGVFVNGK | 835.4309 | [M+H]+ | 835.4309 | 835.4840 | 24.98 |
| T7 | TDPGHLQDFCVAINDPK | 1869.8799 | [M+2H]2+ | 935.4436 | 935.5186 | 29.10 |
| T8 | AEDFFFSGLGKPGNTANR | 1927.9296 | [M+2H]2+ | 964.4684 | 964.4894 | 34.28 |
| T9 | GDVFVFPQGLIHFQFNIGK | 2163.1385 | [M+2H]2+ | 1082.0728 | 1082.1140 | 32.14 |
| T10 | LGVDETDANVEQIPGLNTLGISAFR | 2629.3467 | [M+3H]3+ | 877.1204 | 877.1707 | 38.21 |
| T11 | TNAAAYSALNSQFPGEVTIADTVFGANPSINPDFLGK | 3797.8656 | [M+3H]3+ | 1266.6267 | 1266.7316 | 40.57 |
The analysis of the tryptic peptides indicated that only the tryptic fragment with the potential N-glycosylation site was not detected. To determine if this was caused by the relatively high molecular mass of this peptide (3155.6874 Da) or because it is glycosylated, the tryptic peptides were further digested with chymotrypsin. The fragments which are important for the sequence coverage are shown in Table 2. An unglycosylated peptide containing the potential N-glycosylation site was not detected, which suggested that the occupancy of this site is complete. Indeed only glycosylated forms of this peptide were detected. Most prominently, a doubly-charged ion with m/z 1137.97, glycosylated with an MMXF structure, was found with the sequence VTSDQLNNTL (peptide CT14); an overlapping glycopeptide (CT13) was also found. As shown in Table 3, minor structures, other than MMXF, were also present.
Table 2. Detected tryptic/chymotryptic fragments of the translated Cit s 1 protein.
Peptides resulting from the sequential digestion with trypsin and chymotrypsin are listed which were detected and relevant for an increased sequence coverage. The locations of peptides CT1 - CT12 within the Cit s 1 sequence are shown in Figure 5.
| Peptide | Fragment sequence |
[M+H]+ calc. |
Predominant Ion |
m/z theor. |
m/z found |
Ret. time [min] |
|---|---|---|---|---|---|---|
| CT1 | LVL | 344.2544 | [M+H]+ | 344.2544 | 344.2494 | 31.25 |
| CT2 | DPK | 359.1925 | [M+H]+ | 359.1925 | 359.1686 | 27.85 |
| CT3 | IDY | 410.1922 | [M+H]+ | 410.1922 | 410.1895 | 27.92 |
| CT4 | YVGF | 485.2394 | [M+H]+ | 485.2394 | 485.2000 | 40.39 |
| CT5 | EGTLY | 582.2769 | [M+H]+ | 582.2769 | 582.2640 | 28.41 |
| CT6 | VTSDQL | 662.3355 | [M+H]+ | 662.3355 | 662.3419 | 28.69 |
| CT7 | ASEIFL | 679.3661 | [M+H]+ | 679.3661 | 679.3511 | 34.71 |
| CT8 | VLEGTLY | 794.4294 | [M+H]+ | 794.4294 | 794.4069 | 31.25 |
| CT9 | LARAEDF | 821.4152 | [M+H]+ | 821.4152 | 821.4072 | 28.41 |
| CT10 | VGFVTSDQL | 965.4938 | [M+2H]2+ | 483.2505 | 483.2319 | 32.77 |
| CT11 | APYGQRPPHIHPR | 1525.8134 | [M+2H]2+ | 763.4103 | 763.3263 | 29.86 |
| CT12 | QLDPKIVKDLQNK | 1538.8900 | [M+2H]2+ | 769.9486 | 769.9529 | 29.03 |
Table 3. Glycopeptides of Cit s 1.
Listed are the ions detected after combined trypsin/chymotrypsin treatment and their assignment to forms of the overlapping peptides NNTLIAK (CT13) and VTSDQLNNTL (CT14) modified with various N-glycan structures.
| Glycan structure | [M+H]+ calc. | Pre-dominant Ion |
m/z theor. | m/z found | Ret. time [min] |
|---|---|---|---|---|---|
| CT13 + MMXF | 1943.8690 | [M+2H]2+ | 972.4381 | 972.4393 | 27.16 |
| CT14 + MMXF | 2274.9704 | [M+2H]2+ | 1137.9889 | 1137.9729 | 28.41 |
| CT14 + MMX | 2128.9125 | [M+2H]2+ | 1064.9599 | 1064.9653 | 28.48 |
| CT14 + MMF | 2142.9282 | [M+2H]2+ | 1071.9677 | 1071.9607 | 28.41 |
| CT14 + GnMXF / MGnXF | 2478.0500 | [M+2H]2+ | 1239.5287 | 1239.5316 | 28.27 |
As a final confirmation for the presence of glycosylation, the aforementioned CT14 ion was fragmented by CID (see Figure 6). This tandem MS experiment gave fragments of the peptide alone as well as a set of fragments of the glycopeptide sequentially lacking the individual monosaccharide residues; the intact glycopeptide, which was the peptide VTSDQLNNTL modified with an N-linked MMXF glycan, was also observed. Fragments of the carbohydrate alone were detected between 204 and 822 Da. The glycopeptide and glycan fragments confirm the major N-glycan structure as being, as expected, MMXF. After treatment of the trypsin/chymotrypsin peptides with PNGase A, the deglycosylated peptide was also found with the sequence VTSDQLDNTL; the glycosylated Asn was converted to Asp. The observed m/z for the singly-charged ion was 1105.5687 as compared to the theoretical value of 1105.5372. Similarly, a deglycosylated form of CT13 (m/z 774.3902; DNTLIAK) was also observed in the PNGase A digest; thereby, the exact location of the single N-glycosylation site of Cit s 1 was verified.
Figure 6. CID analysis of the major glycopeptide of Cit s 1.
CID was performed on the form of the peptide VTSDQLNNTL (peptide CT14) putatively modified with an MMXF glycan; the glycan attached to the peptide could be fully sequenced (fragments with m/z between 1104 and 2279). Smaller fragments of the glycan alone were also observed with m/z between 204 and 822. The glycans are depicted using the nomenclature of the Consortium for Functional Glycomics (http://www.functionalglycomics.org); see also Figure 2.
DISCUSSION
The molecular analysis of allergens is a major focus of modern allergology and raises the possibility of more accurate diagnosis and therapy. In the case of plant and insect glycoproteins which bind patients’ IgE, cross-reactions are very common (Aalberse, et al. 1981, Batanero, et al. 1996, Hemmer, et al. 2001, Mari, et al. 1999, Tretter, et al. 1993); this is not unexpected, since the glycan is probably the most conserved aspect of these molecules. The presence of β1,2-xylose and core α1,3-fucose on N-glycans is more-or-less ubiquitous throughout the plant kingdom, whereas these moieties are absent from mammals, thus making them immunogenic. On the other hand, the matter as to whether cross-reactive carbohydrate epitopes are clinically significant has been a matter of controversy and debate (Aalberse, et al. 1997, Altmann 2007, Fötisch, et al. 2001, Van der Veen, et al. 1997). However, a number of cases show that glycans can contribute to the allergenicity of glycoproteins as measured by histamine release with, e.g., Api g 5, Cup a 1, Phl p 11 and Lyc e 2 (Bublin, et al. 2003, Foetisch, et al. 2003, Iacovacci, et al. 2002, Westphal, et al. 2003, Wicklein, et al. 2004) or skin prick tests with horseradish peroxidase (Mari 2002). Such examples of an actual biological activity are at variance with other studies on other glycoproteins, such as Phl p 1 (Wicklein, et al. 2004), which is not active in histamine release assays, or bromelain (Mari 2002), which generally does not elicit positive skin prick tests; it must be noted, though, that the ‘positive’ examples tend to be proteins carrying multiple glycans, while ‘negative’ examples, such as bromelain or Phl p 1, carry only a single N-glycan, and that multivalency is considered a pre-requisite for the antigen-induced cross-linking, of IgE bound to its receptors, necessary for histamine release. This would then imply that, for a solely anti-carbohydrate response, in vivo allergenicity requires that a protein will carry at least two glycan residues.
The major orange allergen, Cit s 1, has now been molecularly proven by us to possess, however, only a single N-glycan and examination of the tryptic and chymotryptic peptides facilitated the final definition of the glycosylation of this protein. The theoretical Mr of the unglycosylated mature protein is 21,943.79 (average mass); with an MMXF type N-glycan, the theoretical mass would be 23,114.56. Previous MALDI-TOF MS of the intact protein demonstrated the existence of three major species of 23,095, 23,298 and 23,504 Da (Ahrazem, et al. 2006); the first corresponds to the protein carrying MMXF, the second to a form carrying an additional non-reducing terminal GlcNAc residue (either the isomers MGnXF or GnMXF, depending on whether the α1,3- or α1,6-arm carries the non-reducing GlcNAc, or a mixture thereof). Thus, our glycopeptide MS data confirm this microheterogeneity of Cit s 1. However, there are, in addition, minor forms lacking either a fucose (MMX) or a xylose (MMF), but these perhaps occur in too low a concentration in order to be observed by mass spectrometry of the intact protein. These types of xylosylated and fucosylated glycan observed have been found on a number of allergens such as Api g 5 (Bublin, et al. 2003), Cup a 1 (Alisi, et al. 2001), Cyn d 24 (Chow, et al. 2005), Hev b 4 (Kolarich, et al. 2006), Lol p 11 (van Ree, et al. 2000), Lyc e 2 (Westphal, et al. 2003) and Phl p 1 (Wicklein, et al. 2004). Interestingly, other allergens have been found to possess only or predominantly solely xylosylated species such as MMX; examples of such allergens include Ara h 1 (Kolarich, et al. 2000, van Ree, et al. 2000), Ole e 1 (Kolarich, et al. 2000, van Ree, et al. 2000) and Cor a 11 (Lauer, et al. 2004). The presence of xylose and fucose residues on Cit s 1 explains its ability to be recognised by anti-horseradish peroxidase.
The matter of the cross-reactivity of IgE from the sera of patients allergic to orange is somewhat more complicated than the situation with the ‘model’ IgG anti-horseradish peroxidase. In one study, a monovalent MUXF glycopeptide (as opposed to the multivalent conjugates used in the present study) did not inhibit binding of IgE to Cit s 1 (Crespo, et al. 2006). Our data, though, indicate that the MUX and MUXF glycans attached to the BSA neoglycoconjugates have approximately the same potency as ligands for the patients’ IgE and as inhibitors of IgE binding to Cit s 1: thereby, it should be noted that there appears no particular bias towards recognition of fucose as being a major decisive element. Certainly, however, our inhibition data, as well as those derived from blots and ELISA previously showing that deglycosylated Cit s 1 does not possess IgE epitopes (Ahrazem, et al. 2006), strongly indicate that the glycan component is important for IgE binding to Cit s 1.
The direct binding to the transferrin neoglycoforms is potentially weaker than to the BSA-neoglycoconjugates - the former carry only two N-glycans and neither the MMF nor the MMF forms has both xylose and fucose; on the other hand, the BSA can carry many bromelain glycopeptides (on average 3-4) and so has a higher degree of valency (Wilson, et al. 1998). It has also been reported that the α1,3-mannose found on, e.g., MMX, may inhibit IgE binding in some cases as compared to glycans lacking this residue, e.g., MUX (van Ree, et al. 2000). Interestingly, in the case of inhibition experiments, it appears that even MM can prevent binding of IgE to Cit s 1, with approximately the same potency as MMX and MMF; thus, the trimannosyl core may, at a sufficient concentration, sterically hinder access by xylose and fucose-containing N-glycans to Cit s 1-specific IgE. This may be similar to the ability of MM to inhibit binding of the monoclonal YZ1/2.23, which recognises plant-type N-glycans, to MMX (Bencúrová, et al. 2004). Other previous data has suggested that the mannose residues do have a role in binding of antibodies to plant-type glycans, but that the trimannosyl core itself is insufficient for recognition by anti-horseradish peroxidase (Bencúrová, et al. 2004, Wilson, et al. 1998); on the other hand, a monoclonal antibody derived from Schistosoma-infected mice could bind MM and MMF equally (van Remoortere, et al. 2003). All our experiments suggest that there is no strong difference between those glycans carrying xylose alone (i.e., MMX and MUX) and those carrying either fucose alone (MMF) or both xylose and fucose (MUXF). Perhaps, there may be a pool of fucose-specific IgE and a more dominant pool of xylose-specific IgE in the sera tested. In some studies, fucose-specific IgE seems to predominate (Bencúrová, et al. 2004), although a role for xylose in IgE binding has been also observed in some cases (Bencúrová, et al. 2004, Garcia-Casado, et al. 1996, van Ree, et al. 2000).
The presence of a single N-glycan on Cit s 1 confers a monovalent status to the protein as regards binding of anti-carbohydrate IgE; together with the apparent lack of polypeptide epitopes, this constitutes an explanation of its previously-determined low in vivo allergenic activity and of its ‘equivocal’ status as an allergen (Ahrazem, et al. 2006). This is not merely an academic problem, since in vitro diagnostics can play a major role in deciding the course of treatment of allergic individuals. Falsely-identified positives can lead to overzealous avoidance measures for patients, with a consequent effect on their quality of life; analytical tools, showing whether a glycoallergen is multivalent and so potentially active in histamine release, are therefore a useful accompaniment to the usual laboratory tests. Cit s 1 is a paradigmatic example of a strong IgE-binding protein that can lead to false-positives in in vitro diagnosis, although the positive skin prick test responses in some patients suggest its potential clinical relevance in some subjects with orange allergy. Nevertheless, its full molecular characterisation opens the way for further studies to determine the role of this protein in complicating the laboratory-based diagnosis of which proteins are important in orange allergy and in facilitating the preparation of recombinant forms of this protein.
MATERIALS AND METHODS
Patients’ sera
A serum pool (n=10) and individual sera (n° 1-4) from orange-allergic patients, corresponding to patients 6, 8, 10 and 11 whose clinical characteristics have been previously described (Ahrazem, et al. 2006), were used for IgE immunodetection, specific IgE determination and ELISA-inhibition assays. All ten sera of the pool showed specific IgE to purified Cit s 1. Furthermore, as shown by Ahrazem et al. (2006), testing sera 2-4 with trifluoromethanesulphonic acid-deglycosylated forms of either Cit s 1 or horseradish peroxidase resulting in abolition of IgE binding as judged by blotting (sera 3 and 4), whereas in the case of serum 2, IgE binding to Cit s 1 in ELISA was inhibited ~80% by horseradish peroxidase.
Orange extract and purified glycoproteins
An orange peel extract was prepared as previously described (Ahrazem, et al. 2006), and its protein content quantified according to Bradford (Bradford 1976). Cit s 1 allergen was isolated as in Ahrazem et al. (2006) and quantified by the commercial bicinchoninic acid test (Pierce, Cheshire, UK). The model neoglycoconjugates, BSA-MUXF and BSA-MUX were prepared as previously described using, respectively, native and defucosylated bromelain glycopeptides (Wilson, et al. 1998). The remodelled forms of transferrin were produced by a modification of previously-published procedures (Bencúrová, et al. 2004, Fabini, et al. 2001) as follows: MMX-transferrin, carrying xylose on the core Man3GlcNAc2 pentasaccharide, was produced by serial incubation of human apo-transferrin with sialidase, galactosidase, a supernatant of Pichia expressing rice β1,2-xylosyltransferase (Léonard, et al. 2004) with UDP-Xyl and finally hexosaminidase; MMF-transferrin, carrying core α1,3-fucose, was produced similarly using sialidase, galactosidase, hexosaminidase and finally partially-purified Pichia-expressed Caenorhabditis elegans core α1,3-fucosyltransferase FUT-1 (Paschinger, et al. 2004) with GDP-Fuc. MM controls (−X or −F), carrying only the core pentasaccharide, were prepared in the presence of the relevant glycosyltransferase but in the absence of a nucleotide sugar donor. The presence of the transferred sugar was demonstrated by MALDI-TOF MS analysis of the tryptic peptides.
SDS-PAGE and immunodetection assays
Samples (20 μg of protein of crude orange extract and 3 μg of purified proteins) were separated by SDS-PAGE on a Bio-Rad Miniprotein II System (Bio-Rad, Hercules, Calif., USA) gels (12.5 or 15% polyacrylamide), following the method of Laemmli (Laemmli 1970), and then electrotransferred onto nitrocellulose membranes (Amersham Biosciences, Little Chalfont, UK, or Pall Corporation). After blocking, membranes were immunodetected with rabbit anti-horseradish peroxidase (Sigma; 1:20000 dilution) and then treated with a goat anti-rabbit IgG alkaline phosphatase-conjugated antibody (Vector Laboratories; 1:2000 dilution) prior to development with SigmaFAST NBT/BCIP. Alternatively, replica blocking membranes were incubated with a pool of sera from orange-allergic patients (1:4 dilution), then with mouse anti-human IgE monoclonal antibody HE-2 ascitic fluid (1:3000 dilution) (Sanchez-Madrid, et al. 1984), and finally with a rabbit anti-mouse IgG peroxidase-conjugated antibody (DAKO A/S; 1:5000 dilution) prior to enhanced chemiluminescence (Amersham Biosciences).
Specific IgE determination and ELISA-inhibition assays
Specific IgE binding to Cit s 1 and model glycoproteins was determined essentially by a method previously described using peroxidase-labeled anti-human IgE (DAKO) for detection (Diaz-Perales, et al. 2003). Cit s 1 and each purified glycoprotein were used as solid phase (3 μg/ml) and the serum pool and 4 individual sera (1:2 dilution) from orange-allergic patients were tested. BSA (1% in PBS buffer), dilution buffer, and 2 sera from patients with orange allergy showing positive specific IgE levels to orange lipid transfer protein (LTP) allergen Cit s 3 but negative to Cit s 1, were used as negative controls. ELISA-inhibition assays were carried out by the same method, except that the sera were preincubated with the appropriate inhibitor (3, 6 and 9 μg/ml) for 3 h at 25°C. All tests were performed in triplicate.
Tryptic digest
About 10 μg of the purified Cit s 1 protein were dried, dissolved in 15 μl 50 mM ammonium acetate, pH 8.4 and denatured for 10 minutes at 95 °C. After cooling to room temperature, 5 μl of a solution of 50 ng/μl trypsin (bovine pancreas; Sigma) were added and the sample was incubated overnight at 37 °C; the trypsin was then deactivated for 20 min at 95°C. For a single MS analysis about one-third of the sample was used.
Trypsin and chymotrypsin digest
An aliquot of the trypsin-digested peptides was further digested with chymotrypsin; dried tryptic peptides were dissolved in 15 μl 50 mM ammonium acetate in water, pH 8.4. Afterwards 5 μl of a 50 ng/μl α-chymotrypsin from bovine pancreas (Sigma) solution (ca. 200 μU) were added and the sample was incubated overnight at 37°C; the sample was heat-inactivated for 20 minutes at 95°C prior to analysis by LC-ESI-MS.
Deglycosylation of the tryptic / chymotryptic peptides
Peptides resulting from combined trypsin and chymotrypsin digestion were dried under reduced pressure in a vacuum centrifuge and dissolved in 20 μl 0.1 M citrate-phosphate buffer, pH 5.0. The deglycosylation was performed overnight with 0.15 mU of PNGase A from almonds (Altmann, et al. 1998). One-hundred μl of 1% acetic acid were then added and the peptides were purified using a Phenomenex Strata C18-E 50 mg cartridge previously washed with 75 % (v/v) aqueous acetonitrile and equilibrated with 1% acetic acid; the deglycosylated peptides were then collected by elution with 75% (v/v) aqueous acetonitrile, dried and dissolved in water.
LC-ESI-MS Analysis
The LC-ESI-MS experiments were carried out using a Q-TOF Ultima Global mass spectrometer (Micromass, Manchester, U.K.) equipped with an atmospheric pressure ionization electrospray interface and an upstream Micromass CapLC. The precolumn was a Thermo Aquastar C18 30 × 0.32 mm and the analytical column a Thermo BioBasic - 18 150 × 0.18 mm column. The flow rate was 2 μl/min, starting with 95% solvent A (aqueous 0.1% formic acid) and 5% solvent B (acetonitrile containing 0.1% formic acid). After loading the sample, the separation gradient (5-50% B; 7-33 minutes) was applied prior to washing the column with 60% solvent B for 5 minutes. The MS instrument was calibrated with [Glu1]-fibrinopeptide B in the mass range of 72-1285 amu. The sampling cone potential was 80 V and the capillary voltage 3.0 kV. The electrospray source temperature was 60 °C, and the desolvation temperature 120 °C. In the MS experiments mass spectra were scanned over the range m/z 100 - 1900. In the CID MSMS experiments the detection range was m/z 50 – 1990 and, for fragmentation, a collision energy of 35 V was used.
ACKNOWLEDGEMENTS
This work was partially supported by Fondo de Investigación Sanitaria, Ministerio de Salud y Consumo (grant RTIC-G03/094 and PI050375) and also in part by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (P18447). The authors also thank project students Claudiu Simionca and Adriane Winkler for helping prepare the transferrin neoglycoforms and Dr. Friedrich Altmann for access to the Micromass Q-TOF Global, whose purchase was made possible by a grant from the Austrian Rat für Forschung und Technologieentwicklung.
ABBREVIATIONS
- CID
collision-induced dissociation
- ELISA
enzyme-linked immunosorbent assay
- HRP
horseradish peroxidase
- LC-ESI-MS
liquid chromatography electrospray-ionisation mass spectrometry
- MM
Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc
- MMX
Manα1-6(Manα1-3)(Xylβ1-2)Manβ1-4GlcNAcβ1-4GlcNAc
- MMF
Manα1-6(Manα1-3) Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc
- MMXF
Manα1-6(Manα1-3)(Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc
- MUX
Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4GlcNAc
- MUXF
Manα1-6 (Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc
- Q-TOF
quadrupole time-of-flight
REFERENCES
- Aalberse RC, Koshte V, Clemens JGJ. Cross-reactions between vegetable foods, pollen and bee venom due to IgE antibodies to a ubiquitous carbohydrate determinant. Int Arch Allergy Appl Immunol. 1981;66(Suppl 1):259–260. [Google Scholar]
- Aalberse RC, Van Ree R. Crossreactive carbohydrate determinants. Clinical Reviews in Allergy and Immunology. 1997;15:375–387. doi: 10.1007/BF02737733. [DOI] [PubMed] [Google Scholar]
- Ahrazem O, Ibañez MD, Lopez-Torrejon G, Sanchez-Monge R, Sastre J, Lombardero M, Barber D, Salcedo G. Lipid transfer proteins and allergy to oranges. Int Arch Allergy Immunol. 2005;137:201–210. doi: 10.1159/000086332. [DOI] [PubMed] [Google Scholar]
- Ahrazem O, Ibañez MD, Lopez-Torrejon G, Sanchez-Monge R, Sastre J, Lombardero M, Barber D, Salcedo G. Orange germin-like glycoprotein Cit s 1: an equivocal allergen. Int Arch Allergy Immunol. 2006;139:96–103. doi: 10.1159/000090384. [DOI] [PubMed] [Google Scholar]
- Alisi C, Afferni A, Iacovacci P, Barletta B, Tinghino R, Butteroni C, Puggioni EMR, Wilson IBH, Federico R, Schininà ME, Ariano R, Di Felice G, Pini C. Rapid isolation, characterization, and glycan analysis of Cup a 1, the major allergen of Arizona cypress (Cupressus arizonica) pollen. Allergy. 2001;56:978–984. doi: 10.1034/j.1398-9995.2001.103125.x. [DOI] [PubMed] [Google Scholar]
- Altmann F. The Role of Protein Glycosylation in Allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- Altmann F, Paschinger K, Dalik T, Vorauer K. Characterisation of peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidase A and its N-glycans. Eur. J. Biochem. 1998;252:118–123. doi: 10.1046/j.1432-1327.1998.2520118.x. [DOI] [PubMed] [Google Scholar]
- Bardor M, Faveeuw C, Fitchette AC, Gilbert D, Galas L, Trottein F, Faye L, Lerouge P. Immunoreactivity in mammals of two typical plant glyco-epitopes, core α(1,3)-fucose and core xylose. Glycobiology. 2003;13:427–434. doi: 10.1093/glycob/cwg024. [DOI] [PubMed] [Google Scholar]
- Batanero E, Crespo JF, Monsalve RI, Martin-Esteban M, Villalba M, Rodríguez R. IgE-binding and histamine-release capabilities of the main carbohydrate component isolated from the major allergen of olive tree pollen, Ole e 1. J. Allergy Clin. Immunol. 1999;103:147–153. doi: 10.1016/s0091-6749(99)70538-5. [DOI] [PubMed] [Google Scholar]
- Batanero E, Villalba M, Monsalve RI, Rodríguez R. Cross-reactivity between the major allergen from olive pollen and unrelated glycoproteins: Evidence of an epitope in the glycan moiety of the allergen. J Allergy Clin Immunol. 1996;97:1264–1271. doi: 10.1016/s0091-6749(96)70194-x. [DOI] [PubMed] [Google Scholar]
- Bencúrová M, Hemmer W, Focke-Tejkl M, Wilson IBH, Altmann F. Specificity of IgG and IgE antibodies against plant and insect glycoprotein glycans determined with artificial glycoforms of human transferrin. Glycobiology. 2004;14:457–466. doi: 10.1093/glycob/cwh058. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bublin M, Radauer C, Wilson IBH, Kraft D, Scheiner O, Breiteneder H, Hoffmann-Sommergruber K. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J. 2003;17:1697–1699. doi: 10.1096/fj.02-0872fje. [DOI] [PubMed] [Google Scholar]
- Chow LP, Chiu LL, Khoo KH, Peng HJ, Yang SY, Huang SW, Su SN. Purification and structural analysis of the novel glycoprotein allergen Cyn d 24, a pathogenesis-related protein PR-1, from Bermuda grass pollen. FEBS J. 2005;272:6218–6227. doi: 10.1111/j.1742-4658.2005.05000.x. [DOI] [PubMed] [Google Scholar]
- Crespo JF, Retzek M, Foetisch K, Sierra-Maestro E, Cid-Sanchez AB, Pascual CY, Conti A, Feliu A, Rodriguez J, Vieths S, Scheurer S. Germin-like protein Cit s 1 and profilin Cit s 2 are major allergens in orange (Citrus sinensis) fruits. Mol Nutr Food Res. 2006;50:282–290. doi: 10.1002/mnfr.200500200. [DOI] [PubMed] [Google Scholar]
- Diaz-Perales A, Sanz ML, Garcia-Casado G, Sanchez-Monge R, Garcia-Selles FJ, Lombardero M, Polo F, Gamboa PM, Barber D, Salcedo G. Recombinant Pru p 3 and natural Pru p 3, a major peach allergen, show equivalent immunologic reactivity: a new tool for the diagnosis of fruit allergy. J Allergy Clin Immunol. 2003;111:628–633. doi: 10.1067/mai.2003.75. [DOI] [PubMed] [Google Scholar]
- Fabini G, Freilinger A, Altmann F, Wilson IBH. Identification of core α1,3-fucosylated glycans and the requisite fucosyltransferase in Drosophila melanogaster. Potential basis of the neural anti-horseradish peroxidase epitope. J. Biol. Chem. 2001;276:28058–28067. doi: 10.1074/jbc.M100573200. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Mallevaey T, Paschinger K, Wilson IBH, Fontaine J, Mollicone R, Oriol R, Altmann F, Lerouge P, Capron M, Trottein F. Schistosome N-glycans containing core α3-fucose and core β2-xylose epitopes are strong inducers of Th2 responses in mice. Eur. J. Immunol. 2003;33:1271–1281. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- Foetisch K, Westphal S, Lauer I, Retzek M, Altmann F, Kolarich D, Scheurer S, Vieths S. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111:889–896. doi: 10.1067/mai.2003.173. [DOI] [PubMed] [Google Scholar]
- Fötisch K, Vieths S. N- and O-linked oligosaccharides of allergenic glycoproteins. Glycoconj J. 2001;18:373–390. doi: 10.1023/a:1014860030380. [DOI] [PubMed] [Google Scholar]
- Garcia-Casado G, Sanchez-Monge R, Chrispeels MJ, Armentia A, Salcedo G, Gomez L. Role of complex asparagine-linked glycans in the allergenicity of plant glycoproteins. Glycobiology. 1996;6:471–477. doi: 10.1093/glycob/6.4.471. [DOI] [PubMed] [Google Scholar]
- Hemmer W, Focke M, Kolarich D, Wilson IBH, Altmann F, Wöhrl S, Götz M, Jarisch R. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honeybee and yellow jacket venom in patients with stinging-insect allergy. J. Allergy Clin. Immunol. 2001;108:1045–1052. doi: 10.1067/mai.2001.120013. [DOI] [PubMed] [Google Scholar]
- Iacovacci P, Afferni C, Butteroni C, Pironi L, Puggioni EM, Orlandi A, Barletta B, Tinghino R, Ariano R, Panzani RC, Di Felice G, Pini C. Comparison between the native glycosylated and the recombinant Cup a1 allergen: role of carbohydrates in the histamine release from basophils. Clin Exp Allergy. 2002;32:1620–1627. doi: 10.1046/j.1365-2222.2002.01516.x. [DOI] [PubMed] [Google Scholar]
- Ibañez MD, Sastre J, San Ireneo MM, Laso MT, Barber D, Lombardero M. Different patterns of allergen recognition in children allergic to orange. J Allergy Clin Immunol. 2004;113:175–177. doi: 10.1016/j.jaci.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Jin C, Bencúrová M, Borth N, Ferko B, Jensen-Jarolim E, Altmann F, Hantusch B. Immunoglobulin G specifically binding plant N-glycans with high affinity could be generated in rabbits but not in mice. Glycobiology. 2006;16:349–357. doi: 10.1093/glycob/cwj071. [DOI] [PubMed] [Google Scholar]
- Kolarich D, Altmann F. N-Glycan analysis by matrix-assisted laser desorption/ionisation mass spectrometry of electrophoretically separated nonmammalian proteins: application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal. Biochem. 2000;285:64–75. doi: 10.1006/abio.2000.4737. [DOI] [PubMed] [Google Scholar]
- Kolarich D, Altmann F, Sunderasan E. Structural analysis of the glycoprotein allergen Hev b 4 from natural rubber latex by mass spectrometry. Biochim Biophys Acta. 2006;1760:715–720. doi: 10.1016/j.bbagen.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Kurosaka A, Yano A, Itoh N, Kuroda Y, Nakagawa T, Kawasaki T. The structure of a neural specific carbohydrate epitope of horseradish peroxidase recognized by anti-horseradish peroxidase antiserum. J. Biol. Chem. 1991;266:4168–4172. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauer I, Foetisch K, Kolarich D, Ballmer-Weber BK, Conti A, Altmann F, Vieths S, Scheurer S. Hazelnut (Corylus avellana) vicilin Cor a 11: molecular characterization of a glycoprotein and its allergenic activity. Biochem J. 2004;383:327–334. doi: 10.1042/BJ20041062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter H, Mucha J, Staudacher E, Grimm R, Glössl J, Altmann F. Purification, cDNA cloning, and expression of GDP-L-Fuc:Asn- linked GlcNAc α1,3-fucosyltransferase from mung beans. J. Biol. Chem. 1999;274:21830–21839. doi: 10.1074/jbc.274.31.21830. [DOI] [PubMed] [Google Scholar]
- Leitner A, Jensen-Jarolim E, Grimm R, Wüthrich B, Ebner H, Scheiner O, Kraft D, Ebner C. Allergens in pepper and paprika. Immunologic investigation of the celery-birch-mugwort-spice syndrome. Allergy. 1998;53:36–41. doi: 10.1111/j.1398-9995.1998.tb03771.x. [DOI] [PubMed] [Google Scholar]
- Léonard R, Kolarich D, Paschinger K, Altmann F, Wilson IBH. A genetic and structural analysis of the N-glycosylation capabilities of rice and other monocotyledons. Plant Mol. Biol. 2004;55:631–644. doi: 10.1007/s11103-004-1558-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Torrejon G, Ibanez MD, Ahrazem O, Sanchez-Monge R, Sastre J, Lombardero M, Barber D, Salcedo G. Isolation, cloning and allergenic reactivity of natural profilin Cit s 2, a major orange allergen. Allergy. 2005;60:1424–1429. doi: 10.1111/j.1398-9995.2005.00903.x. [DOI] [PubMed] [Google Scholar]
- Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129:286–295. doi: 10.1159/000067591. [DOI] [PubMed] [Google Scholar]
- Mari A, Iacovacci P, Afferni C, Barletta B, Tinghino R, di Felice G, Pini C. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergic diseases. J Allergy Clin Immunol. 1999;103:1005–1011. doi: 10.1016/s0091-6749(99)70171-5. [DOI] [PubMed] [Google Scholar]
- Mulder H, Dideberg F, Schachter H, Spronk BA, De Jong-Brink M, Kamerling JP, Vliegenthart JFG. In the biosynthesis of N-glycans in connective tissue of the snail Lymnaea stagnalis of incorporation GlcNAc by β2GlcNAc-transferase I is an essential prerequisite for the action of β2GlcNAc-transferase II and β2Xyl-transferase. Eur J Biochem. 1995;232:272–283. doi: 10.1111/j.1432-1033.1995.tb20809.x. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Rendić D, Lochnit G, Jantsch V, Wilson IBH. Molecular basis of anti-horseradish peroxidase staining in Caenorhabditis elegans. J. Biol. Chem. 2004;279:49588–49598. doi: 10.1074/jbc.M408978200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F, Morago G, Corbi AL, Carreira J. Monoclonal antibodies to three distinct epitopes on human IgE: their use for determination of allergen-specific IgE. J Immunol Methods. 1984;73:367–378. doi: 10.1016/0022-1759(84)90412-5. [DOI] [PubMed] [Google Scholar]
- Strasser R, Mucha J, Mach L, Altmann F, Wilson IBH, Glössl J, Steinkellner H. Molecular cloning and functional expression of β1,2-xylosyltransferase cDNA from Arabidopsis thaliana. FEBS Lett. 2000;472:105–108. doi: 10.1016/s0014-5793(00)01443-5. [DOI] [PubMed] [Google Scholar]
- Tretter V, Altmann F, Kubelka V, März L, Becker WM. Fucose α1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int. Arch. Allergy Immunol. 1993;102:259–266. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- Van der Veen MJ, Van Ree R, Aalberse RC, Akkerdaas J, Koopelman SJ, Jansen HM, Van der Zee JS. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100:327–334. doi: 10.1016/s0091-6749(97)70245-8. [DOI] [PubMed] [Google Scholar]
- van Die I, Gomord V, Kooyman FNJ, van der Berg TK, Cummings RD, Vervelde L. Core α1→3-fucose is a common modifcation of N-glycans in parasitic helminths and constitutes an important epitope for IgE from Haemonchus contortus infected sheep. FEBS Lett. 1999;463:189–193. doi: 10.1016/s0014-5793(99)01508-2. [DOI] [PubMed] [Google Scholar]
- van Ree R, Cabanes-Macheteau M, Akkerdaas J, Milazzo JP, Loutelier-Bourhis C, Rayon C, Villalba M, Koppelman S, Aalberse R, Rodríguez R, Faye L, Lerouge P. β(1,2)-xylose and α(1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J. Biol. Chem. 2000;275:11451–11458. doi: 10.1074/jbc.275.15.11451. [DOI] [PubMed] [Google Scholar]
- van Remoortere A, Bank CMC, Nyame AK, Cummings RD, Deelder AM, van Die I. Schistosoma mansoni-infected mice produce antibodies that cross-react with plant, insect, and mammalian glycoproteins and recognize the truncated biantennary N-glycan Man3GlcNAc2-R. Glycobiology. 2003;13:217–225. doi: 10.1093/glycob/cwg025. [DOI] [PubMed] [Google Scholar]
- Westphal S, Kolarich D, Foetisch K, Lauer I, Altmann F, Conti A, Crespo JF, Rodriguez J, Enrique E, Vieths S, Scheurer S. Molecular characterization and allergenic activity of Lyc e 2 (β-fructofuranosidase), a glycosylated allergen of tomato. Eur J Biochem. 2003;270:1327–1337. doi: 10.1046/j.1432-1033.2003.03503.x. [DOI] [PubMed] [Google Scholar]
- Wicklein D, Lindner B, Moll H, Kolarich D, Altmann F, Becker WM, Petersen A. Carbohydrate moieties can induce mediator release: a detailed characterization of two major timothy grass pollen allergens. Biol Chem. 2004;385:397–407. doi: 10.1515/BC.2004.044. [DOI] [PubMed] [Google Scholar]
- Wilson IBH. Glycosylation of proteins in plants and invertebrates. Curr. Opin. Struc. Biol. 2002;12:569–577. doi: 10.1016/s0959-440x(02)00367-6. [DOI] [PubMed] [Google Scholar]
- Wilson IBH, Harthill JE, Mullin NP, Ashford DA, Altmann F. Core α1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology. 1998;8:651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]