Abstract
The complete small subunit rRNA (SSrRNA) gene sequence (2,142 nucleotides) of the freshwater fish trypanosome Trypanosoma ophiocephali Chen (1964) was determined. The phylogenetic analysis deduced using neighbor-joining, maximum parsimony, and Bayesian methods demonstrated the existence of an “aquatic clade”. T. ophiocephali was revealed to be a member of the freshwater fish trypanosomes and form the sister species with Trypanosoma siniperca and Trypanosoma sp. Carpio with high bootstrap values (98% MP, 100% NJ, 100% Bay). The high similarity of SSrRNA gene sequences and morphometric characters showed that T. ophiocephali, T. siniperca and T. sp. Carpio probably were the same species. The phylogenetic trees further suggested that Chinese freshwater fish trypanosome might be paraphyletic, and fish trypanosomes should have low host specificity.
Introduction
Piscine trypanosomes, transmitted by various bloodsucking aquatic leeches, are widespread parasites of fish (Lom 1979; Letch 1979; Gibson et al. 2005). Since Valentin first discovered a trypanosome from the blood of Salmo fario, more than 100 fish trypanosome species have been described worldwide, mostly on the basis of “one host–one species” paradigm, according to which a new species was assigned for every “new” fish host (Lom 1979). However, clear-cut evidence of strict host specificity is lacking, while several successful cross-transmission experiments have proved that particular trypanosome species are not restricted to the host species from which they were originally recovered (Lom 1973; Letch 1979; Woo and Black 1984). In addition, Karlsbakk et al. (2005) found a trypanosome species transmitted by the marine leech from Northern Norway showed large disparities in morphology and aspects during different infection time and Gu et al. (2007b) found at least two genotype trypanosomes occurred in Pseudobagras fulvidraco from Chinese Niushan Lake. Moreover, most of the early descriptions are simple and inadequate according to current morphological standards (Smit et al. 2004; Karbowiak et al. 2005; Gu et al. 2006a, 2007a, b) and can hardly be used for a safe determination of species. Therefore, the validity of most species should be questioned, and the taxonomic status and phylogenetic relationships of these species need to be revised. Many taxa are considered nomina dubia, and the taxonomic status of piscine trypanosomes remains generally controversial.
Molecular methods, in particular, the determination of small subunit rRNA (SSrRNA) sequences, have been commonly used to re-evaluate the phylogenetic relationships of trypanosomes in recent years (Jakes et al. 2001; Sehgal et al. 2001; Martin et al. 2002; Votypka et al. 2002, 2004; Gibson et al. 2005; Davies et al. 2005; Karlsbakk and Nylund 2006; Gu et al. 2007b). These studies provided many results inconsistent with those from traditional morphological and ontogenetic characters (Sehgal et al. 2001; Davies et al. 2005; Karlsbakk and Nylund 2006; Gu et al. 2007b).
Trypanosoma ophiocephali was previously described from the blood of Ophiocephalus argus captured from Liaoho River, Liaoning Province, and Hwa-ma Lake, Hubei Province, China, by Chen (1964) and Chen and Hsieh (1964), respectively. Recently, Gu et al. (2006a) redescribed this parasite from O. argus and Odontobutis obscura in Niushan Lake, Hubei Province, China, according to current morphological standards (Smit et al. 2004; Karbowiak et al. 2005; Gu et al. 2007a). As a comprehensive analysis of fish trypanosome phylogeny is being carried out in the author’s group, we have recently sequenced the SSrRNA gene of the trypanosomes from the blood of O. argus for the first time in order to determine its systematic position with molecular biological methods and provide more information on phylogenetic relationships within the fish trypanosomes. The results are supplied and discussed here.
Materials and methods
As described previously (Gu et al. 2006a, 2007a), host fishes were collected from Niushan Lake Fishery in Wuhan, Hubei Province (30°19′N, 114°31′E), China, in May 2007, and transported live to the laboratory. Fish samples were anesthetized, and whole blood was gained from the fish caudal vein. Then the flagellates were collected after centrifugation at 3,000×g for 10 min. Observations on living cells were carried out with light microscopy at ×200 magnification. The Giemsa staining method according to Karbowiak et al. (2005) was used to reveal the morphometric characters. Our result showed that these flagellates were morphologically identical to T. sp. III of T. ophiocephali described recently (Gu et al. 2006a). The terminology of morphometric parameters used in the present paper is the same as previously reported by Gu et al. (2006a).
Trypanosomes were isolated from host blood cells by centrifugation in Percoll density gradient and filtration through DEAE cellulose as described by Gu et al. (2006b). DNA was extracted as described by Lynn and Sogin (1988). Primers S-762 and S-763 (Malsov et al. 1996) were used for amplifying the trypanosome SSU rRNA gene. The PCR reaction steps, cloning, and sequencing of the SSrRNA gene with a set of M13 primers and “walking” primers were performed as described previously (Gu et al. 2007a).
The nucleotide sequences in the present paper are available from the GenBank/EMBL databases under the following accession numbers: Trypanosoma siniperca DQ494415, Trypanosoma cobitis AJ009143, Trypanosoma sp. EI-CP L14841, Ts-Se-BL AJ620550, Ts-Ab-Tb AJ620556, Trypanosoma granulosum UK AJ620551, Marv AJ620549, T. granulosum Portugal AJ620552, CLAR AJ620555, Ts-Tt-HOD AJ620553, R6 AJ620554, Trypanosoma boissoni U39580, Trypanosoma triglae U39584, Trypanosoma sp. K&A leech AJ009167, Trypanosoma chelodinae AF297086, Trypanosoma binneyi AJ132351, T. sp. Carpio EF375882, Trypanosoma sp. Pseudobagri EF375883, Trypanosoma sp. EF375884. Trypanosoma lewisi AJ223566, Trypanosoma theileri AB007814, and Trypanosoma avium U39578 were selected as the outgroup species.
All sequences were globally aligned with other SSrRNA gene sequences using the Clustal-X (Thompson et al. 1997) with default settings and then examined by eye. The postulated gaps and ambiguously aligned regions were excluded from phylogenetic analyses. As described previously (Gu et al. 2007a, b), phylogenetic trees were constructed by the neighbor-joining method, the maximum parsimony (MP) method and the Bayesian method, which implemented in the MEGA3 computer package (Kumar et al. 2004), PAUP* computer package (Swofford 2003) and MrBayes version 3.0b4 (Huelsenbeck and Ronquist 2001), respectively.
Results
The complete SSrRNA gene sequence of T. ophiocephali contained 2,142 nucleotides. The G+C content is 49.4%, and it is in the same range as other fish trypanosomes. The sequences obtained from T. ophiocephali and T. siniperca were nearly identical (structural similarity 99.99%), with the exception of one variable site and one gap between them. The sequence of T. ophiocephali differed in four nucleotides from the sequence of T. sp. Carpio, a trypanosome from carp from China (structural similarity 99.82%), while a total of 23 sites were different from that in T. sp. Pseudobagri, a slender form of trypanosome from P. fulvidraco (structural similarity 98.97%).
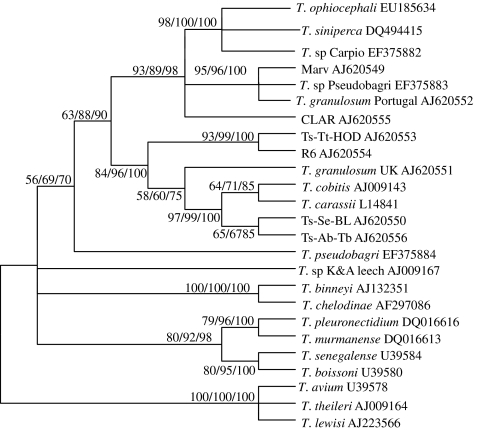
Phylogenetic analyses were based on the final edited alignment which was 2,226 bp in length and contained 25 taxa. The neighbor-joining analysis confirmed the clustering patterns of the maximum parsimony analysis and Bayes analysis in several cases with different bootstrap values (Fig. 1). SSrDNA neighbor-joining tree strongly supported the existence of an “aquatic clade” (Gibson et al. 2005) consisting of trypanosomes from aquatic vertebrates, transmitted by bloodsucking aquatic leeches. All the fish trypanosome isolates fell in the aquatic clade. Four subclades were supported in the aquatic clade: (1) freshwater fish trypanosomes, (2) an unknown species from an aquatic leech (Piscicola geometra) from UK (T. sp. K&A leech), (3) a clade containing two species from an aquatic tortoise (T. chelodinae) and duck-billed platypus (T. binneyi), and (4) marine fish trypanosomes containing two species from Senegal (T. boissoni and Trypanosoma senegalense) and two species from Norway (Trypanosoma pleuronectidium and Trypanosoma murmanense). T. ophiocephali was placed within the subclade of freshwater fish trypanosomes and formed a sister group with T. siniperca and T. sp. Carpio with high bootstrap values (98% MP, 100% NJ, and 100% Bay).
Fig. 1.
The neighbor-joining tree of aquatic trypanosomes constructed from complete small subunit ribosomal RNA (SSrRNA) sequences indicating the systematic position of T. ophiocephali and phylogenetic relationships among the aquatic trypanosomes whose sequences are available. T. lewisi, T. theileri, and T. avium are taken as the outgroup. Bootstrap values are shown for the maximum parsimony/neighbor-joining/Bayes analyses
Discussion
As demonstrated in the present and many other papers (e.g., Gibson et al. 2005; Karlsbakk and Nylund 2006; Gu et al. 2007a, b), the existence of the aquatic clade was strongly supported, and all freshwater fish trypanosomes grouped in a single clade. In our phylogenetic trees, freshwater fish trypanosomes were subdivided into two main groups, and those from China formed a subgroup, excepting that T. sp. Pseudobagri, a larger-form trypanosome from P. fulvidraco was placed at the bottom of freshwater fish trypanosome group and became a single isolate. This result was in consistent with the notion of Gu et al. (2007a, b) and revealed that Chinese freshwater fish trypanosome may be paraphyletic (Fig. 1). In addition, Karlsbakk and Nylund (2006) found T. pleuronectidium and T. murmanense from Norway and T. senegalense and T. boissoni from Senegal did not group together but formed respective clade, and both formed sister clades with freshwater fish trypanosomes. In the present study, all marine fish trypanosomes, though divided into two subclades, however, clustered together and then formed sister clade with freshwater fish trypanosomes (Fig. 1). Analyzing the different results between two papers, we thought the main reason was that a combination of insufficient taxa and an imbalance in the spread of included taxa, especially the absence of Chinese trypanosome species, affected the topology structure of trees in the research of Karlsbakk and Nylund (2006).
In our phylogenetic analysis based on the SSrRNA gene sequence, T. ophiocephali from the blood of O. argus fell within the freshwater fish trypanosome group and formed the sister species with T. siniperca and T. sp. Carpio with high bootstrap values (98% MP, 100% NJ, 100% Bay). Gu et al. (2007a, b) had compared the morphological characters of T. siniperca with T. ophiocephali and T. sp. Carpio, respectively, and found they shared many similar characteristics. Moreover, T. siniperca and T. sp. Carpio were considered to be a same species according to a comprehensive analysis of morphological and molecular data (Gu et al. 2007b). In this paper, we observed their movements, summarized the mensural morphometric values of blood trypomastigotes and found that there were greatly significant similarities among T. ophiocephali, T. siniperca, and T. sp. Carpio such as body length and width, nucleus length and width, PN KN distances. Furthermore, the SSrRNA gene sequences obtained from these trypanosomes were nearly identical (structural similarity 99.99%). Therefore, we concluded that T. ophiocephali, T. siniperca, and T. sp. Carpio may be the same species from same region and different hosts.
So far, more than 30 species of trypanosomes have been reported and described from Chinese freshwater fishes according to the traditional taxonomy (Chen and Hsieh 1964, Zhao 1998), but it is believed the actual number of species is much lower, and the freshwater fish trypanosome species richness in China may be exaggerated. Although we cannot make any conclusive statements about the levels of diversification of Chinese freshwater fish trypanosomes, the data in this study do provide the first molecular-based evidence that a single morphospecies of T. ophiocephali can infect at least three fish species. In addition, our previous study also provided the molecular evidence that a single fish species could be infected with more than one species of trypanosome (Gu et al. 2007b). This phenomenon can be caused by infections of leeches with different trypanosomes; but so far, no infected leech has been collected in freshwater from China.
Acknowledgement
This work was supported by the “Nature Science Foundation of China” (Project Numbers 30770261; 30800096), “New Teacher Foundation of Chinese Education Department” (Project Number 200805041025), and the Open Fund of State Key Laboratory of Freshwater Ecology and Biotechnology (Project Number 2008FB003). We are much grateful to senior research technologist W. S. Feng for his skilled technical assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Jianguo Wang, Phone: +86-27-68780720, FAX: +86-27-68780720, Email: wangjg@ihb.ac.cn.
Xiaoling Liu, Phone: +86-27-68780720, FAX: +86-27-68780720, Email: liuxl@mail.hzau.edu.cn.
References
- Chen CL. Parasitic flagellates of fishes from Liaoho River of China. In: Parasitic organisms of freshwater fish of China (Compiled edited by Institute of Hydrobiology Academia Sinica) Beijing: Agricultural Publishing House; 1964. [Google Scholar]
- Chen CL, Hsieh SR. Parasitic flagellates of fishes from Hwa-ma Lake. Acta Hydrobiol Sin. 1964;5:37–54. [Google Scholar]
- Davies AJ, Gibson W, Ferris VR, Basson L, Smit NJ. Two genotypic groups of morphologically similar fish trypanosomes from the Okavango Delta, Botswana. Dis Aquat Org. 2005;66:215–220. doi: 10.3354/dao066215. [DOI] [PubMed] [Google Scholar]
- Gibson WC, Lom J, Peckova H, Ferris VR, Hamilton PB. Phylogenetic analysis of freshwater fish trypanosomes from Europe using ssu rRNA gene sequences and random amplification of polymorphic DNA. Parasitology. 2005;130:405–412. doi: 10.1017/S0031182004006778. [DOI] [PubMed] [Google Scholar]
- Gu ZM, Wang JG, Zhang JY, Gong XN. Redescription of Trypanosoma ophiocephali Chen 1964 (Kinetoplastida: Trypanosomatina: Trypanosomatidae) and first record from the blood of dark sleeper (Odontobutis obscura Temminck and Schlegel) in China. Parasitol Res. 2006;100:149–154. doi: 10.1007/s00436-006-0247-3. [DOI] [PubMed] [Google Scholar]
- Gu ZM, Wang JG, Zhang JY, Gong XN. Isolation of Trypanosoma monopteri using Percoll reagent and DEAE-cellulose. Acta Hydrobiol Sin. 2006;30:204–208. [Google Scholar]
- Gu ZM, Wang JG, Li M, Zhang JY, Gong XN. Redescription of Trypanosoma siniperca Chang 1964 from freshwater fish of China based on morphological and molecular data. Parasitol Res. 2007;100:395–400. doi: 10.1007/s00436-006-0355-0. [DOI] [PubMed] [Google Scholar]
- Gu ZM, Wang JG, Li M, Zhang JY, Ke XL, Gong XN. Morphological and genetic differences of Trypanosoma in some Chinese freshwater fishes: difficulties of species identification. Parasitol Res. 2007;101:723–730. doi: 10.1007/s00436-007-0536-5. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jakes KA, O’Donoghue PJ, Adlard RD. Phylogenetic relationships of Trypanosoma chelodina and T. binneyi from Australian tortoises and platypuses inferred from small subunit rRNA analyses. Parasitology. 2001;123:483–487. doi: 10.1017/S0031182001008721. [DOI] [PubMed] [Google Scholar]
- Karbowiak G, Wita I, Rychlik L (2005) Trypanosoma (Megatrypanum) ornata sp.n., a parasite of the Eurasia Water Shrew Neomys fodiens (Pennant, 1771). Acta Protozool 44:363–367
- Karlsbakk E, Nylund A. Trypanosomes infecting cod Gadus morhua L. in the North Atlantic: a resurrection of T. pleuronectidium Robertson, 1906 and delimitation of T. murmanense Nikitin, 1927 (emend.), with a review of other trypanosomes from North Atlantic and Mediterranean teleosts. Syst Parasitol. 2006;65:175–203. doi: 10.1007/s11230-006-9049-3. [DOI] [PubMed] [Google Scholar]
- Karlsbakk E, Haugen E, Nylund A. Morphology and aspects of growth of a trypanosome transmitted by the marine leech Johanssonia arctica (Piscicolidae) from Northern Norway. Folia Parasitol. 2005;52:209–215. doi: 10.14411/fp.2005.028. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:50–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Letch CA. Host restriction, morphology and isoenzymes among trypanosomes of some British freshwater fishes. Parasitology. 1979;79:107–117. doi: 10.1017/S0031182000052008. [DOI] [Google Scholar]
- Lom J. Experimental infections of freshwater fishes with blood flagellates. J Protozool. 1973;20:537. [Google Scholar]
- Lom J. Biology of the trypanosomes and trypanoplasms of fish. In: Lumsden WHR, Evans DA, editors. Biology of the Kinetoplastida. Vol.2. London: Academic Press; 1979. pp. 269–337. [Google Scholar]
- Lynn DH, Sogin ML (1988) Assessment of the phylogenetic relationships among ciliated protests using partial ribosomal RNA sequences derived from reverse transcripts. Biosystems 21:249–254. [DOI] [PubMed]
- Malsov DA, Lukes J, Jirku M, Simpson L (1996) Phylogeny of trypanosomes as inferred from the small and large subunit rRNAs: implications for the evolution of parasitism in the trypanosomatid protozoa. Mol Biochem Parasitol 75:197–205 [DOI] [PubMed]
- Martin DS, Wright ADG, Barta JR, Desser SS. Phylogenetic position of the giant anuran trypanosomes Trypanosoma chattoni, T. fallisi, T. mega, T. neveulemaigei, and T. ranarum inferred from 18S rRNA gene sequences. J Parasitol. 2002;88:566–571. doi: 10.1645/0022-3395(2002)088[0566:PPOTGA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sehgal RNM, Jones HI, Smith TB. Host specificity and incidence of Trypanosoma in some African rainforest birds: a molecular approach. Mol Ecol. 2001;10:2319–2327. doi: 10.1046/j.1365-294X.2001.01339.x. [DOI] [PubMed] [Google Scholar]
- Smit NJ, Van As JG, Davies AJ (2004) Fish trypanosomes from the Okavango Delta, Botswana. Folia Parasitol 51:299–303 [DOI] [PubMed]
- Swofford DL. PAUP* Phylogenetic analysis using parsimony (*and other methods) Sunderland: Sinauer Associate; 2003. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votypka J, Obornik M, Volf P, Svobodova M, Lukes J. Trypanosoma avium of raptors (Falconiformes): phylogeny and identification of vectors. Parasitology. 2002;125:253–263. doi: 10.1017/S0031182002002093. [DOI] [PubMed] [Google Scholar]
- Votypka J, Lukes J, Obornik M. Phylogenetic relationship of Trypanosoma corvi with other avian trypanosomes. Acta Protozool. 2004;43:225–231. [Google Scholar]
- Woo PTK, Black GA. Trypanosoma danilewskyi: host specificity and host’s effect on morphometrics. J Parasitol. 1984;70:788–793. doi: 10.2307/3281762. [DOI] [PubMed] [Google Scholar]
- Zhao YJ. Parasitic flagellates of freshwater fishes from Sichuan province of China. Acta Hydrobiol Sin. 1998;22:114–119. [Google Scholar]