Abstract
Background
At 6 months poststroke, most patients cannot incorporate their affected hand into daily activities, which in turn is likely to reduce their perceived quality of life.
Objective
This preliminary study explored change in patient-reported, health-related quality of life associated with robotic-assisted therapy combined with reduced therapist-supervised training.
Design and Setting
A single-blind, multi-site, randomized clinical trial was conducted.
Participants
Seventeen individuals who were 3 to 9 months poststroke participated.
Intervention
Sixty hours of therapist-supervised repetitive task practice (RTP) was compared with 30 hours of RTP combined with 30 hours of robotic-assisted therapy.
Measurements
Participants completed the Stroke Impact Scale (SIS) at baseline, immediately postintervention, and 2 months postintervention. Change in SIS score domains was assessed in a mixed model analysis.
Results
The combined therapy group had a greater increase in rating of mood from preintervention to postintervention, and the RTP-only group had a greater increase in rating of social participation from preintervention to follow-up. Both groups had statistically significant improvement in activities of daily living and instrumental activities of daily living scores from preintervention to postintervention. Both groups reported significant improvement in hand function postintervention and at follow-up, and the magnitude of these changes suggested clinical significance. The combined therapy group had significant improvements in stroke recovery rating postintervention and at follow-up, which appeared clinically significant; this also was true for stroke recovery rating from preintervention to follow-up in the RTP-only group.
Limitations
Outcomes of 30 hours of RTP in the absence of robotic-assisted therapy remain unknown.
Conclusion
Robotic-assisted therapy may be an effective alternative or adjunct to the delivery of intensive task practice interventions to enhance hand function recovery in patients with stroke.
Stroke is the most prevalent cause of adult-onset disability in the United States. An estimated 5.8 million people who have had a stroke have residual neurological deficits.1,2 At 6 months poststroke, about 65% of patients cannot incorporate their affected hand into their usual activities,1 a limitation of distal motor function that understandably is associated with reduced perception of quality of life.3 There has been limited research on quality of life and rehabilitation therapies among people with conditions such as stroke that limit mobility.
Repetitive task practice (RTP) strategies, developed for use in constraint-induced movement therapy (CIMT), can improve hand function and individuals’ perception of their health-related quality of life (HRQOL).4 Support for the use of RTP as a fundamental basis for retraining upper-extremity function has been addressed in 2 excellent reviews.5,6 Briefly, RTP consists of breaking a task down into specific segments. These segments then are practiced individually and require successful completion before the entire task is “put together.” The activities the patient performs and the feedback provided are consistent with models of a massed RTP practice schedule.7
Repetitive task practice–based interventions, however, are labor-intensive therapies. Robotic devices continue to improve in design, control, and usability and may offer a solution to decrease the therapist time demands necessary for the delivery of RTP-type interventions. The ability of several device-oriented approaches to facilitate improvement in movement capability among patients following stroke has been explored.8–11 In this regard, sophisticated upper-extremity robotic systems (eg, MIT-Manus*) have been developed.8,12,13 The vast majority of upper-extremity robotic systems (eg, MIT-Manus/InMotion 2.0,* GENTILE/s,† MIME,‡ and others) are focused on improving gross reaching movements of the upper extremity.14–18 These devices have shown effectiveness in improving proximal upper-extremity function and motor control10 and have yielded useful data for understanding recovery mechanisms.8,9
A systematic review of the rehabilitation literature indicates strong evidence that intensity and task specificity are primary indicators of an effective treatment program following stroke.19,20 In addition, training should be repetitive, functional, meaningful, and challenging to the patient; these are characteristics of task practice interventions that have been shown to improve upper-extremity function in patients with stroke.4,21,22 Despite their effectiveness, widespread implementation of RTP interventions in their current forms faces substantial obstacles (primarily cost of delivering the service and the duration of each session) that limit the potential for clinical acceptance. The use of a robotic device as a therapeutic adjunct to task practice is appealing because this approach may enhance the recovery process and potentially decrease the time spent in therapist-directed task practice.
The robotic device used in this project focuses on improving distal motor function by enhancing active range of motion (AROM) of the wrist and fingers and reducing spasticity (exaggerated reflex response to slow or fast stretch of wrist or finger flexor muscles) about the wrist. The device is based on principles of motor learning, as it engages and provides feedback to the individual during the performance of repetitive activities that are intended to transfer to functional distal motor activities. The robotic device provides consistent and precise therapy for long durations without fatigue. Previous studies have shown that the quantity and quality of afferent information are greater during consistent and larger-amplitude movements.23–25 The resultant increase in the quantity and quality of afferent information provided to the patient during robotic device use may facilitate motor learning or relearning. Augmenting the motor learning or relearning processes with a robotic device may allow for a reduction in the duration of therapist-directed RTP without compromising improvements in motor function or HRQOL in patients with stroke.
A meta-analysis of robot-assisted therapy effects on upper-limb function in patients with stroke concluded that robotic therapy typically improves proximal limb control.18,26 However, there is limited clinical acceptance of robot-assisted therapy.9 Although the motor effects of robotic-assisted therapy are beginning to be documented, there is little evidence to date about the effects on patient-assessed HRQOL when robotic-assisted therapy is used in conjunction with an RTP intervention.
This article reports HRQOL outcomes that were gathered as part of a preliminary randomized clinical trial. The primary aim of the clinical trial was to determine the clinical effectiveness of using a distally focused robotic device in conjunction with an abbreviated duration of RTP to improve upper-extremity motor function in 2 groups of patients with stroke: (1) an RTP-only group whose participants received 60 hours of therapist-supervised RTP and (2) a combined therapy group whose participants received 30 hours of robotic-assisted therapy and 30 hours of therapist-supervised RTP. The well-validated Stroke Impact Scale (SIS)27 was used as the primary outcome measure to assess HRQOL. This preliminary study was conducted to estimate the magnitude of change and standard error between the 2 treatment groups in order to determine the sample size needed for a larger randomized clinical trial. Based on preliminary data that suggested HRQOL benefits associated with the combined therapy, we wanted to explore the relative HRQOL benefits of receiving therapist-supervised RTP only compared with receiving combined therapy among patients with reduced hand function secondary to stroke.
Method
Design Overview
A prospective, parallel group, randomized clinical trial with blinded assessments was conducted. All participants gave written informed consent prior to inclusion in the study.
Setting and Participants
A total of 109 patients with subacute stroke were assessed for eligibility. Participant eligibility criteria included: first clinical stroke diagnosis; status 3 to 9 months poststroke; Mini-Mental Status Examination28 score of >24; ability to stand independently for 2 minutes; passive range of motion (PROM) of ≥45 degrees for abduction, flexion, or external rotation of the shoulder or pronation of the forearm; active extension of the wrist of ≥10 degrees; active extension of the metacarpophalangeal and interphalangeal joints of the thumb; and ≥10 degrees of extension in at least 2 additional digits. Of the 109 patients assessed, 72 did not meet the inclusion criteria (in most cases due to overly high or low functional levels based on the specified criteria), 4 refused to participate, and 12 were excluded for other reasons (eg, lack of transportation, anticipated move to another area). Twenty-one patients with stroke were recruited at Emory University and at the Cleveland Clinic Foundation. Three patients withdrew after randomization due to transportation difficulties, and 1 patient did not complete the 2-month follow-up evaluation. Twelve patients completed training and evaluation at Emory University, and 5 patients were trained and evaluated at the Cleveland Clinic.
Randomization and Interventions
After screening by the study coordinator, participants were randomly assigned by the sealed envelope method to receive either 60 hours of RTP or 30 hours of RTP + 30 hours of robotic-assisted (ie, Hand Mentor§ [HM]) therapy over the course of 3 weeks. Therapists and evaluators had occupational or physical therapy backgrounds. Staff at the 2 research sites were trained to deliver standardized treatment and patient evaluation procedures. Research staff blinded to treatment assignment conducted interview-based outcome assessments. The participants were not blinded to their assignment but were not informed of the study hypotheses or primary outcome measures.
The RTP tasks were selected by each participant, in collaboration with the trainer, on the basis of personal preference, relevance, and interest. Most importantly, the tasks were selected so that the participant was challenged, rather than simply completing activities because of their apparent ease. Impairment training was not part of the RTP protocol for either group. Some typical activities were: ironing, potting a plant, holding cards, and so on. In the present context, the variables manipulated were related to the temporal and spatial domains for task completion. Upon selection, the tasks were broken into segments that required successful completion before the entire task was “put together.” For example, for the activity of potting a plant, the first component was reaching to grasp and release a hand shovel, then raising the shovel to the top of a pail and placing it back on the table. These individual actions were reinforced through RTP. Ultimately, the entire complement of movements included shoveling dirt into the pot and placing seedlings or plant stems in the pot. As can be deduced, this sequence placed progressively greater demands on multijoint control and sequencing. Improvements in spatial control were accomplished by having the therapist progressively move the object farther from the participant, thus imposing greater joint ranges of motion. Temporal domain elements were engaged by requiring the participant to repeat the task components or total task activity as frequently as possible during a defined time interval. The activities that the participant performed and the feedback provided were consistent with models of a massed RTP practice schedule.29 An RTP approach in which summary feedback is provided avoids potential complications related to feedback type and schedule for patients with stroke, especially as the optimal feedback schema for this population is unknown.
Participants in the combined therapy group divided their time equally between the RTP activities with a therapist and supervised HM use. The selection of RTP tasks for this group was identical to task selection for the RTP-only group, and all RTP training was delivered in the same manner (eg, increasing temporal and spatial requirements of the activity over the course of practice based on the participants’ performance). Participants did not wear or use the HM during the performance of any RTP activities.
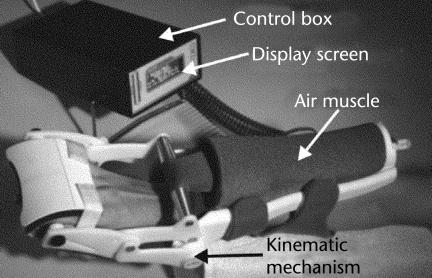
The HM and its components are illustrated in Figure 1, and the system has been described in detail previously.30 Briefly, an air muscle (pneumatic actuator) provides the force necessary to extend the wrist and resist wrist flexion. Activation of the air muscle rotates a bar about a pivot point positioned in line with the axis of rotation about the wrist. This action extends the wrist and fingers. Wrist extension position is measured by a potentiometer, which is centered in-line with the pivot point and the axis of wrist flexion. Resistance to wrist flexion is measured by force-sensitive resistors (FSRs). The FSR output is a measure of the resistance offered by multijoint stiffness attributable to changes in viscoelastic properties and muscle length stretch sensitivity in both the finger and wrist flexor muscle groups. Excessive force on the hand is prevented in several ways. A microcompressor has a maximum output pressure of 28 psi, thus limiting the maximum force produced by the air muscle. To prevent excessive extension of the wrist, a physical stop also is provided that limits motion of the activation bar at 60 degrees of wrist extension. The HM provided biofeedback to the participant during various activities requiring varying levels of wrist control. The therapist monitored HM usage and provided assistance in the donning and doffing of the device. The therapist also adjusted difficulty levels based on participant performance and comfort level.
Figure 1.
Hand Mentor robotic system and its components.
The primary goal of the HM is to improve AROM about the wrist and fingers (flexion-extension), wrist control, and initiation of distal movement. The 3 HM protocols used were: motor control, recruitment, and spasticity reduction.30,31
Briefly, the goal of the motor control protocol was to increase AROM at the wrist. The participants started with the wrist in a neutral position. Real-time wrist position was represented by a horizontal row of light-emitting diodes (LEDs) on the control box. Participants were instructed, via the ARM control box, to extend their wrist to the target LED. Once that target was achieved, the participants actively performed a wrist flexion, past neutral, to obtain the wrist flexion target. There were 2 motor control modes: a wrist flexion-extension mode and an extension-flexion mode. If a participant was unable to achieve the wrist extension goal, the air muscle would be activated and slowly extend the wrist to the target position. The difficulty of the task (eg, range of motion necessary to reach the LED targets) was systematically increased as a participant had multiple consecutive successful trials. By changing the sensitivity, success across the difficulty levels encapsulated a wrist AROM between 11 and 76 degrees.
The aim of the recruitment protocol was to increase active wrist extensor muscle activity via feedback and assistive motion of the fingers and wrist. The participants were asked to relax their wrist and hand; this point was considered the initial starting position. Participants had to actively extend their hand and wrist to their maximum and hold their hand and wrist at that position for approximately 10 seconds. Over the next 20 seconds, the air muscle was inflated; during this time the fingers and wrist were extended to 60 degrees (or less if significant flexor tone [velocity-dependent resistance to stretch] was present). The resulting position was held for 10 seconds, after which air pressure was released and the hand and fingers returned to the initial position. This process was repeated for 10 to 20 cycles. The electromyographic (EMG) activity of the wrist extensors was presented as a line in the LED display. After each cycle, participants were encouraged to increase the height of the EMG and position lines on subsequent cycles using visual biofeedback.
The aim of the spasticity protocol was to decrease flexor tone of the fingers and wrist via feedback and assistive motion of the fingers and wrist. The initial position of the hand was at approximately 30 degrees of flexion. The air muscle was inflated to bring the wrist to approximately half of the participants' available PROM. The amount of force necessary to achieve this position was measured by FSRs within the ARM device while a potentiometer provided wrist position. A flexor stiffness ratio (ratio of force to position) was calculated and displayed on the LCD graph, and the participants’ goal was to decrease the amount of wrist flexor activity during the next 60 seconds. If flexor activity was reduced, the height (number) of illuminated LEDs, which was scaled to each participant, also decreased while wrist extension increased. After 60 seconds, the air pressure was released from the air muscle, and the wrist returned to its initial flexed position. Participants were encouraged to decrease the height of the line through relaxation on subsequent trials. They started in the easiest mode (ie, least amount of active wrist extension and flexion necessary to achieve the goal) and progressed to more difficult levels (ie, more active control of wrist extension and flexion) after consistently achieving an 85% success rate on a specific protocol.
Participants in both groups adhered to all procedures and protocols and completed all of the training sessions. No adverse events were reported.
Outcome Measures
Data for the outcome measures were collected before the intervention, immediately postintervention, and 2 months postintervention.
Primary outcome measure.
Health-related quality of life was assessed by the 8 subscales and overall stroke recovery rating of the Stroke Impact Scale (SIS) version 3.0.32 The SIS version 3.0 comprises 59 items and assesses the domains of strength, memory/thinking, mood, communication, activities of daily living/instrumental activities of daily living (ADL/IADL), mobility, hand function, and social participation. An overall rating of stroke recovery also is included. Each domain contains a general description of the type of questions that follow and a statement with a reference to a specific time period (1, 2, or 4 weeks). Respondents score their performance on a 5-point scale (eg, “no strength” to “a lot of strength”; “none of the time” to “all of the time”). Duncan et al27 have shown the SIS to be valid, reliable, and sensitive to change, and other investigators also have concluded that the SIS has good psychometric properties.33 A 10- to 15-point change in a domain score may represent a clinically significant change.34
Covariates.
Symptoms of depression are prevalent among patients who have had a stroke.35 Depressed mood was assessed by the 20-item Center for Epidemiologic Studies–Depression (CES-D) Scale.36 Additional covariates included in the analyses were age, sex, and race. Depressed mood, age, sex, and race influence reported HRQOL.3,33 Although the randomization process may ensure that intervention groups are not significantly different on these variables at baseline, with a small sample size, it is difficult to ensure that intervention groups are balanced with respect to these characteristics.
Follow-up.
Follow-up was at 2 months postintervention for both groups.
Sample Size
Data to determine sample size were derived from an analysis of grasping kinetics among patients who participated in a previous CIMT study,4 in which an effect size of 1.0 was present from pretreatment to posttreatment. Using 1.0 as the estimated effect size, with a sample of 8 participants in the combined therapy group, pretreatment to posttreatment change could be detected with power of 0.68 using a 2-sided significance level of .05. In addition, assuming the same effect size, the test for time × treatment interaction effects (with treatment being either combined therapy or RTP alone) had power of 0.67 using a 2-sided significance level of .05 with 8 participants per group.
Data Analysis
Internal consistency reliability of each of the 8 SIS domains was evaluated by Cronbach alpha. All domains had adequate reliability (alpha exceeded 0.7). Baseline characteristics of the 2 intervention groups were compared by t test (continuous variables) and chi-square analysis (categorical variables). The HRQOL outcomes for the 2 groups, as measured by average change in SIS scores, were examined in a mixed-effects model, with random effects for patient and the estimate of interest being the time × treatment interaction. The 2 factors—treatment (RTP only, combined therapy) and time (preintervention, postintervention, and follow-up)—were included in the model, and the average response for each combination was modeled. The SIS scores were adjusted for participants’ age, sex, race, and baseline CES-D Scale score. Analyses were performed using SAS proc MIXED in SAS version 9.0.∥
Role of the Funding Source
This study was funded by a grant (R21 HD057020) from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH), to Dr Alberts. The study sponsor had no role in the study's conduct or reporting. Recommendations received from NIH reviewers in the application phase were taken into consideration in the study design.
Results
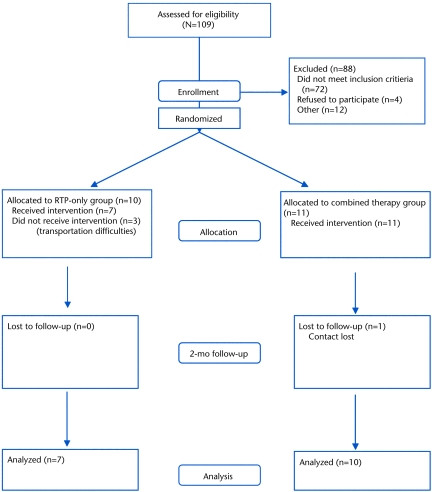
Figure 2 details the recruitment and passage of participants through the trial. At 2 months postintervention, 7 participants in the RTP-only group and 10 participants in the combined therapy group had successfully completed the study.
Figure 2.
Flow of participants through the trial. RTP=repetitive task practice.
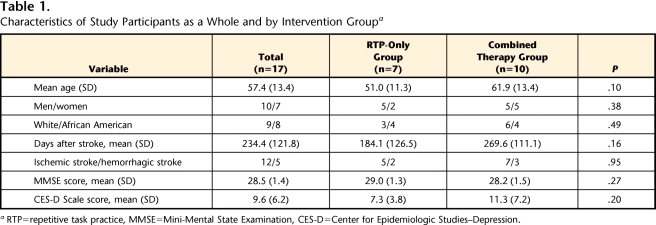
Randomization resulted in a distribution of patient demographic and clinical characteristics that was not statistically different at baseline for the 2 intervention groups (Tab. 1). The average age of the participants who were randomly assigned to the RTP-only group was 51 years, and the average age of the participants who were randomly assigned to the combined therapy group was approximately 62 years (P=.10). Participants in the RTP-only group were, on average, 184.1 days poststroke, and participants in the combined therapy group were, on average, 269.6 days poststroke (P=.16). Average CES-D Scale baseline scores for participants in the 2 groups did not differ significantly (P=.20) and were below the suggested cutoff (16) for possible clinical depression.36 As measured by the Fugl-Meyer Motor Assessment (maximum score=66),37 participants were similar in severity of upper-limb motor impairment at baseline, with a mean (SD) score of 39.4 (6.6) in the RTP-only group and 33.6 (9.7) in the combined therapy group.
Table 1.
Characteristics of Study Participants as a Whole and by Intervention Groupa
RTP=repetitive task practice, MMSE=Mini-Mental State Examination, CES-D=Center for Epidemiologic Studies–Depression.
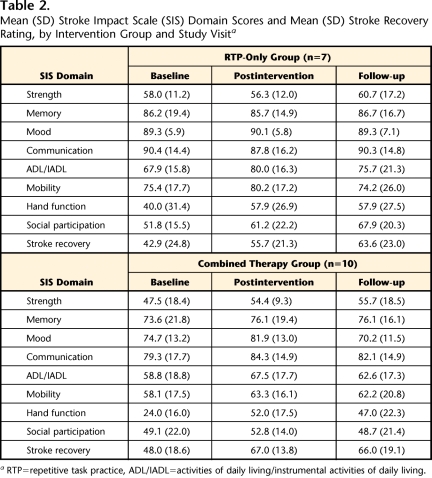
Mean (SD) SIS scores for participants in the 2 groups at baseline, postintervention, and 2-month follow-up are shown in Table 2. Baseline SIS scores were not significantly different for the participants in the 2 treatment groups.
Table 2.
Mean (SD) Stroke Impact Scale (SIS) Domain Scores and Mean (SD) Stroke Recovery Rating, by Intervention Group and Study Visita
RTP=repetitive task practice, ADL/IADL=activities of daily living/instrumental activities of daily living.
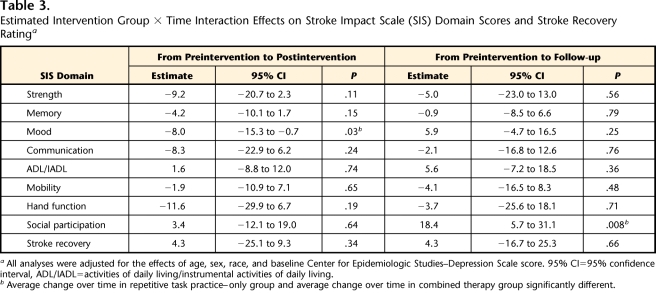
When average SIS score changes observed in the 2 groups were compared, group × time interaction effects were evident for 2 SIS domains (Tab. 3). First, compared with average change in mood ratings in the RTP-only group, the average change in mood ratings in the combined therapy group was greater from preintervention to postintervention. The estimated effect was −8.0 (P=.03). Second, compared with average change in social participation ratings in the combined therapy group, the average change in social participation ratings in the RTP-only group was greater from preintervention to follow-up. The estimated effect was 18.4 (P=.008). The data reported in Table 2 provide information about the nature of these changes in the 2 groups.
Table 3.
Estimated Intervention Group × Time Interaction Effects on Stroke Impact Scale (SIS) Domain Scores and Stroke Recovery Ratinga
All analyses were adjusted for the effects of age, sex, race, and baseline Center for Epidemiologic Studies–Depression Scale score. 95% CI=95% confidence interval, ADL/IADL=activities of daily living/instrumental activities of daily living.
b Average change over time in repetitive task practice–only group and average change over time in combined therapy group significantly different.
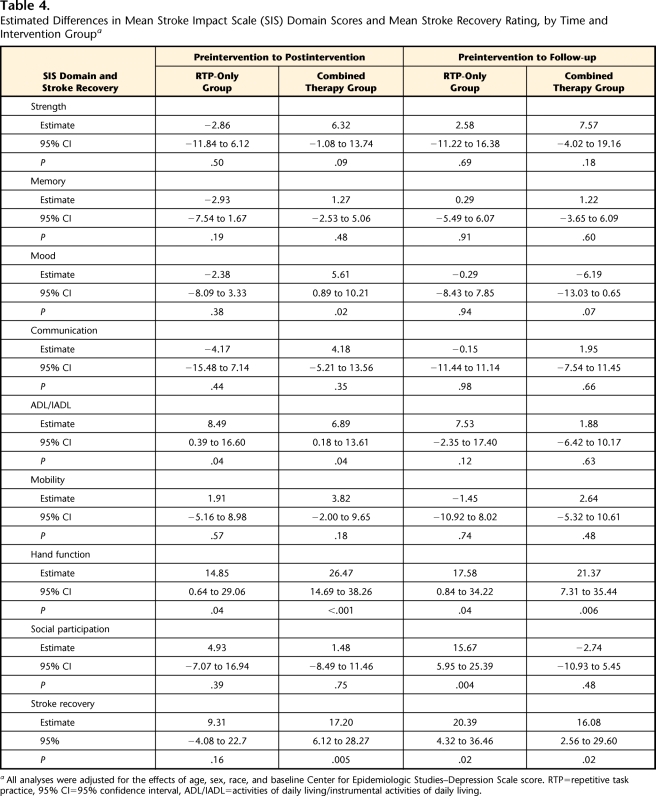
As indicated in Table 4, both RTP only and the combined therapy were associated with a statistically significant improvement in rating of the ADL/IADL domain from preintervention to postintervention, but the estimated changes did not meet the criterion for potential clinical significance. Both RTP only and the combined therapy were associated with a statistically significant improvement in rating of hand function at postintervention and at follow-up, and the magnitude of these changes suggested clinical significance for both intervention groups. Combined therapy was associated with a statistically significant improvement in stroke recovery rating postintervention and at follow-up, and both of these changes suggested clinical significance. The RTP-only intervention was associated with a statistically significant improvement in stroke recovery rating at follow-up, and this change suggested clinical significance.
Table 4.
Estimated Differences in Mean Stroke Impact Scale (SIS) Domain Scores and Mean Stroke Recovery Rating, by Time and Intervention Groupa
All analyses were adjusted for the effects of age, sex, race, and baseline Center for Epidemiologic Studies–Depression Scale score. RTP=repetitive task practice, 95% CI=95% confidence interval, ADL/IADL=activities of daily living/instrumental activities of daily living.
Discussion
Patients who have had a stroke can experience accelerated gains in quality of life after participating in targeted interventions that focus on improving upper-extremity strength.38 Our study indicated improvement in ratings of hand function from baseline to follow-up among patients whose therapy time was divided between the HM and RTP, as well as among patients who received twice as much RTP. Based on ratings obtained with the SIS, assessment of hand function improved over time, with no significant difference between the 2 intervention groups. In addition, the combined therapy group reported improvement in perceived overall stroke recovery postintervention and at follow-up, and the RTP-only group reported improvement in perceived overall stroke recovery at follow-up. The clinical significance of hand function improvement may generalize to a more positive perception of individuals’ poststroke level of functioning.
Among the participants in this study, hand function was rated lower at enrollment than any of the other domains measured by the SIS. This observation also was true of the 222 patients who were enrolled into the EXCITE trial.4 Involuntary activation of flexor muscles coupled with extensor paresis affects hand function, impeding the ability to perform dexterous activities that require skilled hand use (ie, control and coordination of grasping forces and appropriate opening and closing of the hand). A qualitative study of patients who were ≥7 months poststroke showed that residual impairments in ability to handle newspapers, use paper clips, put things in envelopes, and hang clothes in the closet were a source of great distress and reduced perception of life quality.3
Although the neurophysiologic bases for motor recovery after stroke are not completely understood, neuronal cortical connections and cortical representation areas appear modifiable by sensory input, experience, and learning.39–41 Rehabilitation therapies that incorporate principles of motor learning, such as RTP, can facilitate cortical reorganization.42
Robotic devices, such as the HM, that use motor learning principles (ie, engaging the user, providing meaningful feedback during the performance of repetitive activities that are intended to transfer to functional distal motor activities, and utilizing a massed practice training schedule) may be a valuable component of or adjunct to task practice interventions. Although the exact mechanism responsible for improved rating of hand function following an abbreviated RTP intervention while using the HM is unknown, it is likely that an increase in the AROM of the wrist and fingers and a possible reduction in spasticity of the hemiparetic limb may result in an improved perception of hand function. The consistent, repetitive, and progressive nature of the HM may increase the quantity and quality of sensorimotor information provided to the patient, which has been shown to modulate motor cortex function and excitability43,44 and to promote motor learning.45 An increase in quantity and quality of afferent information provided to the patient during robotic device use may facilitate motor learning or relearning.
It is fully acknowledged that sole use of the HM, in the absence of therapist-directed RTP, would not be expected to result in the same magnitude of improvement in hand function rating that was seen in the combined therapy group in the current study. It is not envisioned that the introduction of a robotic system will replace therapist-directed rehabilitation approaches. Rather, the appeal of robotic systems such as the HM is that they can provide consistent and precise therapy for long durations without fatigue while generally requiring relatively minimal supervision. Therefore, we envision the HM as providing a “helping hand” to the therapist, thereby decreasing the time necessary for RTP interventions, which—despite their effectiveness—have not been adopted clinically on a widespread basis.
At the same time, the degree to which hand function and stroke recovery rating outcomes may reflect the particular skills or personality of the therapists who worked with the patients remains unknown. In addition, the relatively small sample size of this study demands that additional studies be conducted to specify the amount of RTP and robotic-assisted intervention time that is both effective and acceptable to patients. Change over time in reported HRQOL of patients who have had a stroke may reflect response shift rather than true change, as Ahmed et al46 have discussed. Based on their study of a large number of patients with stroke and controls, however, Ahmed et al concluded that improvement in HRQOL over time is likely to be real rather than the result of individuals’ reconceptualization or recalibration over the first 6 months poststroke.46
Stroke is increasingly recognized as a significant and expensive medical and societal problem.47 Even patients who are highly recovered following stroke may report significant residual disability in hand function,48 indicating that rehabilitation interventions that target this dimension of upper-extremity functioning should be made widely available. Robotic-assisted therapy has an important role in technologic intervention in the modern neurorehabilitation setting.49 Patients in the combined therapy group in the current study used the HM device under the supervision of a therapist. Based on feedback from the therapists and patients who participated in this study, we believe that the HM device could be used in a home environment or in a group setting in which the therapist oversees a number of patients with stroke who use HM systems simultaneously. A home and group clinical mode of HM use has the potential to dramatically reduce the amount of therapist-directed RTP without compromising perceived gains in hand function associated with a more traditional time- and labor-intensive RTP intervention. Reduced therapist-directed rehabilitation time without loss in quality of life ratings suggests that incorporation of robotic-assisted therapy may be an effective approach to enhancing motor recovery in patients who have had a stroke, while decreasing labor, the most costly aspect of delivering physical therapy interventions. This reduction in cost may promote greater acceptance of RTP approaches in clinical environments and the acceptance of robotic systems that function as adjuncts to therapist-directed interventions.
Limitations
The combined therapy group received 30 hours of RTP compared with 60 hours for the RTP-only group. A limitation of the study was the lack of understanding about the necessary dose of RTP to elicit the current level of improvements in motor function.50 Limiting training to 30 hours of RTP might be sufficient for patients to reach a plateau in terms of improved motor function. In this case, the robotic device may not be contributing to change in the perception of hand function.
We are currently engaged in studies examining the dose response of RTP and RTP+HM to dissociate the contributions of task practice and robotic therapy to enhanced perception of hand function. Although a potential benefit of utilizing a robotic device is the reduction of the amount of therapist-directed RTP, thereby potentially providing a cost savings to the delivery of RTP interventions, the results from the current study do not directly assess the potential cost savings. In the current study, the HM was used in a clinical environment and under the supervision of a therapist. It is unclear whether the same level of intensity and adherence would occur if a patient used the device in a home environment.
Future studies in which the HM is used in a home environment to complete outpatient RTP will be conducted. These subsequent studies will provide important data regarding any cost savings associated with the HM, feasibility of home use, and patient adherence to intended use of the system. It also will be important to continue to assess the potential effect of decreased therapist time on patients’ rating of the social participation dimension of their perceived quality of life. At the same time, the current data are the first to show that HRQOL measures specific to distal hand function can be improved following a combined robotic and RTP therapeutic approach.
Conclusion
Receipt of 30 hours of therapist-supervised repetitive task practice combined with 30 hours of robotic-assisted therapy during the subacute phase of stroke recovery resulted in patient-rated improvement in hand function similar to that observed with receipt of 60 hours of therapist-supervised repetitive task practice. Patients assigned to the 2 interventions also reported a higher level of overall stroke recovery at follow-up compared with baseline, and patients in the combined therapy group reported higher overall stroke recovery postintervention as well. Incorporating robotic-assisted therapy may be an effective approach to enhancing motor recovery in patients who have had a stroke while decreasing labor, which is the most costly aspect of delivering physical therapy interventions. A potential decrease in therapist labor may enhance the clinical acceptance and potential reimbursement of task practice approaches such as CIMT and modified CIMT.
Footnotes
Dr Kutner, Dr Butler, Dr Wolf, and Dr Alberts provided writing. Ms Zhang provided data analysis. Dr Kutner, Dr Butler, Dr Wolf, and Dr Alberts provided concept/idea/research design and project management. The authors thank Veronica Rowe, OTR, Kelly Crabtree, PT, Libby Rosenstein, OTR, and Vanessa Franco, OT/L, for their invaluable assistance in the training and evaluation of participants and Anil Thota for assistance in data processing.
Ethical approval was obtained from the Emory University and Cleveland Clinic Foundation institutional review boards.
Dr Wolf is a paid consultant for Kinetic Muscles Inc. He is not a shareholder or investor in the company.
This study was funded by a grant (R21 HD057020) from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, to Dr Alberts.
Trial NCT00729625 registered at: https://register.clinicaltrials.gov/prs/app/action/FilterOrSelectProtocol/selectaction/View/ts/2/uid/U0000O71.
Interactive Motion Technologies Inc, 37 Spinelli Place, Cambridge, MA 02318.
This system is not commercially available. It has been and is currently being used in research protocols at the University of Reading in the United Kingdom.
This system is not commercially available; developed at the VA-Rehabilitation Research and Development Center at Palo Alto, California.
Kinetic Muscles Inc, 2103 E Cedar St, #3, Tempe, AZ 85281 (http://www.kineticmuscles.com).
SAS Institute Inc, PO Box 8000, Cary, NC 27513.
References
- 1.Dobkin BH. Rehabilitation after stroke. N Engl J Med 2005;352:1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25–e146 [DOI] [PubMed] [Google Scholar]
- 3.Clarke P, Black SE. Quality of life following stroke: negotiating disability, identity, and resources. J Appl Gerontol 2005;24:319–336 [Google Scholar]
- 4.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006;296:2095–2104 [DOI] [PubMed] [Google Scholar]
- 5.Woldag H, Hummelsheim H. Evidence-based physiotherapeutic concepts for improving arm and hand function in stroke patients: a review. J Neurol 2002;249:518–528 [DOI] [PubMed] [Google Scholar]
- 6.Mulder T, Zijlstra W, Geurts A. Assessment of motor recovery and decline. Gait Posture 2002;16:198–210 [DOI] [PubMed] [Google Scholar]
- 7.Wolf SL. Revisiting constraint-induced movement therapy: are we too smitten with the mitten? Is all nonuse “learned”? And other quandaries. Phys Ther 2007;87:1212–1223 [DOI] [PubMed] [Google Scholar]
- 8.Krebs HI, Volpe BT, Aisen ML, Hogan N. Increasing productivity and quality of care: robot-aided neuro-rehabilitation. J Rehabil Res Dev 2000;37:630–652 [PubMed] [Google Scholar]
- 9.Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng 1998;6:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly JJ, Hogan N, Perepezko EM, et al. Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J Rehabil Res Dev 2005;42:723–736 [DOI] [PubMed] [Google Scholar]
- 11.Reinkensmeyer DJ, Kahn LE, Averbuch M, et al. Understanding and treating arm movement impairment after chronic brain injury: progress with the arm guide. J Rehabil Res Dev 2000;37:653–662 [PubMed] [Google Scholar]
- 12.Volpe BT, Ferraro M, Lynch D, et al. Robotics and other devices in the treatment of patients recovering from stroke. Curr Neurol Neurosci Rep 2005;5:465–470 [DOI] [PubMed] [Google Scholar]
- 13.Krebs HI, Ferraro M, Buerger SP, et al. Rehabilitation robotics: pilot trial of a spatial extension for mit-manus. J Neuroeng Rehabil 2004;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraro M, Palazzolo JJ, Krol J, et al. Robot-aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology 2003;61:1604–1607 [DOI] [PubMed] [Google Scholar]
- 15.Volpe BT, Krebs HI, Hogan N, et al. A novel approach to stroke rehabilitation: robot-aided sensorimotor stimulation. Neurology 2000;54:1938–1944 [DOI] [PubMed] [Google Scholar]
- 16.Lum PS, Burgar CG, Shor PC, et al. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil 2002;83:952–959 [DOI] [PubMed] [Google Scholar]
- 17.Lum PS, Burgar CG, Van der Loos M, et al. Mime robotic device for upper-limb neurorehabilitation in subacute stroke subjects: a follow-up study. J Rehabil Res Dev 2006;43:631–642 [DOI] [PubMed] [Google Scholar]
- 18.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair 2008;22:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci 2004;22:281–299 [PubMed] [Google Scholar]
- 20.Van Peppen RPS, Kwakkel G, Wood-Dauphinée S, et al. The impact of physical therapy on functional outcomes after stroke: what's the evidence? Clin Rehabil 2004;18:833–862 [DOI] [PubMed] [Google Scholar]
- 21.Blanton S, Wolf SL. An application of upper-extremity constraint-induced movement therapy in a patient with subacute stroke. Phys Ther 1999;79:847–853 [PubMed] [Google Scholar]
- 22.Taub E, Wolf SL. Constraint induction techniques to facilitate upper extremity use in stroke patients. Top Stroke Rehabil 1997;3:38–59 [DOI] [PubMed] [Google Scholar]
- 23.Brooks VB, Stoney SD., Jr Motor mechanisms: the role of the pyramidal system in motor control. Ann Rev Physiol 1971;33:337–392 [DOI] [PubMed] [Google Scholar]
- 24.Humphrey DR, Schmidt EM, Thompson WD. Predicting measures of motor performance from multiple cortical spike trains. Science 1970;170:758–762 [DOI] [PubMed] [Google Scholar]
- 25.Waldvogel D, vanGelderen P, Ishii K, Hallett M. The effect of movement amplitude on activation in functional magnetic resonance imaging studies. J Cereb Bood Flow Metab 1999;19:1209–1212 [DOI] [PubMed] [Google Scholar]
- 26.Brewer BR, McDowell SK, Worthen-Chaudhari LC. Poststroke upper extremity rehabilitation: a review of robotic systems and clinical results. Top Stroke Rehabil 2007;14:22–44 [DOI] [PubMed] [Google Scholar]
- 27.Duncan PW, Wallace D, Lai SM, et al. The Stroke Impact Scale version 2.0: evaluation of reliability, validity and sensitivity to change. Stroke 1999;30:2131–2140 [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 29.Lee TD, Magill RA, Weeks DJ. Influence of practice schedule on testing schema theory predictions in adults. J Mot Behav 1985;17:283–299 [DOI] [PubMed] [Google Scholar]
- 30.Rosenstein L, Ridgel AL, Thota A, et al. Effects of combined robotic therapy and repetitive-task practice on upper extremity function in a patient with chronic stroke. Am J Occup Ther 2008;62:28–33 [DOI] [PubMed] [Google Scholar]
- 31.Frick EM, Alberts JL. Combined use of repetitive task practice and an assistive robotic device in a patient with subacute stroke. Phys Ther 2006;86:1378–1386 [DOI] [PubMed] [Google Scholar]
- 32.Stroke Impact Scale version 3.0. Available from the Rehabilitation Outcomes Research Center for Veterans (RORC) at: https://www.maa.nsw.gov.au/getfile.aspx?Type=document&ID=36614&ObjectType=3&ObjectID=3322
- 33.Edwards B, O’Connell B. Internal consistency and validity of the Stroke Impact Scale 2.0 (SIS 2.0) and SIS-16 in an Australian sample. Qual Life Res 2003;12:1127–1135 [DOI] [PubMed] [Google Scholar]
- 34.Cook C, McCluskey A, Bowman J. Increasing the Use of Outcome Measures by Occupational Therapists [final research report]. Penrith South, New South Wales, Australia: University of Western Sydney; 2006 [Google Scholar]
- 35.Whyte EM, Mulsant BH, Vanderbilt J, et al. Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc 2004;52:774–778 [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D scale: a new self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 37.Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient: a method of evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31 [PubMed] [Google Scholar]
- 38.Studenski S, Duncan PW, Perera S, et al. Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke 2005;36:1764–1770 [DOI] [PubMed] [Google Scholar]
- 39.Johansson BB. Brain plasticity and stroke rehabilitation: The Willis Lecture. Presented at: 24th American Heart Association International Conference on Stroke and Cerebral Circulation; February 4, 1999; Nashville, Tennessee [Google Scholar]
- 40.Merzenich MM. Variability in hand surface representations in areas 3b and 1 in adult owl and squirrel monkeys. J Comp Neurol 1987;258:281–296 [DOI] [PubMed] [Google Scholar]
- 41.Jenkins WM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res 1987;71:249–266 [DOI] [PubMed] [Google Scholar]
- 42.Kaplon RT, Prettyman MG, Kushi CL, Winstein CJ. Six hours in the laboratory: a quantification of practice time during constraint-induced therapy (CIT). Clin Rehabil 2007;21:950–958 [DOI] [PubMed] [Google Scholar]
- 43.Ridding MC, Brouwer B, Nordstrom MA. Reduced interhemispheric inhibition in musicians. Exp Brain Res 2000;133:249–253 [DOI] [PubMed] [Google Scholar]
- 44.Kaelin-Lang A, Cohen LG. Enhancing the quality of studies using transcranial magnetic and electrical stimulation with a new computer-controlled system. J Neurosci Methods 2000;102:81–89 [DOI] [PubMed] [Google Scholar]
- 45.Rossini PM, Dal Forno G. Integrated technology for evaluation of brain function and neural plasticity. Phys Med Rehabil Clin N Am 2004;15:263–306 [DOI] [PubMed] [Google Scholar]
- 46.Ahmed S, Mayo NE, Corbiere M, et al. Change in quality of life of people with stroke over time: true change or response shift? Qual Life Res 2005;14:611–627 [DOI] [PubMed] [Google Scholar]
- 47.Dion JE. Management of ischemic stroke in the next decade: stroke centers of excellence. J Vasc Interv Radiol 2004;15:S133–S141 [DOI] [PubMed] [Google Scholar]
- 48.Lai S-M, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 2002;33:1840–1844 [DOI] [PubMed] [Google Scholar]
- 49.Rocksmith ER, Reding MJ. New developments in stroke rehabilitation. Curr Atheroscler Rep 2002;4:277–284 [DOI] [PubMed] [Google Scholar]
- 50.Dobkin BH. Confounders in rehabilitation trials of task-oriented training: lessons from the designs of the excite and scilt multicenter trials. Neurorehabil Neural Repair 2007;21:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]