Abstract
Background. We investigated the association between clinical characteristics, angiographic data and ventricular arrhythmia in patients with ST-elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI)
Methods. In patients with STEMI (n=225), a Holter analysis was performed the first 12 hours after primary PCI.
Results. A total of 151 (66%) patients had ≥1 episode of ventricular tachycardia (VT). Age <70 years (RR 4.9, 95% CI 1.8 to 12.7), TIMI 0-1 pre-PCI (RR 2.6, 95% CI 1.1 to 6.1) and peak CK (RR 3.5, 95% CI 1.9 to 5.8) were independent predictors of VT. One-year mortality was 7%, no association between mortality and presence of early VT was found.
Conclusion. Ventricular tachycardia is common in the first 12 hours after primary PCI for STEMI. Independent predictors of VT are younger age, TIMI 0-1 flow prior to PCI and larger infarct size. The presence of early VT was not significantly associated with one-year mortality. (Neth Heart J 2010;18:122–8.)
Keywords: Arrhythmias, Cardiac; Percutaneous Coronary; Myocardial Infarction; Prognosis; Tachycardia, Ventricular; Ventricular Fibrillation
Patients with acute myocardial infarction (AMI) are prone to develop potentially life-threatening ventricular arrhythmias such as ventricular tachycardia (VT) during the acute phase of the myocardial infarction.1 It is still unclear what specific clinical variables are associated with these ventricular arrhythmias.2,3 Studies performed before the era of percutaneous coronary intervention (PCI) demonstrated the clinical relevance of ventricular arrhythmias if occurring >48 hours after AMI.4-6 Factors associated with the occurrence of ventricular activity in these patients were the presence of extensive myocardial damage, reduced LV function and formation of ventricular aneurysm.6,7 Since myocardial ischaemia and necrosis due to coronary occlusion is the key element leading to ventricular arrhythmias, angiographic characteristics of AMI patients are of particular interest. However, studies investigating ventricular arrhythmias in AMI are hampered by the fact that most patients have been treated with thrombolytic therapy or no reperfusion therapy and therefore no detailed angiographic data are available.2,3,8,9 We studied the correlates of clinical characteristics, angiographic data and the prevalence of ventricular arrhythmia, particularly VT, in the first 12 hours in patients with ST-elevation myocardial infarction (STEMI) treated with primary PCI.
Methods
We performed a prospective registry analysis of all patients admitted with STEMI from December 2004 until February 2006. Patients were recruited in a single centre (University Medical Center Groningen). Patients were included on arrival at the coronary care unit immediately following primary PCI for STEMI. STEMI was defined as complaints of chest pain with electrocardiographic signs compatible with acute myocardial infarction (ST elevation >2 mm in precordial leads and >1 mm in limb leads). All patients were directly transported to the cath lab on arrival and acute coronary angiography was performed with subsequent PCI when indicated. Ischaemic time was defined as time between symptom onset and first balloon inflation. All patients were treated with heparin, aspirin, clopidogrel, β-blockers and statins when not contraindicated. The use of other cardiac medication after PCI was at the discretion of the physician. For logistic reasons, stable patients with successful uncomplicated primary PCI, without an indication for invasive therapy for lesions in non-infarct-related arteries, were often referred to regional non-interventional hospitals within 12 hours after the primary PCI procedure. A Holter recording for the duration of at least 12 hours was performed after primary PCI once the patient was admitted to the coronary care unit (CCU). Holter recordings were analysed by trained personnel and scored for ventricular ectopy (isolated ventricular beats), doublet (two consecutive ventricular beats), bigeminy (alternating isolated supraventricular and ventricular beats) and ventricular runs (≥3 consecutive ventricular beats). Slow ventricular tachycardia was considered to be accelerated idioventricular arrhythmia (AIVR) and was defined as a ventricular run with a frequency of <100 beats/min. Ventricular tachycardia was defined as a ventricular run with a frequency of >100 beats/min. Sustained VT was defined as a VT with a duration of more than 30 consecutive seconds or leading to haemodynamic compromise within this period. Angiographic data were analysed by blinded core lab technicians. TIMI flow was scored according to the TIMI flow grading system pre- and post-PCI.10 Myocardial perfusion was assessed using myocardial blush grade as described previously.11 Presence of collaterals was scored according to the Rentrop classification.12 Infarct size was measured by peak levels of creatinine kinase (CK), CK-MB and troponin levels during the admission. Patient characteristics were recorded through the use of case record forms. Patients in whom 12-hour Holter registration could not be obtained were excluded. One-year follow-up was performed on mortality status.
Statistical analysis
Statistical analysis was performed using SPSS 12.0. Differences between group means were tested by two-tailed Student's t-test when data were normally distributed. Mann-Whitney U or Kruskal-Wallis tests were used to compare means when data were not distributed normally. A Χ2 statistic was calculated to test differences between proportions, with calculation of relative risks and exact 95% confidence intervals. Linear-by-linear correlations were calculated when three subsequent tertiles were compared. Statistical significance was defined as a p value of less than 0.05. Binary logistic regression analysis was used as multivariate model to identify independent associates with VT. In the multivariate model patients in the first tertile of VT were compared with patients in the third tertile. Variables included were gender, age and all significant predictors of ventricular tachycardia in univariate analysis. A separate analysis with inclusion of all angiographic parameters was performed. Correlations between numeric variables were calculated using the bivariate Pearson (normal distribution of values) and Spearman (not normal distribution) correlation coefficient.
Results
From 1 December 2004 to 28 February 2006, 651 consecutive patients were treated with primary PCI for STEMI. Within 12 hours, five died and 421 were referred to regional non-interventional hospitals within 12 hours after the PCI procedure. A total number of 225 patients had 12 hours of Holter registration. Mean age was 61.8±12.4 years and 161 patients were male (70%). TIMI 3 flow after PCI was achieved in 181 patients (80%), mean ischaemic time was 228±153 minutes.
Of the included patients, 223 (98%) experienced solitary ventricular extrasystole (mean number of extrasystoles 617±1109, median 181) and 187 (82%) experienced ventricular doublets (mean number of doublets 54±142, median 6.0). AIVR was present in 133 patients (58%) with a mean number of AIVR episodes of 42±177, median 1.0. A total of 151 (66%) patients had an episode of VT, of which 134 patients (59%) had a VT with >120 beats/min. The mean number of VT episodes in all patients was 20±115, median 2.0. Baseline patient characteristics stratified according to the presence of VT (tertiles) are displayed in table 1. There was no consistent correlation between coronary history, haemodynamic parameters on admission, admission medication, ischaemic time and the presence of VT.
Table 1 .
Baseline characteristics according to tertile of ventricular tachycardia.
| Variable | All patients | VT 1st tertile | VT 2nd tertile | VT 3rd tertile | P value |
|---|---|---|---|---|---|
| Number of patients | 225 | 76 | 79 | 70 | |
| Number of VTs (±SD) | 20±115 | 0±0 | 2.2±1.4 | 61.8±202.6 | |
| - Min-max | 0-1657 | 0-0 | 1-5 | 6-1657 | |
| - Median | 2.0 | 0 | 2.0 | 17.0 | |
| Age >70 years | 59 (26) | 26 (34) | 22 (28%) | 11 (16%) | 0.01 |
| Mean age (± SD) | 61.7±12.4 | 63.2±13.1 | 61.5±13.4 | 60.2±10.0 | 0.15 |
| Male sex | 160 (71) | 51 (67) | 56 (71) | 53 (76) | 0.25 |
| Diabetes | 28 (12) | 10 (13) | 15 (19) | 3 (4) | 0.11 |
| Smoking | 92 (41) | 31 (46) | 26 (36) | 35 (56) | 0.27 |
| Heart rate >100 bpm | 18 (8) | 6 (9) | 7 (10) | 5 (9) | 0.98 |
| SBP ≤100 mmHg | 31 (14) | 11 (16) | 5 (7) | 15 (25) | 0.19 |
| DBP <60 mmHg | 27 (12) | 12 (17) | 7 (10) | 8 (13) | 0.47 |
| Coronary history | |||||
| - AMI | 27 (12) | 12 (16) | 8 (10) | 7 (10) | 0.27 |
| - PCI | 17 (8) | 7 (10) | 5 (6) | 5 (7) | 0.59 |
| - CABG | 6 (3) | 2 (3) | 3 (4) | 1 (1) | 0.67 |
| Admission medication | |||||
| - Aspirin | 48 (21) | 23 (30) | 11 (14) | 14 (20) | 0.12 |
| - β-blocker | 54 (24) | 24 (32) | 11 (14) | 19 (27) | 0.49 |
| - Statin | 41 (18) | 18 (24) | 10 (13) | 13 (19) | 0.40 |
| - ACE inhibition | 29 (13) | 6 (8) | 13 (17) | 10 (14) | 0.24 |
| - Loop diuretic | 12 (5) | 7 (9) | 3 (4) | 2 (3) | 0.09 |
| Ischaemic time | |||||
| - >3 hours | 110 (52) | 37 (54) | 38 (51) | 35 (52) | 0.80 |
| Infarct size (peak levels) | |||||
| - CK | 1067±1143 | 703±1018 | 1193±1282 | 1320±1015 | 0.001 |
| - CK-MB | 86.6±83.1 | 57.9±74.4 | 95.6±94.2 | 107.5±70.3 | 0.001 |
| - Troponin | 160.3±312.0 | 102.1±223.7 | 179.1±270.3 | 202.2±416.1 | 0.001 |
P values calculated with linear-by-linear Χ2 statistic and Kruskall-Wallis test. Values are presented as numbers (%) or mean ± SD. SBP=systolic blood pressure, DBP=diastolic blood pressure, AMI=acute myocardial infarction, CABG=coronary artery bypass grafting, PCI=percutaneous coronary intervention, CK (MB)=creatine kinase (myocardial band), SD=standard deviation, VT=ventricular tachycardia.
There was no correlation between infarct-related vessel, presence of multivessel disease, TIMI flow post, myocardial blush grade, thrombus or collaterals and the presence of VT after PCI. However, patients with TIMI 2-3 flow prior PCI had significantly less VTs (table 2).
Table 2 .
Angiographic characteristics according to tertile of ventricular tachycardia.
| Variable | All patients | VT 1st tertile | VT 2nd tertile | VT 3rd tertile | P value |
|---|---|---|---|---|---|
| (n=225) | (n=76) | (n=79) | (n=70) | ||
| LAD (%) | 115 (51) | 45 (59) | 38 (48) | 32 (46) | 0.10 |
| MVD | 145 (64) | 49 (65) | 48 (61) | 48 (69) | 0.62 |
| Collaterals | 56 (25) | 19 (27) | 15 (20) | 22 (34) | 0.34 |
| TIMI pre 0 | 112 (50) | 26 (34) | 40 (52) | 46 (67) | <0.001 |
| -1 | 28 (12) | 17 (13) | 5 (7) | 10 (15) | |
| - 2-3 | 82 (36) | 37 (49) | 32 (42) | 13 (19) | |
| TIMI post 0-1 | 12 (5) | 3 (4) | 6 (8) | 3 (4) | 0.59 |
| -2 | 32 (14) | 9 (12) | 12 (15) | 11 (16) | |
| -3 | 181 (80) | 64 (84) | 61 (77) | 56 (80) | |
| MBG 2/3 | 171 (76) | 57 (77) | 57 (73) | 57 (81) | 0.54 |
| Thrombus post PCI | 6 (3) | 1 (1) | 3 (4) | 2 (3) | 0.55 |
P values calculated with linear-by-linear Χ2 statistic. Values are presented as numbers (%). LAD=left anterior descending artery, MBG=myocardial blush grade, MVD=multivessel disease, PCI=percutaneous coronary intervention, TIMI=Thrombolysis in Myocardial Infarction, VT=ventricular tachycardia.
Mean peak levels for creatine kinase (CK) were 1064±1140 U/l, for CK-myocardial band (MB) 86.2±82.8 U/l and for troponin levels 164±317 U/l. There was a strong relation between increase in infarct size and a higher prevalence of all types of ventricular arrhythmia (figure 1) (p<0.001). The presence of VT was significantly associated with other types of ventricular activity. Patients with more VT also had more ventricular extrasystole, doublet and slow VT activity (table 3) (p<0.001).
Figure 1 .
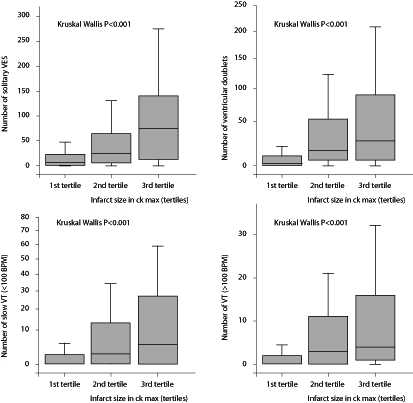
Boxplots showing the incidence of ventricular arrhythmia with regard to infarct size.VES=ventricular extrasystole, CK=creatine kinase.
Table 3 .
Correlation between ventricular tachycardia (>100 beats/min) and other ventricular activity.
| Correlation coefficient | Two-tailed significance | |
|---|---|---|
| Solitary VES | 0.72 | <0.001 |
| Doublet VES | 0.80 | <0.001 |
| Bigeminy VES | 0.52 | <0.001 |
| Slow VT (<100 bpm) | 0.72 | <0.001 |
BPM=beats per minute, VES=ventricular extrasystole, VT=ventricular tachycardia.
After one-year 16 patients had died (7%), 13 from cardiac causes and three from noncardiac causes (table 4). Mortality status could not be obtained in two patients (foreigners). Predictors of overall mortality were age >70 years (15.3 vs. 4.3%, p<0.01), LAD as infarct-related vessel (10.5 vs. 3.7%, p=0.047), TIMI post PCI 0-1 (25.0 vs. 6.2%, p=0.002), post-PCI myocardial blush grade 0-1 (16.0 vs. 4.7%, p=0.007), previous AMI (18.5 vs. 5.7%, p=0.004) and previous PCI (23.5 vs. 5.5%, p=0.005). There was a trend for higher mortality with increasing infarct size (4.2, 6.6 and 10.7% in subsequent CK tertiles). There was no significant difference in mortality between patients in the first tertile of VT (9%), the second tertile of VT (10%) and the third tertile of VT (3%) (p=0.17). The mean number of VT episodes was 21.0±120.5 (median 2.0) in survivors and 5.8±15.6 (median 1.0) in patients who died (p=0.22).
Table 4 .
Per person specific death cause.
| Gender | Age | Categorised death | Death cause | Days after PCI |
|---|---|---|---|---|
| M | 55 | Cardiac | Sudden death | 1 |
| V | 88 | Cardiac | Cardiogenic shock after STEMI | 2 |
| M | 86 | Cardiac | Congestive heart failure | 3 |
| M | 75 | Cardiac | Reinfarction | 3 |
| M | 68 | Cardiac | Congestive heart failure | 3 |
| M | 47 | Cardiac | Sudden death | 4 |
| M | 76 | Cardiac | Congestive heart failure | 4 |
| M | 74 | Cardiac | Congestive heart failure | 8 |
| M | 63 | Cardiac | Congestive heart failure | 9 |
| M | 64 | Cardiac | Congestive heart failure | 18 |
| M | 84 | Cardiac | Congestive heart failure | 21 |
| M | 75 | Cardiac | Congestive heart failure | 25 |
| F | 75 | Cardiac | Sudden death | 43 |
| F | 73 | Noncardiac | Hypoglycaemic coma | 88 |
| F | 56 | Noncardiac | Lung cancer | 186 |
| M | 69 | Noncardiac | Chronic obstructive lung disease | 360 |
Only three patients had one or more episodes of sustained VT. Their mean age was (67±10 years); they all had TIMI 0-1 flow pre-PCI and TIMI 2-3 flow post-PCI with evidence of successful myocardial perfusion (MBG grade 2-3). All patients with sustained VT were alive at one-year follow-up.
Multivariate analysis
In order to identify independent predictors of VT early after PCI, multivariate analysis including age, sex and TIMI flow pre-PCI was performed. Both TIMI 0-1 pre-PCI (RR 4.5, 95% CI 2.1 to 9.8, p<0.001) and age <70 years (RR 3.6, 95% CI 1.5 to 8.8, p=0.004) were associated with the presence of VT. After inclusion of infarct size and angiographic parameters, the following variables were associated with an increase in VT: age <70 years, antegrade TIMI flow 0/1 pre PCI and larger infarct size (table 5).
Table 5 .
Multivariate analysis of clinical and angiographic predictors of ventricular tachycardia after primary PCI
| P value | RR | 95% CI | |
|---|---|---|---|
| < Age 70 | 0.006 | 4.9 | 1.6-15.1 |
| Timi pre 0/1 | 0.004 | 4.1 | 1.6-10.6 |
| Maximum CK level (per 100) | 0.01 | 1.1 | 1.0-1.1 |
| Female | 0.97 | 1.0 | 0.4-2.6 |
| MBG 0/1 | 0.77 | 0.84 | 0.3-2.6 |
| Non-LAD | 0.085 | 2.10 | 0.9-4.9 |
| Non-MVD | 0.20 | 1.82 | 0.7-4.6 |
| TIMI post 0/1 | 0.61 | 0.58 | 0.1-4.7 |
| Collaterals | 0.27 | 1.71 | 0.7-4.5 |
LAD=left anterior descending artery, MBG=myocardial blush grade, MVD=multi-vessel disease, PCI=primary coronary intervention, TIMI=Thrombolysis in Myocardial Infarction.
Discussion
Our study describes the prevalence and predictors of early ventricular arrhythmia in high-risk patients with STEMI treated with primary PCI. Despite the use of effective reperfusion therapy nonsustained ventricular arrhythmia was common during the acute phase of STEMI in our patients. Almost all patients had ventricular ectopy or doublet activity. Approximately two thirds of our patients had at least one VT and one third had more than 6 VTs. However, malignant VT in the first 12 hours after primary PCI was very rare as only 1% of the patients had a sustained VT. Previous studies of cohorts of patients with myocardial infarction report a prevalence of ventricular arrhythmia of about 10%. Unfortunately, comparison of ventricular arrhythmia between studies is hampered significantly by differences in or absence of ventricular arrhythmia definitions and duration and timing of rhythm registration.2,13,14
The only baseline characteristic associated with VT was age. Older patients tended to have less VT compared with younger patients. This association has been described before, although reports are not consistent.2,3 Signs of haemodynamic compromise, such as low systolic tension and tachycardia, did not seem to be related with VT. This is in line with Mehta et al., who investigated VT/VF after primary PCI in the catheterisation laboratory and only found low systolic blood pressure to be predictive of VT/VF but not Killip class or tachycardia.15 Prior studies reporting the importance of haemodynamic compromise and prevalence of VT did not enrol patients treated with primary PCI.2,16 The higher rate of VT in these patients may be a reflection of failure of reperfusion rather than cardiogenic shock.
In our cohort, early antegrade flow prior to PCI was an important predictor for development of VT. In patients with TIMI 2-3 flow prior to PCI the presence of VT was relatively rare, whereas patients without antegrade flow prior to PCI had most VTs. It has been shown that most episodes of VT occur early after reperfusion and therefore it may be that patients with TIMI 2-3 flow on admission already had their peak of ventricular arrhythmia activity before Holter registration. Furthermore, early reperfusion ensues a reduced infarcted and ischaemic area, reducing the risk of associated trigger activity leading to arrhythmia.17,18 Reduction and rapid wash out of ischaemic toxic by-products may also be of importance. Implementation of optimal facilitation of primary PCI is a topic of growing clinical importance. It appears that antegrade flow pre-PCI not only improves LV function, but may also be of importance with regard to improving electrical stability.19 Part of the association between TIMI flow pre-PCI and VT was due to reduced infarct size. However, after correction for infarct size in multivariate analysis TIMI flow pre-PCI remained a strong predictor of VT.
In contrast to antegrade TIMI flow pre-PCI, other angiographic parameters were not associated with the presence of VT. Evidence suggests that infarct-related vessel (IRV) patency after thrombolytic therapy is an important predictor with regard to the susceptibility for VT possibly irrespective of LVEF.20-22 Interestingly, in our patients TIMI flow 0-1 after PCI was not associated with VT. Previous studies, however, primarily investigated late ventricular arrhythmia with regard to IRV patency, whereas we studied only early ventricular arrhythmia. So, ventricular arrhythmia in the absence of IRV patency may develop at later stages and not within 12 hours after presentation. Another explanation could be that patients with TIMI 0-1 flow after PCI represent a different patient population, for example with more chronic total occlusions, compared with failed reperfusion after thrombolysis. As the number of patients with TIMI 0-1 flow after PCI was limited in our cohort, the data should be interpreted with caution.
A prominent finding in our study was the close relation between enzymatic infarct size and all types of ventricular arrhythmia, irrespective of angiographic characteristics. Infarct surface area and infarct mass are organic substrates for developing VT. Indeed, multiple studies have linked VT with increased infarct size or reduced LVEF.2,9,17,23 Our study indicates that infarct size and ventricular arrhythmia are also strongly correlated in the setting of primary PCI.
There was a strong correlation between the occurrence of all types of ventricular arrhythmia. Patients with relatively benign ventricular arrhythmia such as solitary ventricular ectopy also had more episodes of doublets, slow VT and VT. This association suggests that the pathophysiological background of the different types of ventricular arrhythmia occurring in the first 12 hours after myocardial infarction are similar.
In our study no association could be demonstrated between VT during the first 12 hours after primary PCI and one-year mortality. Although patient numbers are small, it appears that early VT (within 12 hours of primary PCI) should not be regarded as a poor prognostic sign for long-term clinical outcome.15 In accordance, previous studies have shown that late VT, but not early VT carries an adverse prognosis.4,16 In order to verify our findings, however, a larger patient cohort with longer follow-up is needed.
In order to reduce hospitalisation and medical costs, early discharge of low-risk STEMI patients has been advocated. Risk scores for stratifying patients with regard to suitability for early discharge have been developed; however, implementation of these risk scores into clinical practice remains cumbersome.24 Our data suggest that early discharge should be discouraged in patients with suspected larger infarction, as they have a higher rate of VT early after PCI. On the other hand, patients with small infarctions, particularly when associated with TIMI 2-3 flow at the initial angiography, are at low risk for VT and do not require rhythm monitoring for prolonged periods.
Currently the Defibrillator After Primary Angioplasty (DAPA) trial is investigating the effect of prophylactic ICD implantation in patients after STEMI with reduced left ventricular function or failed PCI.25 Our finding that early VT is not associated with failed PCI or an adverse prognosis does not change the rationale of this study. Early ventricular tachycardia is not an inclusion criteria of the DAPA study and randomisation takes place 30 days after STEMI, a time period after which the occurrence of ventricular arrhythmias is generally considered more malignant.
Limitations
Holter registration started once patients were admitted on the coronary care unit. Subsequently, VTs occurring out of hospital, in the ambulance and during the PCI procedure in the cath lab were not registered. Furthermore, only patients surviving the initial period of the AMI and admitted to the hospital were included. A small number of high-risk patients who died within 12 hours after primary PCI were excluded. More importantly, when considered safe (stable patients with successful PCI and no severe lesions in noninfarct-related vessels requiring early additional revascularisation), many patients were transported to referral non-interventional hospitals shortly after PCI. This may have resulted in a selection bias. This early discharge and subsequent exclusion of these low-risk patients is reflected by the relatively high percentage of risk features such as multivessel disease, anterior wall infarction and suboptimal PCI success in our study patients. Infarct size was expressed as peak levels of cardiac enzyme release. More accurate measures such as data on LVEF or cumulative cardiac enzyme release were not available. A cut-off value for VT of 100 beats/min can be considered quite low; however, our results did not change when using 120 beats/min as cut-off value. Finally, we have described a limited number of patients from a single centre.
Conclusion
The present findings indicate that early VT in high-risk STEMI patients is quite common despite an optimal reperfusion strategy through primary PCI. Strong predictors of VT were absent antegrade epicardial flow prior to PCI and larger infarct size. Early sustained VT was rare after primary PCI. There was a strong correlation between the prevalence of all types of ventricular arrhythmias. Early ventricular tachycardia was not associated with increased one-year mortality.
References
- 1.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death. Structure, function, and time-dependence of risk. Circulation. 1992;85:I2-10. [PubMed] [Google Scholar]
- 2.Henkel DM, Witt BJ, Gersh BJ, Jacobsen SJ, Weston SA, Meverden RA, et al. Ventricular arrhythmias after acute myocardial infarction: a 20-year community study. Am Heart J. 2006;151:806-12. [DOI] [PubMed] [Google Scholar]
- 3.Newby KH, Thompson T, Stebbins A, Topol EJ, Califf RM, Natale A. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-73. [DOI] [PubMed] [Google Scholar]
- 4.Volpi A, Cavalli A, Turato R, Barlera S, Santoro E, Negri E. Incidence and short-term prognosis of late sustained ventricular tachycardia after myocardial infarction: results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI-3) Data Base. Am Heart J. 2001;142:87-92. [DOI] [PubMed] [Google Scholar]
- 5.Marchlinski FE, Waxman HL, Buxton AE, Josephson ME. Sustained ventricular tachyarrhythmias during the early postinfarction period: electrophysiologic findings and prognosis for survival. J Am Coll Cardiol. 1983;2:240-50. [DOI] [PubMed] [Google Scholar]
- 6.Kleiman RB, Miller JM, Buxton AE, Josephson ME, Marchlinski FE. Prognosis following sustained ventricular tachycardia occurring early after myocardial infarction. Am J Cardiol. 1988;62:528-33. [DOI] [PubMed] [Google Scholar]
- 7.Adhar GC, Larson LW, Bardy GH, Greene HL. Sustained ventricular arrhythmias: differences between survivors of cardiac arrest and patients with recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1988;12:159-65. [DOI] [PubMed] [Google Scholar]
- 8.Engelen DJ, Gressin V, Krucoff MW, Theuns DA, Green C, Cheriex EC, et al. Usefulness of frequent arrhythmias after epicardial recanalization in anterior wall acute myocardial infarction as a marker of cellular injury leading to poor recovery of left ventricular function. Am J Cardiol. 2003;92:1143-9. [DOI] [PubMed] [Google Scholar]
- 9.Popovic AD, Neskovic AN, Pavlovski K, Marinkovic J, Babic R, Bojic M, et al. Association of ventricular arrhythmias with left ventricular remodelling after myocardial infarction. Heart. 1997;77:423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932-6. [DOI] [PubMed] [Google Scholar]
- 11.van 't Hof AWJ, Liem A, Suryapranata H, Hoorntje JCA, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302-6. [DOI] [PubMed] [Google Scholar]
- 12.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587-92. [DOI] [PubMed] [Google Scholar]
- 13.Mont L, Cinca J, Blanch P, Blanco J, Figueras J, Brotons C, et al. Predisposing factors and prognostic value of sustained monomorphic ventricular tachycardia in the early phase of acute myocardial infarction. J Am Coll Cardiol. 1996;28:1670-6. [DOI] [PubMed] [Google Scholar]
- 14.Sala MF, Barcena JP, Rota JI, Roca JV, Lopez CA, Puigdevall JM, et al. Sustained ventricular tachycardia as a marker of inadequate myocardial perfusion during the acute phase of myocardial infarction. Clin Cardiol. 2002;25:328-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta RH, Harjai KJ, Grines L, Stone GW, Boura J, Cox D, et al. Sustained ventricular tachycardia or fibrillation in the cardiac catheterization laboratory among patients receiving primary percutaneous coronary intervention: incidence, predictors, and outcomes. J Am Coll Cardiol. 2004;43:1765-72. [DOI] [PubMed] [Google Scholar]
- 16.Al-Khatib SM, Stebbins AL, Califf RM, Lee KL, Granger CB, White HD, et al. Sustained ventricular arrhythmias and mortality among patients with acute myocardial infarction: results from the GUSTO-III trial. Am Heart J. 2003;145:515-21. [DOI] [PubMed] [Google Scholar]
- 17.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104-8. [DOI] [PubMed] [Google Scholar]
- 18.Hatzinikolaou-Kotsakou E, Tziakas D, Hotidis A, Stakos D, Floros D, Mavridis A, et al. Could sustained monomorphic ventricular tachycardia in the early phase of a prime acute myocardial infarction affect patient outcome? J Electrocardiol. 2007;40:72-7. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW, Cox D, Garcia E, Brodie BR, Morice MC, Griffin J, et al. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation. 2001;104:636-41. [DOI] [PubMed] [Google Scholar]
- 20.Aguirre FV, Kern MJ, Hsia J, Serota H, Janosik D, Greenwalt T, et al. Importance of myocardial infarct artery patency on the prevalence of ventricular arrhythmia and late potentials after thrombolysis in acute myocardial infarction. Am J Cardiol. 1991;68:1410-6. [DOI] [PubMed] [Google Scholar]
- 21.Braunwald E. Myocardial reperfusion, limitation of infarct size, reduction of left ventricular dysfunction, and improved survival. Should the paradigm be expanded? Circulation. 1989;79:441-4. [DOI] [PubMed] [Google Scholar]
- 22.Carnendran L, Steinberg JS. Does an open infarct-related artery after myocardial infarction improve electrical stability? Prog Cardiovasc Dis. 2000;42:439-54. [PubMed] [Google Scholar]
- 23.Heidbuchel H, Tack J, Vanneste L, Ballet A, Ector H, Van de Werf F. Significance of arrhythmias during the first 24 hours of acute myocardial infarction treated with alteplase and effect of early administration of a beta-blocker or a bradycardiac agent on their incidence. Circulation. 1994;89:1051-9. [DOI] [PubMed] [Google Scholar]
- 24.De Luca G, Suryapranata H, van 't Hof AWJ, de Boer MJ, Hoorntje JCA, Dambrink JHE, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109:2737-43. [DOI] [PubMed] [Google Scholar]
- 25.Ottervanger JP, Ramdat Misier AR, Zijlstra F, Schalij MJ, Wever E, Jordaens LJ, et al; for the DAPA Investigators. Implantable defibrillator early after primary percutaneous intervention for ST-elevation myocardial infarction: rationale and design of the Defibrillator After Primary Angioplasty (DAPA) trial. Am Heart J. 2006;152:636-40. [DOI] [PubMed] [Google Scholar]


