Abstract
Background: Vitamin C, vitamin E, and β-carotene are major antioxidants and as such may protect against the development of type 2 diabetes via reduction of oxidative stress.
Objective: The purpose of this study was to investigate the long-term effects of supplementation with vitamin C, vitamin E, and β-carotene for primary prevention of type 2 diabetes.
Design: In the Women's Antioxidant Cardiovascular Study, a randomized trial that occurred between 1995 and 2005, 8171 female health professionals aged ≥40 y with either a history of cardiovascular disease (CVD) or ≥3 CVD risk factors were randomly assigned to receive vitamin C (ascorbic acid, 500 mg every day), vitamin E (RRR-α-tocopherol acetate, 600 IU every other day), β-carotene (50 mg every other day), or their respective placebos.
Results: During a median follow-up of 9.2 y, a total of 895 incident cases occurred among 6574 women who were free of diabetes at baseline. There was a trend toward a modest reduction in diabetes risk in women assigned to receive vitamin C compared with those assigned to receive placebo [relative risk (RR): 0.89; 95% CI: 0.78, 1.02; P = 0.09], whereas a trend for a slight elevation in diabetes risk was observed for vitamin E treatment (RR: 1.13; 95% CI: 0.99, 1.29; P = 0.07). However, neither of these effects reached statistical significance. No significant effect was observed for β-carotene treatment (RR: 0.97; 95% CI: 0.85, 1.11; P = 0.68).
Conclusion: Our randomized trial data showed no significant overall effects of vitamin C, vitamin E, and β-carotene on risk of developing type 2 diabetes in women at high risk of CVD. This trial was registered at clinicaltrials.gov as NCT00000541.
See corresponding editorial on page 253
INTRODUCTION
Oxidative stress, which is characterized by excessive production of reactive oxygen species and reduction of antioxidant defense capacity, has been implicated in the pathogenesis of type 2 diabetes and its complications (1–4). Evidence from basic research and observational studies has suggested that oxidative stress elicits systemic inflammation (3), promotes endothelial dysfunction (5), impairs pancreatic β cell insulin secretion (2, 6), and interferes with glucose disposal in peripheral tissues (2, 3), thereby accelerating the development and progression of type 2 diabetes.
Vitamin C (ascorbic acid), vitamin E (α-tocopherol), and β-carotene are considered important antioxidants in humans. In vitro studies suggest that they protect against free radical–mediated damage by reducing free oxygen radicals and replenishing antioxidant reserves (7–9). In experimental animal models, antioxidant administration has been shown to delay the onset of diabetes (10, 11). It has thus been hypothesized that supplementation with antioxidants may help prevent the development of type 2 diabetes in humans.
Observational epidemiologic studies have shown significant inverse correlations between antioxidant concentrations and several biomarkers of insulin resistance or glucose intolerance in healthy individuals (12). Concentrations of antioxidants in the blood, such as vitamins C (12–14) and E (15, 16) and β-carotene (15–17), were also significantly lower in individuals with type 2 diabetes than in nondiabetic control subjects. Evidence from most previous prospective cohort studies generally supports an inverse association between incidence of type 2 diabetes and dietary, serum, or plasma concentrations of vitamins C (18) and E (19–21) and β-carotene (21, 22) in nondiabetic individuals. Some (23–25), but not all (26–28), of the short-term randomized trials in patients with type 2 diabetes also showed the beneficial effects of oral supplementation of vitamin C or E at high doses on risk factors linked to insulin resistance and diabetes, including oxidative stress, blood pressure (23), lipid metabolism (24), endothelial function (25), and insulin-mediated glucose disposal (24). However, few large trials with long treatment duration have addressed the primary prevention of type 2 diabetes. Two previous randomized clinical trials provided no evidence for significant effects of vitamin E or β-carotene supplementation on the incidence of type 2 diabetes in apparently healthy women (29) and men (30), respectively. To our knowledge, there are no previous trials examining the efficacy of vitamin C in preventing type 2 diabetes. It thus remains unanswered whether vitamins E and C and β-carotene, independently or in combination, protect against the development of type 2 diabetes.
To address this question, we investigated whether long-term supplementation of vitamins C and E and β-carotene reduces the incidence of type 2 diabetes in the Women's Antioxidant Cardiovascular Study (WACS), a randomized, double-blind, placebo-controlled trial conducted over a duration of ≈9.4 y.
SUBJECTS AND METHODS
Study design
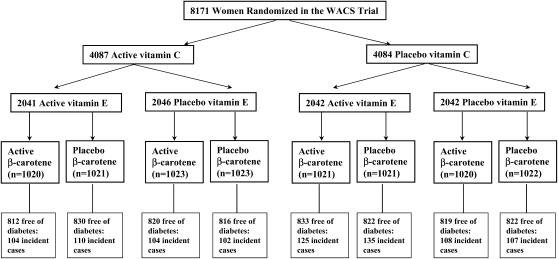
The WACS is a randomized, double-blind, placebo-controlled trial evaluating the effects of vitamin C [500 mg/d synthetic vitamin C (ascorbic acid); provided by BASF Corporation (Mount Olive, NJ], vitamin E [600 IU vitamin E (RRR-α-tocopherol acetate) every other day; provided by Cognis Corporation (La Grange, IL)], and β-carotene (50 mg Lurotin every other day; provided by BASF Corporation) in the secondary prevention of important vascular events in a 2 × 2 × 2 factorial design among women at high risk (either a history of vascular disease or ≥3 cardiovascular risk factors). Details of the design have been reported previously (31, 32). Briefly, a total of 8171 female health professionals, who were willing, eligible, and compliant during a 12-wk run-in period, were entered into the trial from June 1995 through October 1996. They were ≥40 y old, postmenopausal or had no intention of becoming pregnant, and had either a self-reported history of cardiovascular disease (CVD), ie, a history of myocardial infarction, stroke, revascularization procedure, angina pectoris, or transient cerebral ischemia, or ≥3 cardiac risk factors. These cardiac risk factors were self-reported diagnosis of hypertension, high cholesterol concentration, or diabetes mellitus; parental history of premature myocardial infarction (MI) before age 60 y; obesity [body mass index (BMI; in kg/m2) ≥30]; and current cigarette smoking. Women were excluded if they had a self-reported history of cancer (excluding nonmelanoma skin cancer) within the past 10 y, had any serious non-CVD illness, or were currently using warfarin or other anticoagulants. Potential participants also had to be willing to forgo individual supplements of vitamins A, C, and E and β-carotene at amounts beyond the US Recommended Dietary Allowance during the trial. The trial was approved by the institutional review board of the Brigham and Women's Hospital (Boston, MA) and was monitored by an external data and safety monitoring board. From the 8171 women enrolled in the WACS trial, we excluded those with prevalent diabetes at baseline (n = 1597), leaving 6574 nondiabetic women who were randomly assigned to 8 groups for the present analyses (Figure 1).
FIGURE 1.
Flow diagram illustrating diabetes outcomes in the randomly assigned treatment components of the Women's Antioxidant Cardiovascular Study (WACS). A total of 1597 participants who had a diagnosis of type 2 diabetes at baseline were excluded in the analysis.
Study treatment and follow-up
After being randomly assigned to a study group, for every 6 mo for the first year and then annually the women were sent monthly calendar packs containing active agents or placebos along with questionnaires on compliance, adverse effects, and medical events. A semiquantitative food-frequency questionnaire at baseline was used to assess dietary nutrient intake (31, 32). Compliance was assessed through self-report and was defined as taking at least two-thirds of study pills. As previously reported (32), mean compliance over follow-up was ≈73% for all active and placebo agents. In 1999 blood samples were obtained from 30 local participants to evaluate biomarkers for compliance. Blood concentrations were elevated in each active group compared with the placebo group [ascorbic acid: 1.9 compared with 1.3 mg/dL (P = 0.007); vitamin E: 20.2 compared with 12.2 μg/mL (P = 0.007); and β-carotene: 54.4 compared with 19.5 μg/mL (P = 0.003)]. Information was also obtained on outside supplements of study medications for ≥4 d/mo (“drop-ins”). Outside use of vitamin C and vitamin E supplements was <15% and outside use of β-carotene or vitamin A was <5% at both 4 and 8 y and did not differ by β-carotene assignment. When multivitamins containing >100% of the Recommended Dietary Allowance were included in the definition of outside use, rates were 3–5% higher for ascorbic acid, 4–8% higher for vitamin E, and 1–3% higher for β-carotene but, again, did not differ by randomized assignment.
As previously reported (31, 32), there were no significant differences between any of the randomized antioxidant groups in reports of adverse effects, such as bleeding (including gastrointestinal bleeds, hematuria, easy bruising, and epistaxis), gastrointestinal symptoms (including peptic ulcer, gastric upset, nausea, constipation, and diarrhea), or fatigue or drowsiness.
Ascertainment of incident type 2 diabetes
The status of type 2 diabetes was evaluated at baseline, and all of the participants were asked annually whether and when they had been diagnosed with diabetes after randomization. Using the American Diabetes Association diagnostic criteria (33), the self-reported diagnosis of diabetes also was confirmed by using a supplementary diabetes questionnaire on diabetes symptoms, screening test, and hypoglycemic medication. Women who self-reported diabetes during the follow-up (n = 1513) were mailed supplementary questionnaires, and 97% of them responded to the supplementary questionnaires. Among them, the screening rate of WACS health professionals was relatively high (85–90% for blood glucose screening). Self-reported diagnoses of incident diabetes were confirmed in 340 of 354 women (96%) who were free of diabetes at baseline and had a diagnosis of type 2 diabetes during the follow-up. Finally, a total of 895 incident diabetes cases were identified in the WACS on the basis of the combined information from the supplementary questionnaire and annual questionnaires. As previously reported (29), a validation study in a similar cohort of female health professionals has shown that self-reported diabetes has excellent predictive use for identifying true diabetes diagnoses in cohorts of US female health professionals. All of the participants in this study were also health professionals, who are likely to have more robust and valid self-reported diagnostic information. Thus, we believe that self-reported type 2 diabetes is valid in the WACS.
Statistical analysis
Primary analyses were performed on an intention-to-treat basis and included all of the randomly assigned women without self-reported diabetes at baseline. Baseline characteristics were compared by randomized groups by using 2-sample t tests for continuous variables and chi-square tests for categorical variables. Kaplan-Meier survival curves were used to estimate the overall cumulative incidence over time for each active vitamin group and its corresponding placebo group. The log-rank test was computed to compare the curves. We used Cox proportional hazards models to calculate the estimates of relative risks (RRs) and 95% CIs for each antioxidant treatment, after adjustment for age and other randomized treatments. To test the proportionality assumption (ie, that of nonchanging hazards ratios over time), we included an interaction term for treatment with the logarithm of time in the Cox models. The tests showed that the proportional hazard assumption was not violated for any of the models. To examine the effect of actual as opposed to assigned antioxidant use, we carried out a sensitivity analysis according to compliance. Women were censored if and when they stopped taking at least two-thirds of their study pills, reported taking outside supplements containing study agents, or were missing compliance information.
Prespecified subgroup analyses were conducted to examine the effect of vitamins on risk of type 2 diabetes according to major risk factors for type 2 diabetes at baseline, including age groups (45–54, 55–64, and ≥65 y), BMI (continuous), smoking (current, past, or never), alcohol use (never/rarely or ≥1 drink/mo), family history of diabetes (yes or no), physical activity (estimated energy expenditure from leisure activities of <1000 or ≥1000 kcal/wk), menopausal status and hormone therapy (uncertain menopausal status, premenopausal, or postmenopausal including current, past, or never users of hormone therapy), history of hypertension (yes or no), history of hypercholesterolemia (yes or no), and baseline dietary intakes of vitamin C, vitamin E, or total carotenes (tertiles for each). We assessed effect modification by using interaction terms between subgroup indicators and randomized assignment, testing for trend when subgroup categories were ordinal. We also tested interactions of the 3 antioxidant agents by using all of the 2-way and 3-way interaction terms in the Cox model. All of the analyses were conducted by using SAS version 9 (SAS Institute, Cary, NC), and a 2-sided test with a significance level of α = 0.05 (P < 0.05) was used.
RESULTS
Among 8171 participants randomly assigned to a study group, ≈20% of the women (n = 1597) reported prevalent diabetes. After we excluded these 1597 participants, the baseline characteristics remained evenly distributed in the randomized groups without significant statistical differences (Table 1). During a median follow-up period of 9.2 y (mean: 8.45 y), 895 women were diagnosed with type 2 diabetes.
TABLE 1.
Baseline characteristics of 6574 nondiabetic women according to randomly assigned groups in the Women's Antioxidant Cardiovascular Study1
| Vitamin C |
Vitamin E |
β-Carotene |
|||||
| Characteristic | Total n | Active (n = 3278) | Placebo (n = 3296) | Active (n = 3297) | Placebo (n = 3277) | Active (n = 3284) | Placebo (n = 3290) |
| Age (y) | 6574 | 60.8 ± 8.92 | 60.7 ± 8.9 | 60.7 ± 9.0 | 60.7 ± 8.9 | 60.8 ± 9.0 | 60.7 ± 8.9 |
| 45–54 y | 1909 | 29.0 | 29.1 | 29.1 | 29.0 | 28.9 | 29.2 |
| 55–64 y | 2414 | 36.4 | 37.0 | 36.8 | 36.7 | 36.7 | 36.8 |
| ≥65 y | 2251 | 34.6 | 33.9 | 34.1 | 34.4 | 34.4 | 34.0 |
| BMI (kg/m2) | 6570 | 29.6 ± 6.5 | 29.6 ± 6.4 | 29.6 ± 6.3 | 29.6 ± 6.5 | 29.5 ± 6.5 | 29.7 ± 6.4 |
| <25 kg/m2 | 1757 | 26.5 | 27.0 | 26.2 | 27.3 | 27.5 | 26.0 |
| 25–<30 kg/m2 | 1953 | 29.8 | 29.6 | 30.0 | 29.5 | 29.4 | 30.0 |
| ≥30 kg/m2 | 2860 | 43.6 | 43.4 | 43.8 | 43.3 | 43.1 | 44.0 |
| Smoking status (%) | |||||||
| Current | 1043 | 16.0 | 15.8 | 16.0 | 15.8 | 15.7 | 16.1 |
| Past | 2703 | 41.7 | 40.5 | 41.5 | 40.7 | 40.8 | 41.5 |
| Never | 2828 | 42.3 | 43.7 | 42.6 | 43.5 | 43.5 | 42.5 |
| Alcohol use (%) | |||||||
| Never/rarely | 3330 | 51.4 | 49.9 | 50.0 | 51.5 | 51.3 | 50.1 |
| ≥1 drink/mo | 841 | 12.9 | 12.7 | 13.0 | 12.6 | 12.6 | 13.0 |
| 1–6 drinks/wk | 1761 | 26.8 | 26.8 | 27.6 | 26.0 | 26.3 | 27.3 |
| ≥1 drink/d | 642 | 8.94 | 10.6 | 9.58 | 9.95 | 9.81 | 9.73 |
| Physical activity3 (kcal/wk) | 6570 | 855 ± 1158 | 870 ± 1175 | 851 ± 1145 | 873 ± 1188 | 860 ± 1205 | 865 ± 1128 |
| ≤1000 kcal/wk | 4640 | 71.3 | 69.9 | 70.8 | 70.4 | 71.1 | 70.2 |
| >1000 kcal/wk | 1930 | 28.7 | 30.1 | 29.2 | 29.6 | 28.9 | 29.8 |
| Menopause and HT use (%) | |||||||
| Premenopausal | 527 | 8.04 | 8.22 | 8.15 | 8.11 | 7.97 | 8.29 |
| Uncertain | 928 | 13.9 | 14.7 | 14.6 | 14.0 | 14.0 | 14.7 |
| Postmenopausal, current HT use | 2606 | 40.6 | 39.8 | 40.3 | 40.1 | 40.2 | 40.2 |
| Postmenopausal, no HT use | 2421 | 37.5 | 37.2 | 37.0 | 37.8 | 37.9 | 36.8 |
| History of hypertension4 (%) | |||||||
| Yes | 4750 | 72.9 | 71.6 | 71.6 | 73.0 | 72.5 | 72.0 |
| No | 1824 | 27.1 | 28.4 | 28.5 | 27.0 | 27.5 | 28.0 |
| History of hypercholesterolemia5 (%) | |||||||
| Yes | 4808 | 72.8 | 73.5 | 73.2 | 73.1 | 71.5 | 74.8 |
| No | 1766 | 27.2 | 26.5 | 26.8 | 26.9 | 28.5 | 25.2 |
| Parental history of diabetes (%) | |||||||
| Yes | 2282 | 35.9 | 37.0 | 35.8 | 37.2 | 37.0 | 36.0 |
| No | 3975 | 64.1 | 63.0 | 64.2 | 62.8 | 63.0 | 64.0 |
| Parental history of early MI (%) | |||||||
| Yes | 2445 | 37.9 | 36.6 | 37.1 | 37.4 | 37.0 | 37.5 |
| No | 4116 | 62.1 | 63.4 | 62.9 | 62.6 | 63.0 | 62.5 |
| CVD risk6 (%) | |||||||
| Prior CVD | 4373 | 66.3 | 66.4 | 67.1 | 65.6 | 66.0 | 66.7 |
| ≥3 Risk factors | 2219 | 33.7 | 33.6 | 32.9 | 34.4 | 34.0 | 33.3 |
| Current multivitamin use (%) | |||||||
| Yes | 1813 | 27.0 | 28.5 | 27.9 | 27.6 | 27.5 | 27.9 |
| No | 4727 | 73.0 | 71.5 | 72.1 | 72.5 | 72.5 | 72.1 |
| Total energy intake7 (kcal/d) | 6236 | 1733 ± 561 | 1724 ± 545 | 1730 ± 551 | 1728 ± 555 | 1730 ± 554 | 1727 ± 551 |
| Dietary vitamin C intake7 (mg/d) | 6236 | 232 ± 228 | 234 ± 227 | 238 ± 240 | 227 ± 214 | 233 ± 224 | 232 ± 230 |
| Dietary vitamin E intake7 (IU/d) | 6236 | 127 ± 200 | 130 ± 203 | 130 ± 205 | 127 ± 198 | 130 ± 202 | 128 ± 201 |
| Dietary intake of total carotene7 (IU/d) | 6236 | 10,771 ± 8442 | 10,585 ± 8474 | 10,740 ± 8321 | 10,616 ± 8593 | 10,675 ± 8122 | 10,680 ± 8778 |
HT, hormone therapy; MI, myocardial infarction; CVD, cardiovascular disease.
Mean ± SD (all such values).
Total energy expended during physical activity (kcal/wk).
Hypertension was defined as a self-reported systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, physician-diagnosed hypertension, or current antihypertensive treatment.
Hypercholesterolemia was defined as a self-reported total cholesterol ≥240 mg/dL, physician-diagnosed high cholesterol, or current cholesterol-lowering treatment.
All of the participants were at high risk of CVD; they had either a self-reported history of CVD (a history of MI, stroke, revascularization procedure, angina pectoris, or transient cerebral ischemia) or ≥3 cardiac risk factors. These cardiac risk factors were self-reported diagnosis of hypertension, high cholesterol concentration, or diabetes mellitus; parental history of premature MI (before age 60 y); obesity (BMI ≥30); and current cigarette smoking.
Baseline dietary information was collected by using a semiquantitative food-frequency questionnaire.
Overall, there were trends toward a modest reduction in diabetes risk in the vitamin C group and a slightly increased risk in the vitamin E group, as compared with their respective placebo groups; the RRs were 0.89 for vitamin C (95% CI: 0.78, 1.02; P = 0.09) and 1.13 for vitamin E (95% CI: 0.99, 1.29; P = 0.07). There was no significant effect of β-carotene on the risk of type 2 diabetes (RR: 0.97; 95% CI: 0.85, 1.11; P = 0.68). When we subdivided the period of risk into years 1 and 2, years 3 to 5, and years >5 combined (Table 2), it appeared that the effects of both vitamin C and vitamin E became somewhat stronger after 5 y of follow-up, although we did not observe a statistically significant effect of β-carotene in any time period (Table 2). In sensitivity analyses to minimize misclassification due to undiagnosed diabetes at baseline and address the possibility of a latency effect, vitamin C treatment was associated with a significant 14% reduction in the risk of type 2 diabetes when excluding those cases that occurred in the first 2 y (RR: 0.86; 95% CI: 0.75, 1.00; P = 0.04). A nonsignificant 17% increase in diabetes risk associated with vitamin E was observed among women who took at least two-thirds of the study pills and did not use outside antioxidant supplements containing study agents (RR: 1.17; 95% CI: 0.97, 1.40; P = 0.10). Neither vitamin C treatment nor β-carotene had significant associations with type 2 diabetes in our sensitivity analyses taking compliance into account.
TABLE 2.
Relative risks (RRs) and 95% CIs of type 2 diabetes by randomized antioxidant intervention group in the Women's Antioxidant Cardiovascular Study
| Vitamin C |
Vitamin E |
β-Carotene |
||||||||||
| No. of events1 |
No. of events1 |
No. of events1 |
||||||||||
| Active (n = 3278) | Placebo (n = 3296) | RR (95% CI) | P | Active (n = 3297) | Placebo (n = 3277) | RR (95% CI) | P | Active (n = 3284) | Placebo (n = 3290) | RR (95% CI) | P | |
| Follow-up interval | ||||||||||||
| 1–2 y | 74 | 71 | 1.05 (0.76, 1.45) | 0.77 | 77 | 68 | 1.12 (0.81, 1.56) | 0.48 | 66 | 79 | 0.84 (0.61, 1.16) | 0.29 |
| 3–5 y | 156 | 176 | 0.90 (0.72, 1.12) | 0.33 | 169 | 163 | 1.03 (0.83, 1.28) | 0.76 | 168 | 164 | 1.02 (0.83, 1.27) | 0.84 |
| 0–5 y | 230 | 247 | 0.94 (0.79, 1.13) | 0.52 | 246 | 231 | 1.06 (0.89, 1.27) | 0.52 | 234 | 243 | 0.96 (0.81, 1.15) | 0.68 |
| >5 y | 190 | 228 | 0.84 (0.69, 1.01) | 0.07 | 228 | 190 | 1.21 (1.00, 1.47) | 0.05 | 207 | 211 | 0.98 (0.81, 1.19) | 0.87 |
| Total follow-up period | 420 | 475 | 0.89 (0.78, 1.02) | 0.09 | 474 | 421 | 1.13 (0.99, 1.29) | 0.07 | 441 | 454 | 0.97 (0.85, 1.11) | 0.68 |
| Sensitivity analyses excluding first 2 y2 | 346 | 404 | 0.86 (0.75, 1.00) | 0.04 | 397 | 353 | 1.13 (0.98, 1.30) | 0.10 | 375 | 375 | 1.00 (0.87, 1.16) | 0.98 |
| Compliance analyses3 | 240 | 247 | 0.94 (0.79, 1.12) | 0.49 | 243 | 221 | 1.17 (0.97, 1.40) | 0.10 | 264 | 261 | 1.01 (0.85, 1.20) | 0.92 |
The number of cases of type 2 diabetes.
Excluding those cases occurring in the first 2 y of follow-up.
Analyses restricted to those who reported taking at least two-thirds of the study pills and did not use outside antioxidant supplements containing study agents.
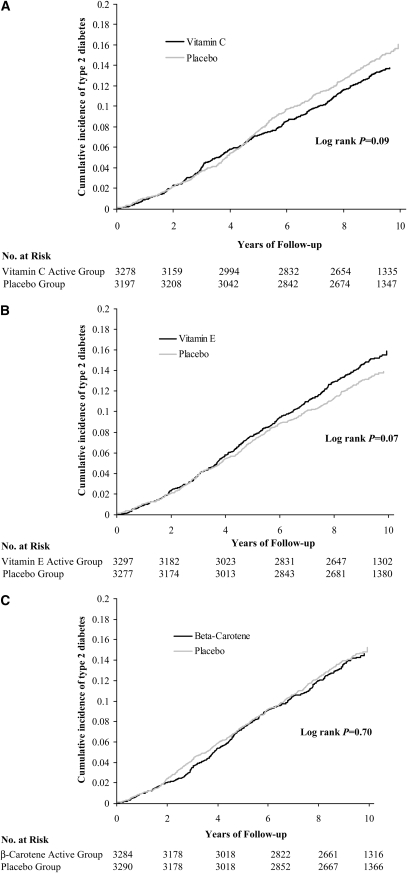
The cumulative incidence of type 2 diabetes events among women in the vitamin C and placebo groups by year of follow-up is shown in Figure 2A. A lower risk of type 2 diabetes in the vitamin C group appeared to emerge after year 5, but the log-rank test for the overall difference did not reach statistical significance (P for log-rank test = 0.09). For vitamin E (Figure 2B), there was a trend toward an elevated diabetes risk in year 6 and beyond, but the excess risk was of small magnitude and did not attain statistical significance (P for log-rank test = 0.07). However, none of the tests for interactions between time and treatment was significant. In contrast, the curves were almost identical in the β-carotene and placebo groups (P for log-rank test = 0.70; Figure 2C).
FIGURE 2.
Cumulative incidence of type 2 diabetes by randomized antioxidant intervention. (A) Active vitamin C compared with placebo; (B) active vitamin E compared with placebo; and (C) active β-carotene compared with placebo in the Women's Antioxidant Cardiovascular Study. Kaplan-Meier survival curves were used to estimate the overall cumulative incidence over time for each active vitamin group and its corresponding placebo group. The log-rank test was performed to compute the P values for the differences.
To determine whether certain subgroups of women were at particularly high or low risk of type 2 diabetes from these interventions, we conducted multiple subgroup analyses stratified by diabetes risk factors. History of high cholesterol concentrations significantly modified the effect of vitamin C on type 2 diabetes (P = 0.01); there was a significant reduction in diabetes risk in women who had no history of high cholesterol concentrations (RR: 0.64, 95% CI: 0.48, 0.87) but a null association among those with a history of high cholesterol concentrations (RR: 0.97, 95% CI: 0.84, 1.13; see Figure 1 under “Supplemental data” in the online supplement). Otherwise, no other significant effect modifications were observed for vitamin C treatment. This subgroup finding may have emerged by chance due to multiple comparison and needs to be confirmed in future investigations. Overall, none of these prespecified diabetes risk factors, including smoking, modified the effects of vitamin E and β-carotene on type 2 diabetes (see Figure 1 under “Supplemental data” in the online supplement).
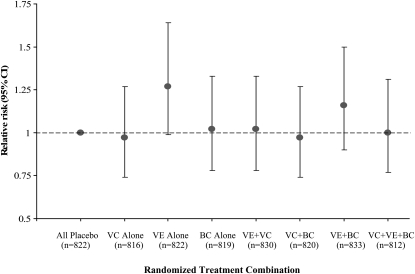
In a separate analysis in which the effects of each of the combinations of active agents were compared with the group receiving all 3 placebos, we found no significant differences in diabetes risk (Figure 3). Women who received any antioxidant alone or in any combination of 2 or 3 had rates of diabetes similar to those of the women receiving all placebos, although there was a trend for a nonsignificant elevation in risk of type 2 diabetes in the group taking vitamin E alone. In addition, there were no significant 2- or 3-way interactions among the agents for diabetes risk.
FIGURE 3.
Relative risks (95% CIs) of type 2 diabetes by 8 combinations of all 3 active antioxidant assignments relative to the all placebo group in the Women's Antioxidant Cardiovascular Study. We used Cox proportional hazards models to calculate the estimates of relative risks and 95% CIs for each antioxidant treatment alone, after adjustment for age and other randomized treatments. We also tested interactions of the 3 antioxidant agents by using all of the 2-way and 3-way interaction terms in the Cox model and found no significant results. VC, vitamin C; VE, vitamin E; BC, β-carotene.
DISCUSSION
In this large, randomized, double-blind, placebo-controlled trial, we found no significant overall effects of vitamins C and E and β-carotene on risk of type 2 diabetes in women at high risk of CVD with >9 y of follow up. In secondary analyses, a protective effect of vitamin C on type 2 diabetes was suggested after longer treatment duration (≥5 y) or after minimizing bias due to misclassification of undiagnosed diabetes in our sensitivity analyses. There was also a trend toward elevated risk associated with vitamin E treatment after 6 y of follow-up, but this was not statistically significant. However, these significant findings may have been due to chance and need to be confirmed in future investigations.
The present study was a secondary analysis for primary prevention of type 2 diabetes within the WACS trial, which was designed primarily to evaluate the efficacy of 3 antioxidant supplements in CVD prevention. To our knowledge, our study provides the first trial data regarding the long-term effect of vitamin C supplementation on risk of type 2 diabetes. Our subgroup analyses showed a significant reduction in diabetes risk with active vitamin C among the prespecified subgroup of women with hypercholesterolemia. Despite the possibility that these results could be explained by chance alone due to low statistical power or multiple comparisons, the potential protective effect on diabetes risk by vitamin C supplementation may be biologically plausible. A large body of evidence has suggested that vitamin C, as a potent water-soluble antioxidant, possesses the ability to scavenge several reactive species and regenerate tocopherols and tocotrenols from their respective radical species (7). Vitamin C may also have a role in the energy-dependent release of insulin from pancreatic islets (34). Although vitamin C is essential for humans, dietary vitamin C intake may be suboptimal in the US general population, especially in the elderly (7). However, there remains much controversy about the optimal vitamin C dose to be administered. High-dose vitamin C (1000–2000 mg/d), widely used in previous short-term trials studying different endpoints, has been shown to improve several lipid and glycemic parameters in some small clinical trials of diabetic patients (23–25); however, the long-term safety of vitamin C with such large doses in the general population has been questioned. The dose of vitamin C supplementation in our trial (500 mg/d) was a relatively high and safe dose. This dose is sufficient to elicit steady state plasma saturation of vitamin C concentrations in healthy people (35) but far below the tolerable upper intake amount of vitamin C ingestion (2000 mg/d) in adults that is associated with adverse effects (36). At present, this finding should serve as a stimulus for further investigation rather than supporting a recommendation for vitamin C supplementation to prevent type 2 diabetes.
For vitamin E, we found a nonsignificant elevation in risk. This finding was unexpected and must be viewed in the context of all of the available evidence of the risks and benefits of vitamin E. Available trial data from another large randomized trial, the Women's Health Study, did not support either a detrimental or beneficial effect of vitamin E (600 IU every other day for 10 y) on the primary prevention of type 2 diabetes in apparently healthy women (29). Although the 2 trials used identical doses and the same natural source of α-tocopherol or α-tocopherol acetate, the present population is a group of women at high risk of CVD. Differences in the study populations could at least partially explain the difference in results between the 2 trials. It is plausible, but unproved, that vitamin E is harmful in special subgroups with high oxidative stress. These populations (including our study population) manifest a high prevalence of metabolic disorders, and vitamin E might exert some prooxidant rather than antioxidant effects. Exogenous vitamin E could interfere with the physiologic progression of oxidative stress owing to its nonantioxidant pleiotropic effects (37). However, we did not find any strong effect modification by categories of CVD risk factors, such as history of hypertension, high cholesterol concentrations, or the number of CVD risk factors that are associated with increased production of reactive oxygen species and lipid peroxidation. Also, there is evidence to show that α-tocopherol supplementation decreases plasma concentrations of γ-tocopherol and could have attenuated the beneficial effects of γ-tocopherol, which is the major dietary form of vitamin E (38). However, due to scant data, it remains controversial whether γ-tocopherol is a more potent antioxidant than α-tocopherol. α-Tocopherol, the most common form used in vitamin supplements (39) in the United States, also has been shown to have effective antioxidant and antiinflammatory activities (40, 41). In addition, concerns have been raised regarding potential adverse effect of high-dose vitamin E supplementation in apparently healthy people. There is evidence that high doses of α-tocopherol may have potential prooxidant effects (37) and that vitamin E supplementation at dosages >400 IU/d may increase all-cause mortality (42). However, there were no differences in either total mortality or CVD events from the dose of 600 IU vitamin E every other day used in our WACS trial (32). Thus, the dose of vitamin E in our trial cannot explain the possible increase in diabetes risk. Given the inconsistencies with other studies, these results may be due to chance and should be interpreted with caution.
Our null finding for the effect of β-carotene supplementation on type 2 diabetes appears congruent with previous null findings in men from the Physicians' Health Study (30). The same dose and formulation were used in both trials, although our study population is different from the Physicians' Health Study in its inclusion of initially healthy men. β-Carotene supplementation at 50 mg on alternate days elevated plasma β-carotene concentrations by ≈4-fold; thus, inadequate dose or duration of treatment was not likely to explain the observed null results in the present study. Taken together, our trial results provide further evidence for the lack of benefit from β-carotene supplementation on type 2 diabetes and on other oxidative stress-related chronic diseases, such as CVD (31). Its routine use in the general population should be discouraged.
Some limitations of our trial deserve consideration. First, declining compliance over time in the WACS may have diluted the findings. However, in sensitivity analyses, although taking into account compliance, the overall null effects and trends were unchanged. Second, case misclassification due to the underdiagnosis of type 2 diabetes is a concern, because the study population was not screened for glucose tolerance, and diagnosis was self-reported. However, all of the participants in this study are health professionals who have been shown to provide reliable self-reported diagnostic information and have a relatively high screening rate for diabetes. The proportions of underdiagnosed cases are likely to be nondifferential between the treatment group and placebo group due to effective randomization and double-blinding strategies but nondifferential misclassification may have attenuated the findings, especially in the earlier follow-up years. Third, we made no direct measures of oxidative stress to assess the effect of antioxidant supplementation. Fourth, vitamins C and E and β-carotene from diet or other sources is an unlikely explanation for our null findings, because dietary intake should have been comparable in the active treatment and placebo groups, and accounting for outside use of vitamins C and E and β-carotene did not make a difference. We also found no evidence of effect modification by dietary intake of these antioxidants at baseline. Finally, our results based on a population at high risk of CVD may not be generalizable to healthy and low-risk populations.
In conclusion, our randomized trial did not find any statistically significant benefit or harm of vitamins C and E and β-carotene supplementation on the primary prevention of type 2 diabetes. Although our sensitivity analyses suggested a modest protective effect of vitamin C, this result could be a chance finding and needs to be confirmed in future investigations. Although additional research is needed, our findings do not support recommending antioxidant supplements for the primary prevention of type 2 diabetes.
Supplementary Material
Acknowledgments
We acknowledge the contributions of the following members of the Data Safety and Monitoring Board: L Cohen, R Collins, T Colton, D DeMets, IC Henderson, A La Croix, R Prentice, and N Wenger (chair) and ex officio members MF Cotch, F Ferris, L Friedman, P Greenwald, N Kurinij, M Perloff, E Schron, A Zonderman. We are grateful for the invaluable contributions of the WACS staff, including Elaine Zaharris, Jean MacFadyen, Ellie Danielson, Marilyn Chown, Shamikhah Curry, Margarette Haubourg, Felicia Zangi, Tony Laurinaitis, Geneva McNair, Philomena Quinn, Harriet Samuelson, Ara Sarkissian, and Martin Van Denburgh. We also thank the endpoints reviewers, including Michelle Albert, Gavin Blake, Claudia Chae, Wendy Chen, Bill Christen, Carlos Kase, Tobias Kurth, I-Min Lee, Aruna Pradhan, Paul Ridker, Jackie Suk, James Taylor, and Simin Liu. Finally, we are indebted to the 8171 dedicated WACS participants.
The authors' responsibilities were as follows—YS and JEM: conceived and designed the study; YS, NRC, and MVD: analyzed the data; YS, NRC, CMA, MVD, and JEM: interpreted the data; YS: drafted the manuscript; and NRC, CMA, MVD, and JEM: critically reviewed the manuscript. None of the authors reported financial conflicts of interest related to the present article.
REFERENCES
- 1.Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V. Abnormal antioxidant status in impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabet Med 1996;13:715–9 [DOI] [PubMed] [Google Scholar]
- 2.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003;52:1–8 [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 2004;24:816–23 [DOI] [PubMed] [Google Scholar]
- 4.Gopaul NK, Manraj MD, Hebe A, et al. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia 2001;44:706–12 [DOI] [PubMed] [Google Scholar]
- 5.Chin JH, Azhar S, Hoffman BB. Inactivation of endothelial derived relaxing factor by oxidized lipoproteins. J Clin Invest 1992;89:10–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bast A, Wolf G, Oberbäumer I, Walther R. Oxidative and nitrosative stress induces peroxiredoxins in pancreatic beta cells. Diabetologia 2002;45:867–76 [DOI] [PubMed] [Google Scholar]
- 7.Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 2003;22:18–35 [DOI] [PubMed] [Google Scholar]
- 8.Handelman GJ. Carotenoids as scavengers of active oxygen species. 1st ed New York, NY: Marcel Dekker, 1996 [Google Scholar]
- 9.Niki E. Alpha-tocopherol. 1st ed New York, NY: Marcel Dekker, 1996 [Google Scholar]
- 10.Slonim AE, Surber ML, Page DL, Sharp RA, Burr IM. Modification of chemically induced diabetes in rats by vitamin E. Supplementation minimizes and depletion enhances development of diabetes. J Clin Invest 1983;71:1282–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy VK, Shipp JC, Hanson C, Shipp DM. Delayed onset and decreased incidence of diabetes in BB rats fed free radical scavengers. Diabetes Res Clin Pract 1992;18:11–6 [DOI] [PubMed] [Google Scholar]
- 12.Sargeant LA, Wareham NJ, Bingham S, et al. Vitamin C and hyperglycemia in the European Prospective Investigation into Cancer–Norfolk (EPIC-Norfolk) study: a population-based study. Diabetes Care 2000;23:726–32 [DOI] [PubMed] [Google Scholar]
- 13.Sinclair AJ, Taylor PB, Lunec J, Girling AJ, Barnett AH. Low plasma ascorbate levels in patients with type 2 diabetes mellitus consuming adequate dietary vitamin C. Diabet Med 1994;11:893–8 [DOI] [PubMed] [Google Scholar]
- 14.Will JC, Ford ES, Bowman BA. Serum vitamin C concentrations and diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 1999;70:49–52 [DOI] [PubMed] [Google Scholar]
- 15.Abahusain MA, Wright J, Dickerson JW, de Vol EB. Retinol, alpha-tocopherol and carotenoids in diabetes. Eur J Clin Nutr 1999;53:630–5 [DOI] [PubMed] [Google Scholar]
- 16.Polidori MC, Mecocci P, Stahl W, et al. Plasma levels of lipophilic antioxidants in very old patients with type 2 diabetes. Diabetes Metab Res Rev 2000;16:15–9 [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Will JC, Bowman BA, Narayan KM. Diabetes mellitus and serum carotenoids: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 1999;149:168–76 [DOI] [PubMed] [Google Scholar]
- 18.Feskens EJ, Virtanen SM, Rasanen L, et al. Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 1995;18:1104–12 [DOI] [PubMed] [Google Scholar]
- 19.Salonen JT, Nyyssonen K, Tuomainen TP, et al. Increased risk of non-insulin dependent diabetes mellitus at low plasma vitamin E concentrations: a four year follow up study in men. BMJ 1995;311:1124–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer-Davis EJ, Costacou T, King I, Zaccaro DJ, Bell RA. Plasma and dietary vitamin E in relation to incidence of type 2 diabetes: The Insulin Resistance and Atherosclerosis Study (IRAS). Diabetes Care 2002;25:2172–7 [DOI] [PubMed] [Google Scholar]
- 21.Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 2004;27:362–6 [DOI] [PubMed] [Google Scholar]
- 22.Reunanen A, Knekt P, Aaran RK, Aromaa A. Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur J Clin Nutr 1998;52:89–93 [DOI] [PubMed] [Google Scholar]
- 23.Mullan BA, Young IS, Fee H, McCance DR. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 2002;40:804–9 [DOI] [PubMed] [Google Scholar]
- 24.Paolisso G, Balbi V, Volpe C, et al. Metabolic benefits deriving from chronic vitamin C supplementation in aged non-insulin dependent diabetics. J Am Coll Nutr 1995;14:387–92 [DOI] [PubMed] [Google Scholar]
- 25.Regensteiner JG, Popylisen S, Bauer TA, et al. Oral L-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med 2003;8:169–75 [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Karne RJ, Hall G, et al. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol 2006;290:H137–45 [DOI] [PubMed] [Google Scholar]
- 27.Darko D, Dornhorst A, Kelly FJ, Ritter JM, Chowienczyk PJ. Lack of effect of oral vitamin C on blood pressure, oxidative stress and endothelial function in Type II diabetes. Clin Sci (Lond) 2002;103:339–44 [DOI] [PubMed] [Google Scholar]
- 28.Bishop N, Schorah CJ, Wales JK. The effect of vitamin C supplementation on diabetic hyperlipidaemia: a double blind, crossover study. Diabet Med 1985;2:121–4 [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Lee IM, Song Y, et al. Vitamin E and risk of type 2 diabetes in the women's health study randomized controlled trial. Diabetes 2006;55:2856–62 [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Ajani U, Chae C, Hennekens C, Buring JE, Manson JE. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA 1999;282:1073–5 [DOI] [PubMed] [Google Scholar]
- 31.Bassuk SS, Albert CM, Cook NR, et al. The Women's Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004;13:99–117 [DOI] [PubMed] [Google Scholar]
- 32.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med 2007;167:1610–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97 [DOI] [PubMed] [Google Scholar]
- 34.Wells WW, Dou CZ, Dybas LN, Jung CH, Kalbach HL, Xu DP. Ascorbic acid is essential for the release of insulin from scorbutic guinea pig pancreatic islets. Proc Natl Acad Sci USA 1995;92:11869–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 1996;93:3704–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Food Nutrition Board, Institute of Medicine Vitamin C. In: Reference dietary intakes for vitamin C, vitamin E, selenium, and carotenoids Washington, DC: National Academy Press, 2000:95–185 [Google Scholar]
- 37.Santanam N, Parthasarathy S. Paradoxical actions of antioxidants in the oxidation of low density lipoprotein by peroxidases. J Clin Invest 1995;95:2594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hensley K, Benaksas EJ, Bolli R, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med 2004;36:1–15 [DOI] [PubMed] [Google Scholar]
- 39.Ford ES, Ajani UA, Mokdad AH. Brief communication: the prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann Intern Med 2005;143:116–20 [DOI] [PubMed] [Google Scholar]
- 40.Meydani M. Vitamin E. Lancet 1995;345:170–5 [DOI] [PubMed] [Google Scholar]
- 41.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr 2005;25:151–74 [DOI] [PubMed] [Google Scholar]
- 42.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.