Abstract
Perfluorooctanoic acid (PFOA) is a ligand for peroxisome proliferator-activated receptor (PPAR) α, which exhibits marked species differences in expression and function, especially between rodents and humans. We investigated the functional difference in PFOA response between mice and humans, using a humanized PPARα transgenic mouse line. Three genotyped mice, 129/Sv wild-type (mPPARα), Pparα-null mice and humanized PPARα (hPPARα) mice (8-week-old males) were divided into three groups: the first was treated with water daily for 2 weeks by gavage (control group), and the remaining two groups were treated with 0.1 and 0.3 mg/kg ammonium perflurooctanate (APFO), respectively, for 2 weeks by gavage. The APFO dosages used did not influence the plasma triglyceride or total cholesterol levels in any mouse line, but the high dose increased both hepatic lipid levels only in mPPARα mice. APFO increased mRNA and/or protein levels of PPARα target genes cytochrome P450 Cyp4a10, peroxisomal thiolase and bifunctional protein only in the liver of mPPARα mice, but not in Pparα-null or hPPARα mice. This chemical also increased expression of mitochondrial very long chain acyl-CoA dehydrogenase only in the liver of mPPARα mice. Taken together, human PPARα may be less responsive to PFOA than that of mice when a relatively low dose is applied. This information may be very valuable in considering whether PFOA influences the lipid metabolism in humans.
Keywords: Ammonium perflurooctanate, Peroxisome proliferator-activated receptor, (PPAR) α, Humanized PPARα mouse, Pparα-null mouse, Lipid metabolism
1. Introduction
Perfluorooctanoic acid (PFOA) (CAS, 335-67-1), an organofluoro compound, is used in industrial surfactants, emulsifiers and numerous consumer products (U.S. Environmental Protection Agency, U.S. EPA, 2006). This chemical is very stable and incombustible at normal temperature, and when released into the environment it is not decomposed in either the ecosystem or the body. Repeated exposure to PFOA may result in accumulation in humans and wildlife (Prevedouros et al., 2006). Thus, PFOA is scheduled to be added to the list of Persistent Organic Pollutants (POPs) in the near future (World Wildlife Fund, 2005).
PFOA is thought to be resistant to clearance in humans, with a biological half-life of 3.5–4.4 years (U.S. EPA, 2002; Olsen et al., 2007). Since there are dramatic sex and species clearance (Kennedy et al., 2004), it is very difficult to evaluate the level of human risk. Several studies of the potential health risk of exposure to PFOA have been conducted. Ammonium perfluorooctanoate (APFO) causes hepatic peroxisome proliferation (Pastoor et al., 1987), liver hyperplasia, liver adenoma, pancreatic acinar cell adenomas (Biegel et al., 2001) and Leydig cell adenomas (Cook et al., 1992; Biegel et al., 2001) in rats and mice. Since PFOA is not mutagenic, the induction of these tumors most likely occurs through nongenotoxic mechanisms (Kennedy et al., 2004).
PFOA is a peroxisome proliferator-activated receptor α (PPARα) agonist in mice and rats (Ikeda et al., 1985; Rosen et al., 2007, 2008a,b), and through PPARα increases peroxisomal and mitochondrial β-oxidation of fatty acids, inhibits the secretion of very low-density lipoproteins and cholesterol from the liver, as well as reduces cholesterol and triglyceride in serum and the accumulation of lipids in the liver (Maloney and Waxman, 1999; Xie et al., 2003; Kennedy et al., 2004). Epidemiological studies have resulted in disparate findings. Olsen et al. (2003a), reported a positive association between PFOA and serum cholesterol (T-Cho) and triglycerides (TG) among participants in a fluorochemical medical surveillance program in some factories. In contrast, no such association was reported in another factory (Olsen et al., 2003b). Since there are species differences in the expression levels of PPARα, it is very difficult to accurately evaluate the exact effects of PFOA on the lipid levels of human from the experiments using mice or rats. To investigate species-specific effects and mechanisms of PPARα, PPARα-humanized (hPPARα) mice were recently established (Cheung et al., 2004). The mice express human PPARα specifically in the liver under the control of the doxycycline (Tet-OFF) system in a PPARα-null background. High-level expression of human PPARα protein achieved in this hPPARα mouse model proved comparable to murine PPARα expression in wild-type mice. This mouse model was shown to be a useful tool in determining human PPARα function in the effects of Wy-14,643 on both the serum and hepatic lipid levels (Cheung et al., 2004).
In this study, we compared the effects of relatively low doses of APFO (0, 0.1, and 0.3 mg/kg) on the PPARα and target gene expression as well as on lipid levels between mouse and human PPARα using wild-type, Pparα-null and hPPARα mice, in order to determine the possible effects of this chemical or its principal metabolite, PFOA, on the lipid metabolism in humans.
2. Materials and methods
2.1. Chemicals
APFO was purchased from Tokyo Kasei Kogyo (Tokyo, Japan).
2.2. Experimental animals
This study was conducted according to the Guidelines for Animal Experiments of the Nagoya University Animal Center. Three genotyped male and female mice, i.e., wild-type (mPPARα), Pparα-null (Lee et al., 1995; Akiyama et al., 2001) and hPPARαTet-Off (Cheung et al., 2004) with a Sv/129 genetic background were provided by Dr. Gonzalez (U.S. National Institutes of Health), and each genotyped male and female was mated in our animal center. All mice were housed in a temperature- and light-controlled environment (25 °C, 12 h light/dark cycle), and maintained on stock rodent chow and tap water ad libitum. At 8 weeks old, the offspring male mice of each strain were assigned to treatment groups as follows: treated with distilled water daily for 2 weeks by gavage (control group); treated with 0.1 or 0.3 mg/kg APFO, respectively, for 2 weeks by gavage (Table 1). On the day following the last dose (18–20 h later), all mice were killed by decapitation, and the blood and livers were removed. The liver samples were stored at −80 °C until use. Plasma was collected after centrifuging blood 3500×g for 10 min and stored at −80 °C until use. All samples were used in all measurements, and the measurements were done in duplicate or triplicate except for the histopathology.
Table 1.
Body and liver weight.
| Total mice | APFO |
|||
|---|---|---|---|---|
| n | 0mg/kg (n) | 0.1 mg/kg (n) | 0.3 mg/kg (n) | |
| Body weight (g) at start of APFO treatments | ||||
| mPPARα | 13 | 21.9 ± 0.7 (4) | 23.3 ± 2.1 (5) | 21.2 ± 1.0 (4) |
| Pparα-null | 18 | 21.5 ± 2.4 (6) | 21.4 ± 1.1 (6) | 19.4 ± 2.0 (6) |
| hPPARα | 15 | 18.4 ± 2.0 (5)$ | 20.6 ± 1.0 (5) | 19.5 ± 1.2 (5) |
| Body weight (g) after APFO treatments | ||||
| mPPARα | 13 | 24.2 ± 0.3 (4) | 24.9 ± 1.6 (5) | 23.8 ± 1.5 (4) |
| Pparα-null | 18 | 24.3 ± 1.7 (6) | 22.1 ± 0.8 (6) | 22.7 ± 1.3 (6) |
| hPPARα | 15 | 20.0 ± 2.2 (5)$ | 21.4 ± 1.4 (5) | 19.7 ± 1.9 (5) |
| Body weight change (g) | ||||
| mPPARα | 13 | 2.3 ± 0.6 (4) | 1.6 ± 0.7 (5) | 2.6 ± 2.9 (4) |
| Pparα-null | 18 | 2.8 ± 0.6 (6) | 0.7 ± 3.4 (6) | 3.2 ± 2.2 (6) |
| hPPARα | 15 | 1.6 ± 0.5 (5) | 0.8 ± 1.0 (5) | 0.1 ± 1.4 (5) |
| Liver weight (g) | ||||
| mPPARα | 13 | 1.17 ± 0.11 (4) | 1.24 ± 0.05 (5) | 1.38 ± 0.15 (4)* |
| Pparα-null | 18 | 1.24 ± 0.07 (6) | 1.05 ± 0.14 (6)* | 1.27 ± 0.15 (6) |
| hPPARα | 15 | 0.90 ± 0.14 (5)$ | 0.94 ± 0.10 (5) | 0.90 ± 0.20 (5) |
| Liver/body ratio (%) | ||||
| mPPARα | 13 | 4.85 ± 0.47 (4) | 5.02 ± 0.18 (5) | 5.78 ± 0.33 (4)* |
| Pparα-null | 18 | 5.12 ± 0.30 (6) | 4.75 ± 0.53 (6) | 5.56 ± 0.43 (6)# |
| hPPARα | 15 | 4.48 ± 0.50 (5) | 4.41 ± 0.30 (5) | 4.55 ± 0.67 (5) |
Data represent mean ± S.D.
Significantly different from respective control (0 mg/kg) group (P < 0.05).
Significantly different from respective low-dose (0.1 mg/kg) group (P < 0.05).
Significantly different from control of Pparα-null mice (P < 0.05).
2.3. Nuclear fraction
A nuclear fraction was extracted from a part of the frozen liver using a CelLytic® NuCLEAR® Extraction Kit (SIGMA, Tokyo, Japan).
2.4. Analysis of protein concentrations
Each tissue was homogenized with a threefold volume of 10 mM phosphate buffer (pH 7.4) containing 0.25 M sucrose. Protein concentrations of the homogenate and nuclear fraction samples were measured using a Protein Assay Kit (Bio-Rad, Tokyo, Japan).
2.5. Western blotting
Liver homogenate or nuclear fraction samples for electrophoresis were subjected to 10% SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 3% skim milk, each membrane was incubated with the respective primary antibody, followed by incubation with alkaline phosphatase-conjugated goat anti-rabbit IgG (Jackson Immuno Research, West Grove, PA). The primary polyclonal antibody was prepared using purified medium-chain acyl-CoA dehydrogenase (MCAD) (Furuta et al., 1981), very long chain acyl-CoA dehydrogenase (VLCAD) (Izai et al., 1992), peroxisomal thiolase (PT) (Furuta et al., 1980) and peroxisomal bifunctional protein (PH) (Osumi and Hashimoto, 1980). These antibodies have been described earlier (Aoyama et al., 1998; Nakajima et al., 2000). The primary polyclonal antibodies of PPARα and RXRα were purchased from Santa Cruz Biotechnology, Inc. (CA). Each band was quantified using the densitometry with the Lane & Spot Analyzer version 5.0 (ATTO Corporation, Tokyo, Japan).
2.6. Real-time quantitative PCR
mPPARα (Accession No. NM_011144) -, hPPARα (BC000052) -, RXRα (NM_011305) -, CYP4A10 (AB018421) -, MCAD (NM_007382) -, VLCAD (NM_017366) -, PT (NM_130864) -, PH (NM_023737) -, diacylglycerol acyltransferase (DGAT) 1 (NM_010046) - and 2 (NM_02638), p65 (XM_342346) -, p50 (AY521463) - and p52 (AF155372) -, tumor necrosis factor (TNF) α (NM_013693) -, TNF receptor (TNFR) 1 (NM_011609) -, and TNFR2 (NM_011610) -mRNA levels were monitored by the ABI PRISM 7000 Sequence Detection system (Applied Biosystems, Foster City, CA). Total RNA was isolated using a RNeasy Protect Mini Kit (QIAGEN, Tokyo, Japan). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using Oligo(dT)20 primer. RNA quantity and quality were checked by a GeneQuant II RNA/DNA Calculator (Pharmacia Biotech, Framingham, MA). The sequences for the forward and reverse primers were as follows: hPPARα forward: 5′-GCGATCTAGAGAGCCCGTTATC-3′ and reverse: 5′-GCCAAAGCTTCCAGAACTATCC-3′; RXRα forward: 5′-CAGTTGCTGCGCATCCAA-3′ and reverse: 5′-GTGCTGCTAAATAACTCTTGCATGA-3′; DGAT1 forward: 5′-GGCGGTCCCCAACCAT-3′ and reverse 5′-GCTCTGCCACAGCATTGAGA-3′; DGAT2 forward: 5′-TGGCCTGCAGTGTCATCCT-3′ and reverse: 5′-GGGCGTGTTCCAGTCAAATG-3′. The sequences of other genes have been described elsewhere (Ito et al., 2007; Ramdhan et al., 2008).
The PCR mixture (25 μl) contained 1×SYBR Green PCR master Mix (Applied Biosystems), 0.1 μM of each primer, and cDNA diluted by TE including 1 μg/ml transfer-RNA. PCR amplification was as follows: an initial step for 2 min at 50 °C, then 10 min at 95 °C, followed by 50 cycles at 95 °C for 15 s and at 60 °C for 1 min. Each mRNA level was normalized to the level of GAPDH (BC096440) mRNA expression in the same preparation.
2.7. Lipid concentrations in plasma and liver
Lipid from livers was extracted using the method of Folch. TG and T-Cho in the liver and plasma were measured using kits of TG-IE and T-Cho IE (Wako, Osaka, Japan), respectively.
2.8. Histopathological analysis
The organs fixed in 10% neutral buffered formalin were embedded in paraffin and sliced into 4 μm sections. Tissue sections of the livers were stained with hematoxylin and eosin, and examined under a light microscope using the BZ-8000 (Keyence, Osaka, Japan). Necrotic cells were measured in a blinded fashion according to the method of Soni et al. (1998). Inflammatory cells, and lipid accumulation were measured in a blinded fashion referring to the methods of Brunt et al. (1999) and Okiyama et al. (2009): (1) grade of steatosis: 0, none; 1, mild (5–33% of parenchymal involvement by steatosis); 2, moderate (33–66%); 3, severe (>66%); (2) intra-acinar (lobular) and portal tract inflammations: 0, none; 1, mild; 2, moderate; 3, severe.
2.9. Alanine aminotransferase (ALT) measurement
Plasma ALT was measured using a Transaminase C II Test kit purchased from Wako (Osaka, Japan).
2.10. Statistical analysis
Comparisons were made using two-way analysis of variance (ANOVA) and the Tukey–Kramer HSD post hoc test among each treated group of each kind of genotyped mice, or also between mPPARα or Pparα-null mice and hPparα mice with the same dose. Values of P < 0.05 were considered to indicate statistical significance.
3. Results
3.1. Body and liver weights
Body and liver weights of hPPARα mice were lower than those of mPPARα and Pparα-null mice, but APFO treatment did not influence the increase in the body weight of all genotyped mice (Table 1). The low dose (0.1 mg/kg) of APFO did not influence either absolute or relative liver weights in any mouse line, while the APFO high dose (0.3 mg/kg) increased the liver weight and the ratio of liver/body weight only in mPPARα mice.
3.2. mRNA and protein levels of PPARα and related genes
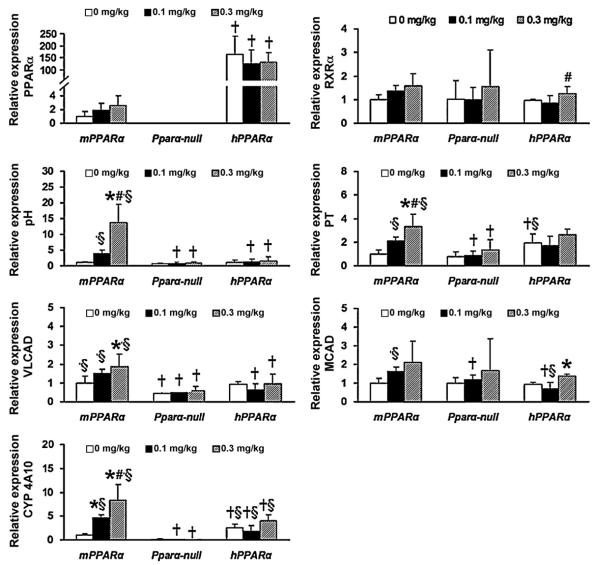
In all groups, the expressions of PPARα protein and mRNA were higher in hPPARα mice than in mPPARα mice (Figs. 1 and 2). APFO treatments did not influence either PPARα mRNA or protein levels in any of the mice regardless of genotype, nor did they influence RXRα, which forms a heterodimer with PPARα in the nucleus, protein and mRNA levels, though a significant increase was observed in RXR-mRNA in hPPARα mice between 0.1 and 0.3 mg/kg APFO exposure.
Fig. 1.
mRNA expression of hepatic PPARα and related genes (n = 4–6) in duplicate or triplicate analyses. Expression of mRNA was analyzed by quantitative real-time PCR. Each mRNA was normalized to the level of GAPDH-mRNA expression in the same preparation, and mean of control in mPPARα mice was assigned a value of 1.0. Each column and bar represents mean and +S.D., respectively. *Significantly different from respective controls (P < 0.05). #Significantly different from respective low dose groups (P < 0.05). †Significantly different from mPPARα mice treated with same dose (P < 0.05). §Significantly different from Pparα-null mice treated with same dose (P < 0.05).
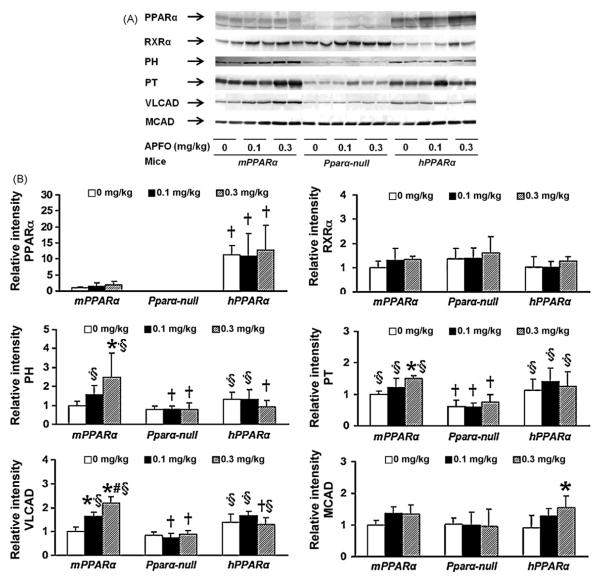
Fig. 2.
(A) Western blot analysis of hepatic PPARα and related genes. All mice from each treatment and genotype were examined across three numbers of gels, and one of them is shown here. (B) Western blot analysis of hepatic PPARα and the related genes (n = 4–6). Each band was quantified by densitometric analysis as described in Section 2, and mean strength of control in mPPARα mice was assigned a value of 1.0. Each column and bar represents mean and +S.D., respectively. A normalizing transformation was applied to PH value before Tukey–Kramer analysis. *Significantly different from respective controls (P < 0.05). #Significantly different from respective low dose groups (P < 0.05). †Significantly different from mPPARα mice treated with same dose (P < 0.05). §Significantly different from Pparα-null mice treated with same dose (P < 0.05).
We then analyzed the effects of APFO treatment on the induction of the PPARα target genes, PH, PT, VLCAD, MCAD and CYP4A10. Among these genes, PH was the most sensitive to APFO treatment; high dose APFO significantly increased PH mRNA and protein expression in mPPARα mice (Figs. 1 and 2). However, APFO increased neither PH mRNA nor protein levels in Pparα-null or hPPARα mice. APFO treatment influenced PT-mRNA and protein levels similarly to PH in mPPARα mice. A high dose of APFO increased mitochondrial VLCAD-mRNA and protein only in mPPARα mice, but the increases seemed to be less than those of the peroxisomal enzymes, PH and PT. MCAD-mRNA and protein levels were slightly increased only in hPPARα mice. CYP4A10-mRNA levels were not detectable in Pparα-null mice. In contrast, CYP4A10 expression was clearly detected in the livers of mPPARα and hPPARα mice (Fig. 1). APFO, however, clearly induced mRNA in the former, but not in the latter.
When the above mentioned gene expressions were compared at the same dosages, there were no significant differences in PH- and MCAD-mRNA and protein expressions among controls of three genotyped mice. In contrast, a significant difference was observed in the expression of VLCAD-mRNA and protein between the control mPPARα or hPparα mice and Pparα-null mice, whereas that of CYP4a10-mRNA was higher in hPparα mice than in mPPARα or Pparα-null mice. PT-mRNA expression was significantly higher in hPparα mice than mPPARα and Pparα-null mice, while the protein expression was higher in mPPARα and hPparα mice than Pparα-null mice.
APFO treatments did not increase hepatic NF-κB subunits, p65-, p50- and p52-mRNAs, TNFα and the receptors, suggesting that the dose used could not cause inflammation (data not shown).
3.3. TG and T-Cho levels in plasma and liver
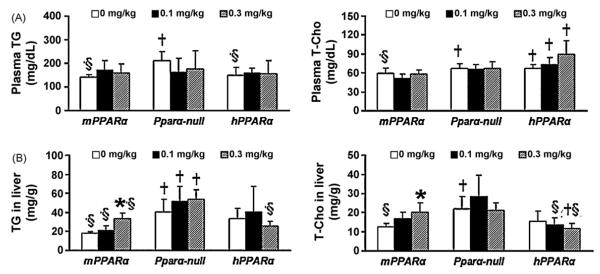
TG and T-Cho are typically decreased upon PPARα activation (Peters et al., 1997). TG and T-Cho in the liver and plasma were measured to investigate whether enzymes involved in lipid metabolism were induced by APFO treatment (Fig. 3). Although plasma TG levels in Pparα-null mice and T-Cho levels in Pparα-null and hPparα mice were significantly higher than those in mPPARα mice, APFO did not influence the levels in any mouse lines (Fig. 3(A)). In hPparα mice, plasma T-Cho levels of all dosage groups were higher than those of the mPPARα group.
Fig. 3.
Plasma and hepatic TG and T-Cho levels. (A) Plasma, (B) liver (n = 4–6). Each column and bar represents mean and +S.D., respectively. *Significantly different from control in mPPARα mice (P < 0.05). †Significantly different from mPPARα mice treated with same dose (P < 0.05). §Significantly different from Pparα-null mice treated with same dose (P < 0.05).
In the liver, TG and T-Cho levels were also significantly higher in Pparα-null mice than in mPPARα mice (Fig. 3(B)). Interestingly, a high dose of APFO significantly increased hepatic TG and T-Cho levels only in mPPARα mice. In Pparα-null mice, hepatic TG levels of all dosage group were higher than those of the mPPARα group.
We also measured mRNA levels of DGAT1 and 2 that are involved in TG synthesis. APFO treatments did not influence expression of these enzymes in any group (data not shown).
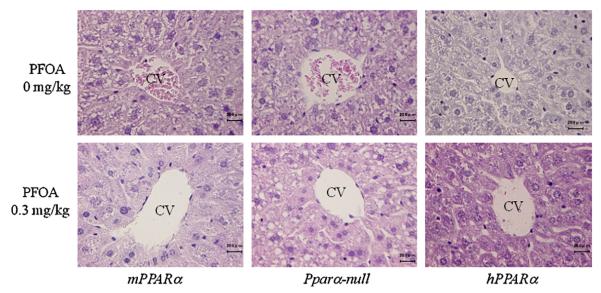
3.4. Histopathological changes
In the control animals, there were no obvious changes in the grade of steatosis, and the inflammatory cells in the lobular and portal area of liver in all genotyped mice, though little or no lipid accumulation was seen in the liver of PPARα-null mice (Fig. 4, score grade of data not shown). A high APFO dose did not cause any histopathological changes in liver except for the quite mild hepatocellular enlargements seen in mPPARα mice and hPPARα mice, and appeared to increase cytoplasmic vacuoles morphologically consistent with the lipid accumulation in zone 2 of hepatic lobule from Pparα-null mice as pointed out by Wolf et al. (2008a).
Fig. 4.
Histological findings were scored by an independent pathologist in a blinded fashion according to the following criteria: (1) grade of steatosis: 0, none; 1, mild (5–33% of parenchymal involvement by steatosis); 2, moderate (34–66%); 3, severe (66>); (2) activity of inflammation: 0, none; 1, mild; 2, moderate; 3, severe.
3.5. ALT levels in plasma
APFO treatment did not affect the ALT levels in any group (data not shown).
4. Discussion
In general, administration of peroxisome proliferators to rodents increases liver weight and the levels of PPARα target genes such as PT, PH, VLCAD (Aoyama et al., 1998; Nakajima et al., 2000) and CYP4A10 (Ramdhan et al., 2008). The present study clearly shows that a relatively low dose of APFO increased liver weight and induced these genes only in mPPARα, but not in Pparα-null or hPPARα mice. These results were quite distinct from an earlier report that another mPPARα ligand, Wy-14,643, induced some of these genes not only in mPPARα mice but also in hPPARα mice (Cheung et al., 2004). One reason for this discrepancy may lie in the different dosage used; the dose of Wy-14,643 was roughly estimated to be 0.37 mmol/kg when mice weighed 25 g and were consuming 3 g feed a day, whereas the dose of APFO (0.3 mg/kg) was calculated to be 0.70 μmol/kg in our study. Indeed, in another study using hPPARα mice, 1 mg/kg APFO, which is about 3 times higher than that in the current study, clearly activated human PPARα judging from the induction of the target genes (in preparation). Recently, Abbott and colleagues measured the lowest effective concentrations of PFOA at which mouse and human PPARα were activated in an in vitro system and showed them to be 10 μM and 30 μM, respectively (Takacs and Abbott, 2007). Wolf et al. (2008b) also reported that activation of mouse PPARα by APFO was generally higher compared to that of human PPARα. Taken together, hPPARα may have a weaker affinity for PFOA than does mPPARα. Foreman et al. investigated the activation of mouse PPARα and human PPARα, by perfluorobutyrate, which has a similar structure to PFOA and is a PPARα agonist, and showed that dosages higher than 35 mg/kg caused activation in both wild-type and humanized mouse strains, and this was the same hPPARα strain as used in the present study. Thus, when a relative high dosage of APFO or perfluorobutyrate is used, PPARα activation may be observed not only in mPPARα mice but also in hPPARα mice.
Expression of PPARα protein was thought not to differ between mPPARα and hPPARα mice, although the mRNA level was greater in the latter than in the former (Cheung et al., 2004). In the current experiment, expression of PPARα protein and mRNA appeared to be greater in hPPARα than in mPPARα mice. Nevertheless, the fact that PFOA-activated mPPARα but not hPPARα suggested that the functional activation of hPPARα itself may also be weak compared to that of mPPARα.
As noted above, although APFO-induced peroxisomal and mitochondrial lipid β-oxidation enzymes in mPPARα mice, it did not reduce TG and T-Cho levels in plasma, and only slightly increased levels in the liver. In contrast, APFO treatment did not increase either of these enzyme levels or plasma and hepatic lipid levels in hPPARα mice. Loveless et al. (2006) reported that the same dose of APFO as the one used in the current study did not affect the plasma TG levels. On the other hand, Kudo et al. (1999) showed that 0.02% APFO in the diet for a week (which corresponded to 20 mg/kg/day for rats with 200 g body weight) reduced plasma TG levels, but increased hepatic TG levels of rats. Similar results were also obtained in our recent study using 1 and 5 mg/kg APFO in mice. In such case, APFO exposure dose-dependently reduced plasma TG level but slightly increased TG in livers of mPPARα mice. Thus, a relatively high dose or long exposure duration might influence both plasma and hepatic TG levels. In the study by Kudo et al., APFO increased the expression levels of glycerol 3-phosphotransferase and DGAT, which are involved in TG synthesis and are thought to induce an increase in hepatic TG levels (Kudo et al., 1999). The expression of these genes may be important for their influence on hepatic TG levels. In the current study, however, APFO affected neither DGAT1 nor DGAT2 expression. After APFO treatment, TG increase in the liver was seen only in mPPARα mice, suggesting that some PPARα target gene(s) might also be involved in the increase. Since fatty acid transport protein is a PPARα target gene (Martin et al., 1997) involved in lipid transport to the liver, the involvement of this protein in TG accumulation should be also be investigated.
In control animals, plasma T-Cho levels were reported to be greater in Pparα-null mice than in mPPARα mice (Peters et al., 1997), a finding which was reconfirmed in the present study. It is thought that endogenous PPARα ligands such as leukotriene B4 and palmitic acid could activate PPARα (Desvergne and Wahli, 1999), which might result in increased cholesterol metabolism, followed by a reduction of T-Cho levels in mPPARα mice. Since human PPARα may be less responsive to these endogenous fatty acids, similar to PFOA, it might contribute to higher levels of plasma T-Cho in hPPARα mice.
Although we did not measure the serum PFOA concentration in mice, Loveless et al. (2006) reported that the serum concentration in mice after a 0.3 mg/kg administration of APFO for 14 days was 13 ± 2.4 μg/ml. In our experiment, we administered the same concentration of APFO for the same period, so that the serum PFOA level might be comparable with those reported by Loveless et al. On the other hand, the mean blood concentration of PFOA among workers engaged in its production was 2.21 μg/ml (95% confidence interval, 1.66–2.77 μg/ml) (Olsen and Zobel, 2007). Because durations of exposure to PFOA differ considerably between animal and epidemiological studies, and half-life of PFOA is 12 days in ddY male mice (Kudo and Kawashima, 2003), which is significantly lower than that of human (U.S. EPA, 2002; Olsen et al., 2007), mouse and human PFOA levels could not be directly compared. Nevertheless, both PFOA levels were not markedly different. Since 0.3 mg/kg APFO did not influence either plasma TG or T-Cho levels and did not affect expression of PPARα and its target genes in hPPARα mice, blood PFOA levels in the workers may not induce the functional activation of PPARα nor alter lipid levels in their blood. The finding that the expression level of PPARα was 10 times lower in humans than in mice or rats (Tugwood et al., 1996; Palmer et al., 1998) may further support our conclusion as noted above. Indeed, although Olsen et al. (2003a) found a relationship between serum PFOA and the TG or T-Cho levels in the workers in two factories, they did not find such an association in another factory (2003b). Then, they re-analyzed the data obtained in two studies (2003a, b) excluding subjects who took antilipemic agents, and reported that serum PFOA concentrations were not associated with T-Cho in the workers (2007). On the other hand, a positive association was seen in the analysis between serum PFOA concentration and TG levels, but they concluded that this association might have been due, at least partially, to residual confounding as a consequence of the dispro-portionate number of employees in the two factories with higher PFOA concentrations than in a remaining factory (Olsen and Zobel, 2007).
Here, we focused on an interaction between PFOA and PPARα in relation to the lipid metabolism. However, Rosen et al. (2008b) demonstrated that PFOA could also activate constitutive androstane receptor, which also has an important role in the lipid metabolism (Maglich et al., 2009). Further study may be required to determine whether or not the dosages used in the current study activate CAR.
In conclusion, the 0.3 mg/kg dose of APFO significantly activated the function of PPARα in wild-type mice, but not in humanized PPARα mice. In addition, APFO increased the hepatic TG and T-Cho in mPPARα mice, but not in hPPARα mice. Since human PPARα expression in hPPARα mice was equal to or even higher than that of mice, the function of human PPARα was significantly lower than that of mice. Thus, PFOA levels such as 1.66–2.77 μg/ml in serum may not influence serum TG or T-Cho levels in human.
Acknowledgements
We wish to thank Mr. Nakagawa, Department of Occupational and Environmental Health, Nagoya University Graduate School of Medicine for his valuable assistance. This study was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (B. 14370121 and B. 17390169).
Footnotes
Conflict of interest None.
References
- Akiyama TE, Nicol CJ, Fievet C, Staels B, Ward JM, Auwerx J, Lee SS, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-alpha regulates lipid homeostasis, but is not associated with obesity: studies with congenic mouse lines. J. Biol. Chem. 2001;276:39088–39093. doi: 10.1074/jbc.M107073200. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Biegel LB, Hurtt ME, Frame SR, O’Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol. Sci. 2001;60:44–55. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C, Gonzalez FJ. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor alpha. Cancer Res. 2004;64:3849–3854. doi: 10.1158/0008-5472.CAN-04-0322. [DOI] [PubMed] [Google Scholar]
- Cook JC, Murray SM, Frame SR, Hurtt ME. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol. Appl. Pharmacol. 1992;113:209–217. doi: 10.1016/0041-008x(92)90116-a. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Furuta S, Miyazawa S, Hashimoto T. Purification and properties of rat liver acyl-CoA dehydrogenases and electron transfer flavoprotein. J. Biochem. 1981;90:1739–1750. doi: 10.1093/oxfordjournals.jbchem.a133651. [DOI] [PubMed] [Google Scholar]
- Furuta S, Miyazawa S, Osumi T, Hashimoto T, Ui N. Properties of mitochondria and peroxisomal enoyl-CoA hydratases from rat liver. J. Biochem. 1980;88:1059–1070. doi: 10.1093/oxfordjournals.jbchem.a133057. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Aiba K, Fukuda K, Tanaka M. The induction of peroxisome proliferation in rat liver by perfluorinated fatty acids, metabolically inert derivatives of fatty acids. J. Biochem. 1985;98:475–482. doi: 10.1093/oxfordjournals.jbchem.a135302. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yamanoshita O, Kurata Y, Kamijima M, Aoyama T, Nakajima T. Induction of peroxisome proliferator-activated receptor alpha (PPARalpha)-related enzymes by di(2-ethylhexyl) phthalate (DEHP) treatment in mice and rats, but not marmosets. Arch. Toxicol. 2007;81:219–226. doi: 10.1007/s00204-006-0141-x. [DOI] [PubMed] [Google Scholar]
- Izai K, Uchida Y, Orii T, Yamamoto S, Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J. Biol. Chem. 1992;267:1027–1033. [PubMed] [Google Scholar]
- Kennedy GL, Jr., Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooc-tanoate. Crit. Rev. Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J. Toxicol. Sci. 2003;28(2):49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- Kudo N, Mizuguchi H, Yamamoto A, Kawashima Y. Alterations by perfluo-rooctanoic acid of glycerolipid metabolism in rat liver. Chem. Biol. Interact. 1999;118:69–83. doi: 10.1016/s0009-2797(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless SE, Finlay C, Everds NE, Frame SR, Gillies PJ, O’Connor JC, Powley CR, Kennedy GL. Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO) Toxicology. 2006;220:203–217. doi: 10.1016/j.tox.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Lobe DC, Moore JT. The nuclear receptor CAR (NR1I3) regulates serum triglyceride levels under conditions of metabolic stress. J. Lipid Res. 2009;50(3):439–445. doi: 10.1194/jlr.M800226-JLR200. [DOI] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J. Biol. Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kamijo Y, Usuda N, Liang Y, Fukushima Y, Kametani K, Gonzalez FJ, Aoyama T. Sex-dependent regulation of hepatic peroxisome proliferation in mice by trichloroethylene via peroxisome proliferator-activated receptor alpha (PPARalpha) Carcinogenesis. 2000;21:677–682. doi: 10.1093/carcin/21.4.677. [DOI] [PubMed] [Google Scholar]
- Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, Aoyama T. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J. Hepatol. 2009;50(6):1236–1246. doi: 10.1016/j.jhep.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, Mandel JH. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 2003a;45:260–270. doi: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL, Mandel JH. U.S. Environmental Protection Agency; Washington, DC: Assessment of lipid, hepatic and thyroid function in relation to an occupational biologic limit value for perfluorooctanoate. 2003b 3M Company, St. Paul. U.S. EPA docket AR-226-1351.
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int. Arch. Occup. Environ. Health. 2007;81:231–246. doi: 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- Osumi T, Hashimoto T. Purification and properties of mitochondrial and peroxisomal 3-hydroxyacyl-CoA dehydrogenase from rat liver. Arch. Biochem. Biophys. 1980;203:372–383. doi: 10.1016/0003-9861(80)90189-7. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol. Pharmacol. 1998;53:14–22. [PubMed] [Google Scholar]
- Pastoor TP, Lee KP, Perri MA, Gillies PJ. Biochemical and morphological studies of ammonium perfluorooctanoate-induced hepatomegaly and peroxisome proliferation. Exp. Mol. Pathol. 1987;47:98–109. doi: 10.1016/0014-4800(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Peters JM, Hennuyer N, Staels B, Fruchart JC, Fievet C, Gonzalez FJ, Auwerx J. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor alpha-deficient mice. J. Biol. Chem. 1997;272:27307–27312. doi: 10.1074/jbc.272.43.27307. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Ramdhan DH, Kamijima M, Yamada N, Ito Y, Yanagiba Y, Nakamura D, Okamura A, Ichihara G, Aoyama T, Gonzalez FJ, Nakajima T. Molecular mechanism of trichloroethylene-induced hepatotoxicity mediated by CYP2E1. Toxicol. Appl. Pharmacol. 2008;231:300–307. doi: 10.1016/j.taap.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Thibodeaux JR, Wood CR, Zehr RD, Schmid JE, Lau C. Gene expression profiling in the lung and liver of PFOA-exposed mouse fetuses. Toxicology. 2007;239:15–33. doi: 10.1016/j.tox.2007.06.095. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol. Sci. 2008a;103:46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Abbott BD, Wolf DC, Corton JC, Wood CR, Schmid JE, Das KP, Zehr RD, Blair ET, Lau C. Gene profiling in the livers of wild-type and PPARalpha-null mice exposed to perfluorooctanoic acid. Toxicol. Pathol. 2008b;36:592–607. doi: 10.1177/0192623308318208. [DOI] [PubMed] [Google Scholar]
- Soni MG, Mangipudy RS, Mumtaz MM, Mehendale HM. Tissue repair response as a function of dose during trichloroethylene hepatotoxicity. Toxicol. Sci. 1998;42:158–165. doi: 10.1006/toxs.1998.2427. [DOI] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 2007;95:108–117. doi: 10.1093/toxsci/kfl135. [DOI] [PubMed] [Google Scholar]
- Tugwood JD, Aldridge TC, Lambe KG, Macdonald N, Woodyatt NJ. Peroxisome proliferator-activated receptors: structures and function. Ann. N.Y. Acad. Sci. 1996;804:252–265. doi: 10.1111/j.1749-6632.1996.tb18620.x. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics, Risk Assessment Division; Revised Draft Hazard Assessment of Perfluorooctanoic Acid and Its Salts. 2002 November 4; 2002.
- U.S. EPA (U.S. Environmental Protection Agency) Basic Information on PFOA. 2006 http://www.epa.gov/opptintr/pfoa/pubs/pfoainfo.htm.
- Wolf C, Takacs M, Schmid J, Lau C, Abbott B. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol. Sci. 2008a;106(1):162–171. doi: 10.1093/toxsci/kfn166. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Moore T, Abbott BD, Rosen MB, Das KP, Zehr RD, Lindstrom AB, Strynar MJ, Lau C. Comparative hepatic effects of perfluorooctanoic acid and WY 14, 643 in PPAR-alpha knockout and wild-type mice. Toxicol. Pathol. 2008b;36:632–639. doi: 10.1177/0192623308318216. [DOI] [PubMed] [Google Scholar]
- World Wildlife Fund Stockholm Convention: “New POPs” Screening Additional POPs Candidates. 2005 http://assets.panda.org/downloads/newpopsfinal.pdf.
- Xie Y, Yang Q, Nelson BD, DePierre JW. The relationship between liver peroxisome proliferation and adipose tissue atrophy induced by peroxisome proliferator exposure and withdrawal in mice. Biochem. Pharmacol. 2003;66:749–756. doi: 10.1016/s0006-2952(03)00386-1. [DOI] [PubMed] [Google Scholar]