INTRODUCTION
Pulmonary tuberculosis (PTB) is a leading cause of death among HIV-infected individuals in sub-Saharan Africa and throughout the world [1]. PTB is of particular concern in South Africa, where an estimated 44% of new adult PTB cases arise among HIV-positive individuals [2]. Prior to the introduction of highly active antiretroviral therapy (HAART), PTB in HIV-positive individuals was identified as a leading cause of death even among those receiving PTB treatment [3-5]. Access to HAART reduces both mortality [6-9] and incidence of PTB [9, 10].
Whether there are differences in prognosis between patients with PTB initiating HAART and patients free of PTB when initiating HAART remains unresolved. Several studies have identified PTB as a leading cause of death among people receiving HAART [11, 12]. A study in Uganda found that among individuals receiving HAART, those who had prevalent or incident PTB died at 4.7 times the rate of those without PTB, although the authors acknowledge that the relationship was likely confounded by body mass index (BMI) and CD4 cell count [13]. In contrast, a large study in Zambia reported no difference in mortality rates by PTB status [14], but these results may have been affected by the substantial numbers of patients lost to follow-up (LTFU).
Controversy also remains over the effect of time between initiation of PTB treatment and initiation of HAART on the risk of death. The South African National Comprehensive Care, Management and Treatment Guidelines for HIV and AIDS recommend waiting two weeks to two months to initiate HAART [15, 16], because of concerns of drug-drug interactions, pill burden, adherence, and immune reconstitution inflammatory syndrome (IRIS) [17]. The WHO suggests separately that while it may be safer to defer the initiation of HAART, concurrent initiation of HAART and PTB treatment may be necessary in patients at high risk of death [1].
In this study of the Themba Lethu Clinical Cohort, a large (>7500 patients) public treatment site in Johannesburg, South Africa, we used robust methods (inverse probability of exposure and censoring weighted marginal structural models to control for confounding and LTFU) to comprehensively assess the impact of treatment for PTB at the time of HAART initiation on subsequent all-cause mortality, both overall and by length of delay between initiation of PTB treatment and initiation of HAART. In addition, we assessed the impact of the combined presence of PTB and other risk factors for mortality on survival during HAART.
METHODS
Study site, clinic procedures, data collection
The Themba Lethu Clinical Cohort is a prospective clinical cohort of adults initiating HAART at the Helen Joseph Hospital in Johannesburg, South Africa. The program is funded by the South African Government, Gauteng Province, PEPFAR, and USAID. Themba Lethu has initiated over 10,000 patients on HAART since April 2004 and is the largest single clinic providing HAART in South Africa, and one of the largest antiretroviral clinics in the world. HAART-eligible patients attend educational and adherence sessions, and are assessed by a physician prior to initiating treatment. The vast majority of patients in South Africa begin a HAART regimen comprising stavudine, lamivudine, and either efavirenz or nevirapine.
After HAART initiation, patients are scheduled for monthly pharmacy visits and clinical visits at month 4 and every 6 months thereafter and whenever needed clinically. Clinical and laboratory (including CD4 count, viral load and hemoglobin) data are collected at all scheduled visits, except for viral load which is not collected at baseline. All data were stored in the TherapyEdge™ (TherapyEdge Inc., USA) database, and analyzed in SAS v9.1.3 (SAS Institute, NC, USA).
South African patients with PTB typically receive two months of isoniazid, rifampicin, pyramizade, and ethambutol followed by four months of isoniazid and rifampicin [15] at primary health care clinics (outside of Themba Lethu). Data on PTB treatment adherence was therefore not available for the majority of patients. Date of PTB treatment initiation was obtained using TB patient clinic card and patient self-report to ensure accuracy.
Study eligibility and definitions
Patients became eligible for this study upon initiation of HAART at Themba Lethu Clinic between April 1 2004 and March 31 2007. The main exposure was prevalent, treated PTB (hereafter, PTB) at the time of HAART initiation; the main outcome was all-cause mortality. Individuals more than three months late for a scheduled clinic appointment and who could not be located after three phone calls and a visit to the last known address were classified as LTFU. These patients were censored midway between the last recorded and the next scheduled visit or at the last recorded date of receipt of therapy.
Statistical analyses
Descriptive analyses were performed using chi-square tests to compare categorical variables, t-tests to compare means, and Wilcoxon rank sum test to compare medians. We assessed the proportional hazards assumption for PTB in univariate and multivariate analysis through visual inspection of survival and log-log-survival curves, as well as Cox tests of continuous and categorical time interactions.
Analysis 1 examined the effect of being on treatment for PTB at time of initiation of HAART on all-cause mortality during follow-up. Analysis 2 examined the effect of time lag between initiation of PTB treatment and initiation of HAART on subsequent mortality, where time lag was categorized by approximate quartile of duration of PTB treatment prior to initiation of HAART: >120 days, 61-120 days, 31-60 days, and ≤30 days. We hypothesized that risk of death from TB would increase with shorter lead-time due drug-drug interactions, non-adherence due to pill burden, and IRIS.
In both analyses we controlled for confounding by baseline factors and LTFU using inverse probability of exposure and censoring weights [18-20]. Analyses were implemented as discrete time hazard models with one observation per person-month and time-updated covariates. Models were evaluated using log-binomial generalized estimating equations, controlling for within-individual correlation using an “independent” correlation matrix for robust standard errors in the presence of repeated observations. Log-binomial regression was preferred to logistic regression because the former directly estimates the hazard ratio (HR) rather than the hazard odds ratio [21].
In both analyses, stabilized weights were estimated separately for exposure and censoring using ordinary (Analysis 1) or polytomous logistic regression (Analysis 2). We considered the following factors as predictors for both the weight and censoring models: gender, ethnicity, employment status, age, history of antiretroviral therapy, history of PTB, baseline measures of pregnancy, peripheral neuropathy, hemoglobin (adjusted for sex, pregnancy status, and altitude), BMI, CD4 cell count, and WHO stage (IV or other), year of enrollment (measuring years starting at baseline), prophylaxis with co-trimoxazole, and whether treatment was initiated before or after October 2006 (when consultation fees of ranging between $2-$5 per consultation were eliminated by the Department of Health). In addition, the time-varying censoring model accounted for time-updated measures of hemoglobin, CD4 count, and BMI, as well as for month of follow-up with both a linear month and cubic spline terms [22] for month of follow up with knots at the approximate 5th, 25th, 50th, 75th and 95th percentiles of survival time [19, 23].
We did not control for HAART adherence, successful virologic suppression, or initial HAART regimen (nevirapine vs. efavirenz) because these factors either occur during follow-up or may be affected by the exposure and therefore would be part of the causal mechanism of the exposure on the outcome. Full descriptions of the components and validation of the stabilized weight models for both exposure and censoring are given in the Appendix.
Sensitivity and secondary analyses
We performed six sensitivity analyses. To limit the potential effects of individuals dying between initiation of PTB treatment and initiation of HAART (i.e., left-truncation), we restricted PTB to cases who initiated HAART within 30 days of start of PTB treatment (S1) and separately to cases who initiated PTB treatment 30 days before or after initiation of HAART (S2). To assess the effect of PTB on early mortality, we restricted follow-up to the first four months of HAART (S3). To determine the effect of the exposure among the exposed, we calculated the weighted standardized mortality ratio for PTB (S4) [24]. We used a traditional proportional hazards regression approach (with adjustment rather than reweighting) (S5). To ascertain the extent of bias due to LTFU, we examined the main effect estimate without censoring weights (S6).
Additionally, we examined effect measure modification of PTB by key baseline risk factors for mortality, including severe immunosuppression (CD4≤50 cells/mm3 or WHO stage IV disease), malnutrition (underweight, BMI<18.5 kg/m2), and anemia.
Ethics approval
This study was approved by the human subjects review boards of the University of the Witwatersrand and the University of North Carolina at Chapel Hill.
RESULTS
There were 7,512 individuals included in this study, contributing a total of 106,795 person-months to this analysis. Of these patients, 1,197 (15.9%) were being treated for PTB at the time of HAART initiation. Individuals who had PTB at baseline were more likely to receive nevirapine, to be male, unemployed, and underweight, and had more severe immunosuppression (lower CD4 counts, more WHO stage IV) than those without PTB at baseline (Table 1). The proportion of patients receiving co-trimoxazole prophylaxis was similar between groups. The risk of death during follow-up was higher in individuals receiving PTB treatment at time of HAART initiation than in individuals not receiving PTB treatment at HAART initiation (6.2% vs. 3.6%, chi-square p<0.0001). Similar proportions of LTFU were observed in the two populations (19.5% and 18.8% respectively, p=0.6150).
Table 1.
Characteristics of 7512 individuals initiating HAART in Themba Lethu Clinical Cohort from 1 April 2004 to 31 March 2007 by pulmonary tuberculosis (PTB) treatment status at baseline.
| Prevalent PTB (n = 1197) |
No PTB (n = 6315) |
p-value | |
|---|---|---|---|
| Female gender | 695 (58.1) | 4300 (68.1) | <0.0001 |
| Age (Median, IQR) | 35 (30-40) | 35 (31-42) | 0.0134 ‡ |
| African ethnicity | 1144 (95.6) | 6022 (95.4) | 0.7483 |
| Employment | <0.0001 | ||
| Employed, student, retired | 358 (29.9) | 2372 (37.6) | |
| Not employed or unknown | 760 (63.5) | 3460 (54.8) | |
| Missing | 79 (6.6) | 483 (7.7) | |
| BMI | <0.0001 | ||
| <18.5 | 393 (32.8) | 1041(16.5) | |
| 18.5-24.9 | 663 (55.4) | 3473 (55.0) | |
| 25.0-29.9 | 110 (9.2) | 1229 (19.5) | |
| ≥30 | 31 (2.6) | 572 (9.1) | |
| Hemoglobin | <0.0001 | ||
| Normal | 330 (27.6) | 3353 (53.1) | |
| Low | 864 (72.2) | 2881 (45.6) | |
| Missing | 3 (0.3) | 81 (1.3) | |
| CD4 count (cells/mm3) | |||
| Median (IQR) | 58 (22-116) | 94 (34-165) | <0.0001 ‡ |
| Mean (95% CI) | 78 (74-83) | 115 (112-118) | <0.0001 † |
| ≤50 | 501 (41.9) | 1865 (29.5) | <0.0001 |
| 51-100 | 271 (22.6) | 1152 (18.2) | |
| 101-200 | 284 (23.7) | 1981 (31.4) | |
| 201-350 | 44 (3.7) | 581 (9.2) | |
| >350 | 12 (1.0) | 183 (2.9) | |
| Missing | 85 (7.1) | 553 (8.8) | |
| WHO stage IV at baseline | 207 (17.3) | 548 (8.7) | <0.0001 |
| History of ART (any) | 11 (0.9) | 211 (3.3) | <0.0001 |
| Pregnant | 20 (1.7) | 552 (8.7) | <0.0001 |
| Peripheral neuropathy | 89 (7.4) | 231 (3.7) | <0.0001 |
| History ofPTB | 60 (5.0) | 796 (12.6) | <0.0001 |
| HAART regimen | |||
| Efavirenz-based | 1139 (95.2) | 5018 (79.5) | <0.0001 |
| Nevirapine-based | 36 (3.0) | 597 (9.5) | <0.0001 |
| Other | 22 (1.8) | 700 (11.1) | <0.0001 |
| Co-trimoxazole | 1067 (89.1) | 5615 (88.9) | 0.8205 |
Figures are expressed as number (% total), except where noted. P-values are 2-sided by chi-square test
t-test or
Wilcoxon rank sum test. After adjustment for altitude, lower limit of normal hemoglobin is 12.35 g/dl for men; 11.35 g/dl for non-pregnant women; and 10.35 g/dl for pregnant women.
Of the 1,197 individuals with PTB at baseline, 254 (21.2%) had been receiving PTB treatment for more than 120 days, 320 (26.7%) for 61-120 days, 289 (24.1%) for 31 to 60 days, and 334 (27.9%) for 30 days or less. Key differences in baseline characteristics by amount of lead-time are summarized in Table 2. Patients with shorter lead time had more advanced disease, including lower CD4 cell counts, hemoglobin, and BMI.
Table 2.
Selected characteristics of 1,197 individuals receiving treatment for pulmonary tuberculosis (PTB) at time of initiation of HAART by length of time between initiation of PTB treatmentand initiation of HAART.
| Characteristic at initiation of HAART |
Time between initiation ofPTB treatment and HAART | p-value | |||
|---|---|---|---|---|---|
| 0-30 days (n=334) |
31-60 days (n=289) |
61-120 days (n=320) |
> 120 days (n=254) |
||
| BMI < 18.5 | 108 (32.3) | 117 (40.5) | 103 (32.2) | 65 (25.6) | 0.0032 |
| Low hemoglobin | 259 (77.5) | 234 (81.0) | 232 (72.5) | 139 (54.7) | <0.0001 |
| CD4 count | |||||
| Median (IQR) | 43 (15-88) | 52 (21-104) | 82 (36-133) | 68 (27-138) | <0.0001† |
| Mean (95% CI) | 60 (53-67) | 69 (62-77) | 95 (86-105) | 92 (80-104) | <0.0001† |
| CD4<50 | 176 (52.7) | 134 (46.4) | 101 (31.6) | 90 (35.4) | <0.0001 |
| WHO stage IV | 63 (18.9) | 49 (17.0) | 60 (18.8) | 35 (13.8) | 0.3525 |
| Peripheral neuropathy | 20 (6.0) | 20 (6.9) | 30 (9.4) | 19 (7.5) | 0.4108 |
| History of PTB | 18 (5.4) | 18 (6.2) | 15 (4.7) | 9 (3,5) | 0.5281 |
Figures are n(% ) unless otherwise noted. P-values are 2-sided by chi-square testor
one-way ANOVA.
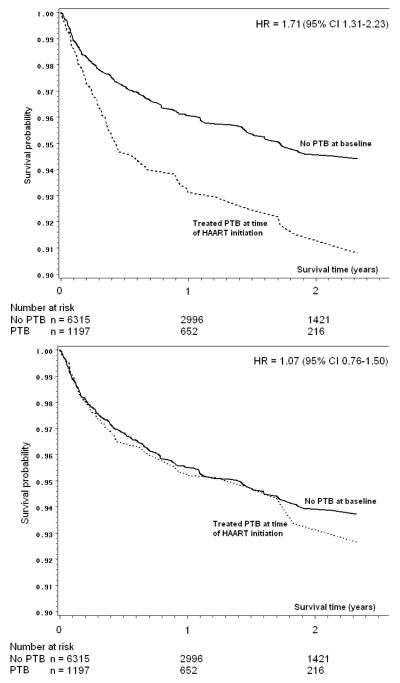
As there was no evidence of violation of the proportional hazards assumption (Figure 1a), we summarized the effect of PTB at time of HAART initiation on mortality as a single estimate. In the overall crude (unweighted and unadjusted) analysis (Table 3), individuals with PTB at baseline were more likely to die during follow-up than those without PTB (crude HR for death 1.71; 95% CI 1.31-2.23). The crude HRs by lead time ranged from 1.34 for those with 60-120 days of lead-time, to 2.02 for those with ≤30 days of lead-time, but all 95% CIs were relatively wide and there were no discernible trends by lead-time. Crude survival curves comparing individuals with PTB to those without PTB are shown in Figure 1a.
Figure 1.
Survival curves by treatment status for pulmonary tuberculosis (PTB) at baseline, unadjusted (1a), and reweighted for confounding only (1b), among 7512 individuals initiating HAART in Johannesburg, South Africa between 1 April
Number at risk changes due to (primarily) administrative censoring (end of follow-up), and losses to follow-up.
Table 3.
Estimates of hazard ratios from marginal structural models of the main analyses of the effect on all-cause mortality of current treatment for pulmonary tuberculosis (PTB) at time of HAART initiation, among 7512 individuals initiating HAART in Johannesburg, South Africa between 1 April 2004 and 31 March 2007.
| Main exposure | Crude (unweighted) estimates |
Adjusted for confounding and censoring |
|||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||
| Model 1 | AllPTB | 1.71 | 1.31-2.23 | 1.06 | 0.75-1.49 |
| Model 2 | > 120 days | 1.89 | 1.17-3.06 | 1.25 | 0.65-2.43 |
| 61-120 days | 1.34 | 0.80-2.23 | 0.89 | 0.48-1.63 | |
| 31-60 days | 1.62 | 0.97-2.72 | 1.08 | 0.59-1.98 | |
| ≤ 30 days | 2.02 | 1.31-3.11 | 1.28 | 0.78-2.10 | |
Model 1estimates the effect of prevalent, treated PTB on outcome of mortality. Model 2 estimates the effect of the same exposure by length delay between initiation of PTB treatment and initiation of HAART. Both models adjust for both confounding by baseline factors and LTFU using inverse probability of exposure and censoring weights; components and specification of weight models are described in the Appendix.
In models which adjusted for confounding and LTFU using inverse probability of exposure and censoring weights, the HR comparing risk of death in those with PTB to risk of death in those without PTB at baseline was 1.06 (95% CI 0.75-1.49). Semi-parametric survival curves adjusted for confounding (but not censoring) with inverse probability of exposure weights [25] are shown in Figure 1b. The HRs in Figure 1 differ slightly from those presented in Table 3 because the figure required the use of weighted Cox proportional hazards models [25]. The results were similar when stratified by lead-time category, with adjusted HRs ranging from 0.89 to 1.28 and confidence intervals including the null (Table 3).
Results of sensitivity and secondary analyses
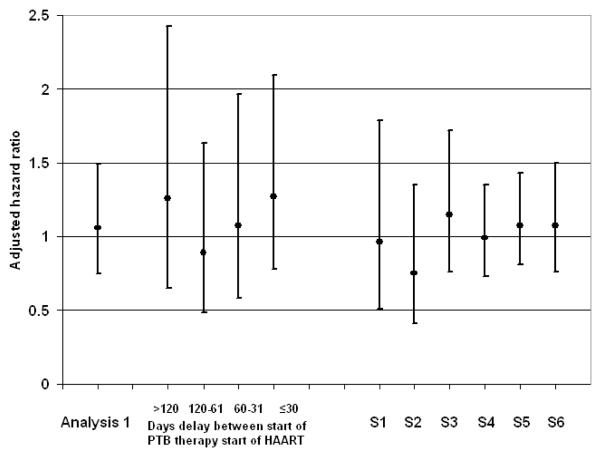
Analyses S1-S6 yielded estimates of effect similar to main analysis, and none yielded an adjusted estimate of effect different from the null (Figure 2). The standard Cox proportional hazards regression (S5) also yielded results similar to the main analysis.
Figure 2.
Estimates of adjusted hazard ratios from main analyses 1 and 2 and sensitivity analysis S1-S6.
Figure 2 footnote. S1: Pulmonary tuberculosis (PTB) treatment started within 30 days before HAART. S2: PTB treatment started within 30 days before or after HAART. S3: restricted follow-up to first four months of HAART. S4: standardized mortality ratio (effect of the exposure among the exposed). S5: Traditional Cox proportional hazards model. S6: Exposure weights only; no censoring weights.
When interaction terms were included in the model, we found several subpopulations in which PTB appeared to increase risk of death. First, this analysis showed that the HR for PTB on mortality among individuals who had CD4>50, BMI≥18.5, normal hemoglobin, and were not in WHO stage IV was 1.70 (95% CI 0.83-3.49), suggesting a raised hazard due to PTB among these otherwise relatively “healthy” individuals. Second, the estimates of the HR for PTB on mortality for individuals who had both PTB and a BMI<18.5, and individuals who had both PTB and CD4≤50 differed when interaction terms were considered. The HR for PTB and BMI<18.5 increased from 2.06 (95% 1.37-3.11) to 4.43 (95% CI 1.91-10.27) when interactions were added to the model and likewise, the HR for PTB and CD4≤50 increased from 2.91 (95% CI 1.95-4.37) to 4.01 (95% CI: 2.09-7.72). No such interactions were observed between PTB and low hemoglobin or WHO stage IV disease. These results suggest a possible synergistic effect of PTB with low BMI and low CD4.
DISCUSSION
In a large urban clinic providing HAART in South Africa, we have shown that treatment for PTB at the time of HAART initiation does increase the risk of death during follow-up and also that initiation of HAART soon after starting PTB treatment does not increase risk of death. Our approach to the problem of LTFU makes it highly unlikely that these results can be explained by differential, informative LTFU among PTB patients. Instead, the increased risk seen in the unadjusted models is due to the fact that PTB patients are more likely than others to have severe immunosuppression (low CD4 counts, WHO stage IV illness), low BMI, and anemia.
Our finding that shorter delay between initiation of PTB treatment and HAART does not increase mortality contradicted our hypothesis that shorter delay would be associated with increased mortality. This finding was reinforced by similar results when analysis was restricted to those who started PTB within 30 days of HAART initiation (S1) or to the first four months of HAART, the period in which the majority of deaths occur (S3). Taken together, these results suggest strongly that starting HAART soon after initiation of PTB treatment is unlikely to result in higher risk of death. While it is tempting to conclude that length of time between initiation of PTB treatment and HAART has no effect on risk of death, our findings must be interpreted cautiously in this respect. Patients in South Africa most often initiate PTB treatment outside of HAART clinics, and may die before starting HAART (in analytic terms, they would be left-truncated). The mortality attributable to PTB could therefore have been underestimated. Consequently, our results must be interpreted conditionally - given that an individual who initiated PTB treatment survived until initiating HAART, there was no effect of current PTB treatment on mortality.
However, the size of this potential left-truncation bias is likely to be small. Preliminary results from a recent randomized controlled trial of time between start of treatment for opportunistic infection other than PTB and initiation of HAART suggested that deferring HAART may increase risk of death or progression to AIDS [26]; as is suggested by universally low CD4 counts and high prevalence of WHO stage III and IV disease in this cohort, many patients in our cohort initiate HAART with an acute opportunistic infection. Therefore, unless left truncation due to PTB acted differently than left truncation due to other opportunistic infections, then, it is unlikely that left truncation due to PTB would substantially bias these results. Additionally, if left-truncation created substantial bias, we would expect individuals with shorter lead-time (and thus, less time to become left-truncated) to be at higher overall risk of death from PTB. We saw no trends in hazard of death with changing lead-time, nor did we observe a significant effect of PTB on mortality among the patients with the shortest lead-time (S1).
Key risk factors for death after HAART initiation include advanced disease with severe immunosuppression, poor nutritional status and anemia [12, 14, 27, 28], conditions which are more prevalent among PTB patients than non-PTB patients. The results of the secondary (hypothesis-generating) analysis suggested that patients with both PTB and one of these key risk factors (low BMI, low CD4 count), may be at increased risk of death in the setting of concurrent treatment for PTB and HAART. Future investigators should rigorously test these hypotheses.
Although a substantial number of people in this cohort became LTFU, rates of LTFU did not differ between those with or without PTB and did not appear to be informative with regard to the PTB-mortality relationship; the effect of PTB on mortality was very similar with or without control for LTFU (see Figure 1b and analysis S6). This should increase our confidence in other reports about the PTB-mortality relationship, e.g., Stringer et al. [14], which suffered from high rates of LTFU but did not model the censoring process explicitly.
This study had several limitations. First, uncontrolled confounding can never be fully excluded in observational cohort studies. Nevertheless, when compared to randomized trials, observational cohort studies may have better generalizability to the population of interest [29]; thus, we believe that these results may be more applicable in practice to people receiving HAART in urban South Africa and possibly throughout sub-Saharan Africa. Second, because TB diagnosis and treatment occurred at primary health care clinics, we lacked culture and smear microscopy results. This may have led to misclassification of the PTB status. However, even if smear microscopy were available, the problem of misclassification would remain due to its low sensitivity among HIV-positive individuals [30]. Drug-resistance status was also not available; the inclusion of drug-resistant PTB cases would likely raise the effect of PTB on death and bias results away from the null. We were also unable to control for adherence to PTB treatment.
In conclusion, this robust analysis of this large South African cohort strongly indicates that the effect of treatment for PTB on mortality following initiation of HAART may be of limited or no clinical importance and that initiation of HAART soon after start of PTB treatment does not increase risk of death. These results complement other emerging evidence, including the randomized trial results (mentioned above) arguing for a earlier initiation of HAART in patients with opportunistic infections other than TB [26], reports suggesting that PTB-associated IRIS is not as dangerous as previously thought [17, 31, 32], and evidence that virologic response can be achieved in the presence of co-administration of rifampicin with efavirenz [33, 34]. While only the results of an ongoing randomized controlled trial (ACTG 5221) can provide a definitive answer to the question of optimal time of initiation of HAART in PTB patients, the results of this study and other recent findings argue that the presence of PTB treatment should not delay initiation of HAART.
ACKNOWLEDGEMENTS
We would like to thank Michele Jonsson Funk, PhD (University of North Carolina Department of Epidemiology) and Jay Kaufman, PhD (University of North Carolina Department of Epidemiology) for their helpful comments on statistical methodology throughout the preparation of this manuscript. They received no compensation for this assistance.
We would like to thank all the staff at TLC for their unyielding efforts on behalf of the patients. We would like to thank the patients of Themba Lethu Clinic for the trust they have placed in receiving treatment at the clinic.
FUNDING The clinical activities of the Helen Joseph Hospital are supported by the National and Gauteng Department of Health and the United States President’s Emergency Plan for AIDS Relief (PEPFAR) in a grant by USAID to Right to Care and the Institution (674-A-00-08-00007-00).
The research activities of this publication are supported by the National Institute for Health in a grant from the NIAID, DAIDS division (CIPRA IU19 AI53217-01, PEPFAR protocol #3 U19 AI 053217-04SI-R2C01). DW also received support from an unrestricted educational training grant from the UNC-GSK Center for Excellence in Pharmacoepidemiology and Public Health, UNC School of Public Health.
The funding sources had no involvement in the design or conduct of the study, in the collection, management, analysis, or interpretation of the data, in the preparation, writing, review or approval of this manuscript, or in the decision to submit this manuscript for publication.
Footnotes
COMPETING INTERESTS
DW received support from an unrestricted educational training grant from the UNC-GSK Center for Excellence in Pharmacoepidemiology and Public Health, UNC School of Public Health. No other competing interests declared.
REFERENCES
- 1.WHO . TB/HIV: A Clinical Manual. Second edition. Geneva: 2004. [(Accessed March 31 2008)]. [Google Scholar]
- 2.WHO . Geneva: 2008. [(Accessed 18 April 2008)]. WHO Report 2008. Global tuberculosis control - surveillance, planning, financing. http://www.who.int/tb/publications/global_report/2008/ [Google Scholar]
- 3.Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5:225–232. [PubMed] [Google Scholar]
- 4.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. Aids. 2001;15:143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 5.Nunn P, Brindle R, Carpenter L, et al. Cohort study of human immunodeficiency virus infection in patients with tuberculosis in Nairobi, Kenya. Analysis of early (6-month) mortality. Am Rev Respir Dis. 1992;146:849–854. doi: 10.1164/ajrccm/146.4.849. [DOI] [PubMed] [Google Scholar]
- 6.Dean GL, Edwards SG, Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. Aids. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 7.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–1676. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 8.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–46. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 9.Meya DB, McAdam KP. The TB pandemic: an old problem seeking new solutions. J Intern Med. 2007;261:309–329. doi: 10.1111/j.1365-2796.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 10.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 11.Etard JF, Ndiaye I, Thierry-Mieg M, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. Aids. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 12.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. Aids. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 13.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. Aids. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 14.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 15.Republic of South Africa Department of Health . HIV and AIDS Policy Guideline. 2004. National Antiretroviral Treatment Guidelines. [Google Scholar]
- 16.WHO . Tuberculosis Care with TB-HIV Co-management: Integrated Management of Adolescent and Adult Illness (IMAI) Geneva: 2007. [(Accessed May 23 2008)]. [Google Scholar]
- 17.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl 1):S63–75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Hernán MA, Anastos K, Jamieson BD, Robins JM. Determining the effect of highly active antiretroviral therapy on changes in human immunodeficiency virus type 1 RNA viral load using a marginal structural left-censored mean model. Am J Epidemiol. 2007;166:219–227. doi: 10.1093/aje/kwm047. [DOI] [PubMed] [Google Scholar]
- 19.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Muthén B, Masyn K. Discrete-Time Survival Mixture Analysis. Journal of Educational and Behavioral Statistics. 2005;30:27–58. [Google Scholar]
- 22.Harrell FE. SAS Macros for Assisting with Survival and Risk Analysis, and Some SAS Procedures Useful for Multivariable Modeling: RCSPLINE. Department of Biostatistics, Vanderbilt University; 2004. RCSPLINE Macro. http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/SasMacros. [Google Scholar]
- 23.Fairall LR, Bachmann MO, Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 24.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14:680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 25.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Zolopa A, Andersen J, Komarow L, et al. Immediate vs Deferred ART in the Setting of Acute AIDS-related Opportunistic Infection: Final Results of a Randomized Strategy Trial, ACTG A5164. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. Paper 142. [Google Scholar]
- 27.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 28.Wools-Kaloustian K, Kimaiyo S, Diero L, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. Aids. 2006;20:41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi M, Ameli N, Bacchetti P, et al. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. Aids. 2005;19:1885–1896. doi: 10.1097/01.aids.0000189866.67182.f7. [DOI] [PubMed] [Google Scholar]
- 30.Tsiouris SJ, Coetzee D, Toro PL, Austin J, Stein Z, El-Sadr W. Sensitivity analysis and potential uses of a novel gamma interferon release assay for diagnosis of tuberculosis. J Clin Microbiol. 2006;44:2844–2850. doi: 10.1128/JCM.02411-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. Aids. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 33.Manosuthi W, Mankatitham W, Lueangniyomkul A, Chimsuntorn S, Sungkanuparph S. Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicin. HIV Med. 2008;9:294–299. doi: 10.1111/j.1468-1293.2008.00563.x. [DOI] [PubMed] [Google Scholar]
- 34.Manosuthi W, Sungkanuparph S, Thakkinstian A, et al. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. Aids. 2005;19:1481–1486. doi: 10.1097/01.aids.0000183630.27665.30. [DOI] [PubMed] [Google Scholar]