Abstract
Objective
To determine the prevalence of the female athlete triad (low energy availability, menstrual dysfunction and low bone mineral density) in high school varsity athletes in a variety of sports compared with sedentary students/controls.
Design
Prospective study.
Setting
Academic medical center in the Midwest.
Participants
Eighty varsity athletes and eighty sedentary students/controls volunteered for this study.
Intervention
Subjects completed questionnaires, had their blood drawn and underwent bone mineral density testing.
Main Outcome Measures
Each participant completed screening questionnaires assessing eating behavior, menstrual status and physical activity. Each subject completed a 3-day food diary. Serum hormonal, TSH and prolactin levels were determined. Bone mineral density (BMD) and body composition were measured by dual energy x-ray absorptiometry (DXA).
Results
Low energy availability was present in similar numbers of athletes (36%) and sedentary/control subjects (39%; p=0.74). Athletes suffered more menstrual abnormalities (54%) compared with sedentary students/controls (21%) (p=<0.001). DXA revealed that 16% of the athletes and 30% of the sedentary/controls had low BMD (p=0.03). Risk factors for reduced BMD include sedentary control student, low BMI and increased caffeine consumption.
Conclusions
A substantial number of high school athletes (78%) and a surprising number of sedentary students (65%) suffer from one or more components of the triad. Given the high prevalence of triad characteristics in both groups, education in the formative elementary school years has the potential to prevent several of the components in both groups, therefore, improving health and averting long-term complications.
Keywords: female athletes, energy deficit, reduced bone mineral density, amenorrhea
INTRODUCTION
Twelve years have passed since ACSM published the first female athlete triad (the “triad”) position stand. Since that time, a plethora of research has been performed nationally and internationally on different aspects of the triad. As a result, a revised ACSM triad position stand was recently published.1 One aspect included in this document is a comprehensive definition of each component of the triad from a spectrum of health to disease including low energy availability (with or without disordered eating), functional hypothalamic amenorrhea and osteoporosis at the extreme end of the spectrum. It also focuses on low energy availability as the common mechanistic factor underlying each of the components.1,2 Updated data on the prevalence of the triad from four current key studies are also included.3–6 Each of these studies used different methods, criteria, patient populations and terminology for defining disordered eating, athletic amenorrhea and low bone density/osteoporosis, making comparisons, generalization, and recommendations difficult. In addition, all the American studies lacked a control group therefore it is unknown if components of the triad are characteristics of American teenage girls.
The new ACSM triad position stand has replaced disordered eating with the concept of low energy availability. No studies to date have attempted to clinically measure energy availability in high school athletes or sedentary students. Another limitation is the lack of appropriate non-athletic controls.
The prevalence of menstrual dysfunction in athletes range from 6 – 79%.1,3–8 The large variation in estimates primarily results from methodological differences among various studies. Recently the American Society of Reproductive Medicine Practice Committee redefined the age of primary amenorrhea from 16 to 15 because menarche is occurring at an earlier age.9 No prevalence studies have evaluated thyroid function or assessed prolactin level to determine if concomitant medical conditions contribute to menstrual dysfunction. In addition, few studies have assessed reproductive hormones to determine if gynecologic pathology is a contributing factor. Finally, many of the prevalence studies have reported on endurance or aesthetic athletes at an elite level and few studies have reported on a range of different athletes with a control group of non-athletes 5,6.
The prevalence of reduced bone mineral density in athletes ranges from 10.7–21.8%.1,3–7 These differences are partly due to different criteria being used from different organizations. The World Health Organization (WHO) uses T-score values, which were designed to predict fracture risk in postmenopausal Caucasian females, not premenopausal or adolescent athletic females.10 Recently, the International Society for Clinical Densitometry (ISCD) attempted to address this issue by making recommendations for younger populations. They recommended using Z-score which is age-matched versus T-score which is compared to young healthy normals.11 They also suggest using the term “low bone density below expected range for age” in premenopausal women and “low bone density for chronological age” in children. The ISCD also recommended that osteoporosis be diagnosed in these populations only when low BMD (Z-score ≤ −2.0) is present with secondary clinical risk factors. These criteria do not attempt to predict traumatic or stress fracture risk in this population. The ISCD recommendations have been endorsed by the American Society for Bone and Mineral Research, the International Osteoporosis Foundation, and the American Association of Clinical Endocrinologists. However, most athletes in weight bearing sports have 12– 15% higher BMD than non-athletes.12–16 Therefore, in the new triad position stand, the term “low BMD” is defined as BMD Z-score of ≤ −1.01 in addition to a history of nutritional deficiencies, hypoestrogenism, and or stress fracture. The ACSM defines “osteoporosis” as secondary clinical risk factors for fracture with BMD Z-scores ≤ −2.0.
Sedentary teenage girls are known to have lower BMD compared with athletes.13–16 However, of equal importance is the unknown prevalence of low energy availability and menstrual irregularities in this group. The purpose of this study was to determine the prevalence of components of the triad in high school varsity athletes in a variety of sports using the energy availability concept compared with a group of sedentary control students.
METHODS
Subjects
This was a cross-sectional study performed on 80 varsity athletes from an all girls’ private high school in the Midwest and 80 sedentary control students. Subjects were recruited from flyers, classroom announcements and mailings. The school consists of 657 students, of which 300 participate in organized sports, 110 in varsity sports. Written informed consent was obtained from each subject and parents and all procedures for recruitment and conduct of the study were approved by the institutional review board.
Inclusion criteria included: students 13–18 years of age and participation on a varsity sports team. Exclusion criteria included known metabolic disease, pregnancy, long term oral steroid use, history of thyroid disorder, pituitary tumor or cardiovascular disease. Students who did not participate in high school, club or recreational sports and exercised less than 2 hours per week were also invited to participate in this study as the control group. Forty-seven subjects were recruited from the study school and 33 from two other private high schools located within a 2 mile radius and with similar demographics.
A self-administered questionnaire was used to assess age, medical history, sports participation and training regimen. Subjects were asked to record the duration and frequency of sport participation over the previous twelve months. Their knowledge of calcium requirements and the triad was ascertained. The principal investigator reviewed all questionnaires and performed individual interviews.
“Energy availability” was defined as the amount of dietary energy remaining for other bodily functions after exercise training 1. Dietary energy was calculated in each subject from a prospective 3-day food diary (two weekdays and one weekend day) 17 using a Nutrient Analysis program (Nutritionist Pro; First Databank, La Jolla, CA, 2nd Ed). All subjects met with the same registered dietitian and explanations were given on how to complete intake in regards to portion sizes and ounces of all drinks. Subjects were encouraged to weigh portion sizes. “Energy expenditure” was calculated from duration of sports participation, intensity of exercise, weight, age and sex from the Ainsworth compendium of physical activity 18–21. No subjects reported a weight change or history of dieting in the last 6 months.
Energy availability was expressed as kcal/kg lean body mass. Normal energy balance is considered > 45 kcal·kg−1 FFM·d−1 in healthy young women 22–24. Amenorrhea is associated with an energy availability ≤30 kcal/kg/LBM 23, however bone formation is suppressed at higher energy availabilities 25, therefore for the purposes for this study, low energy availability was defined as energy availability <45 kcal/kg/LBM. Calcium intake was recorded based upon the 3-day food record and compared to daily requirements for age 26. For the purposes of this study, the high end of recommendations were used (1500mg) 26. Eating habits and attitudes were also assessed by the Eating Attitude Test (EAT-26) which has been validated for disordered eating 27. A history of anorexia nervosa or bulimia nervosa was determined by questionnaire and interview based on DSM-IV criteria 28. The daily amount of milk (8 oz) and caffeinated products (12 oz) consumed was assessed via food records.
Menstrual Status
Subjects completed questionnaires to ascertain age of menarche, number of periods per year, number of days between periods, skipped periods, history of thyroid disease or pituitary microadenoma and birth control use. Menstrual status was defined and correlated with questionnaires during the interview process by the principal investigator. Regular cycling was defined as menstrual periods occurring every 28–30 days. Primary amenorrhea was defined as absence of menarche by age 15 9 and secondary amenorrhea as cessation of menses for ≥3 consecutive cycles after onset of menarche. Oligomenorrhea was defined by menstrual bleeding occurring at intervals longer than 35 days. To exclude endocrinological or gynecologic menstrual dysfunction, a prolactin, thyroid stimulating hormone and reproductive hormone levels were obtained. Subjects were asked if there was a possibility that they were pregnant and offered a pregnancy test.
Bone Mineral Density
Bone mineral density (g/cm2) of the lumbar spine (L1–L4), total left hip, left femoral neck and total body were measured by dual energy x-ray absorptiometry (DXA) (GE Lunar Prodigy densitometer, Version 2.15, Madison, WI) to assess bone mineral density and body composition. The daily coefficient of variation of the calibration phantom over a 6-month period was 0.29%. We used the International Society for Clinical Densitometry (ISCD) 11, the World Health Organization (WHO) 10 and the American College of Sports Medicine (ACSM) 1 classification system to define low bone mineral density based on Z-scores. All subjects also had a left wrist film to compare bone age with chronologic age.
Blood Work
Fasting blood samples were obtained the morning of testing. Thyroid stimulating hormone (TSH) was measured by the Abbott AxSYM System (Abbot Laboratories, Abbott Park, IL), using the AxSYM Ultrasensitive hTSH II Microparticle Enzyme Immunoassay (MEIA). Estradiol was measured by the Abbott AxSYM System, using the AxSYM MEIA. Prolactin was measured by the Abbott AxSYM System, using the AxSYM Prolactin MEIA. Follicle-stimulating hormone (FSH) was measured by the Abbott AxSYM System, using the AxSYM FSH MEIA. Luteinizing hormone (LH) is measured on an ADVIA Centau (Bayer Diagnostics now Siemens) using direct chemiluminometric technology.
Statistics
Student’s t-tests were used to compare continuous variables such as height, weight and BMD Z-scores; the Wilcoxon rank sum test was used to compare ordinal variables such as the eat-26 score and glasses of milk per day. Categorical variables such as energy availability or BMD class were compared with Chi-square or if there were low expected frequencies, such as use of birth control, with Fisher’s Exact or the Fisher-Freedman-Halton test. The Two One-Sided Tests (TOST) procedure was used to test for equivalency (within 6 months) between bone age and chronological age for both the athlete and control groups 29. We also investigated potential factors associated with low bone mineral density. The multivariable ordinal logistic odds ratios in Table 1 gives the independent contribution of the factors that are related to low BMD. For example, the risk for low bone mineral density is 3.04 fold higher for a sedentary control compared to a high school athlete. This odds ratio applies to (1) the risk of a low BMD value compared to a moderately low or normal BMD and (2) the risk of a low or moderately low BMD value compared to a normal BMD. Similarly, an increased number of caffeinated beverages is a risk factor (1.06 times per drink) for low BMD. On the other hand, the odds ratio for BMI was less than 1 (0.72 per kg/m2); this means that low BMI is a risk factor for low BMD. The additional risk of low BMD for a reduced BMI is the reciprocal of the odds ratio, 1.39 fold higher risk for each kg/m2 less BMI. Level of statistical significance was p <0.05. All analyses were performed using SAS v9.1.3 (SAS, Cary, NC). Each table indicates what test was performed.
Table 1.
Factors Associated with Low Bone Mineral Density (BMD) Based on an Ordinal Logistic Regression Model
| Variable | Odds Ratio | P-value | (95% CI)† |
|---|---|---|---|
| Age (year) | 0.88† | NSb | (0.60, 1.28) |
| BMI (kg/m2) | 0.72 | < 0.0001 | (0.60, 0.86) |
| Sedentary control vs. Athlete | 3.04 | 0.02 | (1.17, 7.89) |
| Menstrual Statusb | |||
| Oligomenorrheic | 1.80 | NS | (0.52, 6.29) |
| Primary/secondary amenorrhea | 1.15 | NS | (0.39, 3.38) |
| Eumenorrheicsb | 1.0 | ||
| Calcium Intake‡ | 1.03 | NS | (0.93,1.13) |
| Energy Availabilityb | |||
| > 30 & ≤ 45 kcal/kg/LBM | 0.79 | NS | (0.27, 2.33) |
| ≤ 30 kcal/kg/LBM | 1.71 | NS | (0.26, 11.29) |
| > 45 kcal/kg/LBMb | 1.0 | ||
| # of caffeinated beverages/day | 1.06 | 0.05 | (1.00, 1.12) |
Odds ratio: >1 is a risk factor and <1 is protective against having worse BMD
Represents (actual calcium intake − requirement for age)/100 mg
NS: not significant
Reference group for factor
RESULTS
The physical characteristics of the athletes and sedentary controls are displayed in Table 2. All athletes self reported their ethnicity as Caucasian; whereas two of the sedentary students/controls were African American and two were Hispanic. Representative sports teams included: track (N=24), cross-country (N=25), volleyball (N=13), basketball (N=14), soccer (N=24), tennis (N=7), swimming (N=17), golf (N=3) and softball (N=6). Thirty-four varsity athletes played one sport, thirty nine played 2 sports and seven played three sports. The athletes exercised a mean of 8.66 hours/week compared to 1.02 hours/week among the 13 control subjects that exercised at all. Only 14% (N=11) of the athletes and 11% (N=9) of the sedentary students/controls knew the calcium intake requirement for their age (1300–1500 mg/day). Thirty five percent (N=28) of athletes and 19% (N=15) of controls could identify three components of the triad.
Table 2.
Physical Characteristics
| Characteristic | Athletes (N = 80) | Sedentary Controls (N = 80) | P Value |
|---|---|---|---|
| Age (yr) | 16.53 ± 0.95 | 16.46 ± 1.17 | 0.69 |
| Height (in) | 65.64 ± 2.27 | 64.57 ± 2.49 | 0.01 |
| Weight (lb) | 131.85 ± 16.80 | 137.63 ± 32.14 | 0.16 |
| Body fat (%) | 25.61 ± 5.54 | 32.51 ± 8.05 | <0.001 |
| Body mass index (kg/m2) | 21.60 ± 2.46 | 26.41 ± 27.30 | 0.01 |
| Lean body mass (kcal/kg/LBM) | 50.62 ± 13.74 | 49.22 ± 12.25 | 0.50 |
| Age at menarche, N(%) | (N=75) | (N=79) | 0.02† |
| ≤13 yrs | 52 (69%) | 67 (85%) | |
| 14–15 yrs | 22 (29%) | 10 (13%) | |
| ≥16 yrs | 1 (1%) | 2 (3%) | |
| Training (hours/week) | 8.66 ± 4.54 | 1.02 ± 0.64* | <0.001 |
Values are mean ± SD unless otherwise indicated
P-value from Fisher-Freeman-Halton test
Based on N=13 subjects in the student group that reported training at all on a weekly basis compared to all the student athletes
Energy Availability/Disordered Eating
Among athletes, 29 (36%) had low energy availability (≤45 kcal/kg/LBM) compared to 31 (39%) of the sedentary students/controls (p<0.05). Of the 29 athletes with low energy availability, 6% (N=5) were less than 30 kcal/kg/LBM. Among the sedentary students/controls, 4% (N=3) were less than 30 kcal/kg/LBM (Table 3). Both groups also had low EAT-26 scores and self-reported use of pathogenic weight control, suggesting that this cohort had low energy availability without disordered eating. Seventy four percent of athletes and 93% of sedentary students/controls were in calcium deficit (<1500 mg), with the sedentary students/controls having a larger average difference between actual and recommended calcium intake compared with the athletes (−581 ± 373 vs. −297 ± 517; p<0.001). The sedentary students/controls consumed more caffeinated beverages (12 oz) per day (1.1 compared to 0.5, p=<0.001), drank fewer glasses of milk (8 oz) per day (2.0 versus 2.4, p=0.04) and desired to lose more weight (11.8 lb versus 3.7 lb, p<0.05) than the athletes (Table 4).
Table 3.
Energy Availability
Table 4.
Disordered Eating
| Athletes (N = 80) | Sedentary controls (N = 80) | P Value | |
|---|---|---|---|
| Eat-26 score | 4.59 ± 3.79 | 5.46 ± 6.61 | 0.91‡ |
| Eat-26 score > 15 | 3 (4%) | 5 (6%) | 0.72† |
| Calcium deficit*, N (%) | 59 (74%) | 74 (93%) | 0.002 |
| Mean deficit (mg) | −297.13 ± 516.63 | −580.54 ± 372.77 | <0.001 |
| Glasses of milk/day (8 oz) | 2.21 ± 1.46 | 1.82 ± 1.24 | 0.07‡ |
| Caffeine intake (12 oz) (# beverages/day) | 0.49 ± 6.27 | 1.10 ± 8.65 | <0.001‡ |
| Desired # of lb. to lose | 3.69 ± 8.27 | 11.75 ± 17.85 | 0.002‡ |
| Use of pathogenic weight control, history of anorexia, or history of bulimia, N (%) | 4 (5%) | 6 (8%) | 0.51 |
Values are mean ± SD unless otherwise indicated
P-value from Fisher’s Exact test
P-value from Wilcoxon Rank-Sum Test
Based on 1500mg 1
Amenorrhea/Oligomenorrhea
Menstrual dysfunction was self reported in 43 (54%) of the athletes (Table 5). Five (6%) reported primary amenorrhea, 24 (30%) had secondary amenorrhea and 14 (15%) had oligomenorrhea. For the sedentary students/controls, 21% reported menstrual dysfunction. None had primary amenorrhea, 12 (15%) had secondary amenorrhea and 5 (6%) had oligomenorrhea. The majority of the oligomenorrheic subjects were 2 years post menarche. Menarche occurred at a later age in the athletic group (p=0.02). Two (3%) of the athletes admitted to current oral contraceptive (OC) use compared with 11 (14%) of the sedentary students/controls (p= 0.01). The majority of subjects reported using OC for dermatologic reasons. Both groups had normal TSH and prolactin. There were no differences in FSH (p=0.93), LH (p=0.67) or estradiol (p=0.19).
Table 5.
Menstrual Status
| Athletes (N=80) | Sedentary Controls (N=80) | P Value | |
|---|---|---|---|
| History/Current Secondary Amenorrhea | 24 (30%) | 12 (15%) | <0.001† |
| History/Current Primary Amenorrhea | 5 (6%) | 0 (0%) | |
| History/Current Oligomenorrhea | 14 (18%) | 5 (6%) | |
| Within 2 years of menarche, N (%) | 5 (36%) | 2 (40%) | |
| Number of periods/year | 9.19 ± 3.99 | 10.92 ± 2.74 | 0.01 |
| Using birth control pills | 2 (3%) | 11 (14%) | 0.01 |
Values are mean ± SD unless otherwise indicated
P-value from Fisher-Freeman-Halton test
Bone Mineral Density
Both ISCD (Z-score ≥ −2.0) and ACSM/WHO (Z-score ≥ −1.0) criteria are reported (Table 6). Eleven (13%) athletes had a BMD score between −1.0 and −1.9 either at the lumbar spine, hip or whole body and two (3%) had Z-score ≤ −2.0. Sixteen (20%) sedentary students/controls had a BMD score between −1.0 and −1.9 at the lumbar spine, hip or whole body and 8 (10%) had Z-score ≤ −2.0. Significantly more of the sedentary students/controls had low BMD for chronological age (p=0.04). On average, the bone age for both groups was within six months of their chronologic age (Table 7). Based on the multivariable ordinal generalized linear model (Table 1), whether an individual was an athlete or sedentary control, BMI, and consumption of caffeinated beverages (12 oz) were identified as factors significantly associated with BMD. Higher BMI was indicative of better BMD (Odds ratio=0.71 95% CI: 0.60, 0.84), while being a sedentary control (Odds ratio=2.54 95% CI: 1.09, 5.94) and consuming more caffeinated beverages per week (Odds ratio=1.06 95% CI: 1.01, 1.12) were both associated with worse BMD.
Table 6.
Bone Mineral Density (BMD)
| Athletes (N=80) | Sedentary Controls (N=80) | P Value | |
|---|---|---|---|
| Z-score > −1.0 | 67 (84%) | 56 (70%) | 0.02 |
| Z-score between −1.0 and −1.9a | 11 (13%) | 16 (20%) | |
| Z-score ≤ −2.0b | 2 (3%) | 8 (10%) | |
| History of stress fracture | 15 (19%) | 2 (3%) | 0.001 |
Categories based on Z-scores for lumbar spine, total hip, and total body Ordered Chi-square test for Z-score
At least one Z-score ≤ −1.0, but all > −2.0
At least one Z-score ≤−2.0
Table 7.
Bone Age VS. Chronologic Age
| Bone Age (BA) (N=80) | Chronological Age (CA) (N=80) | Difference μδ = BA − CA (90% CI) † | |
|---|---|---|---|
| Athletes (N=80) | 16.18 ± 1.16 | 16.53 ± 0.95 | −0.34 (−0.49, −0.19) |
| Sedentary Controls (N=80) | 16.43 ± 1.17 | 16.46 ± 1.17 | −0.02 (−0.14, 0.09) |
Tests H0: | μδ | > 6 months using the Two-One Sided Tests procedure. If the 90% CI for μδ falls between −0.5 and 0.5 (i.e. 6 months), then we fail to reject H0 at the 95% level.
Values are mean ± SD.
Prevalence of Triad Components
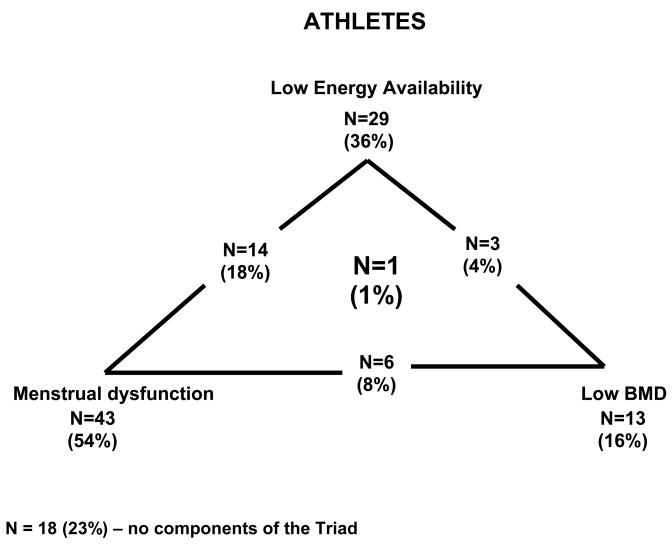
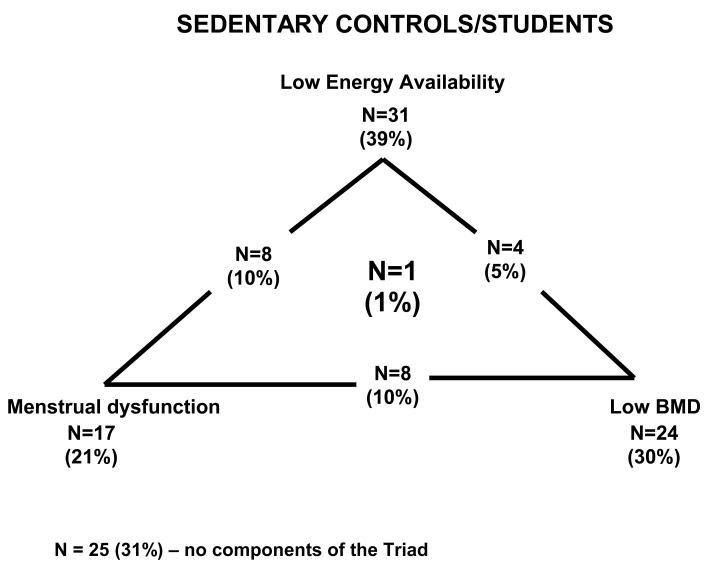
Of the 80 varsity athletes, 29 (36%) met the criteria for low energy availability (< 45 kcal·kg−1 FFM·d−1), 43 (54%) had amenorrhea/oligomenorrhea and 11 (13%) had low bone mineral density, Z-score ≤ −1.0 10, and 2 (3%) had Z-score ≤ −2.0 11. One subject had all 3 components of the triad (soccer athlete) (Figure 1). For the control/sedentary students, 31 (39%) had low energy availability, 17 (21%) had amenorrhea/oligomenorrhea and 16 (20%) had low bone mineral density Z-score ≤ −1.0 and 8 (10%) had Z-score ≤ −2.0. One subject had 3 components of the triad (Figure 2).
Figure 1.
Prevalence of Female Athlete Triad Components in Athletes
N=14 (18%) – number of subjects with Low Energy Availability and Menstrual Dysfunction
N=3 (4%) – number of subjects with Low Energy Availability and Low BMD
N=6 (8%) – number of subjects with Menstrual Dysfunction and Low BMD
N=1 (1%) – number of subjects with Low Energy Availability, Menstrual Dysfunction and Low BMD
Figure 2.
Prevalence of Female Athlete Triad Components in Sedentary Students/Controls
N=8 (10%) – number of subjects with Low Energy Availability and Menstrual Dysfunction
N=4 (5%) – number of subjects with Low Energy Availability and Low BMD
N=8 (10%) – number of subjects with Menstrual Dysfunction and Low BMD
N=1 (1%) – number of subjects with Low Energy Availability, Menstrual Dysfunction and Low BMD
DISCUSSION
This is the first study to report the prevalence of the triad in high school varsity athletes in a variety of sports using the energy availability concept, as well as providing a comparison with sedentary students/controls. We found a high prevalence of several components of the triad in both groups. Although the athletes had a higher prevalence of menstrual dysfunction, the sedentary students/controls had comparable levels of energy availability, and had a higher prevalence of low bone mineral density.
Our results of one subject (1.2%) having all three components of the triad are similar to Nichols et al 4 high school study with two subjects (1.2%) have all three criteria. Both patient populations were approximately the same age and included endurance athletes, but not aesthetic sports or weight category athletes. Our results are also similar to Tortsveit’s 5 study of elite athletes from 66 different sports/events. This study included endurance athletes in addition to aesthetic and weight category athletes. Eight (4.3%) athletes had all three components of the triad whereas five controls (3.4%) did. The controls in Torstveit’s study exercised 5.3 hours per week which is much higher than the controls in our study, who exercised an average of 1 hour per week and more similar to our high school athletes who exercised 6.7 hours per week. However, comparisons between all three studies are difficult as different criteria and patient populations were used.
Disordered Eating/Energy Availability
Normal energy balance is considered > 45 kcal·kg−1 FFM·d−1 in healthy young women22–24. This study revealed that 36% of the athletes and 39% of the sedentary students/controls had low estimated energy availability. None of the athletes and one (1%) sedentary student/control admitted to using pathogenic weight control measures, which is much lower than the 10% reported by Nichols et al 4. Both groups scored low on the EAT-26. This suggests that subjects were not consciously restricting calories. From these data it appears that both cohorts had evidence of low energy availability without disordered eating. In a similar study of high school athletes in a variety of sports, Nichols et al 4 reported elevated EDE-Q scores in 18.2% of high school athletes in a variety of sports. Other studies using the EDE-Q and EDI with different patient populations reported disordered eating to range from 20% – 25% 3,6. From these data it appears that low energy availability has a higher prevalence than disordered eating when assessed by the EDE-Q and EDI. Even more important, it appears that low energy availability is also common in sedentary students/controls. This may have been driven unconsciously by cultural and media pressures to be thin. Even though this group did not make a conscious effort to restrict calories, it is possible that this was inadvertent, as they had evidence of low energy availability without exercising. This raises significant public health concerns for the welfare of adolescent females.
Menstrual Dysfunction
Nichols et al 4 reported menstrual dysfunction in 23.5% of high school athletes. However, most of these athletes reported oligomenorrhea (17.1%). In our study, 54% of the athletes reported menstrual dysfunction, with the majority having amenorrhea (36%). Similar to Nichols et al 4, 18% had oligomenorrhea. Beals and Hill3 reported menstrual dysfunction in 26% of college athletes from a variety of sports and Cobb 6 reported 36% in distance runners. Surprising, the sedentary students/controls also had evidence of menstrual dysfunction (21%) with 15% reporting secondary amenorrhea and 6% reporting oligomenorrhea. The high prevalence of menstrual irregularities in the sedentary students/controls is consistent with the high rate (39%) of low energy availability. Finally, it must be noted that oligomenorrhea is common after menarche. However, 89% of women have normal cycles within 2 years of menarche 30. For subjects that reported oligomenorrhea, the majority were > 2 years from menarche, indicating a true menstrual abnormality, not a normal variation in onset of menstrual function. Our study was the first to attempt to assess for concomitant medical pathology that would affect menstrual function (TSH, prolactin) and reproductive hormones (LH, FSH and estradiol). We found no evidence of thyroid disease or elevated prolactin levels suggestive of a pituitary microadenoma. There were no abnormalities of reproductive hormones suggestive of premature ovarian failure or polycystic ovary disease.
Bone Mineral Density
According to ISCD criteria, 2 athletes had low BMD whereas 8 sedentary students/controls met these criteria. However, if ACSM criteria are used, 13 athletes and 24 sedentary students/controls had evidence of low BMD. The ISCD criteria are worrisome since most athletes that engage in weight bearing sports have a BMD that is 12–15% higher than sedentary students/controls 12–16. If they are not recognized as having low BMD until they are two standard deviations below normal, significant bone loss has occurred at a very critical time period for bone mass accrual. Furthermore, there is no agreement on standards for adjusting BMD for pubertal stage or skeletal maturity in growing individuals. We attempted to address this by comparing bone age with plain x-ray of the wrist and chronologic age, which showed that, on average, the bone ages for each group was within 6 months of their chronologic age. It is not surprising that the sedentary students/controls had lower BMD. This most likely reflects lack of weight bearing exercise, lower calcium intake and increased caffeine consumption. We feel the low BMD in the athletic group is due to the combination of a higher prevalence of menstrual dysfunction (54%) in addition to low energy availability (36%). Although menstrual dysfunction was higher in this group, their BMD was not as low as the sedentary controls, most likely due to the positive weight bearing effects of exercise. The majority of subjects from both groups was unaware of their calcium requirements and could not identify three components of the triad or its implications for current/future consequences. This provides an educational opportunity to improve health and reduce or prevent several or all components of the triad. Such an intervention could include early education of students, parents, teachers and coaches by an experienced physician or healthcare provider. A curriculum explaining each component and signs and symptoms of the triad would be very empowering to young girls. In addition, it is evident from the results of this study that high school girls need formal education about their calcium requirements. More advanced education could focus on learning how to estimate calories expended from exercise and understanding how to supplement with healthy food choices.
Limitations
Measuring energy availability can be a challenge for clinicians and currently no standard clinical approach exists. The most practical way to measure energy availability is to use a prospective 3-day food record, which is currently the gold standard 31 and estimates of energy expenditure from established guidelines 18,21. Both have limitations related to accuracy and honesty of the subjects, of special concern in the adolescent population. Use of a portable metabolic system including a calibrated accelerometer during simulated exercise activity for several days is more accurate, but also more cumbersome and labor-intensive. Continuous monitoring in an inpatient setting obviates problems with documentation bias, but is not practical for the population of subjects in this study.
Subclinical menstrual disorders such as anovulation and luteal phase dysfunction have been reported to be as high as 78% in recreational runners 32. To accurately detect these disorders, reproductive hormones must be measured serially throughout the menstrual cycle which is logistically difficult. Research by De Souza 32 has revealed that measurement of one menstrual cycle at one point in time underestimates the actual incidence of menstrual disturbances. This remains a limitation since such frequent measurements were not practical in the current study population, but its necessity is appreciated to diagnosis all menstrual disorders.
In this study, BMD was measured at one point in time, therefore trends over time cannot be reported, a limitation of the cross sectional design of this study. However, this study is unique in incorporating bone age from a plain x-ray to correlate bone age and chronological age. The BMD database for adolescents is small and future databases should be larger and include age, sex and ethnicity, but also level of physical activity (athlete vs. non-athlete). In addition, it would be valuable to have longitudinal data for BMD over 10–20 years.
CONCLUSION
The American College of Sports Medicine (ACSM) encourages all girls and women to participate in physical activities and sports because the benefits far outweigh the risks. However, the triad or any of its components pose significant health risks to physically active and inactive girls and women. Although several sedentary students and athletes had components of the triad, the majority had low energy availability without disordered eating. Inadvertent low energy availability is much easier to treat than disordered eating or a frank eating disorder. Therefore, a real potential exists to prevent several components of the triad by implementing an early education program with a focus on learning about low energy availability, menstrual abnormalities, low BMD and calcium recommendations in the elementary school years.
Acknowledgments
Grant Information: This study was partially funded by the Cardiovascular Center of the Medical College of Wisconsin and a grant from the General Clinical Research Center, #M01 RR00058.
The authors would like to thank Divine Savior Holy Angels students who participated in this study in addition to Ellen Bartel, school President and Peggy Braun, Athletic Director. The authors would also like to thank Philip Clifford, Ph.D., FACSM, for reviewing/editing this manuscript and David Harder, Ph.D. for supporting this research project. Anne Z. Hoch, DO had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest: There are no conflicts of interest.
Reference List
- 1.Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39:1867–1882. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 2.American Academic of Pediatrics. Committee on Adolescence. Identifying and treating eating disorders. Pediatrics. 2003;111:204–211. doi: 10.1542/peds.111.1.204. [DOI] [PubMed] [Google Scholar]
- 3.Beals KA, Hill AK. The prevalence of disordered eating, menstrual dysfunction, and low bone mineral density among US collegiate athletes. Int J Sport Nutr Exerc Metab. 2006;16:1–23. doi: 10.1123/ijsnem.16.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137–142. doi: 10.1001/archpedi.160.2.137. [DOI] [PubMed] [Google Scholar]
- 5.Torstveit MK, Sundgot-Borgen J. The female athlete triad exists in both elite athletes and controls. Med Sci Sports Exerc. 2005;37:1449–1459. doi: 10.1249/01.mss.0000177678.73041.38. [DOI] [PubMed] [Google Scholar]
- 6.Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity, and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711–719. doi: 10.1249/01.MSS.0000064935.68277.E7. [DOI] [PubMed] [Google Scholar]
- 7.Otis CL, Drinkwater B, Johnson M, et al. American College of Sports Medicine position stand. The Female Athlete Triad. Med Sci Sports Exerc. 1997;29:1669–1671. doi: 10.1097/00005768-199705000-00037. [DOI] [PubMed] [Google Scholar]
- 8.Loucks AB. Methodological problems in studying the female athlete triad. Med Sci Sports Exerc. 2006;38:1020. doi: 10.1249/01.mss.0000218146.91864.b5. [DOI] [PubMed] [Google Scholar]
- 9.Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertility and Sterility. 2004;82:266–272. doi: 10.1016/j.fertnstert.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Technical Report Series 843. Assessment of risk fracture and its application to screening for postmenopausal osteoporosis. [PubMed] [Google Scholar]
- 11.Writing Group for the ISCD Position Development Conference. Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom. 2004;7:17–26. doi: 10.1385/jcd:7:1:17. [DOI] [PubMed] [Google Scholar]
- 12.Lebrun CM. The female athlete triad: what’s a doctor to do? Curr Sports Med Rep. 2007;6:397–404. [PubMed] [Google Scholar]
- 13.Dook JE, James C, Henderson NK, et al. Exercise and bone mineral density in mature female athletes. Med Sci Sports Exerc. 1997;29:291–296. doi: 10.1097/00005768-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 14.To WW, Wong MW, Lam IY. Bone mineral density differences between adolescent dancers and non-exercising adolescent females. J Pediatr Adolesc Gynecol. 2005;18:337–342. doi: 10.1016/j.jpag.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Cassell C, Benedict M, Specker B. Bone mineral density in elite 7- to 9-yr-old female gymnasts and swimmers. Med Sci Sports Exerc. 1996;28:1243–1246. doi: 10.1097/00005768-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Nickols-Richardson SM, Modlesky CM, O’Connor PJ, et al. Premenarcheal gymnasts possess higher bone mineral density than controls. Med Sci Sports Exerc. 2000;32:63–69. doi: 10.1097/00005768-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 17.McKeown NM, Day NE, Welch AA, et al. Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr. 2001;74:188–196. doi: 10.1093/ajcn/74.2.188. [DOI] [PubMed] [Google Scholar]
- 18.Ridley K, Ainsworth BE, Olds TS. Development of a compendium of energy expenditures for youth. Int J Behav Nutr Phys Act. 2008;5:45. doi: 10.1186/1479-5868-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RK. Energy. In: Mahan KL, Escott-Stump S, editors. Krause’s Food, Nutrition and Diet Therapy. Philadelphia, PA: W.B. Saunders Co; 1999. pp. 19–30. [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 22.Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88:297–311. doi: 10.1210/jc.2002-020369. [DOI] [PubMed] [Google Scholar]
- 23.Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84:37–46. doi: 10.1152/jappl.1998.84.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Mulligan K, Butterfield GE. Discrepancies between energy intake and expenditure in physically active women. Br J Nutr. 1990;64:23–36. doi: 10.1079/bjn19900006. [DOI] [PubMed] [Google Scholar]
- 25.Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19:1231–1240. doi: 10.1359/JBMR.040410. [DOI] [PubMed] [Google Scholar]
- 26.NIH Consensus conference. Optimal calcium intake. NIH Consensus Development Panel on Optimal Calcium Intake. JAMA. 1994;272:1942–1948. [PubMed] [Google Scholar]
- 27.Garner DM, Olmsted MP, Bohr Y, et al. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Eating Disorders. In: First M, editor. Diagnostic & Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Publishing, Inc; 1994. pp. 539–550. [Google Scholar]
- 29.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. Longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. World Health Organization Task Force on Adolescent Reproductive Health. J Adolesc Health Care. 1986;7:236–244. [PubMed] [Google Scholar]
- 31.Biro G, Hulshof KF, Ovesen L, et al. Selection of methodology to assess food intake. Eur J Clin Nutr. 2002;56 (Suppl 2):S25–S32. doi: 10.1038/sj.ejcn.1601426. [DOI] [PubMed] [Google Scholar]
- 32.De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]