Abstract
Both individual cells and sheets of cells exert traction forces on the substrate and these forces have been investigated using a wide range of methods. Here we compare the mechanical properties of fibroblasts and epithelial cells using a novel surface geometry. Living cells are added to a thin film of polystyrene [PS] attached to a substrate of crosslinked poly(dimethyl siloxane) [PDMS] microwells. The contractile nature of the cells attached to the surface and the compliance of the PDMS surface geometry allows the PS thin film to buckle, forming arrays of convex microlenses. The resulting curvature of the microlenses allows us to determine the applied strain of growing cell sheets. We report that a monolayer of epithelial cells exerts more stress on the substrate than fibroblasts and attribute this to the collective behavior of the epithelium. By subsequently adding different chemical triggers to the system, the contractile nature of the cells changes, thus modifying the focal length of the microlenses. Together, these findings demonstrate the importance of studying the mechanics of cell sheets and also introduce a new design paradigm for advanced materials, offering great promise for a range of applications.
Keywords: epithelial cells, fibroblasts, traction forces, cell sheets, cell/cell junctions
INTRODUCTION
As a cell adheres to a surface, its contractile nature [Gabbiani et al., 1972; Thoumine and Ott, 1996] causes it to exert traction forces at its points of adhesion [Taylor, 1971; Harris et al., 1980; Thoumine and Ott, 1996; Discher et al., 2005]. This contraction is important for cell migration [Lauffenburger and Horwitz, 1996; Dembo and Wang, 1999], remodeling of extracellular matrix proteins [Burridge et al., 1988], and for the morphological changes resulting from cell spreading onto a surface [Burridge et al., 1988; Reinhart-King et al., 2005]. These forces are generated by the actomyosin network, and transmitted to the extracellular matrix at the sites of adhesion where integrins link the cytoskeleton to the extracellular matrix [Miyamoto et al., 1995]. It has been shown that individual cells can induce traction forces on a surface between 10 and 100 nN [Lee et al., 1994; Tan et al., 2003], but the mechanics of cell sheets have been relatively unexplored. To examine these mechanics, we fabricated PDMS microwells, covered them with a thin film of PS, and treated it to support cell growth. When cells are grown on this surface, they deform the PS, resulting in a well-defined array of microlenses. Using this system, we compare the behavior of epithelial cells and fibroblasts, providing new insight into the collective behavior of monolayers of epithelial cells.
MATERIALS AND METHODS
Fabrication of Surfaces
An SU-8 2100 negative photoresist (Microchem) was spun coat onto a silicon wafer at 1000 rpm. Spin time was varied for varied feature height, 325 or 275 μm. The resist was prebaked for 20 min at 90°C and exposed for 55 sec (OAI 500W DUV, intensity = 20 mJ/cm2) with a mask of circles of varying radii from 200 to 400 μm. The resist was then postbaked for 1 min at 90°C and developed in SU-8 developer (Microchem) to reveal hexagonal arrays of posts. Crosslinked poly(dimethyl siloxane) [PDMS] was prepared by mixing Dow Corning Sylgard 184 with catalyst and degassing for 15 min. The PDMS mixture was then poured onto the substrates prepared via photolithography and subsequently cured at 70°C for 3 h to produce a surface of holes upon separation. The PDMS substrates then placed hole-side down onto thin films of PS that were prepared by spincoating a PS/toluene solution at 4000 rpm for 30 sec on glass slides that had been UV-Ozone treated (Jelight UVO cleaner, 342) for 5 min. The thickness of the dried polystyrene films was measured with an interferometer (Filmetrics). The PS/PDMS pair was floated off in DI-water and placed onto glass bottom culture dishes (MatTek), PS face up. The substrates were then oxygen plasma treated (Harrick Plasma Cleaner, PDC-001) for 8 min to increase the hydrophilicity of the surface. When substrates were made for experiments using the fibroblasts, 2.5 mg/mL of rubrene (Aldrich) was added to the PS/toluene solution to make the surface fluorescent.
Cell Culture and Reagents
NIH/3T3 mouse fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% calf serum and 1% penicillin/streptomycin solution. LLC-Pk1 epithelial cells and LLC-Pk1 epithelial cells that constitutively express GFP-actin were cultured as described previously [Murthy and Wadsworth, 2005]. Cells were treated with inhibitors by adding either a 5 μM solution of latrunculin B (Biomol) or a 33 μM solution of nocodazole (Biomol), both in culture media.
Immunofluorescence Staining and Image Acquisition
Cells were fixed for 10 min in − 20°C methanol, rehydrated in PBS containing 0.1% Tween-20 and 0.02% sodium azide and stained. The primary antibody used was monoclonal anti-pan cadherin (Sigma clone CH-19; 1:500 dilution). Primary staining was followed by incubation in Cy3-labeled goat antimouse (Jackson Immunoresearch, West Grove, PA; 1:400 dilution) secondary antibody. Cells were mounted in Vectashield (Vector laboratories, Burlingame, CA) and sealed with nail polish. Cells were observed on a Nikon Eclipse TE 2000-S inverted microscope with a 100×, oil immersion objective lens. Images were acquired with a Qimaging camera, Micro-Manager 1.1 software through imageJ, and an electronic shutter, Lambda SC, (Sutter Instrument, Novato, CA). A standard filter cube was used for the Cy3 fluorescence.
Measurement of Microlens Properties
The height of the microlenses was measured with confocal microscopy (Zeiss LSM 510 Meta Confocal System). Excess media had been removed and a glass cover slip was placed over the system to facilitate imaging while inverted. Space between the coverslip and the top of the microlenses was fixed to ensure that the two surfaces were not touching. Culture media filled the gap.
RESULTS AND DISCUSSION
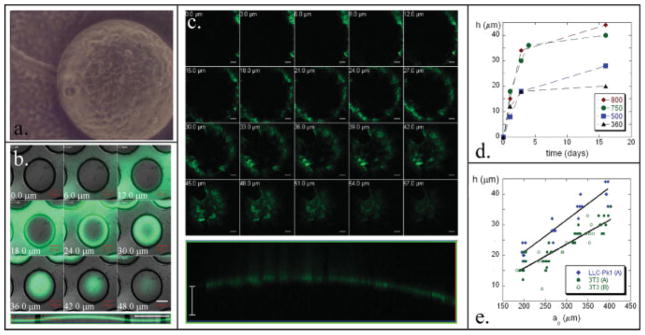
To examine how individual cells and cell sheets exert forces on the substrate, we generated a surface of PDMS microwells from hexagonal arrays of photolithographically fabricated posts. A thin film of PS is placed on the PDMS microwells and treated to make the surface favorable for cell growth (Fig. 1). When cells are then added, they adhere to the PS surface covering the micro-wells and begin to proliferate. With time, the number of cells increases on the surface and collectively the cells apply a contractile force that exceeds the critical force needed to buckle the originally planar PS thin film. This first order buckling gives rise to the microlens formation clearly seen in Fig. 2, where confocal images of microlenses formed from both fibroblasts and epithelial cells are shown (Figs. 2b and 2c, respectively). For the epithelial cells we image the cell sheet (Fig. 2c), which is fluorescent because the cells express GFP-actin; in the case of the fibroblasts, (Fig. 2b) the PS has been doped with rubrene to enable fluorescence. In both views, the convex nature of the microlens is evident.
Fig. 1.
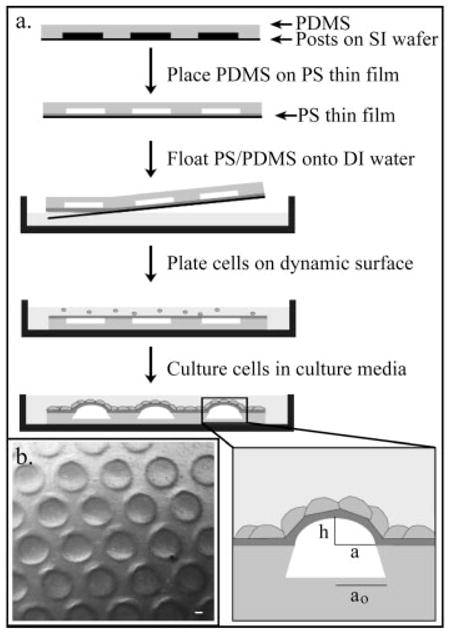
Design of living microlenses. (a) PDMS is molded onto arrays of posts with varying radii (100–800 μm), height (275–325 μm), and spacing. PDMS is placed on a thin film of PS that had been spun coated onto a UVO treated glass slide. The PDMS/PS sample is floated onto water, put in a culture dish, oxygen plasma treated and used as a culture surface. Cell proliferation leads to formation of final microlenses; inset defines variables used. (b) Low magnification DIC image of hexagonal array of microlenses formed by fibroblasts. Scale bar is 50 μm.
Fig. 2.
Features of living microlenses. (a) Low magnification DIC image of a microlens formed by fibroblasts; focus is on the top of the lens. Confocal images of individual fibroblasts (b) and epithelial cells (c) microlenses; both top-view z-stack and side profile. Graph of the height development (d) of four microlenses; initial radii denoted in the inset in μm. Graph of the final microlens height based on the initial well radius (e) for lenses formed by the epithelial cells and fibroblasts; the trend lines are fits using strain values of 0.0055 and 0.0031 respectively. Data points (A) and (B) have well depths of 325 μm and 275 μm respectively. Microlens diameter for (a) is 400 μm. Scale bar for (b) is 200 μm and for (c) is 50 μm.
The buckling of the thin PS layer is an observed preference for the assembly to accommodate the applied in-plane strains exerted by the cell sheet. This development of out-of-plane bending minimizes the in-plane strains, thus the height of the microlens can be estimated by balancing the applied strains with the deformed configuration of the PS/PDMS assembly. Assuming conservation of area in the synthetic assembly and that the initially planar area forms a perfectly spherical cap upon buckling, then the ratio of the microlens height, h, to the initial radius of the microwell, ao, is proportional to the applied strain, ε:
| (1) |
Therefore, higher strains applied to the substrate by the cell sheet lead to microlenses of greater aspect ratios (h/ao). The development of strain is demonstrated through the increase in microlens height as a function of time (Fig. 2d). The kinetics of microlens development is dictated by the growth kinetics related to confluent cell sheet formation. After confluency is achieved, the microlens height approaches a final, steady state value.
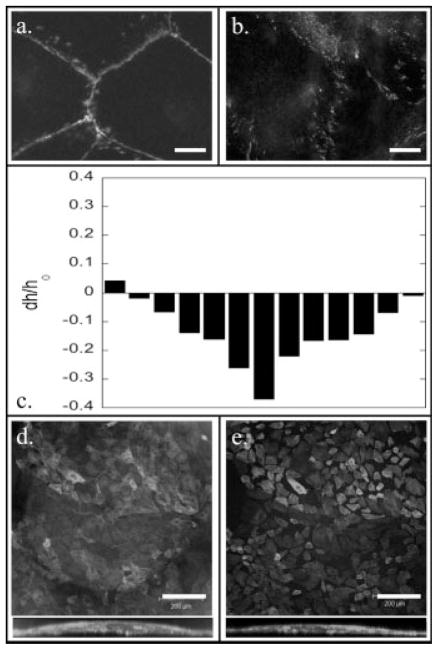
Plotting final h versus ao, and using equation (1) (Fig. 2e), we determine average final strain values of 0.0055 and 0.0031 for the epithelial cells and the fibroblasts respectively. In our experiments, the lateral stiffness of the assemblies is nearly constant, thus differences in applied contractile forces produce different strain values. The different strains developed by the respective cell types is contrary to results found in the literature where single fibroblasts are shown to produce higher traction forces on a substrate than epithelial cells [Dembo and Wang, 1999; Balaban et al., 2001; Tan et al., 2003; du Roure et al., 2005; Li et al., 2007]. du Roure et al. went on to show that the collective nature of subconfluent epithelium actually allows for a higher stress development at the sheet’s edge when compared to their single cell counterparts. Our observations support the hypothesis that as the number of each cell type increases on a given substrate there is a crossover from the single fibroblast exerting more traction forces to the collective nature of epithelial cells exerting higher stresses. One possibility is that the greater force generated by the epithelial cells results from the relatively tight binding of epithelial cells to their neighboring cells in the epithelial sheet, via zonula adherens junctions [Alberts et al., 2002]. These adherens junctions are linked to the intracellular actin network by various accessory proteins that bind to junctional components and the cytoskeleton [Alberts et al., 2002]. Immunofluorescence staining for cadherin shows intense and uniform staining between epithelial cells, but only patchy staining between fibroblasts (Fig. 3). Upon cellular contraction, the greater number of junction points in epithelial, as compared to fibroblasts (Figs. 3a and 3b), leads to the transmission of greater stress to the surface.
Fig. 3.
Effect of the loss of cell/cell junctions in an epithelial cell sheet. Cadherin staining for both the epithelial cells (a) and the fibroblasts (b). (c) Height change of individual microlenses after being in a calcium-free environment. The projection images both looking down and from the side before (d) and after (e) calcium depletion. Scale bar for (a) and (b) is 10 μm and for (d) and (e) is 200 μm.
To test the role of the cell/cell junctions in the increased contractility of the epithelial cells compared to fibroblasts, epithelial cell sheets were incubated with calcium-free phosphate buffered saline, to disrupt cell-cell adhesion via cadherins. Images of the lenses taken both before and after treatment show the disruption of the cell/cell contacts (Figs. 3d and 3e). The decrease in the amount of strain exerted by the cells with depleted junctions is seen in the decrease in microlens height (Fig. 3c).
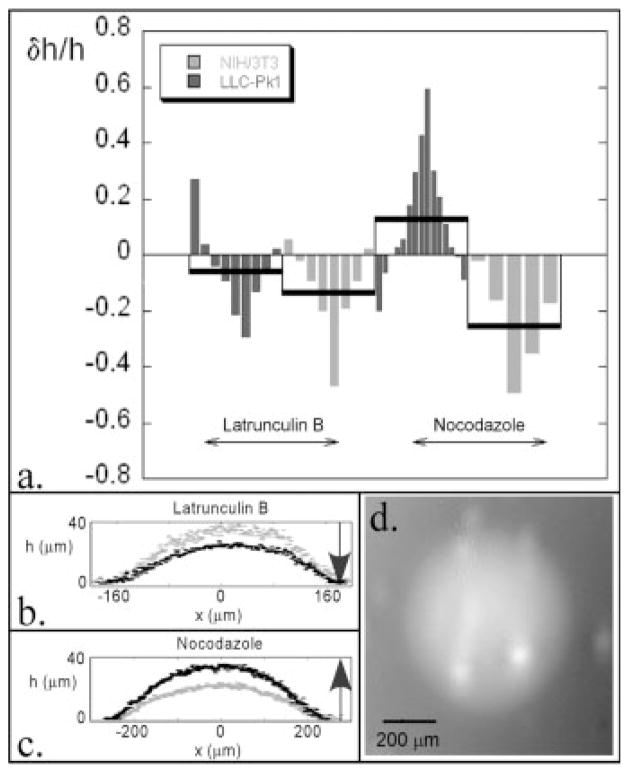
The contractile forces exerted by cells can also be altered by disrupting the cytoskeletal components within the cells. Altering these components with appropriate treatments demonstrates the “living” or dynamic nature of the microlens arrays. We add either latrunculin B or nocodazole to the microlens system to disrupt actin or microtubules, respectively, and observe their effects within minutes after the inhibitors were added. In situ delivery of these agents is currently being developed to quantify the exact response times. When latrunculin B is added to the system, it binds to actin monomers and thus shifts the monomer-polymer equilibrium resulting in the depolymerization of the actin filaments that are responsible, along with myosin, for cellular contractility. The cells then relax as their internal contractile networks are destroyed and the height of the microlenses decreases as shown in Figs. 4a and 4b.
Fig. 4.
Height change of microlenses after drug treatment (a), where the smaller bars are individual samples within a larger average bar. Height profiles of two microlenses of the epithelial cells before (gray) and after (black) treatment with latrunculin B (b) and nocodazole (c). Topographic display of a microlens of fibroblasts that has been treated with nocodazole. Local areas of high strain can be seen as smaller peaks on the microlens (d).
In a similar manner to the depolymerization of the actin filaments, the introduction of nocodazole to the system depolymerizes the microtubules by binding to free tubulin dimers, the subunits of microtubules, in the cells. The addition of nocodazole to the epithelial cells leads to an increased average height of the microlenses (Figs. 4a and 4c). This result is consistent with previous observations of cells grown in culture that demonstrate increased contractility upon the depolymerization of their microtubules [Danowski, 1989; Ingber, 1993; Brangwynne et al., 2006]. This observation led to a model in which microtubules oppose contractility generated by actomyosin, perhaps by acting as struts [Ingber, 1993; Brangwynne et al., 2006] or by weakening actomyosin contractility [Danowski, 1989]. The observation that microtubule disassembly leads to increased myosin phosphorylation further demonstrates the cross-talk between these two cytoskeletal systems [Kolodney and Elson, 1995].
The fibroblasts respond differently to the nocodazole treatment as they show an average decrease in microlens height (Fig. 4a). This microlens height decrease for the fibroblasts in the presence of nocodazole can be explained by the observation of local regions of high strain as seen in a representative contour plot of one of the fibroblast microlenses after treatment (Fig. 4d). These local areas of increased strain may result from the bifurcation of the thin PS shell upon increased compression. These local deformations could accommodate the increase in strain energy due to nocodazole treatment, which would have increased the global height of the microlens in their absence. We believe that these local deformations, which often arise in response to both types of treatments, result because fibroblasts do not form well-connected sheets of cells. This lack of interconnection allows for areas of higher strain to arise that are not transferred across the surface. It should also be noted that these drug induced deformation changes occurred on the time scale of minutes, not longer. The kinetics of these transformations will be investigated in future work.
As microlenses, these structures do transmit optical images at focal lengths defined by their curvature. The optical nature of the microlenses was demonstrated via a projection experiment illustrated in Fig. 5a. As seen in Figs. 5b and 5c, the printed image is projected onto the focal plane of the cell sheet formed microlenses, clearly demonstrating their ability to function as optical devices. The projection of an image array or related diffraction pattern can be advantageous for screening in cell biology and pharmaceutical development where cellular responses to external stimuli can be monitored by focal length changes for convenient tracking.
Fig. 5.
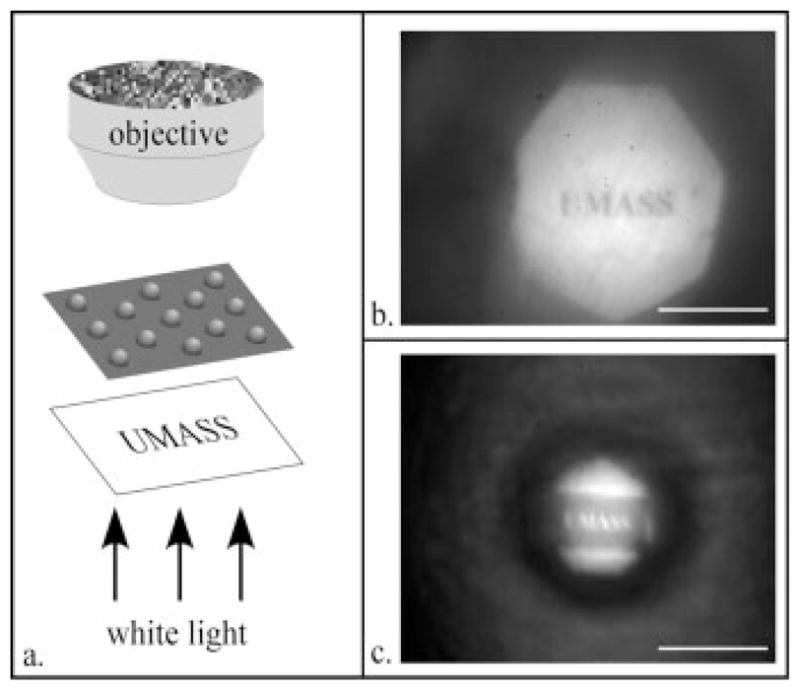
Projection images of the word ‘UMASS’ as produced by two microlenses. (a) Experimental setup with images projected through a lens produced by fibroblasts (b) and epithelial cells (c) Scale bar = 200 μm.
CONCLUSIONS
In summary, we have demonstrated the use of live cells to define a functional topography of a microlens array. The resulting curvature of each microlens is determined by the applied strain of growing cell sheets. Using this design, we experimentally demonstrate the contributions of calcium-dependent adhesion via intercellular junctions in cell sheets to the applied strain. These measurements offer new insight into the collective nature of cells as compared to their single cell counterparts. Specifically, we report that confluent epithelium exert greater stress on the substrate than sheets of fibroblasts and attribute this to the collective behavior of the epithelium. We have shown that the curvature of the living microlenses can be tuned by cell type or by introducing external stimuli, such as different cytoskeletal inhibitors, to which the cells respond over short time scales. In addition to offering a quantitative experimental technique for monitoring the strength of intercellular junctions, these self-formed, living microlenses define a new design paradigm for surface patterning and can be used for numerous advanced applications.
Acknowledgments
The authors would like to thank Professor Todd Emrick and Ms. Kim Wojeck for lab space and materials and information regarding the fibroblasts, Ms. Kausalya Murthy for help with the epithelial cells, Carey Fagerstrom for help with immunofluorescence staining, Professor Thomas McCarthy’s group for use of their oxygen-plasma cleaner, Ms. Stephanie Cole for help with the confocal microscopy images, and Mr. Edwin P. Chan for help with the projection images.
Contract grant sponsors: NSF-IGERT, NIH.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. New York: Garland Science; 2002. [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol. 2006;173(5):733–741. doi: 10.1083/jcb.200601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions - Transmembrane junctions between the extracellular-matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76(4):2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Siberzan P, Ladoux B. Force mapping in epithelial cell migration. Proc Natl Acad Sci USA. 2005;102(7):2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Statkov PR, Hirschel BJ, Majno G. Granulation tissue as a contractile organ - Study of structure and function. J Exp Med. 1972;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone-rubber substrata - New wrinkle in the study of cell locomotion. Science. 1980;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity - Defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Kolodney MS, Elson EL. Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light-chain. Proc Natl Acad Sci USA. 1995;92(22):10252–10256. doi: 10.1073/pnas.92.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994;127(6):1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Xie LK, Starr ZC, Yang ZC, Lin JS, Wang JHC. Development of micropost force sensor array with culture experiments for determination of cell traction forces. Cell Motil Cytoskeleton. 2007;64(7):509–518. doi: 10.1002/cm.20200. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267(5199):883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15(8):724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J. 2005;89(1):676–689. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100(4):1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JF. Changes in nuclear dimensions and orientation during contraction of a cultured fibroblast sheet. J Anat. 1971;108:509–517. [PMC free article] [PubMed] [Google Scholar]
- Thoumine O, Ott A. Influence of adhesion and cytoskeletal integrity on fibroblast traction. Cell Motil Cytoskeleton. 1996;35(3):269–280. doi: 10.1002/(SICI)1097-0169(1996)35:3<269::AID-CM8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]